Open Access Article
Open Access ArticleZIF-8 metal organic framework materials as a superb platform for the removal and photocatalytic degradation of organic pollutants: a review
Aicha Elaouni†
a,
M. El Ouardi†
ab,
M. Zbair
 cd,
A. BaQais
e,
M. Saadi
cd,
A. BaQais
e,
M. Saadi
 a and
H. Ait Ahsaine
a and
H. Ait Ahsaine
 *a
*a
aLaboratoire de Chimie Appliquée des Matériaux, Centre des Sciences des Matériaux, Faculty of Sciences, Mohammed V University in Rabat, Morocco. E-mail: h.aitahsaine@um5r.ac.ma
bUniversité de Toulon, AMU, CNRS, IM2NP, CS 60584, Toulon Cedex 9, F-83041, France
cUniversité de Haute-Alsace, CNRS, IS2M UMR 7361, F-68100 Mulhouse, France
dUniversité de Strasbourg, 67081, Strasbourg, France
eDepartment of Chemistry, College of Science, Princess Nourah Bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia
First published on 7th November 2022
Abstract
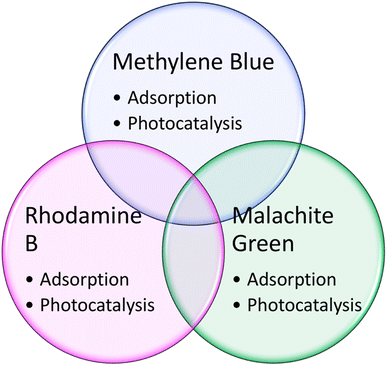
Metal organic frameworks (MOFs) are attracting significant attention for applications including adsorption, chemical sensing, gas separation, photocatalysis, electrocatalysis and catalysis. In particular, zeolitic imidazolate framework 8 (ZIF-8), which is composed of zinc ions and imidazolate ligands, have been applied in different areas of catalysis due to its outstanding structural and textural properties. It possesses a highly porous structure and chemical and thermal stability under varying reaction conditions. When used alone in the reaction medium, the ZIF-8 particles tend to agglomerate, which inhibits their removal efficiency and selectivity. This results in their mediocre reusability and separation from aqueous conditions. Thus, to overcome these drawbacks, several well-designed ZIF-8 structures have emerged by forming composites and heterostructures and doping. This review focuses on the recent advances on the use of ZIF-8 structures (doping, composites, heterostructures, etc.) in the removal and photodegradation of persistent organic pollutants. We focus on the adsorption and photocatalysis of three main organic pollutants (methylene blue, rhodamine B, and malachite green). Finally, the key challenges, prospects and future directions are outlined to give insights into game-changing breakthroughs in this area.
| A. BaQais Amal BaQais is an Assistant Professor at Princess Nourah Bint Abdulrahman University-Riyadh-KSA (PNU), Chemistry Department. She completed her PhD Degree in 2019 under the supervision of Prof. Takanabe at King Abdullah University of Science and Technology (KAUST), Saudi Arabia. She is working on designing materials for energy conversion and storage applications. Dr Amal is currently working at the Research and Innovation Centre at the Engineering College. |
1. Introduction
The increase in industrial activity and development of many key industries (agricultural, textile, and pharmaceutical) has led to serious pollution of the ecosystem. Consequently, pollutants including dyes, heavy metals, pharmaceuticals and agricultural based-chemicals have been found in water.1–6 Accordingly, various technologies have been used for wastewater treatment to degrade or remove these persistent organic pollutants such as adsorption,7 filtration,8 membrane technology,9 coagulation,10 electrochemical oxidation11,12 and photocatalytic degradation.13–16 Besides, the transformation of concentrated or divided contaminants into non-toxic materials is a critical issue. Considering both energy and the economy, it is desirable to treat pollutants through adsorption or photocatalytic reactions.17–19 Accordingly, to achieve an enhanced pollutant removal efficiency, scientists are pursuing new materials to develop adsorbents and photocatalysts.20,21 On account of their large surface area, high crystallinity, chemical adaptability and well-tuned and defined nanometer-scale cavities, metal organic frameworks (MOFs) have been the subject of considerable scientific research as adsorbents and catalysts.22 The structural diversity of MOFs is attributed to the presence of metal ions and bridging organic bonds, making them appealing materials for the elimination of emerging organic pollutants and toxic metals from wastewater.23–26 The zeolite-type imidazole backbone material (ZIF) is a type of MOF having a zeolite skeleton structure, which is created upon reaction in a solvent using Co or Zn as the metal source and imidazole as the organic ligand.27 Due to their unique characteristics, ZIFs have been employed in many fields since their first invention in 2006.28 ZIF-8 is a member of the zeolitic imidazolate family, where the typical MOF comprised of 2-methylimidazole ligands and Zn(II) has been extensively exploited as a catalyst in diverse reactions such as the Knoevenagel reaction,29 photocatalytic reactions30 and adsorption reactions.31 ZIF-8 is also characterized by its ability to retain its crystallinity and porosity when placed in various solutions, such as water and organic solvents.32,33 In addition, the pore size and structure of ZIF-8 are easily tunable, making it suitable for its modification.34 Consequently, the unique chemical characteristics and physical structure of ZIF-8 offer a promising opportunity for its design and application in adsorption and photocatalytic regimes, establishing it as a hot spot for research in the removal of water pollutants.35,36 Interestingly, ZIF-8 demonstrates great thermal stability owing to its unique structure, consisting of Zn bound to imidazolate linkers. Yaghi's group reported the preparation of a stable ZIF-8 MOF at 550 °C in a nitrogen atmosphere, which exhibited structural stability for 7 days in boiling water. The prepared ZIF-8 also maintained the same crystallinity and porosity after being placed in various organic solvents and water.33,37,38 Similarly, Butova and coworkers reported the great thermal stability of a ZIF MOF (400 °C in air and 470 °C in N2) in water.39 Other studies showed that ZIF-8 prepared via a rapid sonochemical synthesis exhibited comparable thermal and structural stability with that prepared by conventional methods, requiring 1 day for their preparation.40However, ZIF-8 presents some drawbacks such as aggregation of its nanoparticles (NPs) in water, which leads to an increase in its particle size, and therefore increased charge transfer resistance and reduction in its interfacial area, thereby reducing its adsorption ability.41 Furthermore, due to its wide band gap, ZIF-8 has low reactivity under visible light, and consequently its photo-activity cannot reach the same degree of traditional semiconductors.42 Regarding their separation and recyclability, the separation of ZIF-8 NPs from the reaction medium and their reusability are difficult. In addition, the need for hybrid materials in various applications has rendered the application of pure ZIF-8 very limited.43 Thus, to overcome these limitations, some modifications have been performed, for example, the controllable association of ZIF-8 with other materials to widen its light adsorption region and to reduce the aggregation of its particles, thus guaranteeing a better adsorption and photocatalysis performance.44,45 Thus far, ZIF-8 hybrids have been successfully developed, together with active materials, such as carbon nanotubes, metals, oxides, polymers, fibers, and metal NPs.46 Compared to pristine ZIF-8, ZIF-8 hybrid materials not only have improved adsorption ability with the photocatalytic efficiency of ZIF-8, but have also expanded the scope of application of ZIF-8. Lately, several research projects have confirmed the validity of this combination strategy. For example, the combination of ZIF-8 with ZnO resulted in molecule size selectivity and excellent photocatalytic efficiency.47,48 Similarly, a ZIF-8@TiO2 composite demonstrated enhanced efficiency for the degradation of rhodamine B (RhB).49 The fibers formed by a combination of ZIF-8 with poly-dopamine and poly-acrylonitrile (ZIF-8@PDA/PAN) showed good flexibility and easy separation from the reactive medium after the adsorption process.50 Compared to both neat ZIF-8 and MoO3 nanowires, the ZIF-8/MoO3 composite exhibited a higher photocatalytic ability towards the reduction of chromium(VI).51
Thus far, many articles and reviews have discussed the different strategies for the synthesis of MOFs and their physicochemical properties.52,53 Nevertheless, there are limited reviews focusing on the application of ZIF-8 hybrids in the photocatalysis and adsorption of water pollutants. Thus, in this review, we discuss the recent progress of ZIF-8-based hybrids in the adsorption and photodegradation of wastewater contaminants (Fig. 1), with the evaluation of some representatives.
2. ZIF-8-based materials for the removal of organic pollutants
Owing to its unique chemical and physical properties, the ZIF-8 MOF is generally implemented to effectively eliminate many types of pollutants from wastewater as both an adsorbent and photocatalyst.48,54–58 ZIF-8 MOFs can be synthesized using different solvents and solvent-free methods including solvothermal, hydrothermal, ionothermal, sonochemical, accelerating agent, mechanochemical and direct solvent-free procedures.15 Their synthesis is dependent on specific catalytic reaction conditions. For instance, solvent-free synthesis leads to the formation of multi-variate ZIF materials and their pores are not partially covered by organic solvents. However, in most cases, the orientation of the metal coordination polyhedra can led to new zeolitic topologies with have high thermal and chemical stability.Nevertheless, ZIF-8 exhibits low recyclability and its specific surface areas considerably decreases due to the aggregation and the packing of its particles.59 Consequently, the construction of ZIF-8-based composites has been proposed to address these problems. Thus, successful synthesis strategies were employed to strengthen the ZIF-8 MOF in wastewater purification60–62
In the following subchapters, we review the current advances in the use of ZIF-8-based materials as adsorbents for the removal of various pollutants from wastewater, focusing on three main organic pollutants (methylene blue (MB), rhodamine B (RhB), and malachite green (MG)).
2.1 ZIF-8 hybrid structures
Adsorption is the most appropriate and promising method for the removal of several contaminants because of its simplicity, low cost, and excellent performance. The adsorption process efficiency is influenced by many factors such as the nature of the adsorbent, the type of pollutant and type interactions occurring between the sorbent and adsorbate.63–67 Compared with the traditional and commercially available adsorbent materials, including coconut shells, layered double hydroxides (LDH), and activated carbon, ZIF-8-based materials possess high porosity and a large specific surface area, endowing this type of material the ability to adsorb several molecules with various sizes.68–70 The specific surface area determined for pure ZIF-8 nanoparticles varies between 1500 and 2500 m2 g−1, which is superior to that of other adsorbents.59,71 Alternatively, in comparison with pristine ZIF-8, ZIF-8 hybrids have shown satisfactory results for the adsorption of organic pollutants owing to their high adsorption capacity and better stability and recyclability.72–74 Also, the synthesis of hybrid systems based on ZIF-8 prevents the aggregation of the adsorbent and facilitates its separation from water.75,76 Various ZIF-8 hybrid materials have been reported for the removal and photocatalytic degradation of several pollutants in aqueous conditions. For instance, the removal of organic dye pollutants (i.e., MB, RhB, MG) was studied in many reports using ZIF-8 materials.54,74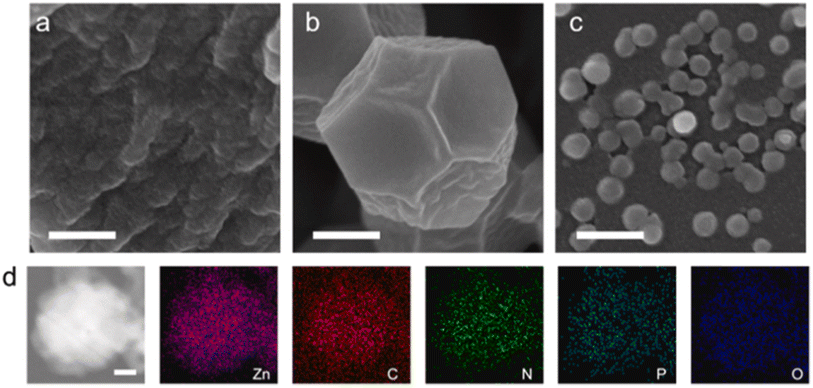 | ||
| Fig. 2 SEM images of ZnAMP (a), ZIF-8 (b) and NZIF (c) (scale bar is 200 nm). (d) HAADF image of NZIF and EDS mapping of Zn, C, N, P and O, where P and O are exclusive to the nucleotide (scale bar is 20 nm). Reproduced with permission from ref. 78 Copyright ©2021, Elsevier. | ||
The adsorption capacity achieved by the NZIF composite for MB was 10 mg g−1, which was 5-fold greater than that of pure ZIF-8. This enhancement was explained by the mesoporous nature of NZIF, which supplied more interaction sites; moreover, the adsorption mechanism investigation revealed that the adsorption capacity of NZIF mainly resulted from the hydrogen bonding and π–π stacking between the incorporated nucleotide monophosphate and the captured molecules. Alternatively, it was found that the inserted nucleoside monophosphate allowed NZIF to preserve its morphology and adsorption capacity after the adsorption process under acidic conditions, while ZIF-8 collapsed. Zhan et al.79 employed the electrospinning technique and a solution containing poly-acrylonitrile (PAN) and 2-methylimidazole to fabricate a composite based on ZIF-8/PAN. The pH and temperature effect were examined to evaluate the performance of the ZIF-8/PAN composite for the adsorption of methylene blue. The authors showed that with an increase in pH to a basic value (11), the adsorption capacity was substantially enhanced, reaching 120.48 mg g−1. This increase in adsorption capacity was attributed to the negative zeta potential value of ZIF-8 at pH = 11, where the cationic nature of methylene blue allowed great attraction between the adsorbent (ZIF-8/PAN) and the adsorbate (MB). Conversely, the adsorption capacity decreased with an increase in temperature, which was due to the fact that an increase in temperature sped up the rate of migration of MB, also boosting the desorption rate. The results of the kinetic study showed that the adsorption of MB obeyed the pseudo-second order kinetic equation; moreover, the adsorption process implied chemical interactions among MB and ZIF-8/PAN polar functional groups. The recyclability tests proved that the adsorption capacity for MB remained higher than 85% after three cycles of regeneration in comparison with the first time.79 In another study, Marsiezade et al.80 developed a composite based on hollow beads containing carboxymethyl-cellulose, ZSM-5 and ZIF-8 (CMC/ZSM-5/ZIF-8) to remove the organic dye methylene blue. Two adsorption modes were evaluated (continuous and discontinuous). The adsorption capacities recorded for both adsorption modes (continuous and discontinuous) for CMC, CMC/ZIF-8, CMC/ZSM-5 and CMC/ZSM-5/ZIF-8 were 10.56 and 12.01, 11.87 and 13.06, 9.29 and 11.53, 8.15 and 10.49, respectively. It is well noted that ZIF-8 exhibited a positive effect compared to ZSM, which had a negative effect on the methylene blue adsorption process. They found that the adsorption of methylene blue was predominantly assigned to the CMC functional groups, which played an extensive role in the adsorption of MB as active sites for the subsequent attachment of MB molecules either by hydrogen bonding or electrostatic interaction. Furthermore, due to the presence of π electrons in the methylene blue molecule, it is advantageous for this molecule to interact with the aromatic rings in the beads by π–π stacking. Alternatively, the creation of an ionic bond between Zn2+ (ZIF-8) and –SO3− (MB) played a major role in the adsorption of methylene blue on ZIF-8. The regeneration of this material was successfully evaluated in five steps, suggesting the recyclability of the composite.80 Recently, Abdelhamid et al.81 reported the growth of ZIF-8 crystal on biopolymers such as cellulose (celloZIF-8). The resulting cello/ZIF-8 materials were employed for the adsorption of methylene blue. In this work, the authors showed that the engineered cello-ZIF-8 synergistically coupled the mechanisms of pore structure adsorption, charge specific adsorption and catalytic hydrogenation, and also the addition of cellulose provided negative functional groups (COOH and OH), creating effective sites for the adsorption of methylene blue. The developed cello-ZIF-8 materials exhibited excellent recyclability during 5 cycles with no appreciable loss in their efficiencies.81 Zhu and co-authors82 confirmed the benefit of adding cellulose to the ZIF-8 framework to prevent the aggregation of the ZIF-8 particles. Furthermore, they showed that the fibrous ZIF-8 aerogels also combined the hierarchical porosities and low density of cellulose aerogels, and hence this combination resulted in improved adsorption capacity and kinetics. Similarly, Zhang et al.83 successfully introduced their developed hybrid (ZIF-8/C3N4) in an agar aerogel, creating a flexible and effective aerogel adsorbent for the elimination of methylene blue from the reaction medium. This ZIF-8/C3N4 composite aerogel displayed an adsorption capacity of 154.87 mg g−1, which was 1.44-times higher than that of the pristine ZIF-8. They explained that this improvement was due to the synergistic effect of carbon nitride (C3N4), which contributed to the regulation of the ZIF-8 crystal growth, leading to small and highly dispersed ZIF-8, resulting in a better performance for the removal of MB. Besides controlling the growth of ZIF-8 crystals, C3N4 also provided the hybrid aerogel (ZIF-8/C3N4) with stable repeatability for over 5 consecutive cycles. Alternatively, Zong et al.84 reported the synthesis of ZIF-8 hybrid aerogels, which were produced by growing MOF crystals on aramid-nanofibril (ANF) aerogels. They indicated that the ANF/ZIF-8 hybrid aerogels showed a better adsorption capacity for cationic dyes such as methylene blue rather than anionic dyes (methyl orange), which implies that the mechanism responsible for the adsorption operation is electrostatic attractions between the formed aerogels and the dye (MB). Moreover, the high surface area, synergistic effect created between the 3D polyhedra of ANF and ZIF-8 also contributed to the adsorption of MB. Li et al.85 exploited a family of transition metal-carbide-carbonitride materials (MXenes) for the development of an MXene/ZIF-8-based hybrid aerogel, which was subsequently used for the adsorption of methylene blue. The prepared composite (MXene/ZIF-8) showed a methylene blue adsorption capacity of 459 mg g−1, while that of MXene alone was 286 mg g−1. This capacity decreased in an acidic environment due to the elevated concentration of H+ ions together with the cationic dyes competing for the adsorption sites, thus hindering the adsorption process. Due to the increase in temperature, the adsorbate mobility also increased, followed by an increase in the rate of diffusion of the adsorbent, where the adsorption capacity of the MXene/ZIF-8 aerogels reached the maximum value. Furthermore, the consistency of the R2 values with the pseudo-second order model compared to the pseudo-first order model suggests that the adsorption of MB on all the MXene/ZIF-8 aerogels follows chemisorption, especially the MXene/ZIF-8-4 aerogel prepared at an MXene mass ratio of 74.2%. The reusability of MXene/ZIF-8 was studied for up to 8 cycles of desorption/regeneration, which indicated the great stability of the developed composite. After eight cycles, the adsorption capacity diminished slightly to 95.8% compared to the pristine adsorbent.85 Similarly, Gu et al.86 reported the preparation of a composite adsorbent based on an MXene (Ti3C2Tx) and ZIF-8 for the uptake of methylene blue (MB) (Fig. 3).
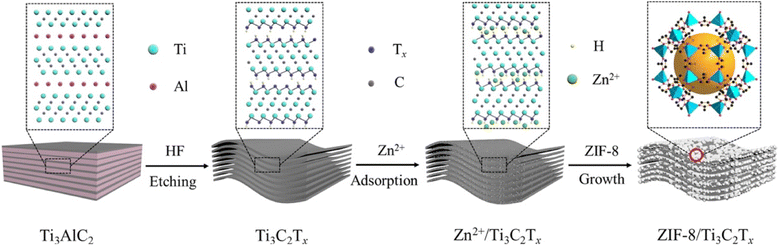 | ||
| Fig. 3 Illustration of the synthesis of ZIF-8/Ti3C2Tx. Reproduced with permission from ref. 86 Copyright ©2022, Elsevier. | ||
Due to the appropriate interstitial space arising from the ZIF-8 arrays in the MXene (Ti3C2Tx) interlayer as well as the ability of Ti3C2Tx to capture cationic dyes such as MB, an adsorption capacity of 107 mg g−1 was obtained by the hybrid ZIF-8/Ti3C2Tx, which was superior to that of ZIF-8 (3 mg g−1) and Ti3C2Tx (9 mg g−1). The low adsorption capacity of Ti3C2Tx was explained by the absence of oxygen groups on its surface and inadequate internal interspace. The addition of ZIF-8 on Ti3C2Tx provided a useful interspace, which was created by the arrangement of a small amount of ZIF-8 inside the interlayer of the MXene (Ti3C2Tx), which improved the ability to absorb methylene blue. In contrast, excess ZIF-8 had a negative effect on the adsorption ability of MB. In another work, Li et al.87 reported another strategy involving the introduction of a family of polyoxometalates (POM) in zeolitic-imidazolate frameworks (ZIF-8) via a mechano-chemical procedure at room temperature. In this study, they evaluated three different hybrids, i.e., H3PW12O40: ZIF-8 (BIT-1), H4SiW12O40: ZIF-8 (BIT-2) and H3PMo12O40: ZIF-8 (BIT-3), for the adsorption of methylene blue. The easy deprotonation of these composites resulted in the formation of poly-anions, which showed great ability to attract methylene blue molecules due to their cationic nature, which led to an increase the affinity of MB particles, and therefore enhanced the adsorption capacity. In addition, these composites served as open hydrophobic conduits, allowing the methylene blue molecules to diffuse inside and outside the POM/ZIF-8 composite. Moreover, the introduction of these POMs in the ZIF-8s generated a synergistic effect and increased the porosity, which resulted in excellent performances compared to ZIF-8. It has been well documented that the maximum adsorption capacity was obtained by the BIT-1 composite (810 mg g−1). Compared to other adsorbents such as activated carbon and pure ZIF-8, the BIT-1 hybrid showed an outstanding efficiency, resulting in the removal of 99% methylene blue (60 ppm) within 5 min. In addition, BIT-1 also showed excellent repeatability without losing its properties such as adsorption capacity.87
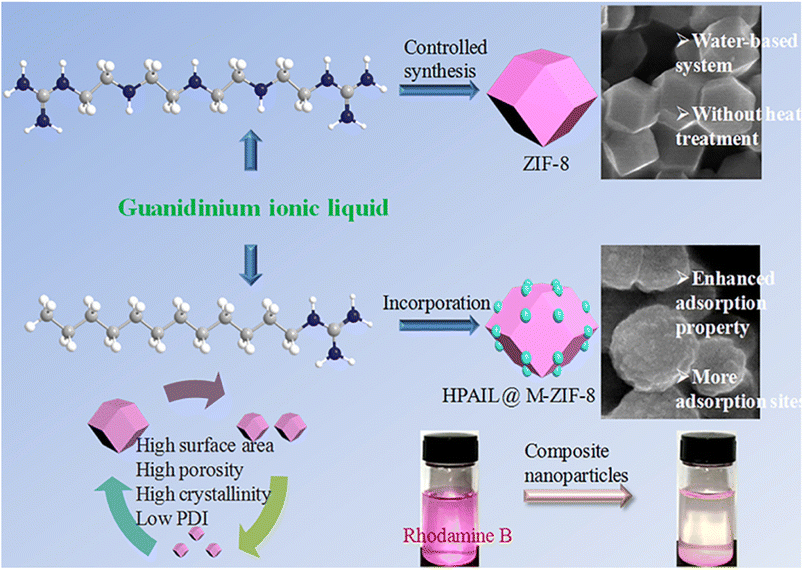 | ||
| Fig. 4 HPAIL@Meso-ZIF-8 composite for the removal of RhB dye. Reproduced with permission from ref. 89 Copyright ©2022, Elsevier. | ||
Chen et al.90 developed a new strategy based on the use of ZIF-8s as a stabilizer for slurry polymerization from hexadecyl acrylate (AA16) in water for the successful preparation of PAA16/ZIF-8 composites, which were employed for the removal of RhB. The UV visible analysis showed that the concentration of RhB decreased by about 24%, 25% and 99% in the presence of PAA16, ZIF-8/PAA16 and ZIF-8, respectively. These results were explained by the BET analysis, which showed that the specific surface area of PAA16/ZIF-8 was about 8 m2 g−1, while that of ZIF-8 alone was 1745 m2 g−1. This large difference was attributed to the low content of ZIF-8s in the surface of PAA16, which implies that the adsorption capacity of RhB is mainly dependent on the content of ZIF-8. The reuse results showed that PAA16/ZIF-8 could be reused for 3 cycles without losing its structural properties and adsorption performance.90 Yin et al.91 employed an in situ method and layer-by-layer assembling method with no solvent for the growth of ZIF-8 sheets on carbon fiber (CF) tissues functionalized with carboxyl groups (–COOH). The developed ZIF-8/CF composite was employed for the adsorption of RhB. The calculated specific surface area for ZIF-8s alone was 865.764 m2 g−1, while that of the ZIF-8/CF composite was 495.297 m2 g−1. This reduction in size was attributed to the non-porous nature of CFs, whereas the HK method showed that ZIF-8s and ZIF-8/CF displayed a similar type and size of microspores, which suggests that the introduction of CF tissue did not alter did not alter the structure and pore size of ZIF-8s in the CF-ZIF-8 hybrid. Although the surface charge of the ZIF-8/CF composite was negative (−2.35 mV), the visible UV analysis showed a slower decrease in the absorption of cationic RhB, which was explained by the high molecular size of RhB. Alternatively, they found that RB19 (anionic dye) and RhB presented a similar UV reduction after the adsorption operation, which was attributed to the similarity in the π–π interaction between the CF-ZIF-8 composite and each dye. The reusability tests revealed that during 5 cycles of reuse, the adsorption capacity of the ZIF-8/CF composite showed only a slight decrease, indicating the outstanding reusability of the ZIF-8/CF hybrid.91 Recently, Yang et al.92 developed an iron-doped ZIF-8 adsorbent (Fe-ZIF-8) for the removal of several pollutants such as rhodamine B. The composite prepared at a temperature of 500 °C (Fe-ZIF-8-500) presented a specific surface area of 1135 m2 g−1, while the Fe-ZIF-8 sample prepared without thermal activation presented a specific surface area of 1536 m2 g−1. This large difference was due to the collapse of the pore channels during high temperature processing. At a pH above the pH at the zero point of charge (pHZPC = 7.53), the surface of the composite (Fe-ZIF-8-500) was negatively charged, and due the cationic nature of RhB, an adsorption capacity of 74 mg g−1 was obtained. Zhang and co-authors tested the combination between two metal organic frameworks such as ZIF-8 and UiO-66-NH2 for the removal of several organic dyes such as RhB. Compared to the removal of methylene blue on the ZIF-8/UiO-66-NH2 composite described in this study, which demonstrated both outstanding selectivity and strong performance, despite the cationic nature of RhB, the ZIF-8-loaded UiO-66-NH2 showed a poor adsorption efficiency for RhB. These results were explained based on the complex structure and large molecular size of RhB compared to that of MB, which could prevent it from penetrating the pores in the ZIF-8-loaded UiO-66-NH2. Specifically, this composite had an insufficient width of 0.5 nm, while the molecule size of RhB was 1.41 × 0.98 nm. Hou et al.93 reported the fabrication of a hybrid based on ZIF-8 and graphene aerogels (ZIF-8/GAs) through a counter diffusion process. Due to the unique integrated characteristics of ZIF-8s and GAs such as large surface area, great interaction between ZIF-8/GA hybrid and analytes and porous structure, the ZIF-8/GA hybrid exhibited an adsorption capacity for RhB of 320.6 mg g−1, while that for GAs was 194.2 mg g−1. The pseudo-second-order model had greater applicability compared to the pseudo-first-order model in describing the adsorption pathway due to its R2 correlation coefficient of 0.999, whereas that for the pseudo-first-order model was 0.9561.93
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 demonstrated a superior adsorptive removal efficiency of malachite green (MG) with a reported maximum adsorption ability of 3056 mg g−1 at 30 °C. Furthermore, due to its high particle size, the 1
4 demonstrated a superior adsorptive removal efficiency of malachite green (MG) with a reported maximum adsorption ability of 3056 mg g−1 at 30 °C. Furthermore, due to its high particle size, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ZIF-8/porous carbon hybrid was readily separated through filtration and reused for 3 cycles with a retention of 88% of its original adsorption.99 Abdi and co-authors100 attempted to remove MG in an aqueous solution, and they studied the efficiency of two hybrids based on ZIF-8/GO and ZIF-8/carbon nanotubes (CNTs) towards the adsorption of MG. After the successful preparation of ZIF-8/CNT and ZIF-8/GO, new pores were observed for both hybrids, which were very useful for the adsorption of pollutant molecules (MG). Furthermore, they showed that the construction of the ZIF-8/CNT and ZIF-8/GO hybrids prevented the generation of aggregates, achieving a high surface area of 830.3 m2 g−1 and 1476.4 m2 g−1, respectively. It was noted that the equilibrium data obtained were in very good accordance with the Langmuir model with MG adsorption capacities of 1667 mg g−1, 3300 mg g−1 and 2034 mg g−1 for ZIF-8, ZIF-8/GO and ZIF-8/CNT, respectively. Moreover, the composites showed great recyclability for 4 cycles.100 Nazir et al.101 reported the development of a porous composite via the in situ growth of ZIF-8s on a layered double zinc hydroxide (ZnAl-LDH).
4 ZIF-8/porous carbon hybrid was readily separated through filtration and reused for 3 cycles with a retention of 88% of its original adsorption.99 Abdi and co-authors100 attempted to remove MG in an aqueous solution, and they studied the efficiency of two hybrids based on ZIF-8/GO and ZIF-8/carbon nanotubes (CNTs) towards the adsorption of MG. After the successful preparation of ZIF-8/CNT and ZIF-8/GO, new pores were observed for both hybrids, which were very useful for the adsorption of pollutant molecules (MG). Furthermore, they showed that the construction of the ZIF-8/CNT and ZIF-8/GO hybrids prevented the generation of aggregates, achieving a high surface area of 830.3 m2 g−1 and 1476.4 m2 g−1, respectively. It was noted that the equilibrium data obtained were in very good accordance with the Langmuir model with MG adsorption capacities of 1667 mg g−1, 3300 mg g−1 and 2034 mg g−1 for ZIF-8, ZIF-8/GO and ZIF-8/CNT, respectively. Moreover, the composites showed great recyclability for 4 cycles.100 Nazir et al.101 reported the development of a porous composite via the in situ growth of ZIF-8s on a layered double zinc hydroxide (ZnAl-LDH).
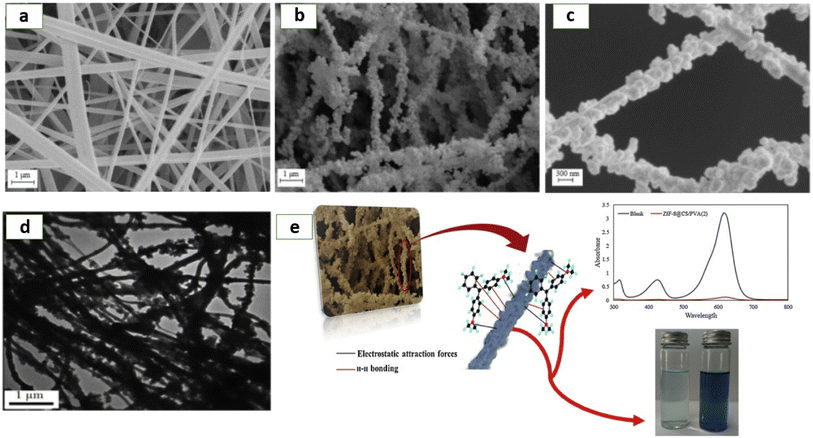 | ||
| Fig. 5 SEM micrographs of engineered composites (a) CS/PVA-ENF and (b and c) ZIF-8-CS/PVA-ENF2. (d) TEM images of ZIF-8-CS/PVA-ENF2 hybrid. (e) Adsorption mechanism of malachite green on ZIF-8-CS/PVA-ENF2. Reproduced with permission from ref. 74 Copyright ©2020, Elsevier. | ||
Compared to ZIF-8 alone, the ZIF-8/ZnAl-LDH hybrid displayed an improved adsorption capacity of 194.5 mg g−1, and with a contact time of 180 min, a removal efficiency of 98% was recorded for MG dye. Moreover, the recyclability tests revealed that after 4 successive cycles, the ZIF-8/ZnAl-LDH composite exhibited an adsorption of 96.27% for MG compared to the initial capacity of the virgin ZIF-8/ZnAl-LDH.101 Wang et al.102 employed in situ polymerization to develop a novel composite aerogel based on egg yolk, ZIF-8 and cross-linked poly-acrylic acid (EY/ZIF-8/CLPAA). To improve the performance of the composite towards the removal of MG, they highlighted the effect of several parameters such as the effect of adsorbent dosage, which showed that when the dosage of the adsorbent increased, the amount of MG molecules adsorbed decreased, and the optimal dosage of the hybrid aerogel (EY/ZIF-8/CLPAA) was 2 mg, achieving an adsorption capacity of 2338 mg g−1. Moreover, the Langmuir model was more applicable than the Freundlich model to describe the adsorption kinetics of MG because of its R2 correlation coefficient, which was 1.00, while the R2 correlation coefficient for the Freundlich model was 0.8916. These results confirmed that the adsorption process belonged to monolayer adsorption without interaction among the adsorbed molecules and without transport adsorption in between the adsorption sites.102
In addition to methylene blue, rhodamine B and malachite green, other pollutants have been removed using different ZIF-8-based composites, as listed in Table 1.
| Adsorbent | Synthesis method | SBET (m2 g−1) | Adsorption capacity (mg g−1) | Pollutant | Ref. |
|---|---|---|---|---|---|
| ZIF-8/CS | In situ growth | — | 922 | CR | 103 |
| ZIF-8/Carbon fibers | In situ self-assembly method | 97.32 | 42.64 | CR | 104 |
| ZIF-8/CoFe2O4 | Solid-phase extraction | 918.9 | 52.04 | CR | 105 |
| ZIF-8/ZnAl-LDH | In situ growth | 963 | 609.7 | MO | 101 |
| ZIF-8/SiO2/MnFe2O | Co-precipitation and modified Stöber method | 830.3 | 78.12 | MO | 106 |
| ZIF-8/graphene aerogels | Counter diffusion method | — | 406.5 | MO | 93 |
| EW/ZIF-8/PAA | In situ polymerization, freezing and freeze drying | — | 297.1 | MO | 107 |
| EY/ZIF-8/CLPAA | In situ polymerization | — | 447 | MO | 102 |
| ZIF8/Fe3O4/BNT | Solvothermal method | 428.7 | 40.5 | RO16 | 108 |
| ACPMG/ZIF-8 | Solvothermal method | 1001.4 | 1250 | SO | 109 |
| ZIF-8/SBA-15 | LPE method | 722 | 116.9 | Bisphenol A | 110 |
| KGM/ZIF-8 | Sol–gel and freeze-drying | — | 812.35 | Ciprofloxacin | 111 |
| ZIF-8/PAM | In situ method | 14.4 | 111.5 | Humic acid | 112 |
| ZIF-8/PDA/PAN | Self-polymerization | 319.38 | 478.18 | Tetracycline | 50 |
| Fe-doped ZIF-8 | Precipitation method | 1135 | 867 | Tetracycline | 92 |
| ZIF-8/wool | In situ method | — | 371.2–391.1 | 2-Naphthol | 113 |
| ZIF-8/GO | In situ method | 946.5 | 171.3 | 1-Naphthylamine | 114 |
| ZIF-8/chitosan-g-PNVCL | Electrospinning method | — | 152.3 | Phenol | 115 |
3. ZIF-8 materials for photocatalytic degradation of organic pollutants
Besides adsorption, other catalytic methods are commonly employed for treating water pollutants including primarily photocatalytic degradation, which represents “green technology” for removing harmful pollutants and other organic pollutants.116–119 Compared to adsorption, photocatalytic processes can decompose organic pollutants such as MB, RhB, and MG into CO2 and H2O in the presence of a photocatalyst (ZIF-8). Thus far, ZIF-8 nanocrystals have been applied in the field of photocatalysis.57,120–123 Nevertheless, due to their wide band gap (4.9–5.1 eV) and poor electron discharge capacity, their photocatalysis capabilities are generally lower than that of traditional semiconductors.59 Consequently, several strategies have been developed to improve the efficiency of ZIF-8 for the removal of organic pollutants (MB, RhB, and MG) such as combining ZIF-8 with other semiconductors or doping ZIF-8 with elements to improve its optical properties and obtain hybrids with improved photocatalytic performance. In this second part, we review the different ZIF-8-based photocatalysts been applied to degrade different organic pollutants such as MB, RhB, and MG.3.1 Photocatalytic degradation of MB
Recently, Faraji et al.124 exploited the magnetic properties of NiFe2O4 to form a ZIF-8/NiFe2O4-based composite, which could be easily separated from the reaction medium. The prepared photocatalyst was evaluated for the photocatalytic decomposition of methylene blue. They showed that the photocatalyst prepared with 30 wt% NiFe2O4 manifested the strongest photocatalytic behavior, resulting in a degradation efficacy of 94% within 120 min. This improvement was attributed to the addition of NiFe2O4, which extended the lifetime of the electron hole pairs. Moreover, its magnetic property allowed the composite to be regenerated and reused for 4 cycles without loss of its properties such as degradation efficiency (90%).124 Chandra and co-authors125 reported the synthesis of a composite named ZIF-8/AgNPs for the photocatalytic degradation of MB. Due to its large specific surface area (1370.91 m2 g−1) and unique synergistic characteristics, the sample prepared with a 300 μL suspension of AgNPs displayed outstanding photocatalytic efficiency towards methylene blue with a degradation rate of 97% at pH ≥ 7. Furthermore, they demonstrated that upon the degradation of MB, the metallic AgNPs acted as electron collectors, which facilitated the transfer of photo-generated electrons into the conduction band (VB) of ZIF-8 as a result of surface plasmon resonance (SPR), and due to the synergistic effect of ZIF-8 and AgNPs, the pairs (e−/h+) readily reacted with water and oxygen to form species capable of mineralizing MB to CO2 and H2O.125 Similarly, He et al.57 reported the fabrication of a ZIF-8/AgBr composite photocatalyst via the solvothermal route, which was used for the photodecomposition of MB. The optical analysis showed that the coupling of ZIF-8 with AgBr resulted in improved light absorption efficiency; moreover, with a high content of AgBr, a red shift in the absorption edge was evident. The remarkable improvement in the charge separation efficiency was a result of the synergistic effect generated when coupling ZIF-8 with AgBr. The recorded MB decomposition rate was 0.0273 min−1, which was 3.59-fold higher than that of AgBr. The stability and recyclability revealed that the photocatalyst with a weight of 40% AgBr (40% AgBr/ZIF-8) displayed very stable photocatalytic activity for 6 successive rounds.57Guan et al.126 synthesized an Ag/AgCl/ZIF-8/TiO2/C-based composite on the surface of cotton fabric using a simple procedure. Under visible light irradiation, the Ag/AgCl/ZIF-8/TiO2/C photocatalyst demonstrated a MB degradation efficiency of 98.5% within 105 min with a first-order kinetic constant of 0.0332 min−1. Moreover, the photocatalytic decomposition capacity of methylene blue was 85% after 3 cycles, which implies the excellent stability of the Ag/AgCl/ZIF-8/TiO2/C hybrid. In this work, they showed that ZIF-8 present in the developed composite acted as an oxygen molecule promoter during the photocatalytic decomposition process, where during this time, the photo-generated holes that existed in the valance band (VB) of the photocatalyst oxidized the water molecules into OH., and therefore, hydroxyl radicals (OH˙) and superoxides (O2˙−) were regarded as the radicals responsible for the photodecomposition of MB. The mechanism of photo-degradation of MB is presented in Fig. 6a.126 Another ternary system based on RGO/black TiO2/2D-ZIF-8 was developed by He et al.,127 which was used for the degradation of MB (Fig. 2b). The EIS analyses presented in Fig. 6c show that after the association of the 2D-ZIF-8 nanosheets, the RGO/black TiO2/2D-ZIF-8 ternary hybrid displayed the lowest semicircle diameter, which means that RGO/BTiO2/2D-ZIF-8 possessed the greatest charge carrier mobility. Moreover, they indicated that the insertion of 2D-ZIF-8 gave the ternary system a strong dye adsorption capacity and monitored the in situ decomposition at the adsorption site. It was found that the rate constant for the photocatalytic decomposition of methylene blue was 0.0232 min−1, which was 3.3-times greater than that of black TiO2. The photocatalytic decomposition mechanism of MB described in Fig. 6d shows that the photo-generated electrons at the surface of B TiO2 cannot easily scavenge O2 to generate O2˙− given that the redox potential of the O2/O2˙− couple was more negative (−0.33 eV) than the conduction band energy of black TiO2 (−0.27 eV). Moreover, it was well noted that the redox potential of the H2O/OH− couple (2.53 eV) was bigger than the valence band energy of ZIF-8 (0.34 eV), thus also rendering it infeasible for the photo-generated holes to generate OH˙ radicals from H2O. Consequently, the holes directly came in contact with MB during the photo-degradation pathway. Due to its great electrical conductivity, RGO also helped to effectively separate the electron/hole pairs, which decreased the recombination rate, and consequently improved the photo-degradation of MB.127 Recently, Yurtsever and co-workers128 investigated the influence of crystallization time and the quantity of zeolitic imidazolate framework-8 (ZIF-8) on the photocatalytic performance of ZIF-8-decorated copper-doped titania powders (Cu–TiO2/ZIF-8). They demonstrated that with a decrease in the ZIF-8 content and growth time, the photocatalytic activity was enhanced, and the nanocomposites prepared at 5% ZIF-8 content and 15 min growth time displayed the highest photocatalytic activity with a 1st order rate constant 1.4- and 4-fold higher than that of pristine Cu–TiO2 and ZIF-8, respectively. Alternatively, an additional increase in ZIF-8 level to 46% induced the clumping of these aggregates, leading to a reduction in photocatalytic activity.128 Outstanding photocatalytic activity was obtained on cotton fabric (CF), which was treated with poly-dopamine (pDA), followed by the in situ deposition of ZIF-8/TiO2 composites, leading to the formation of the ZIF-8/TiO2/pDA/CF hybrid photocatalyst. The formed hybrid was evaluated for the photocatalytic decomposition of MB. The photoluminescence spectra revealed that the incorporation of ZIF-8 enhanced the charge transfer capability and lowered the percentage of pairs recombination (e−/h+), and the photo-degradation efficiency of MB obtained by this hybrid was 93.1% during 130 min of irradiation. This good activity was attributed to the great BET surface area of ZIF-8 and the self-polymerization of pDA, resulting in the formation of melanin, which could absorb the heat, and consequently accelerate the catalytic efficacy.129 Employing an impregnation process, ZIF-8s decorated with carbon quantum dots (ZIF-8@CQDs) were developed and tested to remove methylene blue from the aqueous medium. A greater absorption ability and better response to visible light were obtained by the ZIF-8@CQD hybrid due to its core–shell structure, resulting in an MB removal efficiency of 91% and a 6-fold better rate constant than ZIF-8. Simultaneously, the ZIF-8@CQD photo-catalyst was employed for three cycles of reuse, exhibiting outstanding structural stability without loss in its properties such as catalytic activity.130 Yang et al.131 developed a novel photo-catalyst, where bismuth was doped in the ZIF-8/g-C3N4 heterojunction to form the Bi@g-C3N4/ZIF-8 composite. A decomposition efficiency of 86.6% within 60 min was obtained by the hybrid prepared with 12% of bismuth and a mass ratio of ZIF-8: g-C3N4 = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. They explained that this efficiency was due to the effect of bismuth, which provided a suitable channel for photo-induced carrier transmission. Moreover, the synergistic effect of the inserted bismuth with the ZIF-8/g-C3N4 heterojunction also improved the light absorption performance, thermal stability and recyclability.131 Due to its tight band gap and S–Mo–S sandwich coordination structure, MoS2 was also considered as a semiconductor, which could further boost the catalytic performance of ZIF-8 by forming a ZIF-8/MoS2-based hybrid.132–134 As an example, Chen et al.135 successfully assembled ZIF-8 on a carboxyl cotton cloth, and subsequently grew MoS2 on the surface of ZIF-8 to form an MoS2/ZIF-8/carboxyl cotton composite, which was capable of efficiently absorbing light and degrading MB. The fabric functionalized with 5 ZIF-8 layers (MZ-5/carboxyl-cotton) showed the best methylene blue degradability of about 99.8% for 21 min; moreover, a photocatalytic activity of about 94.8% was retained even 6 recycling cycles.
1. They explained that this efficiency was due to the effect of bismuth, which provided a suitable channel for photo-induced carrier transmission. Moreover, the synergistic effect of the inserted bismuth with the ZIF-8/g-C3N4 heterojunction also improved the light absorption performance, thermal stability and recyclability.131 Due to its tight band gap and S–Mo–S sandwich coordination structure, MoS2 was also considered as a semiconductor, which could further boost the catalytic performance of ZIF-8 by forming a ZIF-8/MoS2-based hybrid.132–134 As an example, Chen et al.135 successfully assembled ZIF-8 on a carboxyl cotton cloth, and subsequently grew MoS2 on the surface of ZIF-8 to form an MoS2/ZIF-8/carboxyl cotton composite, which was capable of efficiently absorbing light and degrading MB. The fabric functionalized with 5 ZIF-8 layers (MZ-5/carboxyl-cotton) showed the best methylene blue degradability of about 99.8% for 21 min; moreover, a photocatalytic activity of about 94.8% was retained even 6 recycling cycles.
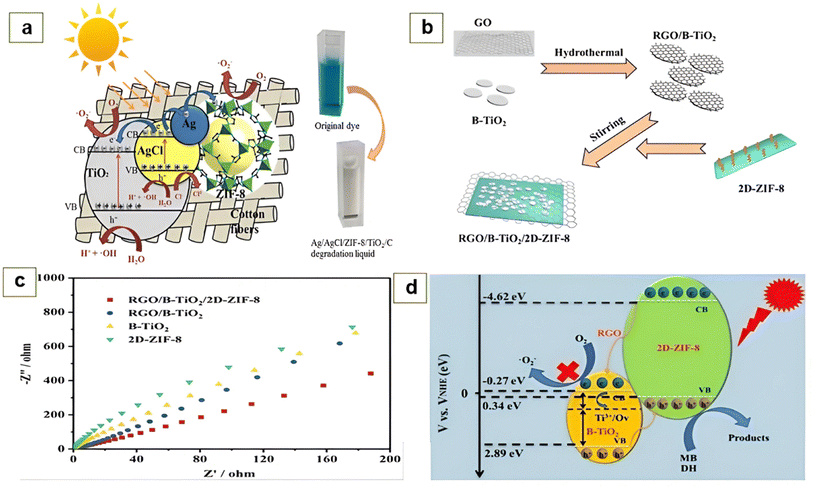 | ||
| Fig. 6 (a) Photo-degradation mechanism of MB with Ag/AgCl/ZIF-8/TiO2/C hybrid. Reproduced from ref. 126 Copyright 2019, Springer. (b) Process for the elaboration of the ternary system RGO/B TiO2/2D-ZIF-8. (c) EIS analysis of B–TiO2, RGO/B TiO2, 2D-ZIF-8 and RGO/B TiO2/2D-ZIF-8. (d) Photo-degradation mechanism of MB with RGO/B TiO2/2D-ZIF-8 ternary system. Reproduced with permission from ref. 127 Copyright ©2020, Wiley VCH. | ||
3.2 Photocatalytic degradation of RhB
Besides the photodegradation of methylene blue, other studies have been conducted to evaluate the potential of ZIF-8-based materials to mineralize rhodamine B (RhB). For example, Cao et al.121 employed an in situ reaction to successfully coat manganese oxide (MnO2) on a zeolitic framework of imidazolate-8 (ZIF-8). The formed ZIF-8@MnO2 hybrid was used as a photocatalyst to decompose RhB in a Fenton-like procedure, yielding a decomposition rate of >96.0% for 120 min, whereas the degradation rate by the pristine ZIF-8s was 63%. These satisfactory results were attributed to the porous nature of ZIF-8s, which allowed the adsorption of more rhodamine B molecules; moreover, the anchoring of the MnO2 nanoparticles (NPs) on ZIF-8 allowed their good dispersion to provide a larger active surface area. Consequently, the formation of the ZIF-8@MnO2 heterojunction resulted in effective charge separation, which led to the high production of free radicals such as OH.. Yang et al.136 developed a novel MOF-derived approach for the fabrication of ZIF-8/ZnO composite photocatalysts through the micro-catalysis process by employing ZIF-8 as the reagent and AgNO3 as the catalyst. The adjustment of the AgNO3 concentration showed that the sample prepared with 50 mM AgNO3 solution (50 ZIF-8/ZnO) gave the highest degradation efficiency of about 98.17% compared to the other composites prepared with AgNO3 (100 mM and 25 mM). Moreover, the ZIF-8/ZnO (50) hybrid exhibited strong photo-stability, translating to a photocatalytic efficiency of 95.54% after 4 cycles of reuse.136 In another paper, Kong and co-authors58 reported the fabrication of a core–shell heterostructure denoted as ZnO/CdS@ZIF8 for the photodegradation of RhB. They showed that spindle-shaped ZnO also delivered Zn2+ to form ZIF-8. Due to the great absorption capacity of ZIF-8s and their unique pore structure, the photocatalytic decomposition rate constant of RhB obtained by the ZnO/CdS@ZIF8 composite was 0.018 min−1, while the rate constant obtained by the CdS/ZnO hybrid was 0.009 min−1.58 Zeng et al.49 employed a sonochemical method and successfully designed a ZIF-8/TiO2 heterojunction, which was intended for the photocatalytic degradation of rhodamine B. In comparison with the commercially available P25, ZIF-8/TiO2 displayed a better degradation efficacy of rhodamine B. They showed that the encapsulation of ZIF-8 accelerated the photodegradation kinetics of RhB, where an N–Ti–O chemical bond was developed between TiO2 and ZIF-8, which favored the efficient separation of electron/hole pairs. The O2 molecules present in the reaction medium were trapped by the photogenerated e− in the conduction band of the ZIF-8/TiO2 composite to form O2˙− superoxide radicals, which contributed to the mineralization of RhB.49 In another study, Liu et al.137 discussed the impact of the incorporation of ZIF-8 on the photocatalytic performance of Ag/AgCl. Firstly, the Ag/AgCl nanoparticles were encapsulated on the surface of ZIF-8 with a content of 50 wt%, resulting in the formation of an Ag/AgCl/ZIF-8 (50%) hybrid. The Ag/AgCl/ZIF-8-50% hybrid exhibited great absorption in the wavelength region of 420–800 nm as a result of the surface plasmon resonance (SPR) effect of the Ag particles formed in situ onto on surface of the AgCl NPs. Compared to Ag/AgCl, the Ag/AgCl/ZIF-8-50% composite showed outstanding photocatalytic activity, which was related to the dual active function of ZIF-8, i.e., adsorption of rhodamine B and production of more O2˙− superoxide radicals, which contributed to the mineralization of RhB.137 Abdi et al.138 successfully used a quick and easy method to fabricate a ZIF-8 photocatalyst doped with various Ag contents (ZIF-8@Ag-X). The photocatalyst was synthesized with 15% Ag content (ZIF-8@Ag-15%) showed the maximum RhB removal efficiency (93%) within 30 min of irradiation, and the rate constant recorded for this photocatalyst was 0.1016 min−1. The inclusion of Ag nanoparticles on the surface of ZIF-8 substantially boosted the photodecomposition efficiency of RhB by scavenging the photogenerated electrons, thereby diminishing the possibility of charge recombination. The trapping investigations revealed that holes (h+) and hydroxyl radicals (OH˙) were the species responsible for the mineralization of RhB into CO2 and H2O.138 Similarly, Jing et al.139 employed a nucleating, precipitating, growing and photo-reducing approach to produce AgCl and Ag co-doped ZIF-8 photocatalysts. As a consequence of the synergistic effect among AgCl, Ag and ZIF-8, great visible light absorption ability was noticed by the hybrid made at an AgNO3 concentration equal to 15 mM, showing a high rhodamine B degradation efficiency of 99.12% for 60 min of illumination, while that for Ag/AgCl was 94.24% and ZIF-8 (5.17%). Moreover, the photocatalytic efficacy of Ag/AgCl-15@ZIF-8 was reduced by only 1.96% after 4 repeated degradation rounds.139 Chang et al.140 constructed a Z-type heterojunction of AgCl/Ag-doped-ZIF-8. They reported the combination of AgCl with Ag-doped-ZIF-8 through a precipitation reaction, forming an AgCl/Ag-ZIF-8 heterojunction. It was noted that doping with Ag on ZIF-8s significantly lowered the gap energy, involving improved light harvesting. Moreover, the formation of the Z-type heterojunction of AgCl/Ag-doped-ZIF-8 also contributed to the improvement in the catalytic performance of the photocatalyst, given that it enabled the effective separation of the photo-generated charges. The photocatalytic activity achieved by the AgCl/Ag-ZIF-8 heterojunction was found to be 12-fold better that the virgin ZIF-8, with high stability and reusability.140 Similarly, He and co-authors57 used a ZIF-8/AgBr hybrid for the photodegradation of RhB. The optical analysis showed that the coupling of ZIF-8 with AgBr resulted in improved light absorption efficiency, and the photodecomposition rate of RhB recorded by the ZIF-8/AgBr hybrid was 98.5% during 60 min irradiation and the rate constant was about 0.115 min−157.3.3 Photocatalytic degradation malachite green
Recently, Abdollahi et al.123 employed a precipitation process to grow a CuO–ZnO/ZiF-8 hybrid photocatalyst. The developed composite was evaluated for the photocatalytic degradation of malachite green. They observed using DRS analysis that the sample developed with a ZIF-8 content equal to 20 wt% (CuO–ZnO/ZiF-8-20) demonstrated a tighter gap energy (1.96 eV) than of ZIF-8 (5.34 eV) and CuO–ZnO (2.89 eV). Also, the inclusion of ZIF-8 on the CuO–ZnO heterojunction resulted in an increase in specific surface area from 12.3 m2 g−1 (CuO–ZnO) to 22.2 m2 g−1 (CuO–ZnO/ZiF-8-20). As a result of this increase, the photocatalyst adsorbed a large quantity of the dye, which was subsequently degraded by visible light. The photocatalytic activity recorded by the CuO–ZnO/ZiF-8-20 photocatalyst towards the removal of MG with a concentration of 80 ppm at pH 7 was 51%.123 Alternatively, Li et al.141 developed an approach based on the conversion of MIL-68(In)@ZIF-8 organometallic frameworks into an In2O3@ZnO heterostructure. They reported that the conversion was performed according to the calcination of MIL-68(In)@ZIF-8 to form core–shell hollow-microtubes of In2O3@ZnO intended for photodegradation of MG. The synthesized heterostructure showed an enhanced photocatalytic efficiency for the decomposition of MG compared to MIL-68(In)-derived In2O3 and ZIF-8-derived ZnO under light irradiation. Moreover, the In2O3@ZnO hybrid formed from MIL-68(In)@ZIF-8 provided a higher BET surface area, exposed more active sites, possessed a suitable amount of oxygen vacancies, better light absorption and efficiently stimulated photo-induced charge carrier transfer and separation. The photocatalytic activity registered by this heterostructure was 80.6% during 200 min of irradiation.141In addition to methylene blue, rhodamine B and malachite green, other pollutants have been removed by different ZIF-8-based composites, as listed in Table 2.
| Photocatalyst | Synthesis method | Gap energy (eV) | Removal efficiency (%) | Pollutants | Ref. |
|---|---|---|---|---|---|
| ZIF-8@CuInS2 | Solvothermal process following by a reaction with methanol and PVP | 1.96 | 90 | Phenol | 142 |
| 87 | Chlorophenol | ||||
| 74 | 2-Naphthalenesulfonic acid | ||||
| ZIF-8/TiO2 | Hydrothermal method | 2.98 | 90 | Tetracycline | 143 |
| ZIF-8/g-C3N4 | Exfoliating-wrapping | 2.73 | 87.6 | Tetracycline | 144 |
| ZIF-8/Cu2O | In situ growth | 4.31 | 84.1 | Tetracycline | 145 |
| Ag-doped ZIF-8 | Fast and facile process | 3.2 | 93 | Methyl orange | 138 |
| ZIF-8@AgNPS | In situ growth | 5.13 | 100 | Congo red | 125 |
| Ag/AgCl@ZIF-8/gC3N4 | Simple process | — | 94.5 | Levofloxacin | 146 |
| ZIF-8/BiOI | Solvothermal process | 1.55–1.88 | 82.5 | Bisphenol A | 147 |
| Pt/TiO2–ZnO@ZIF-8 | Simple process | 3.2 | 99.7 | Phenol | 148 |
| ZIF-8/MoS2 | Solvothermal process | 1.05 | 93.2 | Ciprofloxacin | 149 |
| 75.6 | Tetracycline hydrochloride | ||||
| ZIF-8/CdS | ZIF-8 crystal self-assembled around PVP-embedded CdSNPs | 2.05 | 81.69 | Toluene | 150 |
| ZIF-8/Fe2O3 | Green method | — | 94 | Reactive Red 198 | 151 |
4. Conclusion and prospects
In this review, we discussed the use of the ZIF-8 metal organic framework for the fabrication of composite materials for the removal and photocatalytic degradation of organic pollutants. The adsorption and photocatalysis of persistent organic contaminants are viable and economically feasible methods. Accordingly, the development of stable, selective and robust adsorbents is a promising route to achieve a sustainable future. Particularly, MOFs and ZIF-8 are high-surface area materials, playing a vital role for surface retention of organic pollutants. Improving the pore size and structure of ZIF-8 through metal doping, formation of composites and functionalization is considered necessary to enhance to adsorption capacity due to synergistic effect of ZIF-8 and the doped and composite materials. Additionally, morphological, optical and chemical properties tunability can be an asset for enhancing the photocatalytic degradation efficiency. Chemists and physicists devoting tremendous efforts to the synthesis and application of photo-active ZIF-8 materials for environmental remediation.However, although there have been remarkable achievements in the last decade in this regard for the removal and photocatalytic degradation of organic pollutants, it is still far from real practical application. Thus, herein, we propose the following points for future development:
4.1. Scalability and stability
Pilot-scale synthesis of ZIF-8-based composites with high yields using flow reactors should be considered and stable materials in real world applications should be envisaged.4.2. Cost
Reducing the cost for the synthesis of ZIF-8 materials with a targeted surface area, optical absorption and crystallinity. This includes low energy consumption in synthesis strategies (temperature, pressure) and low-cost reagents to avoid the use of organic solvents.4.3. Real application
Real wastewater experiments in continuous flow reactors (water treatment plants) should be evaluated. Wastewater usually has dye concentrations of 200 ppm, while the lab-scale experiments in adsorption and photocatalysis deal only with concentrations of dyes as low as 50 ppm.4.4. New insights
Exploring the mechanistic fundamental study to assess the stability of adsorbents/photocatalysts and the identification reaction intermediates. DFT calculations can be performed to elaborate a full concise comprehensive study.4.5. Sustainability
The mineralization should be studied and reported. The toxicity of intermediates during the reaction process and the leachability of ZIF-8 adsorbent/photocatalysts should be studied to guarantee the environmental friendliness and sustainability of the materials.Author contributions
Aicha Elaouni: writing the draft, data curation; formal analysis. Mohamed El ouardi: writing the draft, data curation; formal analysis. Mohamed Zbair: writing – review & editing; project administration. Hassan Ait Ahsaine: writing the draft, project administration; supervision and validation. Amal BaQais: writing – review & editing; project administration. Mohamed Saadi: project administration; supervision and validation.Conflicts of interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We thank all the authors and their respective institutions.References
- M. M. S. Abbassy, Mar. Pollut. Bull., 2018, 131, 115–121 CrossRef CAS PubMed.
- P. Xiong, H. Zhang, G. Li, C. Liao and G. Jiang, Sci. Total Environ., 2021, 797, 149179 CrossRef CAS PubMed.
- J. O. Ighalo, S. Rangabhashiyam, C. A. Adeyanju, S. Ogunniyi, A. G. Adeniyi and C. A. Igwegbe, J. Ind. Eng. Chem., 2022, 105, 34–48 CrossRef CAS.
- Md. M. H. Mondol and S. H. Jhung, Chem. Eng. J., 2021, 421, 129688 CrossRef CAS.
- J. Gasperi, C. Sebastian, V. Ruban, M. Delamain, S. Percot, L. Wiest, C. Mirande, E. Caupos, D. Demare, M. D. K. Kessoo, M. Saad, J. J. Schwartz, P. Dubois, C. Fratta, H. Wolff, R. Moilleron, G. Chebbo, C. Cren, M. Millet, S. Barraud and M. C. Gromaire, Environ. Sci. Pollut. Res., 2014, 21, 5267–5281 CrossRef CAS PubMed.
- J. O. Ighalo, O. J. Ajala, G. Umenweke, S. Ogunniyi, C. A. Adeyanju, C. A. Igwegbe and A. G. Adeniyi, J. Environ. Chem. Eng., 2020, 8, 104264 CrossRef CAS.
- K. Kemp, J. Griffiths, S. Campbell and K. Lovell, Journal of Crohn’s and Colitis, 2013, 7, e386–e395 CrossRef PubMed.
- M. F. Abid, M. A. Zablouk and A. M. Abid-Alameer, Iran. J. Environ. Health Sci. Eng., 2012, 9, 1–9 CrossRef PubMed.
- J. Jawad, A. H. Hawari and S. J. Zaidi, Chem. Eng. J., 2021, 129540 CrossRef CAS.
- X. Wang, J. Xu, M. Xu, B. Zhou, J. Liang and L. Zhou, Sep. Purif. Technol., 2021, 258, 118047 CrossRef CAS.
- A. Chennah, Y. Naciri, H. A. Ahsaine, A. Taoufyq, B. Bakiz, L. Bazzi, F. Guinneton, J.-R. Gavarri and A. Benlhachemi, Mediterr. J. Chem., 2018, 6, 255–266 CrossRef CAS.
- H. A. Ahsaine, A. Slassi, Y. Naciri, A. Chennah, C. Jaramillo-Páez, Z. Anfar, M. Zbair, A. Benlhachemi and J. A. Navío, ChemistrySelect, 2018, 3, 7778–7791 CrossRef CAS.
- H. Ait Ahsaine, M. Ezahri, A. Benlhachemi, B. Bakiz, S. Villain, F. Guinneton and J.-R. Gavarri, Ceram. Int., 2016, 42, 8552–8558 CrossRef CAS.
- Y. Naciri, H. Ait Ahsaine, A. Chennah, A. Amedlous, A. Taoufyq, B. Bakiz, M. Ezahri, S. Villain and A. Benlhachemi, J. Environ. Chem. Eng., 2018, 6, 1840–1847 CrossRef CAS.
- M. El Ouardi, A. El aouni, H. A. Ahsaine, M. Zbair, A. BaQais and M. Saadi, Chemosphere, 2022, 136483 CrossRef CAS PubMed.
- S. Lotfi, M. E. Ouardi, H. A. Ahsaine and A. Assani, Catal. Rev., 2022, 1–45 CrossRef.
- A. Bouddouch, E. Amaterz, B. Bakiz, A. Taoufyq, F. Guinneton, S. Villain, J.-R. Gavarri, J.-C. Valmalette and A. Benlhachemi, Minerals, 2021, 11, 1007 CrossRef CAS.
- R. Haounati, F. Alakhras, H. Ouachtak, T. A. Saleh, G. Al-Mazaideh, E. Alhajri, A. Jada, N. Hafid and A. A. Addi, Arabian J. Sci. Eng., 2022, 1–11 Search PubMed.
- K. Aziz, F. Aziz, R. Mamouni, L. Aziz, Z. Anfar, A. Azrrar, B. Kjidaa, N. Saffaj and A. Laknifli, Environ. Sci. Pollut. Res., 2021 DOI:10.1007/s11356-021-16340-w.
- R. Haounati, A. El Guerdaoui, H. Ouachtak, R. El Haouti, A. Bouddouch, N. Hafid, B. Bakiz, D. M. F. Santos, M. Labd Taha, A. Jada and A. Ait Addi, Sep. Purif. Technol., 2021, 277, 119399 CrossRef CAS.
- K. Y. V. Beri, D. P. Barbosa, M. Zbair, S. Ojala and S. B. de Oliveira, Energy Nexus, 2021, 1, 100009 CrossRef CAS.
- C. Du, Z. Zhang, G. Yu, H. Wu, H. Chen, L. Zhou, Y. Zhang, Y. Su, S. Tan, L. Yang, J. Song and S. Wang, Chemosphere, 2021, 272, 129501 CrossRef CAS PubMed.
- J. Wen, Y. Fang and G. Zeng, Chemosphere, 2018, 201, 627–643 CrossRef CAS PubMed.
- J. Huang, D. Huang, F. Zeng, L. Ma and Z. Wang, J. Mater. Sci., 2021, 56, 3127–3139 CrossRef CAS.
- R. Wang, H. Xu, K. Zhang, S. Wei and W. Deyong, J. Hazard. Mater., 2019, 364, 272–280 CrossRef CAS PubMed.
- V. K. Sharma and M. Feng, J. Hazard. Mater., 2019, 372, 3–16 CrossRef CAS PubMed.
- B. Chen, Z. Yang, Y. Zhu and Y. Xia, J. Mater. Chem. A, 2014, 2, 16811–16831 RSC.
- B. Liu, M. Jian, H. Wang, G. Zhang, R. Liu, X. Zhang and J. Qu, Colloids Surf., A, 2018, 538, 164–172 CrossRef CAS.
- V. Anh Tran, L. T. Nhu Quynh, T.-T. Thi Vo, P. A. Nguyen, T. N. Don, Y. Vasseghian, H. Phan and S.-W. Lee, Environ. Res., 2022, 204, 112364 CrossRef CAS PubMed.
- Z. Tang, F. Zhu, J. Zhou, W. Chen, K. Wang, M. Liu, N. Wang and N. Li, Appl. Catal., B, 2022, 309, 121267 CrossRef CAS.
- J. Liu, Y. Chen, Y. Hu, Y. Zhang, G. Zhang, S. Wang and L. Zhang, J. Mol. Liq., 2022, 356, 119057 CrossRef CAS.
- J. Troyano, A. Carné-Sánchez, C. Avci, I. Imaz and D. Maspoch, Chem. Soc. Rev., 2019, 48, 5534–5546 RSC.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939–943 CrossRef CAS PubMed.
- J. Lee, K. Lee and J. Kim, ACS Appl. Mater. Interfaces, 2021, 13, 1620–1631 CrossRef CAS PubMed.
- Q. Jiang, Z. Han, Y. Qian, Y. Yuan, Y. Ren, M. Wang and Z. Cheng, Journal of Water Process Engineering, 2022, 47, 102768 CrossRef.
- A. I. A. Soliman, A.-M. A. Abdel-Wahab and H. Nasser Abdelhamid, RSC Adv., 2022, 12, 7075–7084 RSC.
- H. Bux, F. Liang, Y. Li, J. Cravillon, M. Wiebcke and J. Caro, J. Am. Chem. Soc., 2009, 131, 16000–16001 CrossRef CAS PubMed.
- K. W. Chapman, G. J. Halder and P. J. Chupas, J. Am. Chem. Soc., 2009, 131, 17546–17547 CrossRef CAS PubMed.
- V. V. Butova, A. P. Budnyk, E. A. Bulanova, C. Lamberti and A. V. Soldatov, Solid State Sci., 2017, 69, 13–21 CrossRef CAS.
- E. R. Cooper, C. D. Andrews, P. S. Wheatley, P. B. Webb, P. Wormald and R. E. Morris, Nature, 2004, 430, 1012–1016 CrossRef CAS PubMed.
- W. Lin, J. Gong, W. Ye, X. Huang and J. Chen, Z. Anorg. Allg. Chem., 2020, 646, 1900–1903 CrossRef CAS.
- H. Zheng, D. Wu, Y. Wang, X. Liu, P. Gao, W. Liu, J. Wen and E. V. Rebrov, J. Alloys Compd., 2020, 838, 155219 CrossRef CAS.
- M. P. M. Dicker, P. F. Duckworth, A. B. Baker, G. Francois, M. K. Hazzard and P. M. Weaver, Composites, Part A, 2014, 56, 280–289 CrossRef CAS.
- G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. DuChene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T. Hupp and F. Huo, Nat. Chem., 2012, 4, 310–316 CrossRef CAS PubMed.
- T. Li, W. Zhang, S. Zhai, G. Gao, J. Ding, W. Zhang, Y. Liu, X. Zhao, B. Pan and L. Lv, Water Res., 2018, 143, 87–98 CrossRef CAS PubMed.
- Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC.
- B. Yu, F. Wang, W. Dong, J. Hou, P. Lu and J. Gong, Mater. Lett., 2015, 156, 50–53 CrossRef CAS.
- X. Wang, J. Liu, S. Leong, X. Lin, J. Wei, B. Kong, Y. Xu, Z.-X. Low, J. Yao and H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 9080–9087 CrossRef CAS PubMed.
- X. Zeng, L. Huang, C. Wang, J. Wang, J. Li and X. Luo, ACS Appl. Mater. Interfaces, 2016, 8, 20274–20282 CrossRef CAS PubMed.
- S. Chao, X. Li, Y. Li, Y. Wang and C. Wang, J. Colloid Interface Sci., 2019, 552, 506–516 CrossRef CAS PubMed.
- Y. Zhang and S.-J. Park, Appl. Catal., B, 2019, 240, 92–101 CrossRef CAS.
- H. Zheng, Z. Yang, W. Wang, S. Guo, Z. Li, K. Liu and N. Sui, Plant Physiol. Biochem., 2020, 149, 11–26 CrossRef CAS.
- P. Zhang, S. Wang, B. Y. Guan and X. W. David Lou, Energy Environ. Sci., 2019, 12, 164–168 RSC.
- M. Hu, H. Lou, X. Yan, X. Hu, R. Feng and M. Zhou, Microporous Mesoporous Mater., 2018, 271, 68–72 CrossRef CAS.
- I. Ahmed, B. N. Bhadra, H. J. Lee and S. H. Jhung, Catal. Today, 2018, 301, 90–97 CrossRef CAS.
- Q. T. N. Le and K. Cho, J. Colloid Interface Sci., 2021, 581, 741–750 CrossRef CAS PubMed.
- Y. He, L. Zeng, Z. Feng, Q. Zhang, X. Zhao, S. Ge, X. Hu and H. Lin, Adv. Powder Technol., 2020, 31, 439–447 CrossRef CAS.
- R.-M. Kong, Y. Zhao, Y. Zheng and F. Qu, RSC Adv., 2017, 7, 31365–31371 RSC.
- H. Dai, X. Yuan, L. Jiang, H. Wang, J. Zhang, J. Zhang and T. Xiong, Coord. Chem. Rev., 2021, 441, 213985 CrossRef CAS.
- N. Ouasfi, S. Bouzekri, M. Zbair, H. Ait Ahsaine, S. Bakkas, M. Bensitel and L. Khamliche, Surf. Interfaces, 2019, 14, 61–71 CrossRef CAS.
- H. Ait Ahsaine, M. Zbair, Z. Anfar, Y. Naciri, R. El haouti, N. El Alem and M. Ezahri, Mater. Today Chem., 2018, 8, 121–132 CrossRef CAS.
- M. Zbair, Z. Anfar and H. A. Ahsaine, RSC Adv., 2019, 9, 5756–5769 RSC.
- Z. Anfar, H. Ait Ahsaine, M. Zbair, A. Amedlous, A. Ait El Fakir, A. Jada and N. El Alem, Crit. Rev. Environ. Sci. Technol., 2020, 50, 1043–1084 CrossRef CAS.
- Z. Anfar, M. Zbair, H. A. Ahsaine, A. Jada and N. E. Alem, RSC Adv., 2020, 10, 13430 RSC.
- D. Allouss, Y. Essamlali, O. Amadine, A. Chakir and M. Zahouily, RSC Adv., 2019, 9, 37858–37869 RSC.
- M. Zbair, M. Bottlinger, K. Ainassaari, S. Ojala, O. Stein, R. L. Keiski, M. Bensitel and R. Brahmi, Waste Biomass Valorization, 2020, 11, 1565–1584 CrossRef.
- M. Zbair, A. El Hadrami, A. Bellarbi, M. Monkade, A. Zradba and R. Brahmi, J. Environ. Chem. Eng., 2020, 8, 103667 CrossRef CAS.
- X. Zhao, M. Zheng, X. Gao, J. Zhang, E. Wang and Z. Gao, Coord. Chem. Rev., 2021, 440, 213970 CrossRef CAS.
- I. Cota and F. Fernandez Martinez, Coord. Chem. Rev., 2017, 351, 189–204 CrossRef CAS.
- Z. Chang, L. Zeng, C. Sun, P. Zhao, J. Wang, L. Zhang, Y. Zhu and X. Qi, Coord. Chem. Rev., 2021, 445, 214072 CrossRef CAS.
- D. Yamamoto, T. Maki, S. Watanabe, H. Tanaka, M. T. Miyahara and K. Mae, Chem. Eng. J., 2013, 227, 145–150 CrossRef CAS.
- Q. Zhu, W. Zhuang, Y. Chen, Z. Wang, B. Villacorta Hernandez, J. Wu, P. Yang, D. Liu, C. Zhu, H. Ying and Z. Zhu, ACS Appl. Mater. Interfaces, 2018, 10, 16066–16076 CrossRef CAS PubMed.
- L. Zhao, W. Lv, J. Hou, Y. Li, J. Duan and S. Ai, Microchem. J., 2020, 152, 104425 CrossRef CAS.
- N. M. Mahmoodi, M. Oveisi, A. Taghizadeh and M. Taghizadeh, Carbohydr. Polym., 2020, 227, 115364 CrossRef CAS PubMed.
- W. Dong, D. Wang, H. Wang, M. Li, F. Chen, F. Jia, Q. Yang, X. Li, X. Yuan, J. Gong, H. Li and J. Ye, J. Colloid Interface Sci., 2019, 535, 444–457 CrossRef CAS PubMed.
- J. L. C. Rowsell and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 1304–1315 CrossRef CAS PubMed.
- J. Zheng, C. Cheng, W.-J. Fang, C. Chen, R.-W. Yan, H.-X. Huai and C.-C. Wang, CrystEngComm, 2014, 16, 3960–3964 RSC.
- X. Wu, J. Xiong, S. Liu, J.-H. Cheng, M.-H. Zong and W.-Y. Lou, J. Hazard. Mater., 2021, 417, 126011 CrossRef CAS PubMed.
- Y. Zhan, X. Guan, E. Ren, S. Lin and J. Lan, J. Polym. Res., 2019, 26, 145 CrossRef.
- N. Marsiezade and V. Javanbakht, Int. J. Biol. Macromol., 2020, 162, 1140–1152 CrossRef CAS PubMed.
- H. Nasser Abdelhamid and A. P. Mathew, Chem. Eng. J., 2021, 426, 131733 CrossRef CAS.
- L. Zhu, L. Zong, X. Wu, M. Li, H. Wang, J. You and C. Li, ACS Nano, 2018, 12, 4462–4468 CrossRef CAS PubMed.
- W. Zhang, S. Shi, W. Zhu, L. Huang, C. Yang, S. Li, X. Liu, R. Wang, N. Hu, Y. Suo, Z. Li and J. Wang, ACS Sustainable Chem. Eng., 2017, 5, 9347–9354 CrossRef CAS.
- L. Zong, Y. Yang, H. Yang and X. Wu, ACS Appl. Mater. Interfaces, 2020, 12, 7295–7301 CrossRef CAS PubMed.
- Y. Li, P. Kamdem and X.-J. Jin, J. Electrochem. Soc., 2020, 167, 110562 CrossRef CAS.
- C. Gu, W. Weng, C. Lu, P. Tan, Y. Jiang, Q. Zhang, X. Liu and L. Sun, Chin. J. Chem. Eng., 2022, 42, 42–48 CrossRef.
- R. Li, X. Ren, J. Zhao, X. Feng, X. Jiang, X. Fan, Z. Lin, X. Li, C. Hu and B. Wang, J. Mater. Chem. A, 2014, 2, 2168–2173 RSC.
- X. Meng, C. Duan, Y. Zhang, W. Lu, W. Wang and Y. Ni, Compos. Sci. Technol., 2020, 200, 108384 CrossRef CAS.
- C. Fan, Y. Liang, H. Dong, J. Yang, G. Tang, W. Zhang, D. Kong, J. Li and Y. Cao, Sci. Total Environ., 2018, 640–641, 163–173 CrossRef CAS PubMed.
- X. Chen, Y. Zhang, Y. Zhao, Y. Wu, S. Wang, W. Xu, J. Zhang, S. Yang, L. Liu and Y. Liu, Mater. Lett., 2018, 218, 67–70 CrossRef CAS.
- L. Yin, Z. Liu, Y. Yang, Y. Guo, G. Zhang, F. Gai, Y. Ao, jieran Liu, B. Xin and Y. Liu, Mater. Chem. Phys., 2020, 242, 122563 CrossRef CAS.
- H. Yang, S. Hu, H. Zhao, X. Luo, Y. Liu, C. Deng, Y. Yu, T. Hu, S. Shan, Y. Zhi, H. Su and L. Jiang, J. Hazard. Mater., 2021, 416, 126046 CrossRef CAS PubMed.
- P. Hou, G. Xing, D. Han, H. Wang, C. Yu and Y. Li, J. Solid State Chem., 2018, 265, 184–192 CrossRef CAS.
- M. Hijab, P. Parthasarathy, H. R. Mackey, T. Al-Ansari and G. McKay, Chem. Eng. Process., 2021, 167, 108318 CrossRef CAS.
- X. Fan, L. Deng, K. Li, H. Lu, R. Wang and W. Li, Colloid Interface Sci. Commun., 2021, 44, 100485 CrossRef CAS.
- B. Yan, X. Wang, X. Zhang, S. Liu, H. Lu and R. Ran, Colloids Surf., A, 2022, 639, 128347 CrossRef CAS.
- S. B. Zadvarzi, M. Khavarpour, S. M. Vahdat, S. M. Baghbanian and A. S. Rad, Int. J. Biol. Macromol., 2021, 168, 428–441 CrossRef CAS PubMed.
- J. Ma, J. Hu, Y. Tang, H. Gu, M. Jiang and J. Zhang, J. Colloid Interface Sci., 2020, 572, 160–169 CrossRef CAS PubMed.
- Y. Li, X. Yan, X. Hu, R. Feng, M. Zhou and D. Han, J. Porous Mater., 2020, 27, 1109–1117 CrossRef CAS.
- J. Abdi, M. Vossoughi, N. M. Mahmoodi and I. Alemzadeh, Chem. Eng. J., 2017, 326, 1145–1158 CrossRef CAS.
- M. A. Nazir, M. A. Bashir, T. Najam, M. S. Javed, S. Suleman, S. Hussain, O. P. Kumar, S. S. A. Shah and A. ur Rehman, Microchem. J., 2021, 164, 105973 CrossRef CAS.
- Q. Wang, X. Zhang, F. Wang, Y. Xie, C. Wang, J. Zhao, Q. Yang and Z. Chen, J. Solid State Chem., 2021, 299, 122158 CrossRef CAS.
- Y. Wang, X. Dai, Y. Zhan, X. Ding, M. Wang and X. Wang, Int. J. Biol. Macromol., 2019, 137, 77–86 CrossRef CAS PubMed.
- S.-Q. Xiong, J.-L. Li, Y. Wang, Y.-H. Gao, C.-X. Jin, W.-W. Dong, J. Zhao, M. He, D. Li and H.-B. Shang, ChemistrySelect, 2019, 4, 6429–6436 CrossRef CAS.
- Y. Xu, J. Jin, X. Li, Y. Han, H. Meng, J. Wu and X. Zhang, J. Sep. Sci., 2016, 39, 3647–3654 CrossRef CAS.
- J. Abdi, N. M. Mahmoodi, M. Vossoughi and I. Alemzadeh, Microporous Mesoporous Mater., 2019, 273, 177–188 CrossRef CAS.
- Q. Wang, L. Lei, F. Wang, C. Chen, X. Kang, C. Wang, J. Zhao, Q. Yang and Z. Chen, J. Solid State Chem., 2020, 292, 121656 CrossRef CAS.
- Ü. Ecer, A. Zengin and T. Şahan, Colloids Surf., A, 2021, 630, 127558 CrossRef.
- S. Sharafinia, A. Farrokhnia and E. G. Lemraski, Colloids Surf., A, 2022, 634, 128039 CrossRef CAS.
- J. Peng, Y. Li, X. Sun, C. Huang, J. Jin, J. Wang and J. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 4328–4337 CrossRef CAS.
- Y. Yuan, D. Yang, G. Mei, X. Hong, J. Wu, J. Zheng, J. Pang and Z. Yan, Colloids Surf., A, 2018, 544, 187–195 CrossRef CAS.
- O. Maan, P. Song, N. Chen and Q. Lu, Adv. Mater. Interfaces, 2019, 6, 1801895 CrossRef.
- R. M. Abdelhameed and H. E. Emam, J. Colloid Interface Sci., 2019, 552, 494–505 CrossRef CAS PubMed.
- J. Wang, Y. Li, Z. Lv, Y. Xie, J. Shu, A. Alsaedi, T. Hayat and C. Chen, J. Colloid Interface Sci., 2019, 542, 410–420 CrossRef CAS.
- E. Bahmani, S. Koushkbaghi, M. Darabi, A. ZabihiSahebi, A. Askari and M. Irani, Carbohydr. Polym., 2019, 224, 115148 CrossRef CAS.
- B. Akhsassi, A. Bouddouch, Y. Naciri, B. Bakiz, A. Taoufyq, C. Favotto, S. Villain, F. Guinneton and A. Benlhachemi, Chem. Phys. Lett., 2021, 783, 139046 CrossRef CAS.
- H. A. Ahsaine, A. E. Jaouhari, A. Slassi, M. Ezahri, A. Benlhachemi, B. Bakiz, F. Guinneton and J.-R. Gavarri, RSC Adv., 2016, 6, 101105–101114 RSC.
- H. Ait Ahsaine, Mater. Lett., 2020, 276, 128221 CrossRef CAS.
- A. Bouddouch, E. Amaterz, A. Taoufyq, B. Bakiz, F. Guinneton, S. Villain, J. C. Valmalette, J. R. Gavarri and A. Benlhachemi, Mater. Today: Proc., 2020, 27, 3139–3144 CAS.
- E. Shamsaei, F. Basquiroto de Souza, K. Sagoe-Crentsil and W. Duan, Microporous Mesoporous Mater., 2022, 332, 111702 CrossRef CAS.
- M. Cao, Z. Zhuang, Y. Liu, Z. Zhang, J. Xuan, Q. Zhang and W. Wang, J. Colloid Interface Sci., 2022, 608, 2779–2790 CrossRef CAS PubMed.
- H.-P. Jing, C.-C. Wang, Y.-W. Zhang, P. Wang and R. Li, RSC Adv., 2014, 4, 54454–54462 RSC.
- B. Abdollahi, A. Najafidoust, E. Abbasi Asl and M. Sillanpaa, Arabian J. Chem., 2021, 14, 103444 CrossRef CAS.
- A. Faraji, N. Mehrdadi, N. M. Mahmoodi, M. Baghdadi and A. Pardakhti, J. Mol. Struct., 2021, 1223, 129028 CrossRef CAS.
- R. Chandra and M. Nath, Journal of Water Process Engineering, 2020, 36, 101266 CrossRef.
- X. Guan, S. Lin, J. Lan, J. Shang, W. Li, Y. Zhan, H. Xiao and Q. Song, Cellulose, 2019, 26, 7437–7450 CrossRef CAS.
- J. He, J. Ye, Y. Zhang, L. Kong, X. Zhou, Y. Ma and Y. Yang, ChemistrySelect, 2020, 5, 3746–3755 CrossRef CAS.
- H. A. Yurtsever and A. E. Çetin, Colloids Surf., A, 2021, 625, 126980 CrossRef CAS.
- J. Ran, H. Chen, S. Bi, Q. Guo, C. Yan, X. Tang, D. Cheng, G. Cai and X. Wang, Prog. Org. Coat., 2021, 152, 106123 CrossRef CAS.
- Y. Si, X. Li, G. Yang, X. Mie and L. Ge, J. Mater. Sci., 2020, 55, 13049–13061 CrossRef CAS.
- Q. Yang, W. Lin, Z. Duan, S. Xu, J. Chen and X. Mai, Environ. Technol., 2021, 1–13 CrossRef PubMed.
- L. Lei, D. Huang, G. Zeng, M. Cheng, D. Jiang, C. Zhou, S. Chen and W. Wang, Coord. Chem. Rev., 2019, 399, 213020 CrossRef CAS.
- S. Zhang, C. Liu, G. Zhang, Y. Chen, F. Shang, Q. Xia and W. Yang, Coord. Chem. Rev., 2021, 433, 213742 CrossRef CAS.
- A. A. Ansari and B. D. Malhotra, Coord. Chem. Rev., 2022, 452, 214282 CrossRef CAS.
- H. Chen, Y.-Q. wang, F. Huang, C. Tu and li Cui, Appl. Surf. Sci., 2021, 565, 150458 CrossRef CAS.
- X. Yang, Z. Wen, Z. Wu and X. Luo, Inorg. Chem. Front., 2018, 5, 687–693 RSC.
- J. Liu, R. Li, Y. Wang, Y. Wang, X. Zhang and C. Fan, J. Alloys Compd., 2017, 693, 543–549 CrossRef CAS.
- J. Abdi, Colloids Surf., A, 2020, 604, 125330 CrossRef CAS.
- Y. Jing, Q. Lei, C. Xia, Y. Guan, Y. Yang, J. He, Y. Yang, Y. Zhang and M. Yan, RSC Adv., 2020, 10, 698–704 RSC.
- N. Chang, Y.-R. Chen, F. Xie, Y.-P. Liu and H.-T. Wang, Colloids Surf., A, 2021, 616, 126351 CrossRef CAS.
- J. Li, L. Liu, Q. Liang, M. Zhou, C. Yao, S. Xu and Z. Li, J. Hazard. Mater., 2021, 414, 125395 CrossRef CAS PubMed.
- A. Liu, C. Yu, J. Lin, G. Sun, G. Xu, Y. Huang, Z. Liu and C. Tang, Mater. Res. Bull., 2019, 112, 147–153 CrossRef CAS.
- R. Li, W. Li, C. Jin, Q. He and Y. Wang, J. Alloys Compd., 2020, 825, 154008 CrossRef CAS.
- X. Yuan, S. Qu, X. Huang, X. Xue, C. Yuan, S. Wang, L. Wei and P. Cai, Chem. Eng. J., 2021, 416, 129148 CrossRef CAS.
- Y. Zhou, S. Feng, X. Duan, W. Wu, Z. Ye, X. Dai, Y. Wang and X. Cao, J. Solid State Chem., 2022, 305, 122628 CrossRef CAS.
- J. Zhou, W. Liu and W. Cai, Sci. Total Environ., 2019, 696, 133962 CrossRef CAS PubMed.
- W. Zheng, S. Feng, C. Shao, G. Zhu, Z. Ni, J. Sun and X. Huang, Res. Chem. Intermed., 2020, 46, 2951–2967 CrossRef CAS.
- Y. Jing, H. Yin, C. Li, J. Chen, S. Wu, H. Liu, L. Xie, Q. Lei, M. Sun and S. Yu, Environ. Res., 2022, 203, 111819 CrossRef CAS PubMed.
- W.-Q. Chen, L.-Y. Li, L. Li, W.-H. Qiu, L. Tang, L. Xu, K.-J. Xu and M.-H. Wu, Engineering, 2019, 5, 755–767 CrossRef CAS.
- C. Zhang, J. Zhang, K. Ou, Y. Liu, Z. Guo, X. Chen, G. Cheng and F. Hu, Colloids Surf., A, 2021, 615, 126257 CrossRef CAS.
- N. M. Mahmoodi, S. Keshavarzi, M. Oveisi, S. Rahimi and B. Hayati, J. Mol. Liq., 2019, 291, 111333 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |