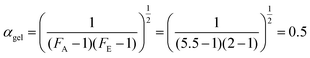A rigid plant oil-based thermoset with a furfural-derived cyclobutane cross-linker†
Jonathan
Tellers
,
Nicolas
Sbirrazzuoli
 and
Nathanael
Guigo
and
Nathanael
Guigo
 *
*
Institut de Chimie de Nice, Université Côte d'Azur, CNRS, UMR 7272, 06108 Nice, France. E-mail: Nathanael.GUIGO@univ-cotedazur.fr
First published on 31st March 2021
Abstract
Bio-based hardeners that are not amine or anhydride based are of particular interest to avoid associated health concerns. In the present study, a recently developed furfural-based cross-linker bearing a semi-rigid cyclobutane moiety is investigated for its capacity to cross-link epoxidized linseed oil, resulting in a wholly bio-based thermoset. The kinetics of the curing process are elucidated, as well as the mechanical properties of the resulting material. The cured thermoset displayed particularly high tensile strength and a high glass transition for a flexible triglyceride-based network, exceeding that of many previously published di-acid thermosets.
Introduction
Spurred on by a paradigm shift in the mindset of consumers, media and policy-makers, researchers have been encouraged to develop sustainable materials that can act as an alternative to the current fossil-based solutions, and thus to design the circular economy process from the ground up.1,2As such, a strong emphasis has been placed on biomass-derived chemicals and entities. The US government has identified key chemicals of the biorefinery process and is encouraging research into these particular chemicals.3 The idea is to use chemicals that are side-products of biofuel production to allow for the establishment of a biobased industry in addition to obtaining materials with unique properties such as their degradability to harmless natural components.4
Furans, such as 5-(hydroxymethyl)furfural (HMF)5 and furfural,6,7 are examples of the key chemicals that have been identified in this manner, having been obtained from the dehydration of sugars and various other biological sources.3,6 They have recently been employed in the development of sustainable and economically viable chemicals and materials.8,9 Furfural is of particular interest, as it is commercially available at a larger scale and is the sole precursor for chemicals comprising a furyl, furfuryl, furoyl or furfuylidene group in the chemical industry.10,11 It has thus been used to make a plethora of precursor molecules for novel polymers and other furyl containing chemicals.12
Recently, furfural-derived trans-3-(2-furyl)acrylic acid (FAA)13,14 has been used to develop a novel type carboxylic di-acid, namely CBDA-2 (cis-cyclobutane-1,2-dicarboxylic acid),15 which can be used as a green, semi-rigid cross-linker. While the researchers mentioned the possibility of using CBDA-2 in conjunction with epoxy resins, they only briefly explored a coating using epoxidized sucrose soyate and, furthermore, relied on methanol as a solvent instead of following a green, solvent-free procedure, as demonstrated in the present paper (vide infra). While sufficient to prove the concept, epoxidized linseed oil (ELO) is by far the predominant choice for bio-based epoxidized matrices, because it can be obtained by green methods, and is both cheap, and industrially available.16–18 To benefit from such a promising natural epoxy matrix, innovation in the field of bio-based hardeners is required, and has gained significant traction in recent years.19–22
In the current study, we investigate the ability of CBDA-2 to cross-link ELO in a hot curing procedure in the absence of any solvent. We analyze the kinetics of the process and the properties of the resulting thermosets, which are compared to the previously described thermosets that have been obtained through sustainable cross-linkers.
Materials and methods
Materials
Epoxidized linseed oil (ELO) was kindly provided by Valtris Chemicals (molecular weight of 980 g mol−1. The average of 5.5 epoxy groups per molecule was determined by the supplier by means of non-aqueous potentiometric titration (ASTM D 1652). Furylacrylic acid (99%) and hexane (puriss, 2.5 L) were purchased from Sigma-Aldrich (now MERCK) and used as received. Two LED UV 50 W black lights were purchased from Amazon.Synthesis of cis-cyclobutane-1,2-dicarbocylic acid (CBDA-2)
The procedure was adapted from the literature.15 The photosynthesis step was carried out in an 500 mL Erlenmeyer flask: 25 g of crystalline 3-(2-furyl) acrylic acid was ground into a fine power using a mortar and pestle. The acid was suspended in 250 mL of hexane in the flask and stirred vigorously. The suspension was irradiated overnight using two 50-watt black lights (395–400 nm). Occasionally, the powder that stuck to the inside wall of the flask was loosened using a spatula. The slurry was then filtered, and an off-white solid was obtained (24.2 g, 97%). The filtered hexane was kept in order to be reused for future reactions. In the case where NMR revealed an uncompleted reaction (observed on one occasion), the powder was suspended again and irradiated for another night, which resulted in complete conversion of the starting material. 1H-NMR (DMSO-d6, 400 MHz) δ 12.56 (s, 2H), 7.40 (m, 2H), 6.25 (m, 2H), 6.11 (d, J = 3.2 Hz 2H), 4.04 (m, 2H), 3.67 (m, 2H); see also Fig. S1† for the NMR spectrum.The precise number of epoxy groups in the ELO and its molecular weight were determined by NMR (Fig. S1†). The integration of the single glycerol CH was set as 1, which allows one to determine the number of epoxy groups per molecule according to eqn (1):
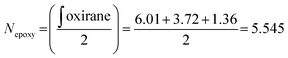 | (1) |
 | (2) |
The amount of required CBDA-2 for 25 g ELO is calculated as follows (eqn (3)):
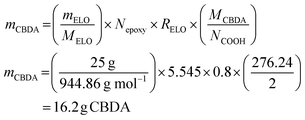 | (3) |
ELO and CBDA-2 were mixed using a spatula, forming a brown sludge with a viscosity similar to a cake dough. This mixture was transferred into the appropriate mold and degassed by floating in a sonicating bath for 1 h. The mixture was then cured in an oven in a two-step curing process. First, 1 h at 110 °C, then 2 h at 130 °C. Full consumption of oxirane groups was confirmed via IR and 13C solid-state NMR.
Nuclear magnetic resonance (NMR)
NMR spectra were recorded on a Bruker AVANCE III (400 MHz). 1H-NMR chemical shifts are given in reference to the residual solvent peak of CDCL3 at 7.26 ppm or DMSO_d6 at 2.50 ppm. The solid-state 13C spectra were obtained on a Bruker Avance-400 MHz NMR spectrometer (magnetic field 9.4 T) using a double channel Bruker probe. About 100 mg of sample was placed in zirconium dioxide rotor of 4 mm outer diameter and spun at a magic angle spinning rate of 10 kHz and 4 kHz for solid and liquid samples, respectively. For the solid samples, the CPMAS technique (Schaefer and Stejskal, 1976) was applied with a ramped 1H-pulse starting at 100% power and decreasing until 50% during the contact time (2 ms) in order to circumvent Hartmann–Hahn mismatches. To obtain a good signal-to-noise ratio in 13C CPMAS experiment 10k scans were accumulated using a delay of 2.5 s. For 13C single pulse experiment (SPE) the acquisition parameters were 3.4 μs 45° pulse, 2 s recycle delay and 1024 scans. The 13C chemical shifts were referenced to tetramethyl silane and calibrated with glycine carbonyl signal, set at 176.03 ppm.Rheology
Curing studies were performed on a Thermo Scientific HAAKE MARS rheometer. Isothermal curing measurements were obtained in plate–plate geometry (25 mm diameter, 1 mm gap, 0.5% strain) at varying temperatures (90, 95, 100, 110, 120, and 130 °C). Variations of complex viscosity and storage and loss modulus with temperature were analyzed.Dynamic mechanical thermal analysis (DMTA)
The dynamic mechanical properties were studied using a Mettler-Toledo DMA-1 in tensile mode. Samples were tested in temperature sweeps from −100 °C to 180 °C with a heating rate of 2 °C min−1. Experiments were done in a single frequency oscillation mode with a frequency of 1 Hz, a force amplitude of 0.1 N and a displacement amplitude of 0.1% in auto-tension offset control. Multi-frequency DMTA experiments were conducted at 1 °C min−1 with a displacement amplitude of 0.1% between 0.1 Hz and 10 Hz.Shore D hardness test
The hardness of the rubbers was determined using Shore D type durometers, which are used for hard rubbers.23 The hardness was determined according to ASTM D2240.Tensile tests
Tensile tests were performed with a Shimadzu EZ-LX tensile tester equipped with a 1 kN load cell. The tests were performed on dog bones (l = 48 mm, w = 5.1–5.3 mm, t = 1.9–3.5 mm) cut from cured 19.6 × 19.6 cm square slabs of the respective material. The exact sample dimensions were determined for each sample before testing. A grip-to-grip separation of 35.92 mm was used. The samples were prestressed to 0.1 N and then loaded with a constant crosshead speed of 20 mm min−1.Differential scanning calorimetry (DSC)
DSC measurements were performed with a Mettler-Toledo DSC823e heat-flux instrument. STAR software was used for data analysis. Temperature, enthalpy, and tau lag calibrations were performed with indium and zinc standards. Freshly mixed CBDA-2/ELO samples (10–20 mg) were placed in a 40 mL aluminum crucible and closed with a punctured pan lid. The experiments were done under air flow (80 mL min−1). Curing of the mixtures was studied under isothermal conditions at 100, 105, 110, 120, 130 °C, followed by a second scan at 10 °C min−1 in order to determine the glass transition (Tg) from the inflection point of the specific heat capacity (Cp) increment. The DSC experiments were conducted according to the ICTAC Kinetics Committee recommendations for collecting thermal analysis data for kinetic computations and were performed using an advanced isoconversional method applied to the dataset obtained from isothermal DSC measurements.24,25Theoretical considerations
Isoconversional methods have been widely used to study complex cure mechanisms.26–28 Polymerizations are frequently monitored by DSC. In this case, the extent of conversion is computed according to eqn (4), as follows: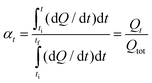 | (4) |
The general form of the basic rate equation to kinetic analysis of the condensed phase processes is usually written as given in literature:24
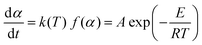 | (5) |
The advanced non-linear isoconversional method (NLN) or Vyazovkin's method used in this study is presented in eqn (6) and (7) and has been derived from eqn (5):29–32
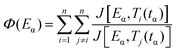 | (6) |
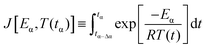 | (7) |
Results and discussion
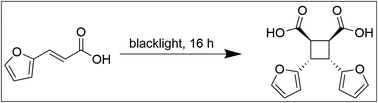
To obtain a potential green alternative to current hardener technologies, it must be possible to obtain all components by green methods. CBDA-2 can be obtained by starting from the furfural-derived trans-3-(2-furyl)acrylic acid through a formation of a four-membered ring facilitated by irradiation using a simple commercially available black light (Fig. 1). For the synthesis described here, a scaled-up version of the procedure described in literature was used,15 employing hexane to float the furylacrylic acid, so that the reaction can proceed in the solid under blacklight irradiation (395–400 nm) with commercially available lights.The hexane that was used during the reaction can be reused in subsequent synthesis, thereby avoiding waste due to discarded solvent. In the future, other liquids could be explored to suspend the acid, including those which are obtained from natural resources. CBDA-2 is obtained as a fine powder after filtering, and can be readily mixed with an epoxy matrix without further need for grinding.
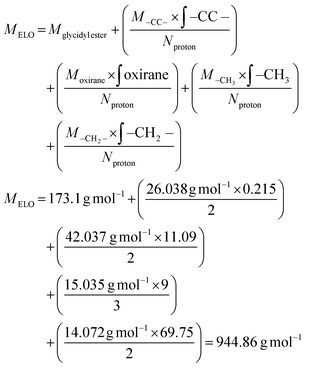
As an epoxy, we chose epoxidized linseed oil (ELO), a green epoxy matrix with high epoxy units content relative to other natural oils.16 From previous investigations,20,34 we know that the highest enthalpy release is observed when a ratio of 0.8 epoxy groups to COOH groups is used. The molecular weight and epoxy group content of ELO was calculated by NMR (spectra in Fig. S2†), and the appropriate amount of CBDA-2 was calculated accordingly (details in the Experimental section). After thorough mixing of ELO and CBDA-2 for a couple of minutes, a brown slush forms (Fig. S3†), which can be poured into a negative mold with the desired shape. The curing behavior of the ELO/CBDA-2 mixture was initially investigated using DSC measurements. A dynamic DSC scan (Fig. S4†) revealed that the reaction starts at around 90 °C, with a peak of DSC heat flow observed at 136 °C, which is proportional to the reaction rate. Furthermore, we observe a Tg of 19 °C for the cured material.
Isothermal curing was subsequently carried out using a rheometer and DSC at multiple temperatures below the observed peak to study curing kinetics and to identify an appropriate curing program to obtain test specimens for mechanical testing. Isothermal curing on a parallel plate rheometer was carried at different temperatures and monitored until the storage modulus (G′) started to plateau. The individual curing experiments are displayed in Fig. S5.† We can see that, even at 90 °C, the curing reaction is initiated. In a previous study,15CBDA-2 is given a melting point of 170–171 °C but according to our DSC analyses, we observed a melting point of 180 °C. Given that the reaction starts much earlier, it is clear that CBDA-2 either dissolves in ELO or, more likely, that they form an eutectic mixture exhibiting a lower melting point, allowing the reaction to proceed at temperatures below the melting point of the acid. This has already been observed in other studies where ELO was cured with the powder of a solid acid.34 The reaction proceeds very quickly at temperatures above 110 °C, reaching a G′ plateau after about 2 h. Using the gel point obtained at different temperatures, the activation energy (EA) at the point of gelation can be determined. Because the conversion α is generally identical at the gel point (tgel), one can calculate the EA using the Arrhenius relation:35–38
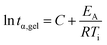 | (8) |
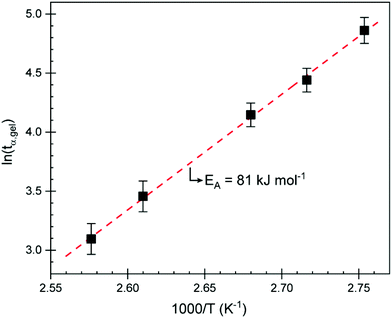 | ||
| Fig. 2 Plot of the logarithm of the time it took to reach the gel point (tα,gel) vs. the inverse isothermal curing temperature. | ||
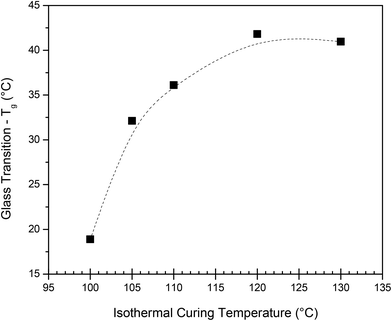
Subsequent isothermal curing tests were carried out on the calorimeter, followed by determination of the Tg reached during the protocol. To achieve this, each sample was subjected to the respective isothermal curing temperature for 2 h, cooled down, followed by immediate determination of the Tg (details in the Experimental section). In Fig. 3, the obtained Tg is plotted as a function of the applied isothermal curing temperature. Two hours at T > 120 °C proved sufficient to obtain full curing, as the Tg of the sample cured at 120 °C and 130 °C gave a similar value. For the lower temperatures, a lower Tg was obtained in the 2 h timeframe, suggesting that it is insufficient to fully cure the system. The Tg obtained during the dynamic curing experiment is about 20 °C lower than that obtained during the isothermal curing experiment performed at 130 °C. It has previously been reported39 that the cyclobutane ring can undergo thermocleavage at high temperatures, which would lead to an overall reduced cross-link density and a Tg of the final polymer. This could be a potential reason for the observed lower Tg and should be considered during sample preparation.
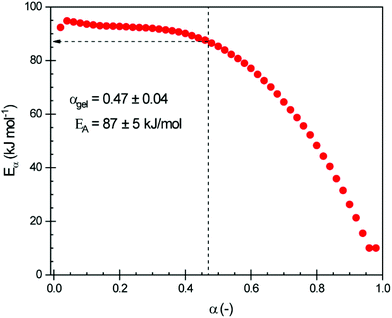
The data obtained from isothermal curing were also used to determine the effective activation energy as a function of the extent of conversion α using the advanced isoconversional method (eqn (2) and (3)). The resulting Eα is plotted in Fig. 4.
According to Flory,40 the conversion at which the gel point is reached is only dependent on the functionality of the epoxy resin and the hardener, according to the following relation:
At low conversions, the curing is controlled by the chemical reactions, and has a high activation energy (around 95 kJ mol−1). Values of around 125–140 kJ mol−1 were reported by Menager et al. for the ring opening of epoxidized linseed oil with three aliphatic biobased dicarboxylic acids (i.e., succinic, suberic and sebacic acids).34 As the reaction processes and the material vitrifies, diffusion becomes the factor that dominates the reaction rate, which lowers the activation energy as diffusion processes have a lower activation energy.37,41 It has been shown that diffusion control can occur for the extent of conversion close to 0.3–0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 and that the onset of the diffusion regime can be associated with gelation rather than vitrification.42
37 and that the onset of the diffusion regime can be associated with gelation rather than vitrification.42
Analysis of the data displayed in Fig. 4 shows that for this system diffusion control starts at around α = 0.4–0.5, i.e., well below vitrification and around gelation. This is explained by the constrained chemical structure of CBDA-2.
To obtain samples without entrapped air that are fully cured, we devised the following curing procedure: specimens were cured in a two step-process, first at 110 °C for 1 h, followed by a post curing step at 130 °C for an additional 2 h. FTIR of samples prepared in this manner revealed full consumption of the epoxy groups, judging from the disappearance of the oxirane group band at 821 cm−1 (Fig. S6†). In addition, solid-state 13C NMR investigations were conducted to highlight structural modifications during the cross-linking. The spectra of both, ELO and the cured CBDA-2/ELO resins are shown in Fig. S7.† Interestingly, the resonances associated to the oxirane groups in ELO (55 < δ < 59 ppm) have disappeared in the spectrum of the cross-linked resin confirming the full consumption of the epoxy group. Instead new resonances appears in the 65–85 ppm region confirming the formation of β-hydroxyester throughout the epoxy ring opening during the cross-linking between ELO and CBDA-2. The resonances observed in the cured sample respectively at δ = 107.4; 110.6; 142.1; 153.2 ppm correspond to the sp2 carbons from the furanic ring in CBDA. In order to obtain suitable sample specimens, a prepared mixture of ELO and CBDA-2 was poured in a rectangular mold with a dimension of 10 × 10 cm and degassed using sonication, followed by curing using the aforementioned curing sequence, yielding solid thermoset slabs. These slabs could be used to cut dog bones and rectangular strips using a CNC router to obtain samples suitable for TGA, tensile testing and DMTA measurements. First, the TGA scan of cured CBDA-2/ELO thermosets is shown in Fig. S8† together with the one of the diacid CBDA-2. It highlights that the diacid starts to decompose above 200 °C. The temperature corresponding to 5% of total decomposition – i.e. T5% = 210 °C for neat CBDA-2 while the T5% increases to 270 °C for the cured resin. It confirms on one hand that the chosen curing conditions (110 °C; 130 °C) are well below 200 °C and thus it should prevent decomposition of CBDA during cross-linking. On the other hand the T5% of CBDA-2/ELO is comparable to those of ELO cured with 2,2′-dithiodibenzoic acid43 and lower compared to systems cross-linked with adipic acid or glutaric anhydride (T5% ∼340 °C) as these linear organic diacid are less prone to decomposition.22
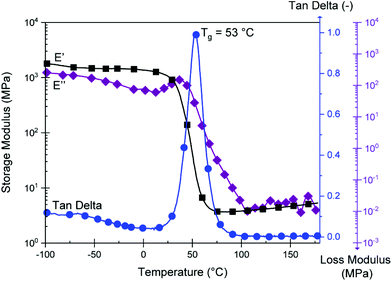
The resulting DMTA data is displayed in Fig. 5. We observe a Tg at 53 °C (peak of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ), explaining the rigid nature of the material at room temperature. This value is significantly above that which can be achieved with green aliphatic multifunctional carboxylic acids19 or common anhydrides such as maleic anhydride (MA).44CBDA-2 is outperformed by more rigid fossil-based anhydrides such as methyl tetrahydrophthalic anhydride (MTHPA),45 and CBDA-2 can thus be employed as a semi-rigid hardener. A plateau modulus of 4.6 MPA at 150 °C is observed. Using the classical theory of rubber elasticity,46,47 one can determine the molecular weight between cross-links (Mc) according to the following relationship:
δ), explaining the rigid nature of the material at room temperature. This value is significantly above that which can be achieved with green aliphatic multifunctional carboxylic acids19 or common anhydrides such as maleic anhydride (MA).44CBDA-2 is outperformed by more rigid fossil-based anhydrides such as methyl tetrahydrophthalic anhydride (MTHPA),45 and CBDA-2 can thus be employed as a semi-rigid hardener. A plateau modulus of 4.6 MPA at 150 °C is observed. Using the classical theory of rubber elasticity,46,47 one can determine the molecular weight between cross-links (Mc) according to the following relationship:
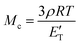 | (9) |
With ρ as the Poisson's ratio (for linseed oil estimated at of 0.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48–51), R as the general gas constant, and E′T as the storage modulus for a temperature T = Tg + 30 within the rubber plateau modulus. We thus calculate a value of Mc = 775 g mol−1, which is similar to findings from other publications where epoxidized linseed oils are used as a matrix.52,53 As shown in Fig. S9,† the variation of E′ and the tan
48–51), R as the general gas constant, and E′T as the storage modulus for a temperature T = Tg + 30 within the rubber plateau modulus. We thus calculate a value of Mc = 775 g mol−1, which is similar to findings from other publications where epoxidized linseed oils are used as a matrix.52,53 As shown in Fig. S9,† the variation of E′ and the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak during the glass transition logically depends on the frequency of solicitation since a shift to higher temperature is observed between 0.1 and 10 Hz. We also observe a high Shore D value (used for hard rubbers) of 73 ± 3 at room temperature, since the material is partially in the glassy state, thus outperforming the hardness of ELO thermosets cured with MTHPA and other hard curing hardeners.54
δ peak during the glass transition logically depends on the frequency of solicitation since a shift to higher temperature is observed between 0.1 and 10 Hz. We also observe a high Shore D value (used for hard rubbers) of 73 ± 3 at room temperature, since the material is partially in the glassy state, thus outperforming the hardness of ELO thermosets cured with MTHPA and other hard curing hardeners.54
The tensile properties of the cured resin were also investigated at room temperature, thus within the glassy region of the material. This resulted in a high tensile strength of 29 ± 2 MPa and observed Young's modulus of 467 ± 28 MPa (Table 1). Compared to linear bio-based diacids previously used to cross-link ELO such as suberic and adipic acid (Table 1), a far stronger material with similar strain at break was obtained. Using succinic acid, a particularly short diacid, a strong material with a relative high elongation at break (εB) can be obtained, which is however still softer and displays a lower tensile strength than CBDA-2 cross-linked ELO. Using a bio-based anhydride such as glutaric anhydride can yield a highly cross-linked thermoset, with a high Young's modulus and tensile strength, yet extremely low εB of 3% compared to 30% with CBDA-2. This CBDA-2 hardener thus competes very well with other petrobased hardeners (Table 1). The tensile strength with CBDA-2 is almost comparable with those obtained when ELO is cured with 4,4′-methylenedianiline (classified in ECHA as a substance of very high concern requiring authorisation before it is used – carcinogenic and suspected to be mutagenic) or with 4-methylhexahydrophtalic anhydride (corrosive and health hazard substance) despite a slightly lower modulus. ELO cured with CBDA largely surpass the ELO resin cured with 2,2′-dithiodibenzoic acid in term of tensile strength and elongation at break suggesting more ductile resins at ambient temperature. Consequently, CBDA-2 positions itself as a suitable cross-linker for strong and durable vegetal oil-based thermosets avoiding health concerns associated with anhydride or amine curing agents.55,56
| Linker | Tensile data | ||||
|---|---|---|---|---|---|
| E (MPa) | σ Y (MPa) | ε B (%) | Toughness (J m−3) | ||
| With E = Young's modulus; σY = tensile strength at yield point; εB = strain at break. | |||||
| Biobased | CBDA-2 | 467 ± 28 | 29 ± 2 | 30 ± 7 | 1.3 ± 0.4 |
| Succinic acid34 | 78 ± 9 | 11 ± 2 | 98 ± 9 | — | |
| Suberic acid34 | 3.9 ± 0.2 | 0.6 ± 0.1 | 22 ± 2 | — | |
| Adipic acid19 | 22 ± 0.1 | 8.8 ± 0.3 | 55 ± 1 | ||
| Glutaric acid anhydride22 | 1477 ± 25 | 24 ± 1 | 3 ± 0.3 | ||
| Petrobased | 2,2′-Dithiodibenzoic acid43 | 811 ± 36 | 10.9 ± 1 | 1.4 ± 0.1 | |
| 4,4′-Methylenedianiline57 | 1500 | 41 | 30 | ||
| 4-Methylhexahydrophtalic anhydride58 | 2200 | 40 | 10 | ||
Conclusions
To meet the current demand to replace conventional fossil-based materials with sustainable alternatives, researchers are encouraged to find new chemicals that perform equally well, or better than conventional chemicals. The current work focused on employing a furfural-based sustainable hardener to cure epoxidized linseed oil (ELO), a widely used and promising epoxy matrix to obtain sustainable materials. Using typical hot curing processes, a furfural-based semi-rigid diacid, namely cis-cyclobutane-1,2-dicarbocylic acid, CBDA-2, was successfully used to cross-link ELO, yielding thermosets with excellent properties.Curing characteristics resembled those of other materials cured with diacid or anhydride hardener, with a significant drop in the activation energy during the progressing reaction due to the diffusion processes becoming the rate limiting factor. The cured materials appeared very solid at room temperature, thanks to a high glass transition of the cured material, which also caused a high Shore D hardness level, characterizing the present material as a hard rubber material. A comparison with other known oil-based thermosets revealed that CBDA-2 can lead to a material with superior strength, while retaining a good degree of toughness and strength at break. By using a simple method, a strong bio-based thermoset was obtained in a convenient manner, providing a potential alternative to fossil-based anhydride curing agents. This is also the first time that CBDA-2 is employed as a 100% sustainable cross-linker to prepare bulky biobased thermoset materials – and not only coatings – which can then expand the potential to processes such as compression molding, resin transfer molding, etc. with many applications in the emerging industry of green composites.
Abbreviations
| DSC | Differential scanning calorimetry |
| DMTA | Dynamic mechanical thermal analysis |
| ELO | Epoxidized linseed oil |
| CBDA-2 | cis-Cyclobutane-1,2-dicarbocylic acid. |
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Funding sources
This work benefited from financial support from the French government, managed by the National Research Agency (ANR) under the UCAJEDI Future Investments project with reference number ANR-15-IDEX-01 and financial support of “La Maison de la Chimie”.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge Dr Fabio Ziarelli from Aix-Marseille Université for the Solid-State NMR spectra. Fruitful collaboration with Mettler-Toledo is acknowledged. Abby Cuttriss is kindly acknowledged for having English edited the manuscript.References
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, 1–5 Search PubMed.
- A. Fothergill, J. Hughes and K. Scholey, David Attenborough: A Life On Our Planet, 2020 Search PubMed.
- T. Werpy and G. Petersen, Top value added chemicals from biomass Volume I—Results of screening for potential candidates from sugars and synthesis gas energy efficiency and renewable energy, 2004 Search PubMed.
- Y. Wang, C. A. Brown and R. Chen, AIMS Microbiol., 2018, 4, 261–273 Search PubMed.
- M. Bicker, J. Hirth and H. Vogel, Green Chem., 2003, 5, 280–284 Search PubMed.
- C. M. Cai, T. Zhang, R. Kumar and C. E. Wyman, J. Chem. Technol. Biotechnol., 2014, 89, 2–10 Search PubMed.
- J. W. Döbereiner, Ann. Pharm., 1832, 3, 141–146 Search PubMed.
- R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádaba and M. López Granados, Energy Environ. Sci., 2016, 9, 1144–1189 Search PubMed.
- G. Machado, S. Leon, F. Santos, R. Lourega, J. Dullius, M. E. Mollmann and P. Eichler, Nat. Resour., 2016, 07, 115–129 Search PubMed.
- H. E. Hoydonckx, W. M. Van Rhijn, W. Van Rhijn, D. E. De Vos and P. A. Jacobs, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2007 Search PubMed.
- S. Thiyagarajan, A. Pukin, J. Van Haveren, M. Lutz and D. S. Van Es, RSC Adv., 2013, 3, 15678–15686 Search PubMed.
- I. Delidovich, P. J. C. Hausoul, L. Deng, R. Pfützenreuter, M. Rose and R. Palkovits, Chem. Rev., 2016, 116, 1540–1599 Search PubMed.
- S. Rajagopalan, Proc. – Indian Acad. Sci., Sect. A, 1942, 16, 163–166 Search PubMed.
- Z. Wang, B. Kastern, K. Randazzo, A. Ugrinov, J. Butz, D. W. Seals, M. P. Sibi and Q. R. Chu, Green Chem., 2015, 17, 4720–4724 Search PubMed.
- Z. D. Wang, Q. Elliott, Z. Wang, R. A. Setien, J. Puttkammer, A. Ugrinov, J. Lee, D. C. Webster and Q. R. Chu, ACS Sustainable Chem. Eng., 2018, 6, 8136–8141 Search PubMed.
- P. Muturi, D. Wang and S. Dirlikov, Prog. Org. Coat., 1994, 25, 85–94 Search PubMed.
- J.-M. Pin, N. Sbirrazzuoli and A. Mija, ChemSusChem, 2015, 8, 1232–1243 Search PubMed.
- W. Knörr, P. Daute, R. Grützmacher and R. Höfer, Fett Wiss. Technol., 1995, 97, 165–169 Search PubMed.
- C. Ding, P. S. Shuttleworth, S. Makin, J. H. Clark and A. S. Matharu, Green Chem., 2015, 17, 4000–4008 Search PubMed.
- J. Tellers, P. Willems, B. Tjeerdsma, N. Guigo and N. Sbirrazzuoli, Green Chem., 2020, 22, 3104–3110 Search PubMed.
- P. Gogoi, M. Boruah, S. Sharma and S. K. Dolui, ACS Sustainable Chem. Eng., 2015, 3, 261–268 Search PubMed.
- C. Ding, G. Tian and A. Matharu, Mater. Today Commun., 2016, 7, 51–58 Search PubMed.
- H. J. Qi, K. Joyce and M. C. Boyce, Rubber Chem. Technol., 2003, 76, 419–435 Search PubMed.
- S. Vyazovkin, A. K. Burnham, J. M. Criado, L. A. Pérez-Maqueda, C. Popescu and N. Sbirrazzuoli, Thermochim. Acta, 2011, 520, 1–19 Search PubMed.
- S. Vyazovkin, A. K. Burnham, L. Favergeon, N. Koga, E. Moukhina, L. A. Pérez-Maqueda and N. Sbirrazzuoli, Thermochim. Acta, 2020, 689, 178597 Search PubMed.
- S. Vyazovkin and N. Sbirrazzuoli, Macromol. Rapid Commun., 2006, 27, 1515–1532 Search PubMed.
- S. Vyazovkin, in Isoconversional Kinetics of Thermally Stimulated Processes, Springer International Publishing, 2015, pp. 27–62 Search PubMed.
- N. Sbirrazzuoli, Polymers, 2020, 12, 1280 Search PubMed.
- S. Vyazovkin, J. Comput. Chem., 1997, 18, 393–402 Search PubMed.
- N. Sbirrazzuoli, L. Vincent and S. Vyazovkin, Chemom. Intell. Lab. Syst., 2000, 54, 53–60 Search PubMed.
- S. Vyazovkin, J. Comput. Chem., 2001, 22, 178–183 Search PubMed.
- N. Sbirrazzuoli, Thermochim. Acta, 2013, 564, 59–69 Search PubMed.
- N. Sbirrazzuoli, L. Vincent and S. Vyazovkin, Chemom. Intell. Lab. Syst., 2000, 52, 23–32 Search PubMed.
- C. Menager, N. Guigo, L. Vincent and N. Sbirrazzuoli, J. Polym. Sci., 2020, 58, 1717–1727 Search PubMed.
- A. Cadenato, J. M. Salla, X. Ramis, J. M. Morancho, L. M. Marroyo and J. L. Martin, J. Therm. Anal., 1997, 49, 269–279 Search PubMed.
- H. Teil, S. A. Page, V. Michaud and J. A. E. Månson, J. Appl. Polym. Sci., 2004, 93, 1774–1787 Search PubMed.
- S. Vyazovkin and N. Sbirrazzuoli, Macromolecules, 1996, 29, 1867–1873 Search PubMed.
- N. Sbirrazzuoli, A. Mititelu-Mija, L. Vincent and C. Alzina, Thermochim. Acta, 2006, 447, 167–177 Search PubMed.
- H. Amjaour, Z. Wang, M. Mabin, J. Puttkammer, S. Busch and Q. R. Chu, Chem. Commun., 2019, 55, 214–217 Search PubMed.
- P. D. Flory, Principles of Polymer Chemistry, Cornell University Press, Ithaca, 1953 Search PubMed.
- N. Sbirrazzuoli, Molecules, 2019, 24, 1683–1699 Search PubMed.
- N. Sbirrazzuoli, S. Vyazovkin, A. Mititelu, C. Sladic and L. Vincent, Macromol. Chem. Phys., 2003, 204, 1815–1821 Search PubMed.
- C. Di Mauro, S. Malburet, A. Genua, A. Graillot and A. Mija, Biomacromolecules, 2020, 21, 3923–3935 Search PubMed.
- J. M. España, L. Sánchez-Nacher, T. Boronat, V. Fombuena and R. Balart, J. Am. Oil Chem. Soc., 2012, 89, 2067–2075 Search PubMed.
- A. R. Mahendran, G. Wuzella, A. Kandelbauer and N. Aus, J. Therm. Anal. Calorim., 2012, 107, 989–998 Search PubMed.
- I. M. Barszczewska-Rybarek, A. Korytkowska-Wałach, M. Kurcok, G. Chladek and J. Kasperski, Acta Bioeng. Biomech., 2017, 19, 47–53 Search PubMed.
- T. Murayama and J. P. Bell, J. Polym. Sci., Part A-2, 1970, 8, 437–445 Search PubMed.
- N. Doroudgarian, L. Pupure and R. Joffe, Polym. Compos., 2015, 36, 1510–1519 Search PubMed.
- L. Pupure, N. Doroudgarian and R. Joffe, Polym. Compos., 2014, 35, 1150–1159 Search PubMed.
- N. Ghasemi Rad, Z. Karami, M. J. Zohuriaan-Mehr, A. Salimi and K. Kabiri, Polym. Adv. Technol., 2019, 30, 2361–2369 Search PubMed.
- H. Miyagawa, A. K. Mohanty, M. Misra and L. T. Drzal, Macromol. Mater. Eng., 2004, 289, 629–635 Search PubMed.
- N. Boquillon and C. Fringant, Polymer, 2000, 41, 8603–8613 Search PubMed.
- S. K. Sahoo, V. Khandelwal and G. Manik, Polym. Adv. Technol., 2018, 29, 2080–2090 Search PubMed.
- K. Thiele, N. Eversmann, A. Krombholz and D. Pufky-Heinrich, Polymers, 2019, 11, 1409 Search PubMed.
- R. Schöneich and G. Wallenstein, Z. Gesamte Hyg., 1990, 36, 164–166 Search PubMed.
- Y. I. Li, F. Xiao, K. S. Moon and C. P. Wong, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1020–1027 Search PubMed.
- J. D. Earls, J. E. White, L. C. López, Z. Lysenko, M. L. Dettloff and M. J. Null, Polymer, 2007, 48, 712–719 Search PubMed.
- T. Tsujimoto, K. Takeshita and H. Uyama, J. Am. Oil Chem. Soc., 2016, 93, 1663–1669 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra and supplementary rheological, FTIR, and DMTA data. See DOI: 10.1039/d0gc04323k |
| This journal is © The Royal Society of Chemistry 2021 |