 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review of II–VI semiconductor nanoclusters for photocatalytic CO2 conversion: synthesis, characterization, and mechanisms
Kai
Li†
a,
Junjun
Ge†
b,
Enhao
Li
b,
Zhe
Li
b,
Hua
Wang
d,
Yuanyuan
Wang
 *b,
Yang
Zhou
*c and
Jun-Jie
Zhu
*b,
Yang
Zhou
*c and
Jun-Jie
Zhu
 *b
*b
aSchool of Science, Wuhan University of Science and Technology, Wuhan 430065, P. R. China
bState Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China. E-mail: wangyy@nju.edu.cn; jjzhu@nju.edu.cn
cKey Laboratory for Organic Electronics & Information Displays (KLOEID), Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications (NJUPT), Nanjing 210046, China. E-mail: iamyangzhou@njupt.edu.cn
dHuzhou Key Laboratory of Medical and Environmental Applications Technologies, School of Life Sciences, Huzhou University, Zhejiang 313000, P. R. China
First published on 7th July 2023
Abstract
The excessive consumption of fossil fuels has caused a severe energy shortage, and the large amount of CO2 released during the combustion process has disrupted the carbon balance in nature. Achieving photocatalytic CO2 reduction to high-value products is of high significance for both the economy and environment. So far, the bottlenecks for photocatalytic reduction of CO2 include low electron–hole separation efficiency and low CO2 productivity. II–VI semiconductor nanoclusters, especially magic-size clusters (MSCs), possess special chemical and physical properties such as an adjustable band gap (broadening the spectral response range), short carrier migration distance (favoring charge separation), and high surface-to-volume ratio (providing more active sites for CO2 adsorption and conversion), making them potent candidates for photocatalysis. This review briefly introduces the research progress in II–VI MSCs. Then, we summarize the recent advances in II–VI MSCs and related composites for photocatalytic CO2 reduction. Finally, the challenges and prospects of MSC-based photoelectron-catalytic systems are also discussed.
Broader contextIn recent years, photocatalytic CO2 conversion has attracted great interest, as it exhibits great theoretical value and application prospects. Notably, proper band structure, wide spectrum responsiveness, high affinity towards CO2 molecules, and high stability during the photocatalytic process are necessities for an ideal semiconductor photocatalyst to conduct photoconversion of CO2 with high efficiency. Besides these, a high turnover frequency of CO2 as well as high selectivity for the products are also crucial for its potential industrial application. However, it is difficult for traditional semiconductor nanostructures to possess all the advantages mentioned above, especially those comprised by single components. Fortunately, seeking innovation in materials is an effective pathway to break through the current bottleneck faced by photocatalytic CO2 reduction, in which II–VI “magic-size” clusters (MSCs) are one of the high-potential candidates. Theoretically, their tunable direct band gaps, short carrier migration distance, and high specific surface area make possible a highly efficient charge separation under a broad spectral irradiation, while providing sufficient active sites for the adsorption and activation of CO2; thus, they are expected to exhibit significantly enhanced photocatalytic performance in comparison with their larger-sized counterparts. Currently, the rational design, controllable synthesis, and precise modification of II–IV MSCs with customized photoelectron performance can be realized, which is attributed to the continuously deepening understanding of their growth mechanism with the assistance of various of in situ characterization techniques. However, studies on their photocatalytic performance for CO2 conversion are still in the computational simulation stage, with little experimental progress reported. Therefore, in this review, we briefly overview the syntheses, compositions, growth mechanisms, in situ characterization, and performance improvement strategies of typical II–VI MSCs. Next, recent advances on the application of II–VI MSCs for photocatalytic CO2 reduction are introduced. Although there are still few reports on the relevant experimental progress, computational simulations predict the feasibility and bright future of MSCs in this field. Finally, the current challenges and future prospects of MSC-based photoelectron catalytic systems are also discussed. |
1. Introduction
As the main factor disrupting the natural carbon balance and causing global warming, excessive CO2 emission from fossil fuel combustion is of particular concern.1–3 Converting CO2 into high-value-added fuels, chemicals, and other target compounds is not only a potential negative carbon emission strategy, but also an effective approach for the resourceful use of CO2, which is consistent with the concept of green and sustainable development.4–8 In recent years, photocatalytic CO2 conversion has aroused great interest, exhibiting great theoretical value and application prospects.9–12 Generally, an ideal material for photocatalytic CO2 conversion should be stable and able to respond to a wide range of solar spectra for the generation of photoinduced electron–hole pairs with high efficiency.9,12,13 In addition, a high turnover frequency towards CO2 and high selectivity for the products are also crucial, as this determines whether photocatalytic CO2 reduction has the potential for industrial application.13–15II–VI semiconductors with tunable direct band gaps have inherent advantages in designing and constructing high-performance photocatalysts.16–18 Among them, the II–VI magic-size clusters (II–VI MSCs), which are series of semiconductor nanoclusters composed of tens to hundreds of atoms from groups II (such as Zn, Cd, etc.) and VI (such as S and Se, etc.), with diameters ranging from 1 to 10 nanometers, are potential candidates for efficient photocatalytic CO2 conversion due to their high specific surface area, adjustable surface ligands, and excellent photoelectric performance.19–21 In Liu et al.'s research,22 magic-size (CdSe)13 was stored at room temperature for a whole year to evaluate its stability. They found that (CdSe)13 displayed better thermodynamic stability in comparison with other MSCs, and its morphology was maintained during the storage tests without any aggregation or decomposition. Besides, the band gap of CdSe nanoclusters can be accurately adjusted according to their size and surface ligand properties, thereby meeting the requirements of wide spectral responsiveness.23 Moreover, the small size and large specific surface area of semiconductor nanoclusters contribute to the exposure of catalytic active sites, making it easier for the combination and conversion of reactant molecules. Compared to the corresponding bulk materials, the average migration distance of photogenerated electron–hole pairs to the surface of MSCs is significantly shortened, which reduces their matrix recombination and increases the probability for them to initiate photoinduced redox reactions. Therefore, MSCs are very promising for the construction of photocatalytic systems with high performance, and they have met success in some pioneering works.24,25
However, there are still several challenges to using II–VI MSCs for photocatalytic CO2 conversion. On the one hand, the high dissociation energy of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in CO2 (about 750 kJ mol−1) means that improving the activation ability of MSCs for CO2 molecules is crucial to enabling efficient photocatalytic reactions over a wide spectral range.26 On the other hand, the tunable band structure of MSCs still needs to be optimized to make the conductive band (CB) more negative than the reduction potential of CO2, as shown in Table 1.27 The stability of MSCs in the appropriate solvent for CO2 is another key point that contributes to the adsorption and conversion of CO2 on their surface. Using MSCs as a cocatalyst to construct a composite photocatalytic system could be a promising solution.28–31 Recently, research on II–VI nanostructure (nanoplates, nanoparticles, quantum dots, etc.)-involved heterojunctions for photocatalytic CO2 reduction has made some progress.28–30 It is reasonable to believe that using II–VI MSCs with clear structure, larger specific surface area, and tunable energy band as a substitute will significantly improve photocatalytic performance, although the related research is still rare.
O in CO2 (about 750 kJ mol−1) means that improving the activation ability of MSCs for CO2 molecules is crucial to enabling efficient photocatalytic reactions over a wide spectral range.26 On the other hand, the tunable band structure of MSCs still needs to be optimized to make the conductive band (CB) more negative than the reduction potential of CO2, as shown in Table 1.27 The stability of MSCs in the appropriate solvent for CO2 is another key point that contributes to the adsorption and conversion of CO2 on their surface. Using MSCs as a cocatalyst to construct a composite photocatalytic system could be a promising solution.28–31 Recently, research on II–VI nanostructure (nanoplates, nanoparticles, quantum dots, etc.)-involved heterojunctions for photocatalytic CO2 reduction has made some progress.28–30 It is reasonable to believe that using II–VI MSCs with clear structure, larger specific surface area, and tunable energy band as a substitute will significantly improve photocatalytic performance, although the related research is still rare.
| Reactions | E 0 (V) vs. NHE at pH 7 | |
|---|---|---|
| 1 | 2H2O + 4h+ → O2 + 4H+ | 1.23 |
| 2 | CO2 + e− → CO2− | −1.9 |
| 3 | CO2 + 2H+ + 2e− → CO + H2O | −0.53 |
| 4 | CO2 + 2H+ + 2e− → HCOOH | −0.61 |
| 5 | CO2 + 4H+ + 4e− → HCHO +H2O | −0.48 |
| 6 | CO2 + 6H+ + 6e− → CH3OH + H2O | −0.38 |
| 7 | CO2 + 8H+ + 8e− → CH4 + 2H2O | −0.24 |
| 8 | 2H+ + 2e− → H2 | −0.41 |
The composition, morphology, and surface ligands of MSCs can be controlled through rational design and targeted synthesis strategies, which are key factors determining their optoelectronic properties and may have significant influence on their potential photocatalytic performance. Therefore, in this review, we first briefly overview the synthesis and compositions, growth mechanisms, and performance improvement strategies of typical II–VI MSCs. Then, recent advances on the application of II–VI MSCs for photocatalytic CO2 reduction are introduced. Although there are still few reports on the relevant experimental progress, computational simulations predict the feasibility and bright future of MSCs in this field. Finally, the current challenges and future prospects of MSC-based photoelectron-catalytic systems are also discussed.
2. Synthesis and growth mechanism of typical II–VI MSCs
Research has shown that MSCs are essential intermediates in the formation of II–VI semiconductor nanostructures, such as nanobelts, nanoribbons, and quantum dots.32–37 As is known, these metal chalcogenide nanomaterials with great potential in the fields of optoelectronic manufacturing,16,38,39 photo/photothermal catalysis,17,18 and new energy40,41 have been studied in depth and seen great progress in the past few decades. In contrast, current topics are more focused on their ultrasmall intermediates due to the quantum confinement that brings about special phenomena and significantly enhances the conventional performance.In relevant research, the first issue that needs to be addressed is the synthesis of MSCs. Except for the isolation of existing MSCs during the formation of larger II–VI semiconductor nanostructures, direct synthesis strategies have been developed to obtain MSCs with the desired stoichiometry and make it possible to regulate and control the performance of the products.33–37,42–44 Since the successful synthesis of (CdSe)33,34 clusters by Kasuya et al. in 2004,44 numerous direct-synthesized MSCs as well as other synthetic methodologies have been reported. Zhou and colleagues are one of the first groups to conduct the synthesis and separation of II–VI semiconductor nanoclusters, pioneering the synthesis of nine II–VI “magic size” inorganic semiconductor nanoclusters.45,46
Among them, the strategy (see Fig. 1(a)–(c)) proposed by Buhro et al. is regarded as a universal one for the synthesis of most (ME)n clusters (M: metal, E: chalcogenide).47,48 Typically, a metal-salt primary-amine bilayer is formed firstly as a template. After adding chalcogenide precursor into the reaction system, (ME)n cluster is generated and released from templates due to the ligand-exchange-induced unbundling process. On this basis, the as-obtained (CdS)13 MSCs could act as a single precursor to form colloidal CdS nanoplatelets with a wurtzite structure.49 In addition, Yu et al.50 demonstrated for the first time how colloidal semiconductor MSCs undergo isomerization at low temperatures under external chemical stimuli, namely, MSC-399 to MSC-422. Notably, ion exchange makes it possible for as-obtained MSCs to transform into their counterparts, indicating that new MSCs can be synthesized via a template-based approach (see Fig. 1(d)).
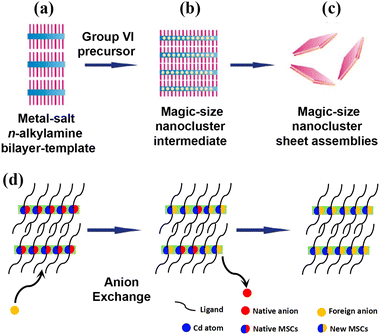 | ||
| Fig. 1 (a) The combination of metal salts and primary-amine solvents forms lamellar, primary-amine-bilayer templates. (b) The addition of group VI precursors results in the growth of magic-size nanoclusters within the templates. (c) Ligand exchange with oleylamine liberates sheetlike aggregates. (d) Formation of new MSCs via an anion exchange pathway. Reproduced from ref. 48 and 49 with permission from the American Chemical Society. | ||
Precursor compounds (PCs) also play a vital role in the formation of MSCs. As reported by Yang et al., two different MSCs (MSC-299 and MSC-328) were transformed from ZnSe PCs by introducing octylamine and acetic acid into the reaction system at room temperature. Their study proposes the composition of PCs for the first time (ZnSe PCs with a formula of Zn16Se32) and determines the formation of the covalent bond Zn–Se in ZnSe PCs, therefore deepening the understanding of the transformation relationship between PCs and MSCs and providing a new path for the synthesis of MSCs. In addition, diphenylphosphine (DPP) can inhibit the decomposition of Zn precursors and reduce the generation of by-products, which is also considered to play a crucial role in the synthesis of other PCs and related MSCs.
It is worth noting that MSCs with special stability always require “jumps” to obtain a series of crystallites with no intermediate sizes. In order to further clarify the growth mechanism of MSCs, nine magic-sized CdSe clusters with increased sizes were synthesized by Mule and coworkers, together with the proposition of a microscopic model based on classical nucleation theory.34 Results show that these nanocrystals grow layer by layer from one size to the next-larger size sequentially, which could be ascribed to the synergism of size-dependent nanoscale effects and the tetrahedral shape, while the surface-reaction-limited conditions take charge. Although this study only focuses on the growth of CdSe MSCs, the general growth mechanism is valuable for the study of related II–VI semiconductor MSCs and can provide guidance for their synthesis.
3. In situ characterization of typical II–VI MSCs
As is known, traditional non-in situ characterization techniques require a certain pre-treatment procedure for the test samples to meet testing requirements. During the process, some important information about their morphology, structure, as well as surface properties may be lost. Moreover, the detection of reaction intermediates is difficult to achieve using non-in situ characterization techniques. Therefore, researchers have turned to in situ characterization techniques for assistance, in order to monitor the reaction process in real time and speculate on the possible reaction mechanism by analyzing the intermediates in each stage of the reaction. To date, in situ characterization technologies run through the synthesis, separation, and performance evaluation of MSCs to determine their composition, structure, and physicochemical properties, while avoiding damage to them during the above process. Typically, time-of-flight mass spectrometry (TOF-MS) can provide exact information about the molecular mass and possible structures of MSCs by relating the molecular mass and the isotope patterns, which also helps to elucidate the formation mechanism of specific MSCs. As reported by Kasuya et al., highly stable mass-to-charge ratios (m/z) related to (CdSe)33 and (CdSe)34 were obtained by using laser desorption ionization mass spectroscopy (LDI-MS).44 Meanwhile, the detachment of surface ligands in MSCs during the ionization process is of great significance for studying the surface properties of MSCs, which has significant impacts on their photoelectric performance.51–53 In addition, this relatively mature technique also contributes to determining the mesostructures formed by the assembly of MSCs.54On this basis, more information about the core and shell of MSCs can be obtained using nuclear magnetic resonance (NMR) technologies.55–57 According to Li's research, different Cd atoms (in the core or on the surface) can be differentiated by comparing the 113Cd cross-polarization magic-angle spinning (CP-MAS) and 113Cd MAS NMR spectra.57
The structure of MSCs is another current focus that can be determined by small-angle X-ray scattering (SAXS). Benefiting from the well-defined peaks, SAXS not only can characterize the crystal structure of MSCs but also demonstrate the existence of intermediates and mesophases during their formation, further elucidating their growth pathways.58,59 Moreover, the local environment, especially the structural changes induced by different capping ligands, can be investigated by extended X-ray absorption fine structure spectroscopy (EXAFS).60
Obviously, through a series of in situ characterization techniques, the composition, structure, size, surface state, and growth mechanism of MSCs have been clearly revealed (see Table 2), providing valuable information for their future targeted design and modification to meet the needs of photocatalytic CO2 reduction.
| Methods | Research object | Applications | Ref. |
|---|---|---|---|
| TOF-MS | Study the molecular mass, possible structures, surface ligands, growth pathways of MSCs | Obtaining highly stable mass-to-charge ratios (m/z) related to (CdSe)33 and (CdSe)34 | 44 and 51–54 |
| NMR | Investigate the inorganic core and organic shell (ligands) of MSCs | Distinguishing 113Cd atoms in the core or on the surface of (CdSe)13 | 55–57 |
| SAXS | Analyze the crystal structure, size and shape, and growth pathways (intermediates and mesophases) of MSCs | Demonstrating the presence of intermediates formed during the induction period of CdTe MSCs | 58–60 |
| EXAFS | Monitor the structural changes of MSCs induced by different capping ligands | Discovering the relationship between Cd–S distance in CdS MSCs and their surface ligands (longer for thiol capping ligands, shorter for polyphosphonate capping ligands) | 61 |
4. Modification on typical II–VI MSCs for enhanced performance
Doping or alloying can provide additional assistance for the property modulation and enhancement of MSCs, although it is more challenging to obtain MSCs with controlled amount of dopant, as compared to implementing the technique on larger semiconductor nanostructures.61–63 As reported by Hyeon, MnxCd13−xSe13 nanoclusters (x = 0–2) with bright-orange photoluminescence (an evidence of Mn2+ doping) were synthesized by taking a modified procedure applied for preparing the Mn2+-doped CdSe nanoribbons.62 The systemic introduction of Mn2+ with controllable amount endows the production of MSCs with unique magneto-optical characteristics. This scalable method can also guide the synthesis of CoxCd13−xSe13 nanoclusters (x = 0–2), although there is a significant mismatch in the radii of Co2+ and Cd2+.63 Notably, ion exchange makes it possible for as-obtained MSCs to transform into their counterparts, indicating that new MSCs can be synthesized via a template-based approach (see Fig. 1(d)). White et al. found that reorganization of the Cd2+ sublattice in (CdSe)33,34 initiated by Cu+ doping (treating with tetrakis(acetonitrile) copper(I) hexafluorophosphate ([(CH3CN)4C-u]PF6) in an oxygen-free and moisture-free glove box) created a meta-stable six-coordinate structure.64 The stronger affinity between this new structure and Cu+ ensured continuous cation exchange, thereby successfully converting (CdSe)33,34 into Cu2Se for the first time. As reported by T. Hyeon,65 the CO2 cycloaddition reaction (from propylene oxide to propylene carbonate) catalyzed by Mn2+:(Cd0.5Zn0.5Se)13 MSCs suprastructures has a conversion rate of up to 95%. This new insight into the dynamics of ion exchange at the solid–liquid interface is a good supplement to the doping strategy, providing new ideas for the introduction of dopants into the MSC matrix with an accurate amount.It should be pointed out that all the synthesis, modification, and performance improvement strategies mentioned above serve the potential applications of MSCs. Based on the scope of this review, we will focus on the application of MSCs in the field of photocatalysis in the next section.
5. II–VI MSCs for photocatalytic CO2 reduction
As mentioned earlier, the shorter carrier migration distance in MSCs increases the probability of photogenerated electron–hole pairs transferring to the surface of the nanoclusters. On this basis, the charge separation efficiency of MSCs can be further improved by constructing heterostructures, thereby enhancing the photocatalytic performance of the nanocomposite. According to Choudhuri's calculations, encapsulating the CdSe nanocluster (Cd6Se6) in NU-1000 (a metal–organic framework, MOF) allows the direct transfer of electrons (generated in the highest occupied crystal orbital [HOCO] on the linker of NU-1000 under visible light irradiation) to the lowest unoccupied crystal orbital (LUCO) of Cd6Se6 (see Fig. 2), leading to effective charge separation and longer lifetime of the excited state, which is beneficial for photocatalytic reactions.66 Another important challenge faced by the chemical conversion of CO2 is the activation process. Since structural irregularity at the atomic scale may endow small anionic metal clusters (Mn−) with superior catalytic performance, theoretical research was conducted by Lim et al. to investigate the anionic activation of CO2 on these tiny nanostructures.67 Results based on density functional theory show that activated complexes (Mn–CO2)− were produced by the magic-numbered Cu, Ag, and Au clusters with 1, 2, and 6 atoms. Owing to the formation of M–C bond between Mn− and CO2 that is partially covalent, completely delocalized electrons were transferred from the HOMO of Mn− to the LUMO of CO2, resulting in the chemical activation of CO2. Obviously, with the deepening of research on the synthesis of doped or alloyed MSCs through ion exchange, it is reasonable to believe that this strategy can effectively introduce the abovementioned metal species into MSCs to enhance their adsorption and activation ability for CO2, thus laying the foundation for high-performance photocatalytic CO2 reduction. On this basis, a concept for efficiently selective reduction of CO2 to CH3OH by the ternary composites (CuN/MoS2/Ag(111), N = 1–8) was theoretically established, demonstrating the feasibility of MSC-included systems to convert CO2 into value-added chemicals.68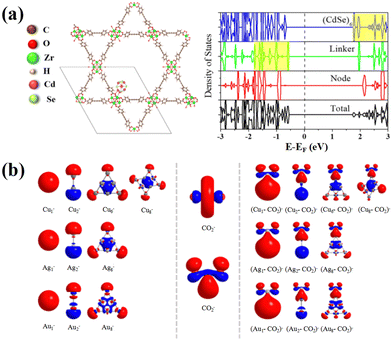 | ||
| Fig. 2 (a) Structure of the Cd6Se6 cluster encapsulated in NU-1000 at a node site (Cd6Se6@NU-1000); total and partial density of states of Cd6Se6@NU-1000. (b) HOMOs of Mn− clusters, LUMO of CO2 (top) and HOMO of CO2− (bottom), and HOMOs of (Mn–CO2)− complexes calculated by B3LYP functional (M = Cu, Ag, Au; n = 1, 2, 6 (and 8 for Cu)). Reproduced from ref. 66 and 67 with permission from the American Chemical Society. | ||
It is worth noting that although theoretical simulations predict the potential of MSCs in photocatalytic CO2 reduction, there are still few reports on the relevant experimental progress. Fortunately, recent advances on the conventional II–VI semiconductor nanostructures for photocatalytic CO2 reduction have great reference significance.28–31,41 In our recent work, CdS/TiO2:Cu hollow spheres were constructed for photocatalytic CO2 reduction with the assistance of H2S (see Fig. 3). The relatively high generation rates of CO (781.3 μmol g−1 h−1) and H2 (5875.1 μmol g−1 h−1) indicate the high performance of the nanocomposite in the synergistic conversion of these two environmental hazards, as well as the huge potential for syngas production. Considering the enormous advantages of MSCs, it is highly anticipated that they can replace traditional II–VI nanostructures to achieve significantly enhanced performance in photocatalytic CO2 reduction.
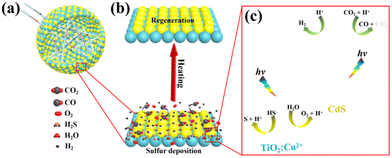 | ||
| Fig. 3 (a) The complex scattering of light in the CdS/TiO2:Cu (CTC) hollow spheres. (b) Regeneration of the catalyst by heat treatment desulfurization. (c) The step-scheme charge transfer diagram between CdS and TiO2:Cu, as well as the redox reactions of the gaseous adsorbates under the full spectrum of xenon lamp. Reproduced from ref. 24 with permission from Wiley-VCH. | ||
6. Conclusions
The advancement of synthesis and isolation methods, combined with modern characterization techniques, has given a deeper understanding of the composition, structure, and optoelectronic properties of II–VI MSCs. Moreover, the determination of growth mechanisms makes possible the rational design, controllable synthesis, and precise modification of II–VI MSCs with customized performance. On this basis, the potential application of II–VI MSCs as a highly efficient photocatalyst was developed.In view of the increasingly severe energy and environmental crises, as well as the steady progress of carbon peaking and carbon neutrality goals, it seems that the use of II–VI MSCs for photocatalytic CO2 conversion can give full play to its advantages. In our opinion, progress still needs to be made in the following aspects before this can be realized:
(1) A comprehensive consideration of various factors, such as mass production of high-purity MSCs, regulation of their spectral response range, stability in CO2-rich media, adsorption and activation capacity of CO2, and product selectivity is in great demand, which also points to the lack of experimental verification at this stage.
(2) It is worth noting that the main product of photocatalytic CO2 reduction now is still CO. Since C–C coupling starting with CO is relatively mature during the electrocatalytic process, combining photocatalysis and electrocatalysis to construct an optoelectronic coupling cathode (realizing the cascade of photocatalytic CO2 reduction by II–VI MSCs and electrocatalytic C–C coupling by noble metals or Cu) to achieve the conversion of CO2 to high-value multicarbon compounds is extremely attractive.69–73
(3) Combining the cathode with a high-efficiency photo anode to construct an all-light (solar)-driven self-biasing catalytic system, in which green and efficient CO2 conversion without conventional energy consumption can be realized, is expected to become the ultimate goal in this field.74,75
It is worth noting that there are still many challenges to using II–VI MSCs for photocatalytic CO2 reduction and constructing efficient CO2 conversion systems driven by all-solar energy. However, with the continuous improvement of theoretical simulations, it is reasonable to believe that breakthroughs in related experimental research will be made in the near future. We hope that this review can inspire some constructive ideas and provide practical guidance and assistance for related work.
Author contributions
J. Ge, E. Li, Z. Li, H. Wang, Y. Wang, and J. Zhu provided important and constructive suggestions for this review. K. Li and J. Ge designed and wrote this review. E. Li edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Natural Science Foundation of Jiangsu Province (BK20220405), National Natural Science Foundation of China (22276100, 22171132), Research Fund for Jiangsu Distinguished Professor (RK030STP22001), and Natural Science Foundation of Shandong Province (ZR2020ZD37).Notes and references
- P. C. Frumhoff, R. Heede and N. Oreskes, Clim. Change, 2015, 132, 157–171 CrossRef.
- H. D. Matthews, N. P. Gillett, P. A. Stott and K. Zickfeld, Nature, 2009, 459, 829 CrossRef CAS PubMed.
- J. Fu, P. Li, Y. Lin, H. Du, H. Liu, W. Zhu and H. Ren, Eco-Environ. Health, 2022, 1, 259–279 CrossRef.
- T. Gasser, C. Guivarch, K. Tachiiri, C. D. Jones and P. Ciais, Nat. Commun., 2015, 6, 7988 CrossRef PubMed.
- Z. J. Wang, H. Song, H. M. Liu and J. H. Ye, Angew. Chem., Int. Ed., 2020, 59, 8016–8035 CrossRef CAS PubMed.
- S. Uden, P. Dargusch and C. Greig, Joule, 2021, 5, 1956–1970 CrossRef.
- J. Liu, J. Fu, Y. Zhou, W. Zhu, L. P. Jiang and Y. Lin, Nano Lett., 2020, 20(7), 4823–4828 CrossRef CAS PubMed.
- L. X. Liu, J. Fu, L. P. Jiang, J. R. Zhang, W. Zhu and Y. Lin, ACS Appl. Mater. Interfaces, 2019, 11(29), 26024–26031 CrossRef CAS PubMed.
- G. Chen, G. I. N. Waterhouse, R. Shi, J. Zhao, Z. Li, L.-Z. Wu, C.-H. Tung and T. Zhang, Angew. Chem., Int. Ed., 2019, 58(49), 17528–17551 CrossRef CAS PubMed.
- Y. Zhao, G. I. N. Waterhouse, G. Chen, X. Xiong, L.-Z. Wu, C.-H. Tunga and T. Zhang, Chem. Soc. Rev., 2019, 48(7), 1972–2010 RSC.
- A. Wagner, C. D. Sahm and E. Reisner, Nat. Catal., 2020, 3, 775–786 CrossRef CAS.
- X. Yang, K. Li, G. Wang, X. Li, P. Zhou, S. Ding, Z. Lyu, Y. C. Chang, Y. Zhou and W. Zhu, Chem. – Eur. J., 2022, 28(26), e202201881 CAS.
- X. Li, J. Yu, M. Jaroniec and X. Chen, Chem. Rev., 2019, 119, 3962–4179 CrossRef CAS PubMed.
- S. Navarro-Jaén, M. Virginie, J. Bonin, M. Robert, R. Wojcieszak and A. Y. Khodakov, Nat. Rev. Chem., 2021, 5, 564–579 CrossRef PubMed.
- S. Yoshino, A. Iwase, Y. Yamaguchi, T. M. Suzuki, T. Morikawa and A. Kudo, J. Am. Chem. Soc., 2022, 144, 2323–2332 CrossRef CAS PubMed.
- B. T. Diroll, B. Guzelturk, H. Po, C. Dabard, N. Y. Fu, L. Makke, E. Lhuillier and S. Ithurria, Chem. Rev., 2023, 123(7), 3543–3624 CrossRef CAS PubMed.
- A. Vaneski, J. Schneider, A. S. Susha and A. L. Rogach, J. Photochem. Photobiol., C, 2014, 19, 52–61 CrossRef CAS.
- Q. Guo, S. G. Xia, Z. K. Xin, Y. Wang, F. Liang, X. L. Nan, Z. S. Lin, X. B. Li, C. H. Tung and L. Z. Wu, J. Mater. Chem. A, 2023, 11, 3937–3941 RSC.
- J. J. Ge, J. Liang, X. F. Chen, Y. L. Deng, P. W. Xiao, J. J. Zhu and Y. Y. Wang, Chem. Sci., 2022, 13, 11755–11763 RSC.
- X. F. Chen, J. J. Ge, P. W. Xiao, Y. L. Deng and Y. Y. Wang, Nano Res., 2023, 16, 3387–3394 CrossRef CAS.
- A. Vartanian, Nat. Rev. Mater., 2022, 7, 596 CrossRef.
- T. E. Hsieh, T. W. Yang, C. Y. Hsieh, S. J. Huang, Y. Q. Yeh, C. H. Chen, E. Y. Li and Y. H. Liu, Chem. Mater., 2018, 30, 5468–5477 CrossRef CAS.
- V. Singh, Priyanka, P. V. More, E. Hemmer, Y. K. Mishra and P. K. Khanna, Mater. Adv., 2021, 2, 1204–1228 RSC.
- P. Wang, Q. Q. Yang, C. Xu, B. Wang, H. Wang, J. D. Zhang and Y. D. Jin, Nano Res., 2022, 15, 3106–3113 CrossRef CAS.
- R. Shi, Y. H. Cao, Y. J. Bao, Y. F. Zhao, G. I. N. Waterhouse, Z. Y. Fang, L. Z. Wu, C. H. Tung, Y. D. Yin and T. R. Zhang, Adv. Mater., 2017, 29, 7 Search PubMed.
- K. H. Kim, S. Kim, B. C. Moon, J. W. Choi, H. M. Jeong, Y. Kwon, S. Kwon, H. S. Choi and J. K. Kang, J. Mater. Chem. A, 2017, 5, 8274–8279 RSC.
- L. F. Wei, C. L. Yu, Q. H. Zhang, H. Liu and Y. Wang, J. Mater. Chem. A, 2018, 6, 22411–22436 RSC.
- Y. Huo, J. F. Zhang, K. Dai and C. H. Liang, ACS Appl. Energy Mater., 2021, 4, 956–968 CrossRef CAS.
- A. Li, T. Wang, C. C. Li, Z. Q. Huang, Z. B. Luo and J. L. Gong, Angew. Chem., Int. Ed., 2019, 58, 3804–3808 CrossRef CAS PubMed.
- H. Cho, W. D. Kim, K. Lee, S. Lee, G. S. Kang, H. L. Joh and D. C. Lee, Appl. Surf. Sci., 2018, 429, 2–8 CrossRef CAS.
- K. Li, Y. M. Cai, X. H. Yang, S. Wang, C. Teng, Y. Tian, Q. H. Min and W. L. Zhu, Adv. Funct. Mater., 2022, 32, 2113002 CrossRef CAS.
- Z. J. Jiang and D. F. Kelley, ACS Nano, 2010, 4, 1561–1572 CrossRef CAS PubMed.
- M. S. Bootharaju, W. Baek, S. Lee, H. Chang, J. Kim and T. Hyeon, Small, 2021, 17, 2002067 CrossRef CAS PubMed.
- A. S. Mule, S. Mazzotti, A. A. Rossinelli, M. Aellen, P. T. Prins, J. C. van der Bok, S. F. Solari, Y. M. Glauser, P. V. Kumar, A. Riedinger and D. J. Norris, J. Am. Chem. Soc., 2021, 143, 2037–2048 CrossRef CAS PubMed.
- Y. Kwon and S. Kim, NPG Asia Mater., 2021, 13, 37 CrossRef CAS.
- J. Zhang, X. Y. Hao, N. Rowell, T. Kreouzis, S. Han, H. S. Fan, C. C. Zhang, C. W. Hu, M. Zhang and K. Yu, J. Phys. Chem. Lett., 2018, 9, 3660–3666 CrossRef CAS PubMed.
- C. R. Luan, J. B. Tang, N. Rowell, M. Zhang, W. Huang, H. S. Fan and K. Yu, J. Phys. Chem. Lett., 2019, 10, 4345–4353 CrossRef CAS PubMed.
- K. Pal, A. Si, G. S. El-Sayyad, M. Abd Elkodous, R. Kumar, A. I. El-Batal, S. Kralj and S. Thomas, Crit. Rev. Solid State Mater. Sci., 2021, 46, 385–449 CrossRef CAS.
- M. F. Peng, Z. Wen and X. H. Sun, Adv. Funct. Mater., 2023, 33, 2211548 CrossRef CAS.
- Y. Xu, G. D. Li, R. S. Li, Y. Jing, H. Y. Zhang, X. Wang, Z. B. Du, J. H. Wu and Z. Lan, Nano Energy, 2022, 95, 106973 CrossRef CAS.
- K. H. Li, X. K. Yang, Y. Lu, J. Y. Xue, S. C. Lu, J. J. Zheng, C. Chen and J. Tang, Adv. Energy Mater., 2022, 12, 2200725 CrossRef CAS.
- W. Baek, M. S. Bootharaju, K. M. Walsh, S. Lee, D. R. Gamelin and T. Hyeon, Nat. Mater., 2021, 20, 650–657 CrossRef CAS PubMed.
- X. X. Yang, M. Zhang, Q. Shen, Y. Li, C. R. Luan and K. Yu, Nano Res., 2022, 15, 465–474 CrossRef CAS.
- A. Kasuya, R. Sivamohan, Y. A. Barnakov, I. M. Dmitruk, T. Nirasawa, V. R. Romanyuk, V. Kumar, S. V. Mamykin, K. Tohji, B. Jeyadevan, K. Shinoda, T. Kudo, O. Terasaki, Z. Liu, R. V. Belosludov, V. Sundararajan and Y. Kawazoe, Nat. Mater., 2004, 3, 99–102 CrossRef CAS PubMed.
- Y. Zhou, F. D. Wang and W. E. Buhro, J. Am. Chem. Soc., 2015, 137, 15198–15208 CrossRef CAS PubMed.
- Y. Zhou, F. D. Wang and W. E. Buhro, Chem. Mater., 2020, 32, 8350–8360 CrossRef CAS.
- Y. Y. Wang, Y. H. Liu, Y. Zhang, F. D. Wang, P. J. Kowalski, H. W. Rohrs, R. A. Loomis, M. L. Gross and W. E. Buhro, Angew. Chem., Int. Ed., 2012, 51, 6154–6157 CrossRef CAS PubMed.
- Y. Y. Wang, Y. Zhou, Y. Zhang and W. E. Buhro, Inorg. Chem., 2015, 54, 1165–1177 CrossRef CAS PubMed.
- Y. L. Deng, J. Liang, X. K. Kong, P. W. Xiao, Y. Zhou and Y. Y. Wang, Chem. Mater., 2023, 35, 2463–2471 CrossRef CAS.
- Y. S. Yang, Q. Shen, C. C. Zhang, N. Rowell, M. Zhang, X. Q. Chen, C. R. Luan and K. Yu, ACS Cent. Sci., 2023, 9, 519–530 CrossRef CAS PubMed.
- Y. Wang, Y. Zhang, F. Wang, D. E. Giblin, J. Hoy, H. W. Rohrs, R. A. Loomis and W. E. Buhro, Chem. Mater., 2014, 26, 2233–2243 CrossRef CAS PubMed.
- S. Dolai, P. R. Nimmala, M. Mandal, B. B. Muhoberac, K. Dria, A. Dass and R. Sardar, Chem. Mater., 2014, 26, 1278–1285 CrossRef CAS.
- M. Li, J. Ouyang, C. I. Ratcliffe, L. Pietri, X. Wu, D. M. Leek, I. Moudrakovski, Q. Lin, B. Yang and K. Yu, ACS Nano, 2009, 3, 3832–3838 CrossRef CAS PubMed.
- D. Wurmbrand, J. W. A. Fischer, R. Rosenberg and K. Boldt, Chem. Commun., 2018, 54, 7358–7361 RSC.
- R. Wang, J. Ouyang, S. Nikolaus, L. Brestaz, M. B. Zaman, X. Wu, D. Leek, C. I. Ratcliffe and K. Yu, Chem. Commun., 2009, 962–964 RSC.
- R. Wang, C. I. Ratcliffe, X. Wu, O. Voznyy, Y. Tao and K. Yu, J. Phys. Chem. C, 2009, 113, 17979–17982 CrossRef CAS.
- T. E. Hsieh, T. W. Yang, C. Y. Hsieh, S. J. Huang, Y. Q. Yeh, C. H. Chen, E. Y. Li and Y. H. Liu, Chem. Mater., 2018, 30, 5468–5477 CrossRef CAS.
- B. Abecassis, C. Bouet, C. Garnero, D. Constantin, N. Lequeux, S. Ithurria, B. Dubertret, B. R. Pauw and D. Pontoni, Nano Lett., 2015, 15, 2620–2626 CrossRef CAS PubMed.
- M. Liu, K. Wang, L. Wang, S. Han, H. Fan, N. Rowell, J. A. Ripmeester, R. Renoud, F. Bian, J. Zeng and K. Yu, Nat. Commun., 2017, 8, 15467 CrossRef CAS PubMed.
- J. Rockenberger, L. Tröger, A. Kornowski, T. Vossmeyer, A. Eychmüller, J. Feldhaus and H. Weller, J. Phys. Chem. B, 1997, 101, 2691–2701 CrossRef CAS.
- J. H. Yu, X. Y. Liu, K. E. Kweon, J. Joo, J. Park, K. T. Ko, D. Lee, S. P. Shen, K. Tivakornsasithorn, J. S. Son, J. H. Park, Y. W. Kim, G. S. Hwang, M. Dobrowolska, J. K. Furdyna and T. Hyeon, Nat. Mater., 2010, 9, 47–53 CrossRef CAS PubMed.
- J. Yang, R. Fainblat, S. G. Kwon, F. Muckel, J. H. Yu, H. Terlinden, B. H. Kim, D. Iavarone, M. K. Choi, I. Y. Kim, I. Park, H. K. Hong, J. Lee, J. S. Son, Z. Lee, K. Kang, S. J. Hwang, G. Bacher and T. Hyeon, J. Am. Chem. Soc., 2015, 137, 12776–12779 CrossRef CAS PubMed.
- F. Muckel, J. Yang, S. Lorenz, W. Baek, H. Chang, T. Hyeon, G. Bacher and R. Fainblat, ACS Nano, 2016, 10, 7135–7141 CrossRef CAS PubMed.
- S. L. White, P. Banerjee, I. Chakraborty and P. K. Jain, Chem. Mater., 2016, 28, 8391–8398 CrossRef CAS.
- W. Baek, M. S. Bootharaju, K. M. Walsh, S. Lee, D. R. Gamelin and T. Hyeon, Nat. Mater., 2021, 20, 650–657 CrossRef CAS PubMed.
- I. Choudhuri and D. G. Truhlar, J. Phys. Chem. C, 2020, 124, 8504–8513 CrossRef CAS.
- E. Lim, J. Heo, X. X. Zhang, K. H. Bowen, S. H. Lee and S. K. Kim, J. Phys. Chem. A, 2021, 125, 2243–2248 CrossRef CAS PubMed.
- Y. W. Wang, Y. D. Zhu, X. W. Zhu, J. L. Shi, X. Y. Ren, L. L. Zhang and S. F. Li, ACS Catal., 2023, 13(1), 714–724 CrossRef CAS.
- H. J. Lu, Z. L. Wang and L. Z. Wang, ACS EST Engg., 2022, 2(6), 975–988 CrossRef CAS.
- C. B. Chen, Y. F. Li, S. Yu, S. Louisia, J. B. Jin, M. F. Li, M. B. Ross and P. D. Yang, Joule, 2020, 4, 1688–1699 CrossRef CAS.
- J. F. Huang, M. Mensi, E. Oveisi, V. Mantella and R. Buonsanti, J. Am. Chem. Soc., 2019, 141, 2490–2499 CrossRef CAS PubMed.
- R. Kortlever, J. Shen, K. J. P. Schouten, F. Calle-Vallejo and M. T. M. Koper, J. Phys. Chem. Lett., 2015, 6, 4073–4082 CrossRef CAS PubMed.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Y. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Norskov, T. F. Jaramillo and I. Chorkendorff, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS PubMed.
- V. Andrei, B. Reuillard and E. Reisner, Nat. Mater., 2020, 19, 189–194 CrossRef CAS PubMed.
- Y. J. Jang, I. Jeong, J. Lee, J. Lee, M. J. Ko and J. S. Lee, ACS Nano, 2016, 10, 6980–6987 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |
