Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Separation and purification of fluorescent carbon dots – an unmet challenge
Namratha
Ullal
,
Riya
Mehta
and
Dhanya
Sunil
 *
*
Department of Chemistry, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal-576104, Karnataka, India. E-mail: dhanyadss3@gmail.com
First published on 26th February 2024
Abstract
Literature reports demonstrate versatile optical applications of fluorescent carbon dots (CDs) in biological imaging, full-color solid-state lighting, optoelectronics, sensing, anticounterfeiting and so on. The fluorescence associated with CDs may originate significantly from byproducts generated during their synthesis, which need to be eliminated to achieve error-free results. The significance of purification, specifically for luminescence-based characterizations, is highly critical and imperative. Thus, there is a pressing demand to implement consistent and adequate purification strategies to reduce sample complexity and thereby realize reliable results that can provide a tactical steppingstone towards the advancement of CDs as next-generation optical materials. The article focuses on the mechanism of origin of fluorescence from CDs and further demonstrates the different purification approaches including dialysis, centrifugation, filtration, solvent extraction, chromatography, and electrophoresis that have been adopted by various researchers. Furthermore, the fundamental separation mechanism, as well as the advantages and limitations of each of these purification techniques are discussed. The article finally provides the critical challenges of these purification techniques that need to be overcome to obtain homogeneous CD fractions that demonstrate coherent and reliable optical features for suitable applications.
Introduction
Carbon dots (CDs) are zero-dimension quasi-spherical allotropes of carbon having a size below 10 nm that generally exhibit tunable photoluminescence characteristics. After their discovery as a byproduct during the synthesis of carbon nanotubes,1 CDs are extensively studied for diverse applications including biosensing,2–4 bioimaging,5,6 optoelectronic devices,7,8 solar cells9,10etc. The term CD is broad and is classified into different types, carbon quantum dots (CQDs),11 graphene quantum dots (GQDs),12 carbon nanodots (CNDs),13 and carbonized polymer dots (CPDs),14 based on their unique carbon core structure, surface functionalities, chemical, physical, and photophysical properties (Fig. 1).15 CQDs are crystalline carbon nanoparticles with a core comprising a combination of sp2 and sp3 carbons, whereas GQDs are single- or multi-layered graphene particles with <10 nm size having chemical groups and graphene lattices at their edges. GQDs are composed dominantly of sp2 carbon formed from π-conjugated graphene sheets, and display quantum confinement when these conjugated domains are separated by defects at the level of the graphene sheet. The edge effect is caused because of several chemical functional moieties present within the edge or interlayer defect.16 Furthermore, graphene-like 2D graphitic carbon nitride quantum dots (g-CNQDs) are frequently referred to as equivalents of GQDs. The quasi-spherical CNDs are mostly made up of an amorphous structural core, devoid of a crystal lattice, whereas CPDs are carbonaceous nanoparticles with a central carbonized core surrounded by polymer chains or functional groups produced by the aggregation or crosslinking of linear monomers or polymers.17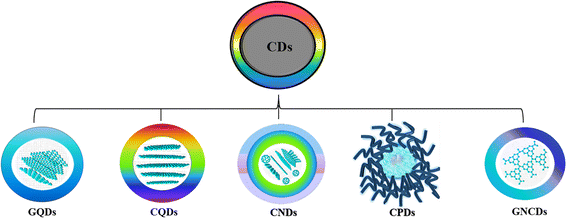 | ||
| Fig. 1 Classification of CDs.16 | ||
Although there are dedicated and systematic reviews that focus on the synthesis methods, properties, and applications of CDs,18–23 a distinct compilation of separation and purification techniques for CDs that influence their optical features is not available. Through this article, we aim to spotlight the mechanism of fluorescence origin in CDs, which is of prime importance as the optical properties are vital for their biological and optoelectronic applications. Furthermore, a sincere attempt has been made to specifically present the need for the purification of CDs and the various techniques adopted by several research groups for the separation of the as-synthesized carbonaceous nanoparticles. Besides, the article focuses not only on the fundamental separation mechanism, but also the advantages and drawbacks of each of the implemented purification strategies. Finally, the review extends to provide critical insights into the influence of purification techniques on the fluorescence of CDs and proposes future research that could be undertaken towards this direction.
Synthesis routes and fluorescence properties of CDs
The use of less expensive precursors and facile synthesis to realize CDs with different functionalization possibilities has garnered immense research attention.22–26 The synthesis techniques adopted for CDs are broadly divided into ‘top-down’ and ‘bottom-up’ depending on the carbon source and the method adopted. The fluorescence emission of the CD sample generated is one of the most fascinating features, and has been exploited in versatile applications. The surface functional groups of the CDs could be changed by utilizing different synthesis routes to achieve tunable photoluminescence (PL).The top-down method depends on cutting, stripping off or exfoliating bulk carbon into luminous nanoscale carbon materials of <10 nm size with high crystallinity, which can be further modified post-preparation. Top-down techniques include chemical oxidation, laser ablation, arc discharge, electrochemical approaches, and ultrasonic passivation. The chemical exfoliation process makes use of a variety of bulk carbon precursors, such as carbon fibers, graphene oxide, and graphite or multi-walled carbon nanotubes cleaved by oxidizing agents or strong acids. Making luminous CDs with different fluorescence emission and excitation-wavelength-dependent emission using this method is simple, rapid, and inexpensive because it does not require sophisticated equipment.27–29 Laser ablation is practical and simple in using an intense laser pulse, which causes carbon vapors to crystallize into a range of nanostructures. Laser passivation has a significant influence on the PL performance and generates CDs with stable/visible/dual/tunable emission.30 In arc-discharge methods the decomposition of the carbon precursor to form carbon vapors that assemble to fluorescent nanostructures occurs at high temperatures (>4000 K) when an electrical arc-discharge is formed between two metallic electrodes. Though CDs with high quality are obtained in this method, their yield is less. Electrochemical deposition occurs at the interface of an electrolyte solution containing the metal to be deposited and an electrically conductive metal substrate. In this case, strong emission arises from the quantum-sized graphite structure, and small, medium, and large CDs emit in the ultraviolet (UV), visible region, and near-infrared (NIR) regions.11 In the low-cost and simple ultrasonic-assisted process, strong hydrodynamic shear forces generated from the cavitation of small bubbles cut macroscopic carbon materials into nanoscale pieces. The as-prepared CDs generally display good PL features with superior photostability.31
In the “bottom-up” approach, single atoms and molecules assemble into larger nanostructures of the required size with distinct optical characteristics and high quantum yield (QY). The bottom-up approaches including hydrothermal, microwave, plasma, pyrolysis/carbonisation etc. are more economical, have adjustable reaction conditions and are less harmful to the environment. Hydrothermal/solvothermal is one of the extensively used approaches, wherein the reactants in water/solvent are subjected to a high temperature and pressure to obtain CDs of a homogeneous size distribution. Natural and biowaste materials are used as precursors to obtain CDs with multifunctional PL properties, good QY, low toxicity and high photostability.32 Microwave-assisted synthesis involves the use of intense energy to heat carbon precursors in a single step, to produce luminous CDs having good biocompatibility as well as high QY and photostability with excellent yield, but with poor regulation of the nanoparticle size.33,34 Molecular precursors such as carbohydrates can be burned or heated at high temperature in the pyrolysis process to produce CDs. This process is advantageous in terms of easy operation, shorter reaction time, use of solvent-free methods, scalable production, and low cost. However, the resulting CDs have a wide size range and produce excitation-wavelength-dependent fluorescence emission, down- and up-conversion fluorescence, good PL stability and high solubility.35–38
Origin of fluorescence emission from CDs
Literature evidence provides exhaustive studies exploiting the fascinating PL behavior of CDs including excellent photostability, fluorescence in the visible or NIR region, tunable fluorescence, excitation wavelength emission, efficient up-converted PL, and photoinduced electron transfer ability.39 The facile synthesis of CDs as described in the previous section enables researchers to use different precursors such as synthetic molecules, plant and animal products, bio wastes, etc. Hence, the structure and chemical composition of CDs are complex and vary with the different precursors, solvents and post-treatment methods used. A natural precursor contains several active ingredients, and sometimes with unknown composition upon subjecting to different reaction conditions produces complex carbonaceous nanoparticles and byproducts with non-uniform chemical compositions, which can also contribute to fluorescence.40–44 Moreover, the use of dopants generates CDs with diverse surface functional groups that possess their own definitive PL mechanism. Consequently, a unified explanation for the origin of fluorescence is yet to be reported.Presently, three main perspectives are proposed for the fluorescence emission of CDs: (i) quantum captivity or core-state/size-dependent emission, induced by perfect carbon crystals with modified groups and fewer defects, (ii) surface-state emission based on the hybridization of the carbon framework and associated functional moieties and (iii) molecular fluorescence due to free or bound fluorescent impurities formed from the byproducts during the synthesis of CDs.45,46
Core emission (quantum confinement effect or size-dependent emission)
It is well-established that the emission behavior of a material having the size of a Bohr radius (<10 nm) is governed by quantum confinement, and therefore displays unique PL properties.47 The PL peaks are narrow and excitation independent with a definite size-dependent emission property. The crystalline carbon core of CDs in the nanometer scale size significantly influences the electron distribution, resulting in bandgap- and size-dependent energy relaxation dynamics, consequently affecting the emission phenomenon.48,49Kim et al. concluded that the red-shifted emission behavior of CDs is associated with the increase in the nanoparticle size and tentatively suggested that the fluorescence is because of the quantum confinement effect.49 Furthermore, Li et al. also demonstrated that the fluorescence of CDs arises from the quantum confinement effects and is size-dependent.11 In the meantime, Sun et al. attributed the fluorescence emission from CDs to the presence of surface energy traps that became emissive upon surface passivation due to their quantum confinement effect.47 Yuan et al. synthesized CDs with multi-colour emission by using a nitrogen (N)-rich source under solvothermal conditions. The bandgap (3.02 to 2.12 eV) dependent emission was confirmed via transmission electron microscope (TEM) imaging, wherein the CDs with average size distributions of 1.95, 2.41, 3.78, 4.90 and 6.68 nm displayed corresponding blue, green, yellow, orange, and red fluorescence.50 The bandgap-dependent emission was also proved through lifetime measurement to display a mono-exponential decay nature for all CDs with the QY estimated as 75%, 73%, 58%, 53%, and 12%, respectively. Another research group synthesized size-dependent emissive CDs from citric acid and urea by employing different solvent systems including water (CDw: 1.7 nm), glycerol (CDg: 2.8 nm size) and DMF (CDd: 4.5 nm size).51 They proposed the full color emission mechanism for the CDs based on the extent of graphitization of the carbon core, suggesting a higher degree of dehydration and graphitization of CDs upon using aprotic solvents, resulting in a greater proportion of sp2 than sp3 carbon (Fig. 2a). In yet another report, multi-colour emissive CDs were prepared through solvothermal synthesis by varying the composition of the precursors. Blue and green (3.6 nm size) emitting CDs were obtained from m-phenylenediamine, whereas yellow and red emissive CDs (4.8 nm size) were produced from the same source in the presence of tartaric acid, which suggested that size plays a critical role in the characteristic emission behavior.52
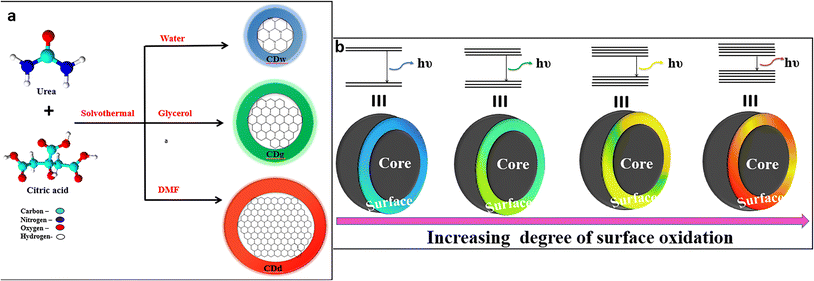 | ||
| Fig. 2 (a) Full-color fluorescent CDs depicting size-dependent emission51 and (b) full-color luminescent CDs with a surface-state-controlled emission.57 | ||
Surface state of CDs
The surface state including diverse functional groups and degree of surface oxidation serves as energy-trapping sites and is intently linked to the emission of CDs, which is one of the most widely accepted mechanisms for fluorescence. Light of a specific wavelength corresponding to the bandgap of the CDs induces excitation of electrons to a higher energy level, which upon relaxation are trapped on the surface defective sites. Later, when they return to the ground state, the energy difference falls in the visible region, shifting the emission to a longer wavelength range.53 Wang et al. demonstrated the effect of surface functionalities on the emission centers of the CDs.54 Liang's group reported N-doped CDs having same oxygen content with tunable multicolor fluorescence from dark blue to red or even white that originated from the surface functional groups.55 The return of electrons in the N-related defect states to the highest occupied molecular orbital (HOMO) causes a red-shifted emission.A few reports suggest that as the degree of surface oxidation increases, the amount of surface defects also rises, these serving as exciton capture centers leading to a red-shifted emission in the CDs.56 Xiong and team obtained CDs purified through column chromatography, which showed excitation-independent emission from blue to red.57 The band gap reduced with increasing surface oxygen content to showcase the red-shifted fluorescence. Liu et al. prepared green-emissive CNDs and yellow-emissive GQDs with a similar size distribution as well as chemical groups, but showed a bathochromic shift in the emission due to different degrees of surface oxidation.58 This observation is because of the decreased band gaps between the lowest unoccupied molecular orbital (LUMO) and the HOMO. The reports by various groups including Zhu et al. and Ding et al. also proved that the oxygen-related surface states are responsible for the red-shifts in the emission of CDs obtained by hydrothermal treatment and subsequent purification using column chromatography57,59 (Fig. 2b). Similar observations were reported wherein diverse surface states were introduced on CDs either by a controlled pyrolysis temperature or different combination of dopants. Miao and team developed B-CDs (3.96 nm size, λem = 440 nm, QY = 52.6%), G-CDs (4.12 nm size, λem = 540 nm, QY = 35.1%) and R-CDs (4.34 nm size, λem = 600 nm, QY = 12.9%,) indicating that size-dependent emission cannot be used to relate to the PL of the CDs.60 However, surface state-induced emissions can explicitly explain the introduction of a cocktail of chemical groups, with increased oxygen groups in R-CDs in comparison with G-CDs and B-CDs.
Core emission and surface state of CDs: synergistic effect
A few pieces of evidence suggest the involvement of both the quantum confinement effect and the surface state in the fluorescence emission of CDs.60,61 Pang and research group demonstrated that the fluorescence originates from the surface state emission, wherein the surface properties, the π-electron system and the size of the CDs determine the surface state energy gaps.62 The increase in the amount of surface oxidation or particle size results in a red-shifted emission of CDs. Huang and group proposed that the confinement effect and the surface state synergistically affect the CD emission.63 They also demonstrated that the carbon core size can be adjusted, and the surface functional groups of the shell can be manipulated to tune the fluorescence properties of the CDs.Molecular fluorophores
During the formation of CDs, fluorescent impurities or molecular fluorophores are also generated. The presence of these fluorescent impurities, either attached to the CD surface or found freely floating in the as-prepared sample solution, is inevitable and contributes to the emissions of CDs with reportedly higher QY values, but the emission intensity diminishes over time as they are prone to photobleaching.64,65 Although several mechanisms are hypothesized, the molecular fluorophores differ majorly with the precursor and the reaction conditions. Krysmann et al. synthesized carbon nanoparticles at 180, 230, 300 and 400 °C and compared their PL properties.66 The QY measured for CDs corresponding to 230 °C (15%) is less in comparison with that obtained at 180 °C (50%), which indicates the predominance of molecular fluorophores at a lower temperature and moreover, with a gradual increase in temperature the carbonaceous core is developed at the expense of these fluorogenic particles (Fig. 3a).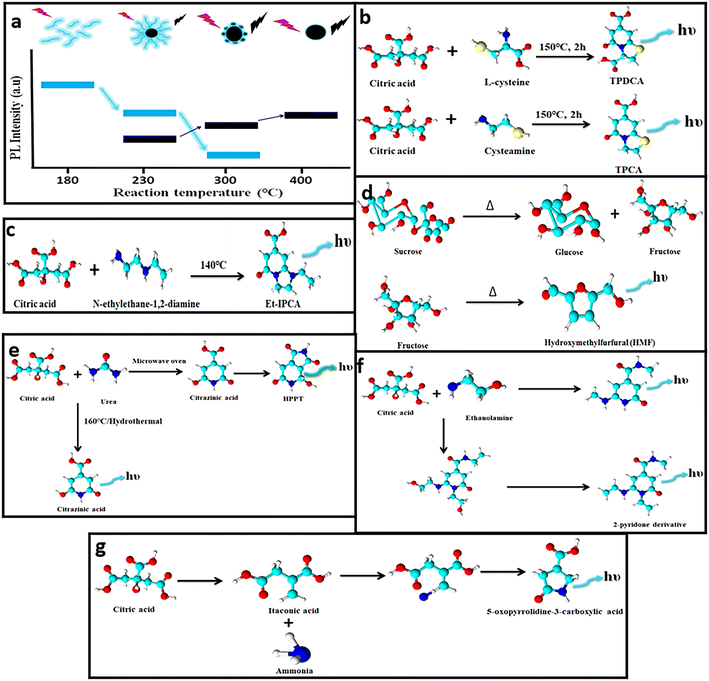 | ||
| Fig. 3 (a) Emission intensity of CDs using citric acid and ethylenediamine as precursors during synthesis under different temperatures.66 (b–f) Different types of molecular fluorophoric species71,73–76 and (g) unconventional aliphatic fluorophores identified that contribute to the fluorescence of CDs.77 | ||
To identify the chemical composition of molecular luminophores, nuclear magnetic resonance (NMR) spectroscopy coupled with mass spectrometry (MS) is performed. Duan et al. demonstrated a high fluorescence QY of CDs prepared from citric acid and ethylenediamine.67 However, they proved that the as-prepared mixture contains imidazo[1,2-a]pyridine-7-carboxylic acid (IPCA), isopropyl chloroacetate, polymers and carbon cores, wherein isopropyl chloroacetate contributes to the intense blue fluorescence and high QY. Similarly, Rogach's and Baker's groups proposed that the molecular fluorophores attached to the CDs contribute distinctly to the optical features of the CDs.68,69 Furthermore, Righetto et al. established that small emitters in the excitation range from 320 to 450 nm that are dispersed in solution contribute to the fluorescence of CDs,70 whereas weak fluorescence observed above 480 nm excitation is majorly due to the poor-emitting carbon cores. Therefore, although carbon cores exist, the fluorescence origin is dominated by small organic molecules that are free in solution.
Zhang and team identified two fluorophores, 5-oxo-3,5-dihydro-2H-thiazolo[3,2-a]pyridine-7-carboxylic acid (TPCA) and 5-oxo-3,5-dihydro-2H-thiazolo[3,2-a]pyridine-3,7-dicarboxylic acid (TPDCA), which were found to display similar emission behavior to the CDs (Fig. 3b).71 Besides, the QY of TPCA (66%) and TPDCA (76%) was closer to the QY of the CDs (64%). The contribution of these fluorophores was evaluated further by subjecting them to dialysis (1 kDa) wherein the dialysate demonstrated a higher QY in comparison with the residual retentate solution. Both the solutions exhibited similar emission attributes indicating the presence of these fluorophores. In addition, both the solutions were exposed to a UV source, which displayed nearly no fluorescence as these fluorophores are more prone to photobleaching.71 Several fluorophores including IPCA, 5-(hydroxymethyl)furfural (HMF), 4-hydroxy-1H-pyrrolo[3,4-c]pyridine-1,3,6(2H,5H)-trione (HPPT), citrazinic acid, and a 2-pyridone derivative (Fig. 3c–f) were identified and separated, which induced fluorescence increments in CDs.72–76 To date reports show the formation of fluorophores with aromatic structures but recently, a new kind of fluorophore was identified by Yao et al.77 The blue-emissive fluorophore was produced during the synthesis of CDs using urea and citric acid (Fig. 3g). The hydrothermal product was dialyzed and subjected to reversed-phase column chromatography to obtain various fluorophore fractions. The NMR analysis showed numerous peaks in the aliphatic region. In a separate experiment, itaconic acid was prepared when citric acid undergoes dehydration and decarboxylation, and further subjected to hydrothermal treatment after adding urea. The raw product obtained possessed similar NMR spectroscopy and electron spray ionisation-MS spectra to the dialysate obtained from citric acid and urea. Hence, it was established that the new fluorophore was 5-oxopyrrolidine-3-carboxylic acid and possessed similar emission properties to itaconic acid. Thus, it can be concluded that fluorophores are the main species that induce changes in the fluorescence of CDs and their identification is possible by efficient separation and characterization via NMR and MS techniques.
Significance of purification and characterization of CDs
The presence of small molecular weight or oligomeric luminescent impurities and byproducts that result from incomplete reaction of the precursor contributes mostly to the emission of CDs and may strongly affect the optical properties, which demands the inevitable need to purify the synthesized products. Elucidating the true nature of these emergent nanocarbonaceous materials is of vital importance as inadequate purification poses a primary setback in their versatile applicability. Moreover, the complexity increases when plant/animal-based precursors are used, and their chemical composition remains unknown. Fluorescent impurities that originate during synthesis create an enormous obstacle to the analysis of the PL properties of CDs. They can change or even mask the actual optical features of the CDs to show dramatic differences in the fluorescence performances.78 The molecular fluorophores that are present along with CDs contribute to most of the PL emission, suggesting towards their removal for reliable fluorescence results that originate only from the homogeneous CDs. Bartolomei and Prato briefly discussed the importance of purification and chemical analysis to identify the molecular fluorophores.79 Therefore, the role of purification is imperative to separate the CDs from the constituting byproducts to rule out the possible errors associated with interference by these fluorophores.Bottom-up methods are extensively utilized for CD synthesis due to their easy synthetic protocol and wide choice of precursors. The high temperatures involved results in not only the carbonization of the precursor, but also yield small molecular weight luminophores. Though various purification techniques are utilized to separate the fluorescent CDs from the remnant fluorescent byproducts, the extent of purification is unknown and challenging to prove.69,80 Though TEM and atomic force microscope (AFM) imaging enable us to view the CDs with definitive lattice d-space values, the presence of molecular fluorophores cannot be examined. Therefore, inaccurate understanding is gained with respect to the purification of the small carbonaceous luminophores. The PL emission as well as QY measured for the purified sample is interpreted as the inherent emission from CDs alone.81,82 The most definitive and effective characterization technique to confirm the presence of CDs is NMR spectroscopy.67 Bartolomei et al. emphasize the necessity to incorporate NMR spectroscopy as a standard and an essential tool to minimize the risk in analyzing the fluorescence that originates from residual molecular species in the final sample, rather than the target CDs.83 It can unveil the occurrence of molecular species in a rapid and easy-to-operate approach. The chemical shift peaks for the nanocarbons appear as broad and unresolved due to their complex chemical environment. In contrast, the appearance of sharp and resolved peaks is indicative of the presence of small molecules with specific groups.67
Despite the purification techniques adopted, these luminophores are not eliminated due to non-optimization of the purification procedure. For instance, Essner et al. pointed out the importance of the usage of appropriate molecular weight cut-off (MWCO) dialysis bags, as the smaller dimension can hinder the flux of CDs.69 Yang and group reported the formation of small fluorophores during the synthesis of CDs using citric acid at lower temperatures, and upon a gradual increase in temperature the carbon cores are formed at the expense of these fluorophores.72 They also proved that the CDs were composed of IPCA, polymer and a carbonaceous core which contributed to the blue fluorescence. Moreover, they identified IPCA as one of the fluorophores, which is highly emissive and obtained after column chromatography separation. The fluorophore was prepared separately while the same fluorophore was separated from the CDs via column chromatography and confirmed via NMR spectroscopy. Based on these findings the PL mechanism and structural relationship between the fluorophore and CDs could be elucidated; the excitation-independent emission and high QY were mainly due to the presence of the IPCA moiety.
When small molecules like sucrose, glucose and fructose are subjected to hydrothermal treatment, CDs are formed along with HMF derivatives as the condensation product. Gude et al. identified these fluorophores after solvent extraction and column chromatography separation of the synthesized product.74 However, the NMR spectrum of the purified CDs matched with the NMR spectrum of the HMF except for the presence of an aldehyde group instead of a hydroxyl group. Additional confirmation is obtained via MS analysis where the m/z peak corresponds to the HMF dimer in agreement with the molecular weight of the HMF derivative. Besides, they display mono-exponential decay along with excitation-independent emission indicating that the CDs are composed of aggregates of HMF. The same research group prepared CDs from citric acid and ethanolamine and purified via a column chromatography method using a suitable elution system.76 It was confirmed via NMR and MS analysis that the fluorophore obtained is a 2-pyridone derivative. Furthermore, the single-particle spectroscopy suggested otherwise that CDs constitute aggregates of fluorophore due to a gradual decay with respect to time along with greater photostability. Kasprzyk and team attempted to associate the blue and green emissions of CDs to the molecular fluorophore generated during the synthesis process. The product sample was purified via a high-resolution liquid chromatography electron spray ionization MS method enabling the efficient separation of different fractions. The blue emission observed was due to the formation of citrazinic acid as a condensation product, which converts to green-emissive HPPT when subjected to a dehydration step.75 Bartolomei et al. demonstrated the importance of NMR spectroscopy to differentiate chiral emissive CDs from emissive molecular species by performing dialysis and high-performance liquid chromatography (HPLC).83 These demonstrative studies highlight the importance of optimizing the purification protocol for the reaction mixture obtained after the synthesis of carbonaceous nanomaterials. In addition, the role of NMR and mass spectra evaluation as effective characterization tools to identify the different kinds of fluorophore generated during the synthesis of carbon dots is also evident.
Though numerous studies on CDs briefly explain the different purification procedures adopted in various research investigations, their impact on the fluorescence properties is not reviewed in detail. This unveils the need for a critical and concise appraisal of the use of appropriate purification techniques and their optimization to obtain reproducible and consistent fluorescence features from the CDs. The different purification techniques and their intricacies involved are briefly discussed.
Purification and separation methods for CDs
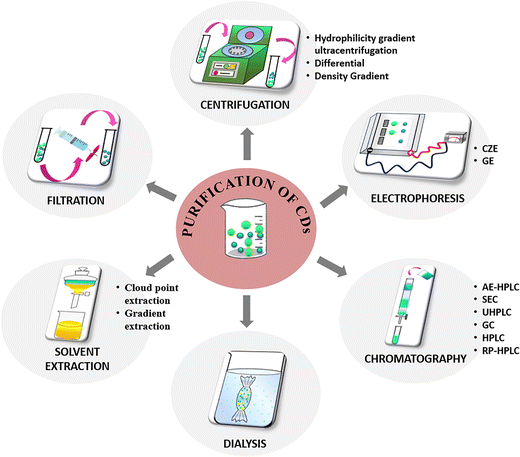
It is to be noted that a universal method for the purification and separation of CDs does not exist, but specific protocols can be established according to the nature of the products obtained after the synthesis procedure. The following section attempts to summarize the different techniques reported (Fig. 4) by various research groups to separate the CDs from other reaction products and provides a comprehensive understanding of the extent of purification attempted to separate the nanocarbonaceous material.Filtration
Filtration is a simple method of purification where the as-synthesized CD product comprising different-sized nanoparticles is allowed to pass through filters of fixed pore sizes. The larger particles that are unable to penetrate the pore remain on the filter surface while the smaller particles are obtained as filtrate. This method can serve as a preliminary purification step to separate the insoluble or suspended particles or agglomerates in the CD sample obtained after synthesis. However, the pore diameter of the filter ranging between 0.1–1 μm needs to be optimised as there is tendency to clogging of the pores. Moreover, the concentrated sample needs dilution prior to filtration to avoid clogging. Previous reports on the purification of CDs state either the use of filters with definite pore sizes or the use of syringe filters. Sun et al. synthesized CDs from human hair fiber via heat-assisted sonication, wherein the remaining strands of hair were separated from the product utilizing a porous membrane of 0.22 μm pore diameter.84 Lan et al. prepared carbon nanoparticles from melamine and trisodium citrate dehydrate and the resultant product was purified using a 0.22 μm pore size membrane.85 Another study reported the synthesis of CDs using chitosan and ethylenediamine as precursors by microwave-assisted heating to induce dehydration.86 The resultant powder was re-dissolved in water and filtered utilizing a syringe filter of 0.45 μm dimension to eliminate dissolved salts and unreacted chitosan. Although filtration is a less time-consuming approach for purification, the product requires additional purification as the separation does not isolate specific-sized particles.Centrifugation
Centrifugation is yet another simple and inexpensive technique used in the initial purification to segregate small sized carbonaceous nanoparticles from large aggregates or suspended particles from the product.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for a few minutes. Briefly, the sample is added on the top of a density gradient established by solutions of different densities layered sequentially. Upon centrifugation, the particles deposit in the density gradient at distinct speeds based on their shapes, sizes, or densities and ultimately form diverse bands. Several literature reports mention the use of centrifugation employing different radial speeds to remove the less-fluorescent and insoluble particles, while the highly fluorescent supernatant is collected for further purification.87–92 The advantage of centrifugation is that both organic/aqueous soluble constituents can be separated to collect concentrated samples.
000 rpm for a few minutes. Briefly, the sample is added on the top of a density gradient established by solutions of different densities layered sequentially. Upon centrifugation, the particles deposit in the density gradient at distinct speeds based on their shapes, sizes, or densities and ultimately form diverse bands. Several literature reports mention the use of centrifugation employing different radial speeds to remove the less-fluorescent and insoluble particles, while the highly fluorescent supernatant is collected for further purification.87–92 The advantage of centrifugation is that both organic/aqueous soluble constituents can be separated to collect concentrated samples.
Sucrose, glycerol, cesium chloride, and other aqueous solutions are usually used to prepare the density gradient. Sucrose density gradient centrifugation (SDGC) enables the isolation of the nanodots of desired size and shape by varying the sucrose concentrations in the gradient for diverse applications, especially biological. Pandey et al. reported the fabrication of crystalline CDs from highly alkaline sugar cane juice at 28 °C and the separation from amorphous carbonaceous materials using the lucid and non-toxic SDGC technique.93 A gradient was achieved in the test tube by overlaying 50–100% of pure sucrose, commencing from the highest concentration at the bottom. About 2 mL of the sample to be fractionated was top-layered and spun at 6000 rpm for 30 min. Fine separation was accomplished based on nanoparticle size due to the sharp density gradients (Fig. 5a).
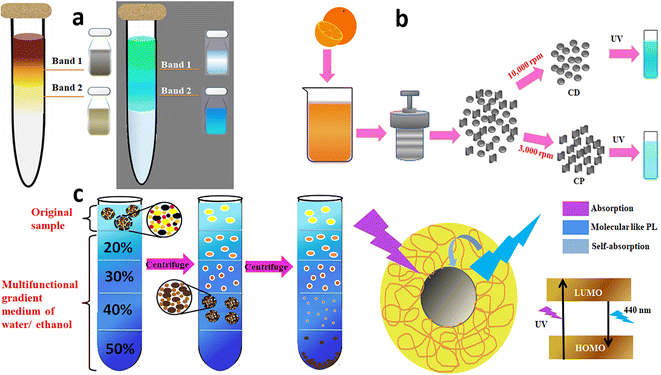 | ||
| Fig. 5 (a) SDGC separation of CDs from the sonication of sugar cane juice and the separated CD bands under ambient and UV light,93 (b) differential centrifugation of hydrothermally obtained orange juice-derived CDs to deposit the less-fluorescent coarse nanoparticles and highly fluorescent CDs.95 (c) Hydrophilicity gradient ultracentrifugation wherein the CDs are clustered at the starting point and de-clustered at successive layers with increasing water content during sedimentation, and the proposed PL mechanism of the CDs.96 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min after adding excess acetone to obtain highly fluorescent CDs (1.5–4.5 nm size) in the supernatant (Fig. 5b).
000 rpm for 15 min after adding excess acetone to obtain highly fluorescent CDs (1.5–4.5 nm size) in the supernatant (Fig. 5b).
Dialysis
Dialysis is one of the purification techniques used extensively to remove low molecular weight residual precursors and polymers to separate the CDs. Water-soluble CD samples can be easily filtered and dialyzed. The process relies on the diffusion of small fluorescent molecules from a higher concentration region to a lower concentration area across a semipermeable membrane. During this process, the as-synthesized CDs are dissolved in deionised water and dialyzed with stirring against an appropriate MWCO dialysis bag (semipermeable membrane) in deionised water. Gradually, the low molecular weight components diffuse out due to the concentration gradient and constitute the dialysate, while the remnant solution inside the bag called the retentate comprises high molecular weight species. The constituent of the dialysate depends upon the sample concentration, duration and MWCO of the dialysis bag. Maintenance of the sample pH is tedious, and the buffer solution must be recharged or changed periodically with fresh deionised water at various time intervals to hold the dialysate dilute.97 The MWCO of the dialysis membrane, the stepwise process in terms of water replacement and the duration of dialysis require suitable optimization to realize the best purification outcome. Though hydrophobic samples cannot be separated via this technique, dialysis is employed extensively as one of the purification techniques in several research studies.69Kang and group synthesized blue-emissive CDs from PEG via a hydrothermal approach. The dark-coloured sample was subjected to dialysis (MWCO 1 kDa) until the solution changed to golden yellow (Fig. 6a) to provide the initial indication of the completion of purification of the CDs.98 Similarly, Liu et al. synthesized CDs from PEG and NaOH and the resultant brown solution was dialyzed (MWCO 1 kDa) until a clear golden solution was obtained.99 Yet another research group used a similar MWCO dialysis bag to obtain hydrophilic and blue-emissive CDs. The dialysis was carried out for 3 days to completely remove the molecular precursor. Despite the extensive purification carried out, the CDs displayed a QY of 2.03%, 10.28%, 19.72%, and 27.66% corresponding to a pyrolysis period of 1, 2, 3 and 4 h respectively. Besides, TEM analysis confirmed the presence of small and narrow-distribution-sized CDs.100
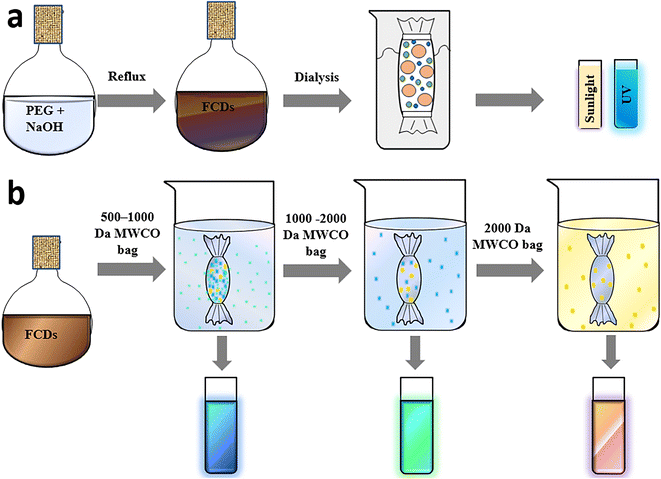 | ||
| Fig. 6 (a) Simple dialysis for the purification of PEG-derived CDs,98 (b) concentrated dialysate obtained for 500–2000 Da dialysis bags and illustration of samples when exposed to 365 nm UV irradiation.108 | ||
Most studies report the dialysis of CDs using a low MWCO of 1 kDa, performed with the intention of separating only the smaller sized CDs from the remnant precursor and byproducts.59,101–104 Therefore, an attempt was made by Yuan et al.105 and Feng et al.106 to use dialysis bags of MWCO of 3.5 kDa. The TEM images of the CDs present in the dialysate depicted a narrow distribution indicating successful separation and optimisation of the dialysis procedure. Noun and team reported that the initial dialysate of the CD sample had a lower QY, indicating the presence of less-emissive fluorophore.107 However, upon further dialysis there was an increment in QY by many fold, suggesting that highly emissive fluorophores formed can be removed via an organic solvent wash, thereby reducing the QY. Thus, the dialysis process must be monitored at every stage via photophysical spectroscopic analysis to evaluate the accuracy of purification for a specific system.
Although the composition and population of fluorescent species formed along with CDs vary based on the precursor used and reaction conditions maintained, these highly fluorescent small-sized species can pass through the permeable dialysis membranes. Hence, the rationale behind choosing the appropriate MWCO is a matter of curiosity, inviting numerous trials to separate CDs as the sample has an unknown product composition. Zhang et al. used smaller (500 Da) to bigger dimension (2000 Da) dialysis bags (Fig. 6b) with the intention of separating the smaller fluorophores initially, and then the CDs.108 After subsequent dialysis the decrease in QY of the retentate indicated that the small-dimension fluorophores are highly emissive in comparison with the CDs. Essner et al. used dialysis bags of different molecular weight; accordingly, the retentate from an MWCO of 1 kDa showed the presence of larger particles during TEM imaging, while dialysis with a 20 kDa membrane displayed a better separation of CDs.69 Chang et al. reported a detailed investigation on the purification of citric acid-derived CDs using dialysis.109 Dialysis was performed for different durations to completely remove the molecular luminophores and the retentate was analysed via HPLC coupled with fluorescence spectrophotometry. With time, the fluorescence QY of the sample inside the dialysis bag diminished from 2.2% to 0.6% and the retentate concentration declined from 580 to <1 mg mL−1. This suggested that the sample solution is largely composed of small molecule byproducts and the actual CDs formed are very low. Besides, the UV–vis absorption and fluorescence peaks of both the retentate and dialysate obtained via HPLC demonstrated remnants of many byproducts in the retentate even after 24 h, suggesting ∼72–120 h of dialysis is essential to eliminate the highly emissive molecular impurities from the CD solution. Besides, the use of MWCO <1 kDa was not necessary as most of the species were trapped within the dialysis bag. This study vividly highlights not only the significance of the purification of the CDs, but also stresses the necessity to customize the dialysis parameters according to the sample under investigation. In contrast, if nanocarbonaceous materials had better solubility in organic solvents, the residual impurities could have been removed through solvent extraction and/or precipitation.
Though these findings do not hold absolute claim, they encourage researchers to re-think about the impact of different-MWCO dialysis bags and the dialysis duration on the separation of nanoparticles. Furthermore, these dialysis parameters need to be suitably customized in accordance with the investigated sample to achieve a complete separation of CDs for further characterisation and practical application.
Solvent extraction
Liquid–liquid partitioning or solvent extraction has been widely utilised in the separation of CDs from the product mixtures after synthesis. This method is used when the precursor is hydrophobic, and dialysis cannot be employed. The sample consisting of hydrophobic/hydrophilic components can be separated based on its solubility in two immiscible solvents (an organic solvent and water), and the components distribute among the solvents. Chloroform, ethyl acetate, n-hexane, acetone, and toluene are the generally used solvents to extract the amphiphilic CDs and completely retain the impurities in aqueous solution.110–112The disadvantage of this method is that although a separation of components in two or more immiscible liquids is obtained, each separated solution may contain more than one type of species. The validity of separation can be checked on TLC plates, which could suggest the presence of different compounds. Moreover, it is difficult to distinguish and purify the reactants from the products when both have the same solubility. Depending upon the solubility of the CDs obtained during the hydrothermal/solvothermal process, different solvent systems are used. Goncalves and research group synthesized CDs by laser ablation and concentrated them using ethyl acetate to eliminate the remaining unreactants.110 In another report, CDs were synthesized from acrylic acid and 1,2-ethanediamine, and the further addition of glycidyl methacrylate induced polymerization.113 The polymerised product was separated from the oily phase via the addition of water and the latter was given an n-hexane wash to remove the unreacted glycidyl methacrylate. Yu et al. used ascorbic acid to prepare blue-emissive CDs by a solvothermal approach to obtain a dark brown product and the organic byproducts were removed via dichloromethane extraction.114 The water fraction was further dialysed to eliminate the impurities to obtain CDs as small as 3 nm in size. In another synthesis process, the precursor was subjected to carbonisation and the CDs were extracted using chloroform in the form of a brown dispersion. The remnants of the reactants were removed through a hexane wash. Another research group reported the microwave-mediated synthesis of CDs from resorcinol and H2SO4.115,116 The product was provided with an n-butanol wash to remove the unreacted impurities, and the CDs were concentrated via double distilled water extraction. The CDs separated via solvent extraction demonstrated better QY and excitation-independent emissions in comparison with those obtained through a dialysis process. This indicates that the solvent interactions with CDs can confer enhancement in the emissive behaviour.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and dichloromethane (3.4), were exploited for extraction to collect the CD fractions. The four fractions obtained exhibited dissimilar surface polarities due to the different polar surface functional groups and showed, hence, different surface polarity-dependent PL (Fig. 7a). Red-shifted PL peaks with greater excitation-dependency and a longer lifetime were observed with increasing surface polarity of the CDs. The increasing amount of auxochromes with increasing polarity leading to an internal energy transfer process was proposed to explain the distinct PL properties of each CD fraction. The study also showed that the organic solution dispersion process has no evident influence on the PL behaviour and originated entirely from the separated CDs itself.
2), and dichloromethane (3.4), were exploited for extraction to collect the CD fractions. The four fractions obtained exhibited dissimilar surface polarities due to the different polar surface functional groups and showed, hence, different surface polarity-dependent PL (Fig. 7a). Red-shifted PL peaks with greater excitation-dependency and a longer lifetime were observed with increasing surface polarity of the CDs. The increasing amount of auxochromes with increasing polarity leading to an internal energy transfer process was proposed to explain the distinct PL properties of each CD fraction. The study also showed that the organic solution dispersion process has no evident influence on the PL behaviour and originated entirely from the separated CDs itself.
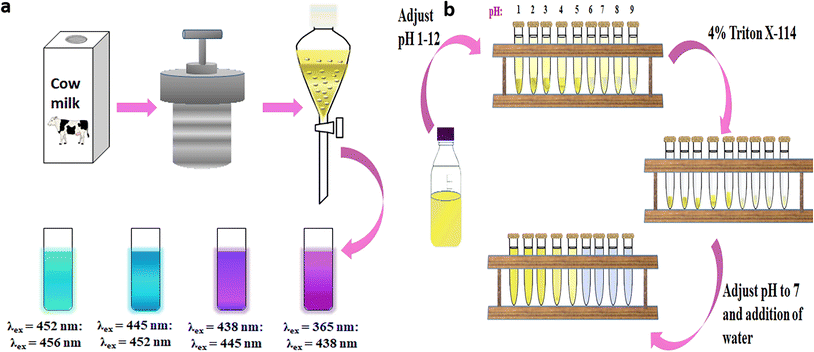 | ||
| Fig. 7 (a) Schematic illustration of fabrication, gradient extraction, and surface polarity-dependent PL of isolated CDs,117 and (b) cloud point extraction of CDs depicting the two phases separated during the first step of extraction, the separated micellar phase containing CDs and the second step of purification which returns the CDs to the aqueous phase.118 | ||
Chromatography techniques
The efficiency of separation of CDs is greatly enhanced when column chromatography is adopted. Chromatographic techniques that are available for the separation of CDs include size exclusion chromatography (SEC), HPLC, anion-exchange high-performance liquid chromatography (AE-HPLC), reversed-phase high-performance liquid chromatography (RP-HPLC), and gravitational column chromatography.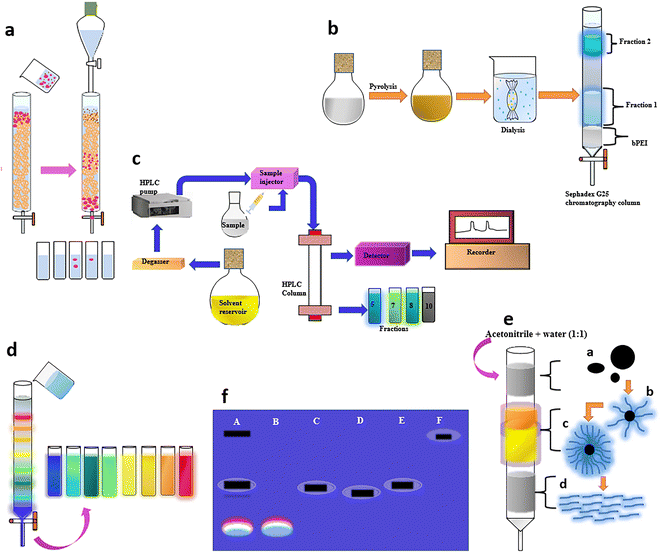 | ||
| Fig. 8 (a) Schematic representation of particle size-based separation using SEC.119 (b) Dialysis and subsequent SEC to obtain two CD fractions, which demonstrated characteristic emissions.122 (c) Isolation of brighter CDs from an apparently low QY mixture using AE-HPLC126 and (d) GC using a silica column to obtain a series of full-color light-emitting excitation-independent CDs with a surface-state-controlled luminescence mechanism.57 (e) Purification of a citric acid and cysteine-derived CD mixture by GC to obtain three different fluorescent species.131 (f) GE in a 1% agarose gel viewed under 365 nm UV light; A – crude suspension of single-walled carbon nanotubes suspension, B – fluorescent carbon, C – short tubular carbon, D and E – further separation of C, and F – cut single-walled carbon nanotubes. | ||
It is well-established that the surface state plays a prime role in the fluorescence behaviour of CDs, resulting in size-independent emission. Wang et al. fractionated surface passivated (PEG1500N) fluorescent CDs through an aqueous column packed with Sephadex G-100 gel using water as the eluent to achieve CDs free of any non-fluorescent material for an enhanced QY close to 60%.120 Jiang and team mixed Nescafe® coffee powder obtained via a hydrothermal method in distilled water at 90 °C and centrifuged it for 15 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The large or agglomerated particles in the supernatant were filtered off through a 0.22 μm membrane and the filtrate was loaded onto the Sephadex G-25 gel column with distilled water as eluent.121 The isolated CDs displayed excitation-wavelength-dependent multiple emission colors, and fascinating up-conversion PL. Liu and team initially adopted dialysis to separate the precursor from the product and further obtained two fractions via a Sephadex gel column, which demonstrated characteristic emissions (Fig. 8b).122 The PL properties were related to the surface passivation and not based on a quantum confinement effect. The second fraction with smaller particles that eluted last had a greater oxygen content and possessed a 17.4% QY in comparison with the first fraction containing slightly bigger sized CDs with a QY of 8.2%.
000 rpm. The large or agglomerated particles in the supernatant were filtered off through a 0.22 μm membrane and the filtrate was loaded onto the Sephadex G-25 gel column with distilled water as eluent.121 The isolated CDs displayed excitation-wavelength-dependent multiple emission colors, and fascinating up-conversion PL. Liu and team initially adopted dialysis to separate the precursor from the product and further obtained two fractions via a Sephadex gel column, which demonstrated characteristic emissions (Fig. 8b).122 The PL properties were related to the surface passivation and not based on a quantum confinement effect. The second fraction with smaller particles that eluted last had a greater oxygen content and possessed a 17.4% QY in comparison with the first fraction containing slightly bigger sized CDs with a QY of 8.2%.
Electrophoresis
Electrophoresis is a method of purification performed in a conductive medium where the separation of particles takes place under an applied electric field. The migration of these particles is governed by the size, charge, and shape along with the prevailing temperature. This technique is cumbersome and can be employed when dialysis or column chromatography does not efficiently separate the particles. Moreover, it enables the physical segregation of positive and negatively charged particles. Electrophoretic separations of water-soluble CDs can be achieved through gel electrophoresis (GE) and capillary zone electrophoresis (CZE).Kokorina and group utilised two-stage GE to study the constituent fragments of a CD sample obtained hydrothermally from ethylenediamine and citric acid.133 The blue fluorescence with a higher QY confined to discrete bands that originates from the negatively charged small-sized light molecular fraction with the highest mobility and that shows no excitation-dependent light emission corresponds to IPCA. In contrast, bands having a lower QY, and displaying excitation-dependent emission are commonly observed for CDs with different emissive centres. Similarly, Sachdev and research group applied an SDS-PAGE method for the separation of polyethylene glycol and polyethyleneimine passivated CDs in a 12% denaturing gel under an electric field of 120 V.134 Multicolour fluorescence was observed from the excised CD–polyethyleneimine band, whereas no fluorescence was visualised from the excised piece of CD–polyethylene glycol gel. The surface charge-dependent mobility of CDs was studied using GE in a 1.2% agarose gel run using Tris acetate–EDTA buffer under 85 V. Fluorescent bands of positively charged CDs–polyethyleneimine and negatively charged CDs–polyethylene glycol that migrated towards the negative and positive terminal, respectively, were visualised under UV illumination. PAGE can identify the correlation between the mobility and color of the fluorescent CDs. However, it has a low separation efficiency due to the ∼3–5 nm pore size of the gel, which restricts its application in the separation of CDs with a wide size range.
Though CZE is a simple, but powerful technique to realize an acceptable separation efficiency, the benefits offered by the high number of theoretical plates are dominated by the low sensitivity of the UV detection systems due to the small sample injection volumes.136 To overcome this limitation, Hu et al. used a commercial CZE apparatus in combination with a diode array and laser-induced fluorescence detectors to separate and identify the composition of 1,2-ethylenediamine and citric acid-derived CDs synthesized via microwave-assisted pyrolysis.137 The separation was achieved using a 40 cm capillary at an applied voltage of 15 kV with 30 mM sodium acetate–acetic acid as the run buffer of pH 3.6. The CZE technique was also effectively used to investigate the reaction time-associated kinetics of CD formation and identify the functional group-associated neutral, positive, or negative charge states, which influences the emission features. Wu and Remcho reported CZE coupled with UV absorbance detection for the rapid and reliable analysis of CDs using an alkaline working buffer of 100 mM Tris acetate at pH 8.4.138Table 1 highlights the different purification techniques adopted by researchers for the separation of CDs from various sources for diverse applications, along with their benefits and limitations.
| Purification method | Separation device | Separation principle | Advantages | Challenges | Analytical applications |
|---|---|---|---|---|---|
| Filtration | Syringe filter | Filter with fixed pore size allows passage of smaller particles | • Simple and less expensive | • Though separation is selective, unable to separate fluorophores completely | Cell viability assay, Hg2+ & mercaptothiol sensing |
| • Separation of large agglomerates from CD solution | • Tendency to clogging of filter pores | ||||
| Density gradient centrifugation | Tubular centrifuge | Separation based on density gradient | • Simple and inexpensive method to separate CDs from larger particles | • Low molecular weight fluorophores and precursors may not be separated from CD solution | Bioimaging of RBCs, in vitro imaging & fluorescent labels |
| • Fine separation can be achieved | |||||
| • Separation of CDs both in organic and aqueous phase | |||||
| Differential centrifugation | Centrifuge | Separation based on size and density | • Multiple fractions within a CD sample can be separated | • Mixture needs to be centrifuged multiple times | Cellular imaging |
| Hydrophilicity gradient ultracentrifugation | Centrifuge | Separation based on hydrophilicity and solubility of the environmental media | • Efficient approach for the separation of non-sedimental species with extremely high colloidal stability | • Proper choice of solvent system and optimisation of their volume ratios to realise the suitable gradient medium | — |
| Dialysis | Dialysis bags with different MWCOs | Permeable membrane with specific MWCO allows passage of CDs with specific size | • Can remove precursor species from CD solution | • Time-consuming, tedious, and expensive | Hg2+, uric acid sensing, α-amanitin in serum detection, catalytic reduction, fluorescent inks, in vitro & in vivo imaging in the presence of highly reactive oxygen species, anti-bacterial & cytotoxicity assays, & fabrication of solar cells |
| • Can separate CDs of uniform size based on specific MWCO | • Process often affected by solvents | ||||
| • Low molecular weight flurophores can easily diffuse out from dialysis bag | • Appropriate bag selection is crucial | ||||
| • Process duration needs optimisation | |||||
| • Cannot remove hydrophobic components present along with CDs | |||||
| • Requires HPLC to monitor the separated fluorophores | |||||
| • Dialysed sample gets diluted and needs to be concentrated for further experiments | |||||
| • CDs with dissimilar sizes or surface functional groups cannot be separated | |||||
| • Unsuitable for large-scale separation of pure CDs | |||||
| Solvent extraction | Separating funnel or separating column | Transfer of CDs and impurities based on their solubility in two immiscible solvents | • Relatively simple | • Separation of two-component systems rather than multi-component ones | Hg2+ & Fe3+ sensing in water, cell imaging & photocatlytic removal of organic pollutants from water |
| • CDs transferred to miscible solvents show characteristic PL properties | • Separated solutions may contain more than one type of species | ||||
| • Cannot separate CDs of the same type with another | |||||
| Gradient extraction | Separating funnel | Separation of CD fractions with gradient surface polarities with increase in polarity of the extraction solvent | • Simple and cheap | • Selection of solvents based on the surface functional groups | — |
| • Cannot separate CDs and impurities with similar surface polarities | |||||
| Cloud point extraction | Centrifuge tubes | Separation based on pH-induced polarity changes of CDs | • Simple, inexpensive, fast, and green method | — | — |
| • Potential use for large-scale separation of pure CDs | |||||
| • Excludes any toxic solvents or reagents | |||||
| • Can separate CDs into fractions of uniform optical behavior | |||||
| SEC | Column/reversed phase/anionic exchange/HPLC systems | Inclusion or exclusion of CDs from the pores within the gel matrix based on size | • Conditions can be varied to suit the type of CD sample or further purification, analysis, or storage requirements | • Needs optimisation of conditions to achieve sufficient selectivity | In vitro & in vivo imaging, cytotoxicity assays |
| HPLC | Distribution of compounds between mobile and stationary phases | High peak resolution | High cost | — | |
| Collection of CD fractions with scale-up capabilities | Solvents and columns are expensive | ||||
| Regular maintenance and calibration needed | |||||
| UHPLC | Separates components of a mixture by using high pressure to push solvents through the column | • Shorter retention time and better resolution | • Small sample loading | — | |
| • Superior to HPLC in terms of efficiency, sample loading, separation speed, and solvent consumption | • Less collected CD fractions are insufficient for further characterization | ||||
| • Higher concentration and larger sample size loading may lead to peak broadening and tailing, resulting in poor separation of CD fractions | |||||
| AE-HPLC | Separation of negatively charged ions/molecules/CDs based on their affinity for positively charged stationary phase groups of the ion exchange resin | • High-resolution analysis | • Separates charged components, and not suitable to handle neutral CDs | — | |
| • Separation of negatively charged CDs | • Fraction collection is time-consuming | ||||
| • Significant amounts of CDs can be collected | |||||
| • Provides valuable insight into the complexity and composition of the CD sample | |||||
| • Separates charged species to facilitate studies on the influence of surface charge on PL features | |||||
| RP-HPLC | Separation based on the more polar mobile phase compared with stationary phase | • Can deal with a complex mixture of both charged and neutral CDs | • Unable to separate highly polar or ionic compounds from the column void volume, but retaining good peak symmetry | Cytotoxicity assay & bioimaging | |
| • More popular compared with AE-HPLC due to higher separation efficiency, but less expensive | |||||
| Gravity chromatography | Adsorption of CD solution using a stationary phase and separation into discrete components based on polarity difference | • Simple and used for laboratory-scale separation | • Slow and time-consuming | — | |
| • Involves minimal instrumentation | • Manual process requires constant monitoring | ||||
| • Not a high-resolution technique | |||||
| • Reproducibility issue if care is not taken to normalize methods | |||||
| • Optimisation of sample loading and adjusting the flow rate | |||||
| GE | GE system | Mobility of bands and segregation of components by charge-to-size ratio under the influence of an electric field. Smaller and oppositely charged species migrate at a faster rate than larger species | • Efficient physical separation of constituents | • Large-scale separations not possible; need for scale up | Bioimaging of A549 & BHK-21 cells |
| • Requires only a small amount of sample (20–30 μL) | • Limited application in the separation of CDs with a wide range of size | ||||
| • Separated components can easily be physically extracted by cutting the gel and washing out the samples or by freeze-drying | |||||
| CZE | Fused silica capillary and electrophoresis unit | Different electrophoretic mobilities based on charge/size ratio through an electrolyte contained in a fused silica capillary under an electric field | • Good separation efficiency | • Low sensitivity of UV detection systems due to low sample injection volumes | Ketamine detection |
| • Simple to perform | • Collection of sufficient amounts of CD fractions is extremely time-consuming | ||||
| • Separates positively, neutral and negatively charged CDs simultaneously | • Can separate only the charged CDs rather than the neutral ones, which elute out as one single intense peak |
Limitations and future perspectives
The facile synthesis of CDs through various physicochemical strategies from different carbon-based sources to realise desirable PL properties has led to a significant rise in the publications in various fields. However, the composition of as-synthesized CD samples, especially those formed through bottom-up chemical synthesis involving molecular precursors, is highly complex as they exist as mixtures of several CD fractions along with other molecular intermediates and side products with significantly different properties. Presently, no specific techniques are developed to certify the exact composition of these CD mixtures, which significantly complicates their targeted applications. The heterogeneous constitution hampers the explicit analysis of the CD sample for practical application as it can only represent the average features of the individual CD species. For instance, a less understood structure and relatively low fluorescence QY are the major drawbacks that restrict their usage in the life sciences. Moreover, highly fluorescent molecular fluorophores can also contribute to the PL properties along with CDs, leading to inconsistent, erroneous results and interpretation of data. The review provides clear evidence that the fluorescent impurities and byproducts produced during the synthesis of CDs must be completely removed to achieve reliable results. Although TEM/AFM cannot serve the purpose, a few characterisation approaches such as NMR spectroscopy and MS can help detect the presence of these fluorophores.The true chemical nature and insights into the origin of PL properties can be comprehended only upon obtaining pure CDs, devoid of various impurities. Therefore, a careful separation of various CD fractions, which can be confirmed by MALDI-TOF MS and TEM techniques, is vital for structural analysis. The morphology, chemical composition, and fundamental properties of CDs can be better understood to establish their applications in different fields. The isolation of pure homogeneous fluorescent CDs could significantly increase the QY, sensitivity, and, consequently, the operational efficiency of CD-based nanosensors. In contrast to the top-down synthesis of CDs that is associated with fewer molecular byproducts, the fluorescence emission of CDs is dominated by the contribution from small molecular weight organic fluorophores in the case of unpurified product samples obtained by bottom-up methods. The unaccounted presence of these organic molecular fluorophores will have a pronounced effect on the fluorescence-based applications of CDs including heavy metal detection, nanosensors and cellular imaging. Though many reports demonstrate centrifugation and filtration for the purification of CDs, these methods have been frequently demonstrated as inadequate separation techniques. Moreover, performing dialysis with inappropriate membranes with too low an MWCO or adopting a shorter procedure duration results in incomplete purification. In addition, the complete removal of fluorescent byproducts formed alongside the CDs is not guaranteed even when performing dialysis with a membrane MWCO of 50 kDa. Thus, the complex and multicomponent nature of the as-synthesized CDs suggests that dialysis, despite its convenience, may not be a satisfactory mode of purification in some instances. The complete removal of fluorophores might require using more than one technique.
Based on previous reports, studies focussing on analytical separation techniques of CDs are limited. A few researchers have used the most analytically sound chromatographic techniques to reduce the CD mixture complexity through fractionation, but these reports currently constitute only <10% of the reported CD purification protocols. Though robust HPLC- or AE-HPLC-assisted separation techniques that can fractionate CDs may benefit in evaluating the synthetic parameters and product generated, the scale-up ability of this process to the preparative range is an attractive opportunity to attain homogeneous fluorescent CDs in milligram quantities for a wide range of applications. Although AE-HPLC is used to separate negatively charged nanocarbon species, cation-exchange HPLC could be a similar applicable tool for positively charged CDs. Such separations are increasingly important as it becomes more apparent that surface charge rather than size can be a more effective norm for the favourable fractionation of CDs. Furthermore, although the separations obtained during electrophoresis cannot be obtained on a large scale, the sensitivity to both size and charge in nanoscale systems can provide insights into the kinds of fluorophore generated and, hence, demands a scale-up process. Although individual CD fractions that showcase distinct optical properties from the as-synthesized sample including excitation-wavelength-independent emission and QY are realised, a scalable, vetted separation process to achieve CDs of high purity for practical applications remains an unmet challenge.
Several techniques are adopted for the purification of CDs, such as filtration, centrifugation, solvent extraction, dialysis, and chromatography techniques. Various reports state the use of a dialysis method followed by centrifugation and filtration owing to its lesser requirement for technical skills. Filtration is a less time-consuming technique, but the separation of nanoparticles is not explicit and bigger particles tend to clog the pores of the filter. Centrifugation offers a better separation of smaller sized particles as bigger sediments settle at the bottom and less dense particles remain in the supernatant. However, recent studies reveal that smaller sized fluorophores cannot be separated by this method. Dialysis is extensively used as it offers a better separation of smaller sized CDs from larger nanoparticles. The utmost importance lies in the use of appropriate molecular weight cut-off dialysis bags, and hydrophobic CDs cannot be separated by this technique. The solvent extraction method is useful to separate amphiphilic CDs from the hydrophobic components. It is a relatively simpler method used in the separation of two-component/species systems. Column chromatography techniques are less explored and can offer a better alternative for the separation of multi-component CDs with better accuracy. A size-exclusion based column ensures the size-based separation of nanoparticles. High- and ultra-high-performance chromatography can be used in large-scale purification with a shorter elution time and better resolution. The regular optimisation and maintenance along with the high cost limit its application.
Though the PL mechanism and application of CDs are thoroughly investigated, the impact of purification on the emission characteristics of CDs needs to be explored further. Moreover, the use of different precursors introduces uncertainty and therefore, a unified methodology and not an absolute standard protocol has to be adopted for the purification and separation of the CD sample. The sole reason that the as-synthesized CDs are complex mixtures which are far ideal in terms of purity leaves plenty of room for sound analytical separation approaches to harvest precise CD fractions for target applications. There is an urgent need for quality fundamental studies on the purification and separation of CDs and the emission features that vary with size, surface charge, presence of heteroatoms etc., but are unique to a specific CD fraction with a homogeneous composition. Efficient analytical separation techniques must be developed to isolate high-purity CD fractions with unique physicochemical properties. The next step in the research related to carbonaceous nanomaterials should be aimed at a complete shift towards the synthesis of engineered CDs for targeted real-life utility, which calls for an in depth understanding not only of the chemical composition of the final product and its optical properties, but also to establish a clear relationship between the precursor materials used and the reaction conditions. Thus, rigorous and consistent purification steps need to be uniformly implemented, facilitating a tactical change that will unlock the full potential of these carbonaceous nanoparticles as next-generation smart materials for full-color solid-state lighting, photovoltaics, catalysis, biological imaging and sensing.
Abbreviations
| AE-HPLC | Anion-exchange high-performance liquid chromatography |
| AFM | Atomic force microscope |
| CDs | Carbon dots |
| CNDs | Carbon nanodots |
| CPDs | Carbonized polymer dots |
| CQDs | Carbon quantum dots |
| CZE | Capillary zone electrophoresis |
| GE | Gel electrophoresis |
| GQDs | Graphene quantum dots |
| HMF | 5-(Hydroxymethyl)furfural |
| HOMO | Highest occupied molecular orbital |
| HPLC | High-performance liquid chromatography |
| HPPT | 4-Hydroxy-1H-pyrrolo[3,4-c]pyridine-1,3,6(2H,5H)-trione |
| IPCA | Imidazo[1,2-a]pyridine-7-carboxylic acid |
| LUMO | Lowest unoccupied molecular orbital |
| MS | Mass spectrometry |
| MWCO | Molecular weight cut-off |
| N | Nitrogen |
| NIR | Near-infrared |
| NMR | Nuclear magnetic resonance |
| PAGE | Polyacrylamide gel electrophoresis |
| PL | Photoluminescence |
| RP-HPLC | Reversed-phase high-performance liquid chromatography |
| SDGC | Sucrose density gradient centrifugation |
| SDS | Sodium dodecyl sulfate |
| SEC | Size exclusion chromatography |
| TEM | Transmission electron microscope |
| TPCA | 5-Oxo-3,5-dihydro-2H-thiazolo[3,2-a]pyridine-7-carboxylic acid |
| TPDCA | 5-Oxo-3,5-dihydro-2H-thiazolo[3,2-a]pyridine-3,7-dicarboxylic acid |
| UHPLC | Ultra-high-performance liquid chromatography |
| UV | Ultraviolet |
| QY | Quantum yield |
Conflicts of interest
There are no conflicts to declare.References
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed
.
- J. Wang, R. Sheng Li, H. Zhi Zhang, N. Wang, Z. Zhang and C. Z. Huang, Biosens. Bioelectron., 2017, 97, 157–163 CrossRef CAS PubMed
.
- J. Jana, H. J. Lee, J. S. Chung, M. H. Kim and S. H. Hur, Anal. Chim. Acta, 2019, 1079, 212–219 CrossRef CAS PubMed
.
- J. Hu, F. Tang, Y.-Z. Jiang and C. Liu, Analyst, 2020, 145, 2184–2190 RSC
.
- M. L. Liu, B. Bin Chen, C. M. Li and C. Z. Huang, Green Chem., 2019, 21, 449–471 RSC
.
- K. Qin, D. Zhang, Y. Ding, X. Zheng, Y. Xiang, J. Hua, Q. Zhang, X. Ji, B. Li and Y. Wei, Analyst, 2019, 145, 177–183 RSC
.
- L. Cui, J. Wang and M. Sun, Rev. Phys., 2021, 6, 100054 CrossRef
.
- F. Yuan, Y. K. Wang, G. Sharma, Y. Dong, X. Zheng, P. Li, A. Johnston, G. Bappi, J. Z. Fan, H. Kung, B. Chen, M. I. Saidaminov, K. Singh, O. Voznyy, O. M. Bakr, Z. H. Lu and E. H. Sargent, Nat. Photonics, 2019, 14, 171–176 CrossRef
.
- C. Hu, M. Li, J. Qiu and Y. P. Sun, Chem. Soc. Rev., 2019, 48, 2315–2337 RSC
.
- Y. Cao, Y. Cheng and M. Sun, Appl. Spectrosc. Rev., 2023, 58, 1–38 CrossRef CAS
.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed
.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed
.
- C. Lee, W. Kwon, S. Beack, D. Lee, Y. Park, H. Kim, S. K. Hahn, S. W. Rhee and C. Kim, Theranostics, 2016, 6, 2196–2208 CrossRef CAS PubMed
.
- J. Xia, S. Chen, G. Y. Zou, Y. L. Yu and J. H. Wang, Nanoscale, 2018, 10, 22484–22492 RSC
.
- C. Xia, S. Zhu, T. Feng, M. Yang, B. Yang, C. Xia, T. Feng, M. Yang, B. Yang and S. Zhu, Adv. Sci., 2019, 6, 1901316 CrossRef CAS PubMed
.
- S. Mandal and P. Das, Appl. Mater. Today, 2022, 26, 101331 CrossRef
.
- S. Zhu, J. Zhang, L. Wang, Y. Song, G. Zhang, H. Wang and B. Yang, Chem. Commun., 2012, 48, 10889–10891 RSC
.
- R. Tabaraki and O. Abdi, J. Fluoresc., 2019, 29, 751–756 CrossRef CAS PubMed
.
- M. Tian, Y. Wang and Y. Zhang, J. Nanosci. Nanotechnol., 2018, 18, 8111–8117 CrossRef CAS PubMed
.
- J. Luo, Z. Sun, W. Zhou, F. Mo, Z. chao Wu and X. Zhang, Opt. Mater., 2021, 113, 110796 CrossRef CAS
.
- C. Li, X. Sun, Y. Li, H. Liu, B. Long, D. Xie, J. Chen and K. Wang, ACS Omega, 2021, 6, 3232–3237 CrossRef CAS PubMed
.
- Y. Liu, H. Huang, W. Cao, B. Mao, Y. Liu and Z. Kang, Mater. Chem. Front., 2020, 4, 1586–1613 RSC
.
- M. Pirsaheb, S. Mohammadi and A. Salimi, TrAC, Trends Anal. Chem., 2019, 115, 83–99 CrossRef CAS
.
- H. Barhum, T. Alon, M. Attrash, A. Machnev, I. Shishkin and P. Ginzburg, ACS Appl. Nano Mater., 2021, 4, 9919–9931 CrossRef CAS PubMed
.
- W. Shi, H. Lv, S. Yuan, H. Huang, Y. Liu and Z. Kang, Sep. Purif. Technol., 2017, 174, 282–289 CrossRef CAS
.
- X. He, P. Chen, J. Zhang, T. Y. Luo, H. J. Wang, Y. H. Liu and X. Q. Yu, Biomater. Sci., 2019, 7, 1940–1948 RSC
.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J. J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS PubMed
.
- P. Zhao, M. Yang, W. Fan, X. Wang, F. Tang, C. Yang, X. Dou, S. Li, Y. Wang and Y. Cao, Part. Part. Syst. Charact., 2016, 33, 635–644 CrossRef CAS
.
- W. Shi, Q. Han, J. Wu, C. Ji, Y. Zhou, S. Li, L. Gao, R. M. Leblanc and Z. Peng, Int. J. Mol. Sci., 2022, 23, 1456 CrossRef CAS PubMed
.
- X. Li, H. Wang, Y. Shimizu, A. Pyatenko, K. Kawaguchi and N. Koshizaki, Chem. Commun., 2010, 47, 932–934 RSC
.
- S. Y. Park, H. U. Lee, E. S. Park, S. C. Lee, J. W. Lee, S. W. Jeong, C. H. Kim, Y. C. Lee, Y. S. Huh and J. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 3365–3370 CrossRef CAS PubMed
.
- S. Mitra, S. Chandra, S. H. Pathan, N. Sikdar, P. Pramanik and A. Goswami, RSC Adv., 2013, 3, 3189–3193 RSC
.
- Q. Wang, X. Liu, L. Zhang and Y. Lv, Analyst, 2012, 137, 5392–5397 RSC
.
- M. K. Kumawat, R. Srivastava, M. Thakur and R. B. Gurung, ACS Sustainable Chem. Eng., 2017, 5, 1382–1391 CrossRef CAS
.
- M. Xue, Z. Zhan, M. Zou, L. Zhang and S. Zhao, New J. Chem., 2016, 40, 1698–1703 RSC
.
- B. Yin, J. Deng, X. Peng, Q. Long, J. Zhao, Q. Lu, Q. Chen, H. Li, H. Tang, Y. Zhang and S. Yao, Analyst, 2013, 138, 6551–6557 RSC
.
- L. Cui, X. Ren, M. Sun, H. Liu and L. Xia, Nanomaterials, 2021, 11, 3419 CrossRef CAS PubMed
.
- G. Ge, L. Li, D. Wang, M. Chen, Z. Zeng, W. Xiong, X. Wu and C. Guo, J. Mater. Chem. B, 2021, 9, 6553–6575 RSC
.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC
.
- S. Schenker, C. Heinemann, M. Huber, R. Pompizzi, R. Perren and F. Escher, J. Food Sci., 2002, 67, 60–66 CrossRef CAS
.
- T. S. Samaras, P. A. Camburn, S. X. Chandra, M. H. Gordon and J. M. Ames, J. Agric. Food Chem., 2005, 53, 8068–8074 CrossRef CAS PubMed
.
- D. O. Carvalho, L. H. Øgendal, M. L. Andersen and L. F. Guido, Eur. Food Res. Technol., 2016, 242, 1545–1553 CrossRef CAS
.
- H. Yahya, R. S. T. Linforth and D. J. Cook, Food Chem., 2014, 145, 378–387 CrossRef CAS PubMed
.
- H. X. Wang, Z. Yang, Z. G. Liu, J. Y. Wan, J. Xiao and H. L. Zhang, Chem. – Eur. J., 2016, 22, 8096–8104 CrossRef CAS PubMed
.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2014, 44, 362–381 RSC
.
- K. J. Mintz, Y. Zhou and R. M. Leblanc, Nanoscale, 2019, 11, 4634–4652 RSC
.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed
.
- X. Yan, B. Li and L. S. Li, Acc. Chem. Res., 2013, 46, 2254–2262 CrossRef CAS PubMed
.
- S. Kim, S. W. Hwang, M. K. Kim, D. Y. Shin, D. H. Shin, C. O. Kim, S. B. Yang, J. H. Park, E. Hwang, S. H. Choi, G. Ko, S. Sim, C. Sone, H. J. Choi, S. Bae and B. H. Hong, ACS Nano, 2012, 6, 8203–8208 CrossRef CAS PubMed
.
- F. Yuan, Z. Wang, X. Li, Y. Li, Z. Tan, L. Fan and S. Yang, Adv. Mater., 2017, 29, 1604436 CrossRef PubMed
.
- Z. Tian, X. Zhang, D. Li, D. Zhou, P. Jing, D. Shen, S. Qu, R. Zboril and A. L. Rogach, Adv. Opt. Mater., 2017, 5, 1700416 CrossRef
.
- K. Jiang, X. Feng, X. Gao, Y. Wang, C. Cai, Z. Li and H. Lin, Nanomaterials, 2019, 9, 529 CrossRef CAS PubMed
.
- L. Cao, M. J. Meziani, S. Sahu and Y. P. Sun, Acc. Chem. Res., 2013, 46, 171–182 CrossRef CAS PubMed
.
- L. Wang, S. J. Zhu, H. Y. Wang, S. N. Qu, Y. L. Zhang, J. H. Zhang, Q. D. Chen, H. L. Xu, W. Han, B. Yang and H. B. Sun, ACS Nano, 2014, 8, 2541–2547 CrossRef CAS PubMed
.
- Y. Zhang, R. Yuan, M. He, G. Hu, J. Jiang, T. Xu, L. Zhou, W. Chen, W. Xiang and X. Liang, Nanoscale, 2017, 9, 17849–17858 RSC
.
- L. Bao, Z. L. Zhang, Z. Q. Tian, L. Zhang, C. Liu, Y. Lin, B. Qi and D. W. Pang, Adv. Mater., 2011, 23, 5801–5806 CrossRef CAS PubMed
.
- H. Ding, S. B. Yu, J. S. Wei and H. M. Xiong, ACS Nano, 2016, 10, 484–491 CrossRef CAS PubMed
.
- M. L. Liu, L. Yang, R. S. Li, B. Bin Chen, H. Liu and C. Z. Huang, Green Chem., 2017, 19, 3611–3617 RSC
.
- H. Liu, Z. He, L. P. Jiang and J. J. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 4913–4920 CrossRef CAS PubMed
.
- X. Miao, D. Qu, D. Yang, B. Nie, Y. Zhao, H. Fan and Z. Sun, Adv. Mater., 2018, 30, 1704740 CrossRef PubMed
.
- S. Zhu, Y. Song, J. Wang, H. Wan, Y. Zhang, Y. Ning and B. Yang, Nano Today, 2017, 13, 10–14 CrossRef CAS
.
- L. Bao, C. Liu, Z. L. Zhang and D. W. Pang, Adv. Mater., 2015, 27, 1663–1667 CrossRef CAS PubMed
.
- Z. Liu, H. Zou, N. Wang, T. Yang, Z. Peng, J. Wang, N. Li and C. Huang, Sci. China: Chem., 2018, 61, 490–496 CrossRef CAS
.
- Y. Xiong, J. Schneider, C. J. Reckmeier, H. Huang, P. Kasák and A. L. Rogach, Nanoscale, 2017, 9, 11730–11738 RSC
.
- W. Wang, B. Wang, H. Embrechts, C. Damm, A. Cadranel, V. Strauss, M. Distaso, V. Hinterberger, D. M. Guldi and W. Peukert, RSC Adv., 2017, 7, 24771–24780 RSC
.
- M. J. Krysmann, A. Kelarakis, P. Dallas and E. P. Giannelis, J. Am. Chem. Soc., 2012, 134, 747–750 CrossRef CAS PubMed
.
- P. Duan, B. Zhi, L. Coburn, C. L. Haynes and K. Schmidt-Rohr, Magn. Reson. Chem., 2020, 58, 1130–1138 CrossRef CAS PubMed
.
- J. Schneider, C. J. Reckmeier, Y. Xiong, M. Von Seckendorff, A. S. Susha, P. Kasak and A. L. Rogach, J. Phys. Chem. C, 2017, 121, 2014–2022 CrossRef CAS
.
- J. B. Essner, J. A. Kist, L. Polo-Parada and G. A. Baker, Chem. Mater., 2018, 30, 1878–1887 CrossRef CAS
.
- M. Righetto, A. Privitera, I. Fortunati, D. Mosconi, M. Zerbetto, M. L. Curri, M. Corricelli, A. Moretto, S. Agnoli, L. Franco, R. Bozio and C. Ferrante, J. Phys. Chem. Lett., 2017, 8, 2236–2242 CrossRef CAS PubMed
.
- L. Shi, J. H. Yang, H. B. Zeng, Y. M. Chen, S. C. Yang, C. Wu, H. Zeng, O. Yoshihito and Q. Zhang, Nanoscale, 2016, 8, 14374–14378 RSC
.
- S. Zhu, X. Zhao, Y. Song, S. Lu and B. Yang, Nano Today, 2016, 11, 128–132 CrossRef CAS
.
- Y. Song, S. Zhu, S. Zhang, Y. Fu, L. Wang, X. Zhao and B. Yang, J. Mater. Chem. C, 2015, 3, 5976–5984 RSC
.
- V. Gude, A. Das, T. Chatterjee and P. K. Mandal, Phys. Chem. Chem. Phys., 2016, 18, 28274–28280 RSC
.
- W. Kasprzyk, T. Świergosz, S. Bednarz, K. Walas, N. V. Bashmakova and D. Bogdał, Nanoscale, 2018, 10, 13889–13894 RSC
.
- A. Das, V. Gude, D. Roy, T. Chatterjee, C. K. De and P. K. Mandal, J. Phys. Chem. C, 2017, 121, 9634–9641 CrossRef CAS
.
- X. Yao, Y. Wang, F. Li, J. J. Dalluge, G. Orr, R. Hernandez, Q. Cui and C. L. Haynes, Nanoscale, 2022, 14, 9516–9525 RSC
.
- W. Zhang, X. Fang, F. He, J. Bai, Y. Cheng, K. Weerasinghe, X. Meng, H. Xu and T. Ding, J. Phys. Chem. C, 2021, 125, 5207–5216 CrossRef
.
- B. Bartolomei and M. Prato, Small, 2023, 19, 2206714 CrossRef CAS PubMed
.
- Y. Xiong, J. Schneider, E. V. Ushakova and A. L. Rogach, Nano Today, 2018, 23, 124–139 CrossRef CAS
.
- C. J. Reckmeier, J. Schneider, Y. Xiong, J. Häusler, P. Kasák, W. Schnick and A. L. Rogach, Chem. Mater., 2017, 29, 10352–10361 CrossRef CAS
.
- S. Khan, A. Sharma, S. Ghoshal, S. Jain, M. K. Hazra and C. K. Nandi, Chem. Sci., 2017, 9, 175–180 RSC
.
- B. Bartolomei, A. Bogo, F. Amato, G. Ragazzon and M. Prato, Angew. Chem., Int. Ed., 2022, 61, e202200038 CrossRef CAS PubMed
.
- D. Sun, R. Ban, P. H. Zhang, G. H. Wu, J. R. Zhang and J. J. Zhu, Carbon, 2013, 64, 424–434 CrossRef CAS
.
- M. Lan, J. Zhang, Y. S. Chui, H. Wang, Q. Yang, X. Zhu, H. Wei, W. Liu, J. Ge, P. Wang, X. Chen, C. S. Lee and W. Zhang, J. Mater. Chem. B, 2014, 3, 127–134 RSC
.
- Y. Choi, G. H. Ryu, S. H. Min, B. R. Lee, M. H. Song, Z. Lee and B. S. Kim, ACS Nano, 2014, 8, 11377–11385 CrossRef CAS PubMed
.
- S. Chandra, P. Das, S. Bag, D. Laha and P. Pramanik, Nanoscale, 2011, 3, 1533–1540 RSC
.
- P. C. Hsu, Z. Y. Shih, C. H. Lee and H. T. Chang, Green Chem., 2012, 14, 917–920 RSC
.
- M. X. Gao, C. F. Liu, Z. L. Wu, Q. L. Zeng, X. X. Yang, W. B. Wu, Y. F. Li and C. Z. Huang, Chem. Commun., 2013, 49, 8015–8017 RSC
.
- S. S. Liu, C. F. Wang, C. X. Li, J. Wang, L. H. Mao and S. Chen, J. Mater. Chem. C, 2014, 2, 6477–6483 RSC
.
- S. Xie, H. Su, W. Wei, M. Li, Y. Tong and Z. Mao, J. Mater. Chem. A, 2014, 2, 16365–16368 RSC
.
- K. Wei, J. Li, Z. Ge, Y. You and H. Xu, RSC Adv., 2014, 4, 52230–52234 RSC
.
- S. Pandey, A. Mewada, G. Oza, M. Thakur, N. Mishra, M. Sharon and M. Sharon, Nanosci. Nanotechnol. Lett., 2013, 5, 775–779 CrossRef CAS
.
- Q. Hu, X. Gong, L. Liu and M. M. F. Choi, J. Nanomater., 2017, 1804178, DOI:10.1155/2017/1804178
.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC
.
- L. Deng, X. Wang, Y. Kuang, C. Wang, L. Luo, F. Wang and X. Sun, Nano Res., 2015, 8, 2810–2821 CrossRef CAS
.
- S. D. Dsouza, M. Buerkle, P. Brunet, C. Maddi, D. B. Padmanaban, A. Morelli, A. F. Payam, P. Maguire, D. Mariotti and V. Svrcek, Carbon, 2021, 183, 1–11 CrossRef CAS
.
- R. Liu, H. Li, W. Kong, J. Liu, Y. Liu, C. Tong, X. Zhang and Z. Kang, Mater. Res. Bull., 2013, 48, 2529–2534 CrossRef CAS
.
- R. Liu, J. Liu, W. Kong, H. Huang, X. Han, X. Zhang, Y. Liu and Z. Kang, Dalton Trans., 2014, 43, 10920–10929 RSC
.
- B. Wang, A. Song, L. Feng, H. Ruan, H. Li, S. Dong and J. Hao, ACS Appl. Mater. Interfaces, 2015, 7, 6919–6925 CrossRef CAS PubMed
.
- H. Zhang, P. Dai, L. Huang, Y. Huang, Q. Huang, W. Zhang, C. Wei and S. Hu, Anal. Methods, 2014, 6, 2687–2691 RSC
.
- E. Ju, Z. Liu, Y. Du, Y. Tao, J. Ren and X. Qu, ACS Nano, 2014, 8, 6014–6023 CrossRef CAS PubMed
.
- Y. Yan, W. Kuang, L. Shi, X. Ye, Y. Yang, X. Xie, Q. Shi and S. Tan, J. Alloys Compd., 2019, 777, 234–243 CrossRef CAS
.
- J. J. Huang, Z. F. Zhong, M. Z. Rong, X. Zhou, X. D. Chen and M. Q. Zhang, Carbon, 2014, 70, 190–198 CrossRef CAS
.
- C. Yuan, B. Liu, F. Liu, M. Y. Han and Z. Zhang, Anal. Chem., 2014, 86, 1123–1130 CrossRef CAS PubMed
.
- L. Feng, L. Tan, H. Li, Z. Xu, G. Shen and Y. Tang, Biosens. Bioelectron., 2015, 69, 265–271 CrossRef CAS PubMed
.
- F. Noun, J. Manioudakis and R. Naccache, Part. Part. Syst. Charact., 2020, 37, 2000119 CrossRef CAS
.
- Q. Zhang, X. Sun, H. Ruan, K. Yin and H. Li, Sci. China Mater., 2017, 60, 141–150 CrossRef CAS
.
- C. Y. Chen, Y. H. Tsai and C. W. Chang, New J. Chem., 2019, 43, 6153–6159 RSC
.
- H. M. R. Gonçalves, A. J. Duarte and J. C. G. Esteves da Silva, Biosens. Bioelectron., 2010, 26, 1302–1306 CrossRef PubMed
.
- S. Mitra, S. Chandra, T. Kundu, R. Banerjee, P. Pramanik and A. Goswami, RSC Adv., 2012, 2, 12129–12131 RSC
.
- J. F. Y. Fong, S. F. Chin and S. M. Ng, Sens. Actuators, B, 2015, 209, 997–1004 CrossRef CAS
.
- P. Zhang, W. Li, X. Zhai, C. Liu, L. Dai and W. Liu, Chem. Commun., 2012, 48, 10431–10433 RSC
.
- B. Y. Yu and S. Y. Kwak, J. Mater. Chem., 2012, 22, 8345–8353 RSC
.
- J. Wang, C. Cheng, Y. Huang, B. Zheng, H. Yuan, L. Bo, M.-W. Zheng, S.-Y. Yang, Y. Guo and D. Xiao, J. Mater. Chem. C, 2014, 2, 5028–5035 RSC
.
- J. Zhu, C. Wu, Y. Cui, D. Li, Y. Zhang, J. Xu, C. Li, S. Iqbal and M. Cao, Colloids Surf., A, 2021, 623, 126673 CrossRef CAS
.
- S. Han, H. Zhang, J. Zhang, Y. Xie, L. Liu, H. Wang, X. Li, W. Liu and Y. Tang, RSC Adv., 2014, 4, 58084–58089 RSC
.
- A. Beiraghi and S. A. Najibi-Gehraz, J. Nanostruct., 2020, 10, 107–118 CAS
.
- O. E. Trubetskaya, O. A. Trubetskoj, C. Richard, A. M. Vervald, S. A. Burikov, V. V. Marchenkov, O. A. Shenderova, S. V. Patsaeva and T. A. Dolenko, J. Chromatogr. A, 2021, 1650, 462251 CrossRef CAS PubMed
.
- X. Wang, L. Cao, S. T. Yang, F. Lu, M. J. Meziani, L. Tian, K. W. Sun, M. A. Bloodgood and Y. P. Sun, Angew. Chem., Int. Ed., 2010, 49, 5310–5314 CrossRef CAS PubMed
.
- C. Jiang, H. Wu, X. Song, X. Ma, J. Wang and M. Tan, Talanta, 2014, 127, 68–74 CrossRef CAS PubMed
.
- D. D. Liu, H. Su, Q. Cao, X. Y. Le and Z. W. Mao, RSC Adv., 2015, 5, 40588–40594 RSC
.
- N. Fuyuno, D. Kozawa, Y. Miyauchi, S. Mouri, R. Kitaura, H. Shinohara, T. Yasuda, N. Komatsu and K. Matsuda, Adv. Opt. Mater., 2014, 2, 983–989 CrossRef CAS
.
- X. Gong, M. Chin Paau, Q. Hu, S. Shuang, C. Dong and M. M. F. Choi, Talanta, 2016, 146, 340–350 CrossRef CAS PubMed
.
- J. C. Vinci and L. A. Colon, Anal. Chem., 2012, 84, 1178–1183 CrossRef CAS PubMed
.
- J. C. Vinci, I. M. Ferrer, S. J. Seedhouse, A. K. Bourdon, J. M. Reynard, B. A. Foster, F. V. Bright and L. A. Colón, J. Phys. Chem. Lett., 2013, 4, 239–243 CrossRef CAS PubMed
.
- J. C. Vinci and L. A. Colón, Microchem. J., 2013, 110, 660–664 CrossRef CAS
.
- X. Gong, Q. Hu, M. Chin Paau, Y. Zhang, L. Zhang, S. Shuang, C. Dong and M. M. F. Choi, Talanta, 2014, 129, 529–538 CrossRef CAS PubMed
.
- Q. Hu, M. C. Paau, M. M. F. Choi, Y. Zhang, X. Gong, L. Zhang, Y. Liu and J. Yao, Electrophoresis, 2014, 35, 2454–2462 CrossRef CAS PubMed
.
- X. Gong, Q. Hu, M. C. Paau, Y. Zhang, S. Shuang, C. Dong and M. M. F. Choi, Nanoscale, 2014, 6, 8162–8170 RSC
.
- V. Hinterberger, C. Damm, P. Haines, D. M. Guldi and W. Peukert, Nanoscale, 2019, 11, 8464–8474 RSC
.
- H. Liu, T. Ye and C. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473–6475 CrossRef CAS PubMed
.
- A. A. Kokorina, A. A. Bakal, D. V. Shpuntova, A. Y. Kostritskiy, N. V. Beloglazova, S. De Saeger, G. B. Sukhorukov, A. V. Sapelkin and I. Y. Goryacheva, Sci. Rep., 2019, 9, 1–8 CrossRef CAS PubMed
.
- A. Sachdev, I. Matai and P. Gopinath, RSC Adv., 2014, 4, 20915–20921 RSC
.
- J. S. Baker and L. A. Colón, J. Chromatogr. A, 2009, 1216, 9048–9054 CrossRef CAS PubMed
.
- H. P. Jen, Y. C. Tsai, H. L. Su and Y. Z. Hsieh, J. Chromatogr. A, 2006, 1111, 159–165 CrossRef CAS PubMed
.
- Q. Hu, M. C. Paau, Y. Zhang, W. Chan, X. Gong, L. Zhang and M. M. F. Choi, J. Chromatogr. A, 2013, 1304, 234–240 CrossRef CAS PubMed
.
- Y. Wu and V. T. Remcho, Talanta, 2016, 161, 854–859 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |