Open Access Article
Open Access ArticleControlled isolation and stabilisation of pure metastable carbamazepine form IV by droplet-confinement via a continuous manufacturing route†
Alice
Parkes
 ,
Ahmad
Ziaee
,
Gavin
Walker
and
Emmet
O'Reilly
,
Ahmad
Ziaee
,
Gavin
Walker
and
Emmet
O'Reilly
 *
*
Department of Chemical Sciences, SSPC The SFI Research Centre for Pharmaceuticals, Bernal Institute, University of Limerick, Ireland. E-mail: emmet.oreilly@ul.ie
First published on 14th September 2022
Abstract
This study outlines a systematic approach to control the polymorphism of carbamazepine (CBZ) and isolate the metastable polymorph CBZ form IV as a function of droplet size using spray drying as a continuous method of manufacture. In this controlled process, CBZ molecules were confined within spray dried droplets of specific size during the nucleation process to investigate the effect of confinement space on the polymorphic outcome.
Controlling the polymorphism of an active pharmaceutical ingredient (API) through continuous manufacturing is critical in pharmaceutical processing. Greater than 50% of medicines are rejected during the drug approval process due to safety concerns arising from undesired polymorphic transitions according to Jean-Paul Garnier, former CEO of GlaxoSmithKline.1 Individual polymorphic forms exhibit different physiochemical properties such as stability, solubility and bioavailability; therefore, their therapeutic effect or side effects can vary significantly with reduced therapeutic outcomes for patients.2 Moreover, the lack of a systematic approach to control polymorphic transitions has significant financial implications for the pharmaceutical industry alike.3 Therefore, there is a collective aim to selectively obtain polymorphs of APIs with increased stability.
Polymorphs are different structural arrangements of the same molecular unit in a crystal lattice4 and the first example of a polymorphic organic compound was discovered in 1832 by Wöhler and Liebig.5–8 Carbamazepine (CBZ) is an anti-convulsant polymorphic API with five known polymorphs. Its catameric polymorph, form V, however, has only been obtained by a computationally assisted approach.9 As the nomenclature for each polymorph has varied in the literature, the following nomenclature is employed in this study: the triclinic form will be referred to as form I, the trigonal form as form II, the p-monoclinic form as form III and the c-monoclinic form as form IV.10 The relative thermodynamic stability of CBZ polymorphs is in the following order: form III > form I > form IV > form II.10 Therefore, form III is regarded as the most stable and only commercially available form of CBZ.11 Confinement has been identified as a way of selectively controlling the polymorphic transitions of an API.12 It has been proposed that confining crystals within small volumes leads to higher control over the nucleation process and possible stabilisation of metastable polymorphs with higher solubilities.13 Different methods of confinement such as confinement in mesoporous silica,14 porous glass,12 inkjet printing15 or small vials16 have been used for isolating polymorphic APIs.
In this study we demonstrate polymorphic control using a droplet as the confinement space rather than a physical barrier. This is achieved through the optimisation of spray drying parameters to control droplet size. Spray drying is a continuous method for converting a liquid feed solution or suspension to powder in a single step.17 This method can be used to rapidly isolate CBZ form IV by spray drying without the use of additives.18 The addition of additives is undesirable as it can lead to increased final dosage form weight and the need for additional processing to isolate the pure polymorph from the additive.19 Some modifications of conventional spray drying e.g., supercritical CO2 antisolvent-assisted nano spray drying (SASD) have been used for crystallisation of polymorphic APIs; however, in previous spray drying studies of CBZ, varying the droplet size to exert control on the polymorphic form obtained was not considered.18,20 A study controlling the polymorphism of CBZ-saccharin cocrystals using SASD, spray drying and electrospraying concluded that pure polymorphs, form I and form II, were obtained via both SASD and electrospraying; however, a mixture of polymorphic form I and II was obtained for spray drying.21 This was attributed to the anti-solvent effect of SASD and the charge applied in electrospraying, however, nano-sized droplets are generated via SASD and electrospraying whereas micron-sized droplets are generated via spray drying.21 In these studies, the focus has been on exploring different techniques while the effects of droplet size and the subsequent control over the confinement level have been overlooked.
This study presents a controlled method to produce pure metastable CBZ form IV as a function of droplet size by using optimised spray drying parameters. This approach to controlling the polymorphism of CBZ by spray drying has the potential to be applied to control the polymorphism and obtain pure forms of other polymorphic single component crystals, cocrystals and multicomponent pharmaceuticals by continuous manufacturing.
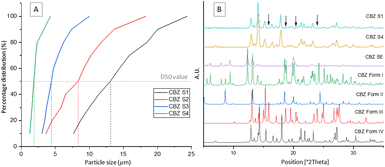
Table 1 displays the process parameters used for spray drying four different samples with various mean particle sizes in the range of 1–13 μm. Particle size distribution analysis shows the particles produced using the lowest atomisation gas flowrate, hence the largest droplet size, S1, have a much larger distribution than the particles produced using the highest atomisation gas flowrate, S4 (Fig. 1A). An inverse correlation can clearly be observed between the atomisation gas flowrate and the mean particle size of the spray dried samples. The final yield of CBZ S1–S4 were 10.48%, 22.11%, 53.21 and 13.64% respectively. This yield could be maximised by optimising the process conditions which will be carried out in future work.
| Formulation | Process parameters | Particle properties | ||||
|---|---|---|---|---|---|---|
| Atomising gas flowrate (L h−1) | Feed flowrate (ml min−1) | Inlet temperature (°C) | Outlet temperature (°C) | Particle size (D50) (μm) (Std. dev.) | Droplet size* (μm)22 (Std. dev.) | |
| CBZ size 1 (S1) | 192 | 1.5 | 65 | 50 | 13.09 (4.72) | 38.55 (13.89) |
| CBZ size 2 (S2) | 246 | 1.5 | 68 | 51 | 8.33 (3.84) | 24.53 (11.31) |
| CBZ size 3 (S3) | 601 | 1.5 | 71 | 52 | 4.42 (1.64) | 13.02 (4.84) |
| CBZ size 4 (S4) | 742 | 1.5 | 74 | 51 | 1.83 (0.71) | 5.39 (2.09) |
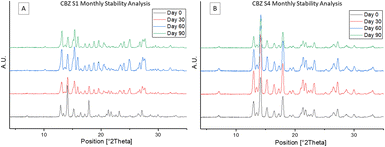
Sample S4 with the smallest particle size distribution was confirmed to consist of CBZ form IV by PXRD.10,23 The CBZ S1 sample with the largest particle size distribution was confirmed by PXRD to consist of a mixture of polymorphic forms IV and III. The arrows in Fig. 1B show characteristic peaks of form III present in CBZ S1.24 For more information on the PXRD diffractograms of each polymorph see ESI.† Based on the PXRD results, a direct correlation between the crystallised polymorphs and the particle size distribution could be identified. Accordingly, the largest particles (D50 = 13.09 μm) contain a mixture of form IV and form III polymorphs, while the smallest particles (D50 = 1.83 μm) contain pure form IV. Based on these results it is likely that the mixture of two polymorphic forms found to be present in S1 is solely due to the larger distribution of particle sizes and that the smaller particles crystallised as form IV, while the larger particles crystallised as the more stable form III. The reproducibility of these results has been verified by three replicate studies for each droplet size. To confirm the polymorphic transition was due to the processing technique outlined, a slow evaporation crystallisation experiment was performed which produced a different polymorph, confirmed by PXRD to be pure CBZ form I.10 PXRD analysis of the initial CBZ starting material (CBZ Int) confirmed the most stable polymorph, form III, was present before processing (Fig. S1†). To check the stability of the spray dried samples at various process conditions and their subsequent polymorphic forms, all samples were tested under accelerated stability test conditions. The stability test was carried out for 90 days at 40 °C/75RH. Fig. 2A shows that the amount of form III present in samples S1–S3 increased over the testing period, as more characteristic peaks of form III are present in the diffractograms at day 90. The smallest droplet size S4, however, was the only sample in which its polymorphic form was fully preserved during the stability test with only form IV being detected by PXRD analysis. This result shows that this controlled processing technique has selectively produced a metastable polymorph in pure form which remains stable.
A plausible explanation for this observation is the high purity of this sample which did not facilitate the formation or transition to other polymorphs. However, for larger droplet sizes, a longer drying process provides sufficient time for solution mediated transformation to occur that may have initially produced a mixture of the two polymorphs, form III and IV. The presence of form III could act as an impurity that can lead to a greater amount of form IV polymorphs transforming to form III. This hypothesis agrees with the seeding phenomenon where the presence of form III potentially facilitates the continual transformation of form IV to the more stable form III.3
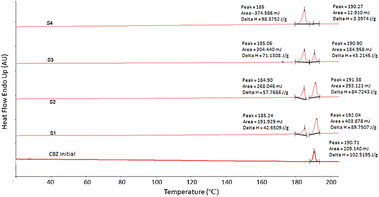
Fig. 3 shows a significant change in the morphology of the spray dried particles. Sample S1 with the largest particle size distribution consisted of spherically packed particles of prism-like crystals that were confirmed to be a mixture of the p-monoclinic (form III) and c-monoclinic (form IV) crystal structures (Fig. 3A). On the other hand, sample S4 with the smallest particle size distribution consisted of agglomerated spherical particles, confirmed by PXRD to be c-monoclinic in pure form (Fig. 3D). This is in agreement with the work of Grzesiak et al. which shows the packing diagrams of the carboxamide dimer units for both monoclinic polymorphs leading to these observed morphologies.10 The differential scanning calorimetry (DSC) results in Fig. 4 show the correlation between the size of the droplet and the relative amount of CBZ form IV present. The first melting peak for all samples regardless of their particle size distribution occurs at 185 °C and corresponds to the melting of form IV.10 As the area under each peak corresponds to the amount of that polymorph melting, the relative amounts of form IV are evidently increasing as the droplet size is decreasing; S4 contains the most amount of form IV. The thermogram of the starting material, CBZ initial, form III, shows only one melting point at 190.71 °C. This shows that the second peak present in the thermograms of samples S1–S3 is representing the relative amounts of form III present in these particles. This confirms a mixture of both form IV and form III are present in CBZ S1–S3, whereas sample S4 only contains form IV. Fourier transform infrared spectroscopy (FTIR) was also used to characterise the spray dried formulations. For more information refer to Fig. S2.†
 | ||
| Fig. 3 SEM images of (A) CBZ S1, (B) CBZ S2, (C) CBZ S3, (D) CBZ S4, (E) CBZ produced from slow evaporation crystallisation and (F) CBZ starting material (CBZ initial) at ×5.00k. | ||
This study shows that the size of the droplets produced in the spray dryer directly influences the final polymorphic form of CBZ. There was a clear transition from a mixture of forms III and IV to pure form IV when the size of particles was reduced from D50 13.09 μm to 1.83 μm. The effect of size and confinement within physical barriers on the polymorphic transition of APIs has been investigated in the literature before, for instance, Dela Cruz et al. conducted a study in which a solution of acetaminophen and a solution of glycine were crystallised in a confined space of 1 ml vials. The study discussed the effect of volume reduction on nucleation induction times, on the polymorphic form and on the probability of a nucleation event occurring.16 Other studies have looked at the confinement of CBZ in porous media, such as porous silica.14 Confinement of CBZ in a droplet during spray drying can influence the nucleation direction of crystal growth and the morphology of the final dried powder. Reducing the volume at which the material is confined to the size of a droplet, allows for metastable polymorphs to be obtained which could not be achieved otherwise. The shape of the confined space can also alter the obtained polymorph as crystal nucleation and growth occurs in a favoured direction.25 In this study, the spray drying process only produced form III, p-monoclinic, and form IV, c-monoclinic, with prism-like morphologies.18,26 Form I and form II, however, exhibit needle-like morphologies.26,27 These observations could be due to the spherical shape of the confinement space i.e., the droplet, favouring the nucleation of prism-like morphologies over needle-like crystals.
In general, polymorphs with the fastest growth rate will crystalise; however, the growth rate changes as a function of the crystallisation conditions:28 the degree of supersaturation, the temperature and the interfacial tension. The resulting polymorph, therefore, depends on each of these conditions. In the spray drying process, the droplets are atomised into the drying chamber at the set inlet temperature of 70 °C. Methanol, with a boiling temperature of 64.7 °C, immediately starts to evaporate from the surface of the droplet. Thereafter, the amount of solvent at the surface is replenished with excess solvent from the droplet core. This process continues until the surface of the droplet becomes supersaturated by solute and crystallisation occurs. A shorter time is required for the solvent to be evaporated from the surface of smaller droplets due to higher surface area being exposed to the inlet temperature of the spray drying chamber. Droplet confinement, therefore, controls the induction time for nucleation by limiting the amount of solvent and accessible space in which the nucleation occurs. In this case, the rapid isolation and drying of a particle in spray drying allows for the metastable polymorph of CBZ, form IV, to be obtained before it dissolves and transforms to a more stable form by solution mediated transformation.
Solution mediated transformation arises due to the solubility differences between polymorphs.28 According to Ostwald's rule of stages, the least stable polymorph will crystallise first. From this point on there is a possibility for solution-mediated transformation to occur. As a result, in samples with larger spray dried droplets, it can be postulated that the least stable CBZ polymorph (form II) nucleates initially followed by solution mediated transformation to form IV. Droplets with remaining methanol that have not fully evaporated after the nucleation of CBZ form IV have the potential to partially dissolve and transform to the more stable polymorph, form III. Therefore, the shorter the drying time, the shorter the available time for solution mediated transformation to occur and the greater the prospect of achieving metastable polymorphs. This is a likely explanation for the results of the study herein in which the formation of pure form IV in the smallest droplet size (CBZ S4) and a mixture of form IV and form III in the largest droplet size (CBZ S1) were observed. The same phenomenon has been observed by Buanz et al. where they obtained the metastable polymorph of B-mannitol through inkjet printing.29
In this study, a process of obtaining both a mixture of stable and metastable polymorphs of CBZ, form III and IV, and isolating pure metastable form IV using spray drying as a continuous method of manufacture has been demonstrated. Crystallisation theories, such as supersaturation and solution mediated transformation, support and explain our findings. A stability investigation was also conducted in which it was found the smaller the level of confinement, the more stable the metastable polymorph CBZ form IV. As pharmaceutical industries look to move in the direction of continuous manufacture, methods of increased process control such as the approach discussed herein are essential.
The research conducted in this publication was funded by the Irish Research Council under grant number [GOIPG/2020/1648].
This work was supported by the Synthesis and Solid-State Pharmaceutical Centre (SSPC), the SFI Research Centre for Pharmaceuticals under grant number [12/RC/2275_P2].
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. R. Gardner, C. T. Walsh and Ö. Almarsson, Drugs as materials: valuing physical form in drug discovery, Nat. Rev. Drug Discovery, 2004, 3(11), 926–934 CrossRef CAS PubMed.
- A. J. Cruz-Cabeza and J. Bernstein, Conformational polymorphism, Chem. Rev., 2014, 114(4), 2170–2191 CrossRef CAS PubMed.
- D.-K. Bučar, R. W. Lancaster and J. Bernstein, Disappearing Polymorphs Revisited, Angew. Chem., Int. Ed., 2015, 54(24), 6972–6993 CrossRef.
- A. J. Cruz-Cabeza, N. Feeder and R. J. Davey, Open questions in organic crystal polymorphism, Commun. Chem., 2020, 3(1), 142 CrossRef CAS.
- A. J. Cruz-Cabeza, S. M. Reutzel-Edens and J. Bernstein, Facts and fictions about polymorphism, Chem. Soc. Rev., 2015, 44(23), 8619–8635 RSC.
- M. À. Cuevas-Diarte and H. A. Oonk, Molecular Mixed Crystals, Springer, 2021 Search PubMed.
- J. Thun, L. Seyfarth, C. Butterhof, J. Senker, R. E. Dinnebier and J. Breu, Wöhler and Liebig Revisited: 176 Years of Polymorphism in Benzamide - and the Story Still Continues!, Cryst. Growth Des., 2009, 9(5), 2435–2441 CrossRef CAS.
- J. Bernstein, Polymorphism in Molecular Crystals 2e, International Union of Crystal, 2020 Search PubMed.
- J.-B. Arlin, L. S. Price, S. L. Price and A. J. Florence, A strategy for producing predicted polymorphs: catemeric carbamazepine form V, Chem. Commun., 2011, 47(25), 7074–7076 RSC.
- A. L. Grzesiak, M. Lang, K. Kim and A. J. Matzger, Comparison of the four anhydrous polymorphs of carbamazepine and the crystal structure of form I, J. Pharm. Sci., 2003, 92(11), 2260–2271 CrossRef CAS PubMed.
- D. Huang, H. C. S. Chan, Y. Wu, L. Li, L. Zhang and Y. Lv, et al., Phase solubility investigation and theoretical calculations on drug-drug cocrystals of carbamazepine with Emodin, Paeonol, J. Mol. Liq., 2021, 329, 115604 CrossRef CAS.
- F. C. Meldrum and C. O'Shaughnessy, Crystallization in Confinement, Adv. Mater., 2020, 32(31), 2001068 CrossRef CAS.
- L. Dwyer, V. Michaelis, M. O'Mahony, R. Griffin and A. Myerson, Confined crystallization of fenofibrate in nanoporous silica, CrystEngComm, 2015, 17(41), 7922–7929 RSC.
- A. V. Gandhi, P. Thipsay, B. Kirthivasan and E. Squillante, Adsorption onto Mesoporous Silica Using Supercritical Fluid Technology Improves Dissolution Rate of Carbamazepine—a Poorly Soluble Compound, AAPS PharmSciTech, 2017, 18(8), 3140–3150 CrossRef CAS PubMed.
- A. B. M. Buanz and S. Gaisford, Formation of Highly Metastable β Glycine by Confinement in Inkjet Printed Droplets, Cryst. Growth Des., 2017, 17(3), 1245–1250 CrossRef CAS.
- I. J. C. Dela Cruz, J. V. Perez, B. G. Alamani, G. Capellades and A. S. Myerson, Influence of Volume on the Nucleation of Model Organic Molecular Crystals through an Induction Time Approach, Cryst. Growth Des., 2021, 21(5), 2932–2941 CrossRef CAS.
- A. Ziaee, A. B. Albadarin, L. Padrela, T. Femmer, E. O'Reilly and G. Walker, Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches, Eur. J. Pharm. Sci., 2019, 127, 300–318 CrossRef CAS PubMed.
- R. A. Halliwell, R. M. Bhardwaj, C. J. Brown, N. E. B. Briggs, J. Dunn and J. Robertson, et al., Spray Drying as a Reliable Route to Produce Metastable Carbamazepine Form IV, J. Pharm. Sci., 2017, 106(7), 1874–1880 CrossRef CAS.
- L. Schenck, A. Koynov and A. Cote, Particle engineering at the drug substance, drug product interface: a comprehensive platform approach to enabling continuous drug substance to drug product processing with differentiated material properties, Drug Dev. Ind. Pharm., 2019, 45(4), 521–531 CrossRef CAS PubMed.
- B. Long, G. M. Walker, K. M. Ryan and L. Padrela, Controlling Polymorphism of Carbamazepine Nanoparticles in a Continuous Supercritical-CO2-Assisted Spray Drying Process, Cryst. Growth Des., 2019, 19(7), 3755–3767 CrossRef CAS.
- L. M. Padrela, B. Castro-Dominguez, A. Ziaee, B. Long, K. M. Ryan and G. Walker, et al., Co-crystal polymorphic control by nanodroplet and electrical confinement, CrystEngComm, 2019, 21(18), 2845–2848 RSC.
- R. Vehring, Pharmaceutical particle engineering via spray drying, Pharm. Res., 2008, 25(5), 999–1022 CrossRef CAS PubMed.
- M. Lang, J. W. Kampf and A. J. Matzger, Form IV of Carbamazepine, J. Pharm. Sci., 2002, 91(4), 1186–1190 CrossRef CAS PubMed.
- V. L. Himes, A. D. Mighell and W. H. De Camp, Structure of carbamazepine: 5H-dibenz [b, f] azepine-5-carboxamide, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1981, 37(12), 2242–2245 CrossRef.
- B. D. Hamilton, J.-M. Ha, M. A. Hillmyer and M. D. Ward, Manipulating crystal growth and polymorphism by confinement in nanoscale crystallization chambers, Acc. Chem. Res., 2012, 45(3), 414–423 CrossRef CAS PubMed.
- F. Tian, J. A. Zeitler, C. J. Strachan, D. J. Saville, K. C. Gordon and T. Rades, Characterizing the conversion kinetics of carbamazepine polymorphs to the dihydrate in aqueous suspension using Raman spectroscopy, J. Pharm. Biomed. Anal., 2006, 40(2), 271–280 CrossRef CAS PubMed.
- M. A. O'Mahony, D. M. Croker, Å. C. Rasmuson, S. Veesler and B. K. Hodnett, Measuring the Solubility of a Quickly Transforming Metastable Polymorph of Carbamazepine, Org. Process Res. Dev., 2013, 17(3), 512–518 CrossRef.
- E. H. Lee, A practical guide to pharmaceutical polymorph screening & selection, Asian J. Pharm. Sci., 2014, 9(4), 163–175 CrossRef.
- A. Buanz, M. Gurung and S. Gaisford, Crystallisation in printed droplets: understanding crystallisation of d-mannitol polymorphs, CrystEngComm, 2019, 21(13), 2212–2219 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ce01041k |
| This journal is © The Royal Society of Chemistry 2022 |