Open Access Article
Open Access ArticleA novel self-healing power cable insulating material based on host–guest interactions†
Lei
Peng
 *ab,
Manjun
Zhang
a,
Musong
Lin
a and
Qiang
Fu
a
*ab,
Manjun
Zhang
a,
Musong
Lin
a and
Qiang
Fu
a
aElectric Science and Research Institute of Guangdong Power Grid Co. Ltd, China. E-mail: penglei1025@whu.edu.cn
bSchool of Electrical Engineering, Chongqing University, China
First published on 16th July 2018
Abstract
The insulating materials used in power cables are susceptible to damage and cracks during installation and operation. To solve this problem, we have prepared a self-healing material PVP/p(HEMA-co-BA), which is synthesized by radical polymerization using HEMA, BA, PVP and a host–guest assembly. The host–guest assembly is constructed through interactions between host and guest molecules (CD–Al2O3 NPs act as the host, and HEMA–Ad acts as the guest). The characterization results of the materials show that there are two kinds of supramolecular interactions, namely, the host–guest interaction and the hydrogen bonding. The material possesses good thermal stability (heat-resisting temperature can reach 200 °C) and good electrical performance. The storage modulus of the material can be increased up to 432 MPa using a cross-linking agent at 20 °C. Furthermore, the material exhibits self-healing property, and it can self-heal several times; its self-healing efficiency is relative to the dosage of the cross-linking agent.
1 Introduction
Insulating material of a power cable is the outermost protective layer of the cable core, and it is exposed to the external environment directly. During the process of installation and operation, damages caused by aging and external forces affect the service life and even cause leakage and blackout accidents, thereby incurring tremendous economic losses and compromising safety.1,2 For example, in 2011, across China, 15 of 48 (31%) cable accidents resulted from external damages.3 At present, methods available for repairing the damage to the cable insulating layer mainly include injected liquid method, heat-sealing method, and thermal contractible tube method; however, these three types of repair methods have disadvantages such as complex steps and poor repairing effect, and they also require the fault location technique to find out the fault points, due to which they are difficult to operate.4 Therefore, there is a need to develop new techniques to repair damaged cable insulation.Self-healing materials (SHMs) are a type of smart materials that can automatically identify damages or structural defects (perceive) and repair them (respond) partially or completely.5 Considering this feature of automatic identification and repair, we have attempted to introduce such self-healing supramolecular monomers into the insulating material of power cables for damage repair.
At present, one type of self-healing material (the filling type) exhibits self-healing property due to the repair agent carriers such as hollow fibers,6,7 microcapsules,8–10 or microvasculatures,11,12 which are dispersed in the polymer matrix. When the material is damaged, the carriers break down, and the repair agents release and diffuse to the crack location. The repair agents can initiate cross-linking reactions to achieve the purpose of repairing. Although such materials can self-repair, it is difficult to achieve repeated repairs due to the limited capacity of the repair agents. However, another kind of self-healing materials repair the defects through the intermolecular interactions of their components. These have the advantages of repeated repair times, which are not restricted by the capacity of the repair agents. The mechanism of such kind of self-healing materials is based on dynamic covalent bonds. Under the stimulation of external factors such as thermal energy,13 light14 and pH,15 reversible cleavage and generation of intermolecular covalent bonds occur. In the process of covalent bond regeneration, the cracks can be repaired. However, in the actual environment in which the power cable insulation sheath exists, the above-mentioned external stimulating factors are not present, which hinders the self-healing efficiency of these materials.
Based on the research of this kind of covalent bond-based self-healing model, scientists have widened the research field to non-covalent bond models. The self-healing property is gained mainly due to the presence of non-covalent bonds such as hydrogen bonds,16 π–π bonds,17,18 and metal coordination bonds19 as well as topology structure.20 The most important advantage of such materials is that they do not require external stimulatory factors to activate the self-repair property; among these, a particular kind of supramolecular material based on a host–guest interaction model is of great interest.
Currently, basic research on the host–guest interaction model has been carried out, and researchers have also developed materials with excellent repairing effects. For example, the group led by Professor Huang at Zhejiang University has developed two kinds of supramolecular gels based on host–guest reactions; these gels exhibit excellent self-healing performance, and they can rapidly self-heal even under 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000% strain.21 Professor Mynar's group from Tokyo University has prepared a gel that can self-heal completely, and it is synthesized using clay and telechelic dendritic macromolecules with multiple adhesive termini.22 The group of Zhang in Sichuan University has developed an elastomer that can restore 90% of its mechanical strength rapidly.23 However, the mechanical properties of these self-healing materials have been proven to be unsatisfactory (some materials are even “jelly-like”).24 The Young's modulus of self-healing materials based on non-covalent bonds is generally in the range of 1–10 MPa.16,25 Therefore, to meet the requirements for use as a cable material, enhancing the mechanical strength of the self-healing material is of great significance.
000% strain.21 Professor Mynar's group from Tokyo University has prepared a gel that can self-heal completely, and it is synthesized using clay and telechelic dendritic macromolecules with multiple adhesive termini.22 The group of Zhang in Sichuan University has developed an elastomer that can restore 90% of its mechanical strength rapidly.23 However, the mechanical properties of these self-healing materials have been proven to be unsatisfactory (some materials are even “jelly-like”).24 The Young's modulus of self-healing materials based on non-covalent bonds is generally in the range of 1–10 MPa.16,25 Therefore, to meet the requirements for use as a cable material, enhancing the mechanical strength of the self-healing material is of great significance.
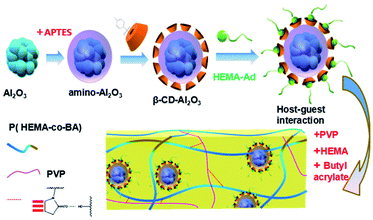
Therefore, in this paper, we proposed a new PVP/p(HEMA-co-BA) self-healing material with suitable mechanical strength. The material adopted a host–guest interaction topology model, and the guests were grafted by special reinforcements. The host–guest assembly was then prepared by free-radical copolymerization with HEMA, BA, and PVP to obtain the self-healing material having suitable mechanical strength.
2 Principle
In this study, the host–guest inclusion model is used for the synthesis of a self-healing material with β-cyclodextrin as the host and adamantane as the guest. The β-cyclodextrin molecule has a lipophilic cavity inside, whereas adamantane shows excellent lipophilicity. Moreover, the size of the cavity in β-cyclodextrin is 0.7–0.8 nm, and the diameter of adamantane molecule is about 0.7 nm.26 Thus, the host and guest molecules match perfectly in size, which means that adamantane can fit in the cavity of cyclodextrin firmly. When the material is damaged, based on the lipophilic cavity of β-cyclodextrin and the oleophilic structure of the adamantyl group, the adamantane group is stuck inside the cavity of the cyclodextrin molecule by the same polar attractive force, resulting in a stable supramolecular network. Thus, the damage is healed. The process of preparation of the PVP/p(HEMA-co-BA) self-healing material is shown in Fig. 1.3 Materials and methods
3.1 Materials
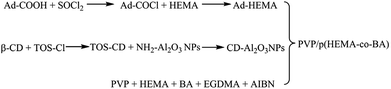
2-Hydroxyethyl-methacrylate (HEMA), β-cyclodextrin (β-CD), butyl acrylate (BA), azobisisobutyronitrile (AIBN, 99%, recrystallization), polyvinylpyrrolidone (PVP-K30, Mn = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), Al2O3 nanoparticles (NH2–Al2O3 NPs, 30 nm, α-phase), ethylene glycol dimethacrylate (EGDMA), (3-aminopropyl) triethoxysilane (APTES), and adamantanecarboxylic acid (Ad-COOH) were obtained from Aladdin (Shanghai) Reagent Co., Ltd. Hydrochloric acid, dichloromethane (CH2Cl2), dimethyl sulfoxide (DMSO), p-toluenesulfonyl chloride (TOS-Cl), thionyl chloride (SOCl2), and concentrated sulfuric acid (98%) were obtained from Chengdu Kelong Chemical Reagent Factory. All the above-mentioned materials were analytically pure.
000), Al2O3 nanoparticles (NH2–Al2O3 NPs, 30 nm, α-phase), ethylene glycol dimethacrylate (EGDMA), (3-aminopropyl) triethoxysilane (APTES), and adamantanecarboxylic acid (Ad-COOH) were obtained from Aladdin (Shanghai) Reagent Co., Ltd. Hydrochloric acid, dichloromethane (CH2Cl2), dimethyl sulfoxide (DMSO), p-toluenesulfonyl chloride (TOS-Cl), thionyl chloride (SOCl2), and concentrated sulfuric acid (98%) were obtained from Chengdu Kelong Chemical Reagent Factory. All the above-mentioned materials were analytically pure.
3.2 Instruments and characterization
1H and 13C NMR spectra were recorded using a Bruker AVANCE AV II-600 NMR spectrometer. Mass spectrometry measurements were conducted on LCQ Deca LC-MS. One-dimensional Fourier infrared (FT-IR) spectra were recorded with a Nicolet 560 Fourier transform infrared spectrometer, and two-dimensional Fourier infrared (2D-IR) characterization was conducted by a Nicolet 710 FT-IR spectrometer (coupled with a control temperature instrument) and a DTGS detector. The storage modulus and glass transition temperature of samples were determined using a Q800 Dynamic mechanical thermal analyzer. Tensile tests were performed on an AG-10TA tensile testing machine. Thermogravimetry analysis of Al2O3 NPs, NH2–Al2O3 NPs, and CD–Al2O3 NPs was carried out using a Q-500 thermal analyzer of TA. The self-healing property of the damage was observed under VHX-1000C Digital Microscopes, and the tensile stresses of the sample before and after self-healing were tested by an AG-10TA tensile testing machine.3.3 Fabrication method
| Sample | HEMA/mL | BA/mL | PVP/g | HEMA–Ad/g | CD–Al2O3 NPs/g | EGDMA/μL |
|---|---|---|---|---|---|---|
| 1 | 0.5 | 2.37 | 0.66 | 0.6 | 0.3 | 0 |
| 2 | 0.5 | 2.37 | 0.66 | 0.6 | 0.3 | 0.5 |
| 3 | 0.5 | 2.37 | 0.66 | 0.6 | 0.3 | 1 |
| 4 | 0.5 | 2.37 | 0.66 | 0.6 | 0.3 | 2 |
| 5 | 0.5 | 2.37 | 0.66 | 0.6 | 0.2 | 1 |
| 6 | 0.5 | 2.37 | 0.66 | 0.6 | 0.1 | 1 |
| Ref. sample 1 | 0.5 | 2.37 | 0.66 | 0.6 | 0 | 1 |
| Ref. sample 2 | 0.5 | 2.37 | 0 | 0.6 | 0.3 | 1 |
4 Results and discussion
4.1 Grafting effects of host molecule and guest molecule
The grafting effects of host molecule CD–Al2O3 NPs and guest molecule HEMA–Ad were analyzed by 1H NMR and 13C NMR spectra, and the results are shown in S1a and b and S2a and b.†4.2 Host molecule CD–Al2O3 NPs
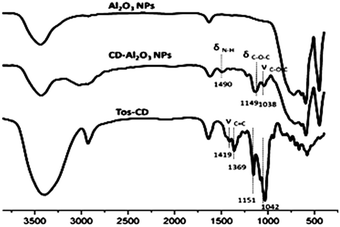
The FT-IR spectra of Al2O3 NPs, CD–Al2O3 NPs, and TOS–CDs were examined; the results are shown in Fig. 3. Compared to the peaks in the FT-IR spectra of Al2O3 NPs, the three new characteristic peaks of CD–Al2O3 NPs at 1038, 1149, and 1490 cm−1 corresponded to νC–O–C and δC–O–C on cyclodextrin and δNH on the amide groups. In addition, compared to the peaks in the FT-IR spectra of TOS–CD, νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (at 1369 cm−1 and 1419 cm−1) on the benzene ring was not observed in the infrared spectrum of CD–Al2O3 NPs, confirming that Al2O3 NPs were successfully modified by cyclodextrin.
C (at 1369 cm−1 and 1419 cm−1) on the benzene ring was not observed in the infrared spectrum of CD–Al2O3 NPs, confirming that Al2O3 NPs were successfully modified by cyclodextrin.
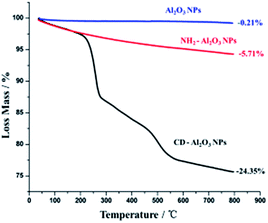
On the other hand, thermogravimetric analyses (TGA) of Al2O3 NPs, NH2–Al2O3 NPs, and CD–Al2O3 NPs are shown in Fig. 4. From Fig. 4, it can be seen that the loss mass of Al2O3 NPs in the region between 50 °C and 800 °C is 0.21% and that of NH2–Al2O3 NPs is 5.71%, thus indicating that organics are modified on the nanoparticles. The difference between the loss mass of CD–Al2O3 NPs (24.35%) and that of NH2–Al2O3 NPs is 18.64%, which proves that cyclodextrin is successfully modified on Al2O3 NPs. According to previous reports,27 an estimated total of 3710 cyclodextrins can be modified on each nanoparticle.
4.3 PVP/p(HEMA-co-BA) self-healing material
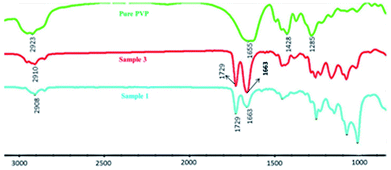
To establish that the crosslinked network in PVP/p(HEMA-co-BA) material is formed by PVP and p(HEMA-co-BA) through hydrogen bonding between the carbonyl groups on polyvinylpyrrolidone and the hydroxyl groups, we used infrared spectroscopy to characterize the hydrogen bonds of PVP and PVP/p(HEMA-co-BA) material (sample 3 and 1 in Table 1). The results are shown in Fig. 5.
Table 2 dynamic thermo-mechanical analysis of PVP/p(HEMA-co-BA)The peaks of PVP at 2923, 1655, 1458, and 1285 cm−1 correspond to the stretching vibration of O–H, the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, the bending vibration of CH2, and the vibration of C–N. In the spectrum of PVP/p(HEMA-co-BA) material, it can be seen that the stretching vibration peak of C
O, the bending vibration of CH2, and the vibration of C–N. In the spectrum of PVP/p(HEMA-co-BA) material, it can be seen that the stretching vibration peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shifts from 1655 cm−1 to 1663 cm−1, indicating that the carbonyl and hydroxyl groups in the material form hydrogen bonds.
O shifts from 1655 cm−1 to 1663 cm−1, indicating that the carbonyl and hydroxyl groups in the material form hydrogen bonds.
| Sample | Storage modulus/MPa | r E′1 | r E′1 | T g/°C | ||
|---|---|---|---|---|---|---|
| 20 °C | 50 °C | 90 °C | (E′20°C/E′50°C) | (E′50°C/E′90°C) | ||
| 2 | 300.8 | 48.8 | 1.1 | 6.16 | 43.32 | 78.1 |
| 3 | 399.0 | 79.1 | 2.3 | 5.04 | 34.21 | 81.3 |
| 4 | 432.3 | 64.8 | 1.0 | 6.67 | 62.08 | 95.8 |
To verify the host–guest interaction between cyclodextrin and adamantane in the PVP/p(HEMA-co-BA) material, it (sample 3 in Table 1) was tested by two-dimensional infrared spectroscopy, which is an effective tool to study molecular interactions in many systems.28 The results are shown in Fig. 6a and b (a and b are the synchronous and asynchronous 2D infrared signals of the material, respectively). According to the theory of 2D IR spectroscopy developed by Noda,29 a correlation peak is formed when two dipole transition moments associated with the molecular vibrations of different functional groups are reorienting simultaneously. The cooperative motion of local structures is expected when strong interactions exist among different functional groups. In Fig. 6a, the peaks at 1161 cm−1 and 1082 cm−1 correspond to the C–O–C bending vibration and the C–C–C–O bending vibration of cyclodextrin. Moreover, the peak at 1103 cm−1 is due to the bending vibration of CH2 on adamantane. It is clear that the characteristic peaks of β-CD (at 1161 cm−1 and 1082 cm−1) and Ad (at 1103 cm−1) show clear correlations (shown in the red box in Fig. 6a). The results indicate that β-CD and Ad in the material interact with each other strongly due to the host–guest interactions.
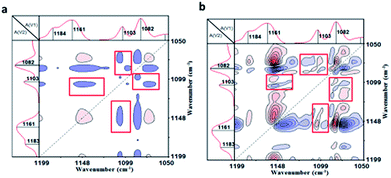 | ||
| Fig. 6 Two-dimensional infrared spectra of the material: (a) synchronous infrared signal, (b) asynchronous infrared signal. | ||
4.4 Thermal properties of PVP/p(HEMA-co-BA)
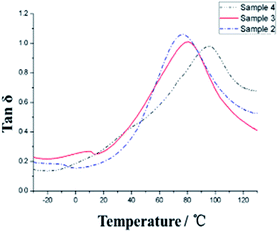
To study the glass transition temperature (Tg) of PVP/p(HEMA-co-BA) material, dynamic thermo-mechanical analysis (DMA) was performed, and the results were used to obtain the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve, as shown in Fig. 7 and Table 2.
δ curve, as shown in Fig. 7 and Table 2.
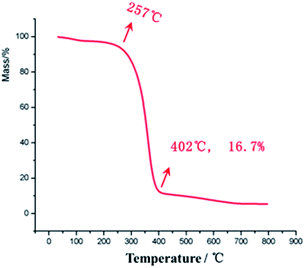
The glass transition temperature of sample 4 is the highest (Tg = 95 °C), whereas the Tg values of sample 3 and sample 2 are 81 °C and 78 °C, respectively, indicating that the glass transition temperature of the sample increases with the increasing amount of the crosslinking agent as the chemical crosslinking points limit the segmental motion of the material. At the same time, it can be observed that all the three samples have a very wide glass transition temperature, e.g., sample 3 with Tg of 20–140 °C. This is because of the presence of two kinds of supramolecular forces in the material, namely, host–guest interaction of adamantane and cyclodextrin and hydrogen bonding of hydroxyl and carbonyl groups, which make the material micro-phase separated, and the different segmental motions of the micro-phases give rise to different Tg temperatures, ultimately resulting in very wide Tg peak overall. In addition, at 20 °C, the storage moduli of the three samples increase (308, 399 and 432 MPa, respectively) with the increasing amount of cross-linking agents. The resistance temperature of the material was obtained through thermogravimetric analysis (TGA), as shown in Fig. 8.
There is no clear mass loss of the material till 257 °C, indicating that the PVP/p(HEMA-co-BA) material can withstand temperatures up to 257 °C. At the same time, it can also be seen that the mass percentage of Al2O3 NPs in the material is 16.7%, which is consistent with the ratio present in sample 3.
4.5 Insulation properties of PVP/p(HEMA-co-BA)
The insulation properties of PVP/p(HEMA-co-BA) were tested. The results are shown in Table 3. It can be seen from Table 3 that the volume resistivity of the material reached 107–108, and the surface resistivity reached 1010, which can meet the insulation requirements for general engineering use (usually greater than 106). The relative permittivity of the material under normal state was 21.7, which was higher than that of polyvinyl chloride (4.8). The material also exhibited good AC and DC electrical strength values (its values were as high as 12.5 MV m−1 and 14.7 MV m−1, respectively), which were greater than the limit of general cable sheath insulation materials (the requirement is greater than 1 MV m−1).| No. | Properties | Unit | Results | |
|---|---|---|---|---|
| 1 | Volume resistivity | Normal | Ω m | 3.2 × 107 |
| 50 °C, vacuum 2 h | 4.1 × 108 | |||
| 2 | Surface resistivity | Normal | Ω | 7.2 × 1010 |
| 50 °C, vacuum 2 h | 3.5 × 1010 | |||
| 3 | Relative permittivity | Normal | — | 21.7 |
| 50 °C, vacuum 2 h | 18.7 | |||
| 4 | AC electrical strength (in oil) | MV m−1 | 12.5 | |
| 5 | DC electrical strength (in oil) | MV m−1 | 14.7 | |
4.6 Self-healing property of PVP/p(HEMA-co-BA)
To investigate the self-healing property of PVP/p(HEMA-co-BA) material, the sample was cut into two pieces with a blade, and the broken ends were brought together immediately. After 2 hours at room temperature, the pieces were found to have rejoined by self-healing (shown in Fig. 9a). Moreover, the sample was cut off at the original fracture to investigate if it could self-heal again; the results showed that the material had achieved the ability to self-heal multiple times.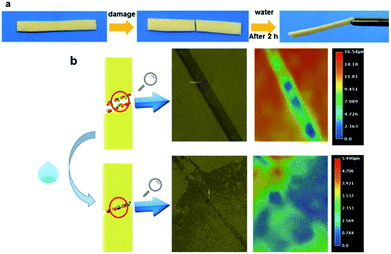 | ||
| Fig. 9 Self-healing of PVP/p(HEMA-co-BA) material: (a) self-healing process of the material; (b) 3D ultra-depth of focus images of the material before and after self-healing. | ||
To observe the healing more clearly, a 3D digital microscope was used for detection. The result is shown in Fig. 9b. The material in Fig. 9b has a damage 28.5 μm in width and 16.54 μm in depth. After self-healing, only a trace of the damage is retained on the surface, indicating that the PVP/p(HEMA-co-BA) material indeed exhibits self-healing properties.
To investigate the self-healing efficiency of the PVP/p(HEMA-co-BA) material and the effect of the cross-linker dosage on self-healing properties, a tensile testing machine was used to study the mechanical properties of the samples before and after self-healing at the drawing speed of 5 mm min−1. The results (including Young modulus EY, breaking elongation ε, breaking strength σ, and self-healing efficiency η) of the samples before and after self-healing are listed in Table 4; as the cross-linker dosage in the material increased (the irreversible cross-linking point increased), the Young modulus EY and the breaking strength σ increased significantly, whereas the breaking elongation ε decreased rapidly; this indicated that within a certain range, the less the cross-linker, the higher the self-healing efficiency (up to 85%).
| Sample | E r/MPa | ε/% | σ/MPa | Self-healing efficiency (η) | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| 1 | 0.36 | 337 | 225 | 0.61 | 0.52 | 85% |
| 2 | 7.64 | 173 | 24 | 0.81 | 0.59 | 73% |
| 3 | 9.42 | 161 | 70 | 1.45 | 0.99 | 68% |
| 4 | 15.0 | 98 | 29 | 2.04 | 1.34 | 66% |
| 5 | 9.30 | 165 | 43 | 1.48 | 0.77 | 52% |
| 6 | 9.22 | 159 | 24 | 1.40 | 0.46 | 33% |
| Ref. 1 | 9.14 | 158 | 0 | 1.43 | 0 | 0 |
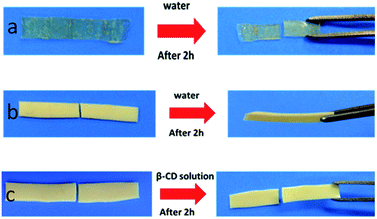
To prove that the self-healing property of PVP/p(HEMA-co-BA) material is derived from the inclusion effect of the host (β-CD group) and the guest groups (Ad group), three groups of control experiments were designed: (1) samples without CD–Al2O3 NPs were prepared. The results showed that the material had no self-healing property (Fig. 10a); (2) samples without PVP were prepared. The results showed that the material had self-healing property (Fig. 10b); (3) the PVP/p(HEMA-co-BA) material was cut, and cyclodextrin solution was smeared on the cut surface; then, the self-healing properties were measured. The results showed that the material could not self-heal (Fig. 10c).
The two sets of experiments shown in Fig. 10a and b demonstrate that the self-healing property does not rely on the interaction between the carbonyl group on PVP and the hydroxyl group on p(HEMA-co-BA) but rather on the host–guest interaction between cyclodextrin and adamantane. The sample shown in Fig. 10c cannot self-heal because the cyclodextrin molecule in the cyclodextrin solution rather than cyclodextrin molecule in material complex with adamantane molecule on the cutting surface, which means that the cyclodextrin molecule in the solution acts as a competitive molecule. Consequently, the host and guest molecules at the cutting surface cannot form inclusion complexes, thus preventing self-healing of materials.
5 Conclusion
Based on the inclusion effect of the host and guest molecules, this paper presented a novel self-healing material synthesized by free radical copolymerization. The 1H and 13C NMR spectra verified that HEMA was successfully grafted on Ad, and Al2O3 NPs were successfully grafted on CD. One-dimensional and two-dimensional infrared spectra showed the existence of two kinds of supramolecular interactions in the material: the host–guest interaction and the hydrogen bonding interaction. The PVP/p(HEMA-co-BA) self-healing material showed good thermal stability with the heat resisting temperature of 200 °C. The thermogravimetric analysis revealed that the storage modulus of the sample at 20 °C increased with the increase in cross-linker dosage, the highest being 432 MPa. The results from 3D ultra-depth of focus imaging and tensile tests showed that the material possessed self-healing property and it can self-heal multiple times; the self-healing efficiency increased with a decrease in cross-linker dosage. Moreover, the tensile test showed that the mechanical strength (Young modulus EY) of the material was up to 15 MPa, and the mechanical strength was satisfactory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by China Postdoctoral Science Foundation Grant (2018M630920) and China Southern Power Grid project (number GDKJQQ20152049).References
- T. Seguchi, K. Tamura, T. Ohshima, A. Shimada and H. Kudoh, Radiat. Phys. Chem., 2011, 80, 268 CrossRef CAS.
- A. Tzimas, S. Rowland, L. A. Dissado, M. Fu and U. H. Nilsson, IEEE Trans. Dielectr. Electr. Insul., 2009, 16, 1436 CAS.
- Z. Jiao and Q. Xia, Distribution & Utilization, 2011, 28, 64 Search PubMed , (in Chinese).
- D. E. Vokey and M. Loewen, US Pat. No. 6,181,140, 2001.
- J. L. Mynar and T. Aida, Nature, 2008, 451, 895 CrossRef CAS.
- S. M. Bleay, C. B. Loader, V. J. Hawyes, L. Humberstone and P. T. Curtis, Composites, Part A, 2001, 32, 1767 CrossRef.
- R. S. Trask, G. J. Williams and I. P. Bond, J. R. Soc., Interface, 2007, 4, 363 CrossRef CAS PubMed.
- S. R. White, N. R. Sottos, P. H. Geubelle, J. S. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown and S. Viswanathan, Nature, 2001, 409, 794 CrossRef CAS.
- M. Keller, S. White and N. Sottos, Adv. Funct. Mater., 2010, 17, 2399 CrossRef.
- J. D. Rule, E. N. Brown, N. R. Sottos, S. R. White and J. S. Moore, Adv. Mater., 2005, 17, 205 CrossRef CAS.
- K. S. Toohey, N. R. Sottos, J. A. Lewis, J. S. Moore and S. R. White, Nat. Mater., 2007, 6, 581 CrossRef CAS.
- K. S. Toohey, C. J. Hansen, J. A. Lewis, S. R. White and N. R. Sottos, Adv. Funct. Mater., 2009, 19, 1399 CrossRef CAS.
- X. Chen, F. Wudl, A. K. Mal, H. Shen and S. R. Nutt, Macromolecules, 2003, 36, 1802 CrossRef CAS.
- J. Ling, M. Z. Rong and M. Q. Zhang, J. Mater. Chem., 2011, 21, 18373 RSC.
- M. Krogsgaard, M. A. Behrens, J. S. Pedersen and H. Birkedal, Biomacromolecules, 2013, 14, 297 CrossRef CAS.
- P. Cordier, F. Tournilhac, C. Soulié-Ziakovic and L. Leibler, Nature, 2008, 451, 977 CrossRef CAS.
- S. Burattini, H. M. Colquhoun, J. D. Fox, D. Friedmann, B. W. Greenland, P. J. F. Harris, W. Hayes, M. E. Mackay and S. J. Rowan, Chem. Commun., 2009, 44, 6717 RSC.
- S. Burattini, B. W. Greenland, D. H. Merino, W. Weng, J. Seppala, H. M. Colquhoun, W. Hayes, M. E. Mackay, I. W. Hamley and S. J. Rowan, J. Am. Chem. Soc., 2010, 132, 12051 CrossRef CAS.
- S. Bode, L. Zedler, F. H. Schacher, B. Dietzek, M. Schmitt, J. Popp, M. D. Hager and U. S. Schubert, Adv. Mater., 2013, 25, 1634 CrossRef CAS.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef.
- M. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng and F. Huang, Angew. Chem., 2012, 51, 7011 CrossRef CAS.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339 CrossRef CAS.
- D. L. Zhang, X. Ju, L. H. Li, Y. Kang, X. L. Gong, B. J. Li and S. Zhang, Chem. Commun., 2015, 51, 6377 RSC.
- R. Hoogenboom, Angew. Chem., Int. Ed., 2012, 51, 11942 CrossRef CAS.
- J. L. Wietor and R. P. Sijbesma, Angew. Chem., 2008, 47, 8161 CrossRef CAS.
- J. P. Morel and N. Morel-Desrosiers, J. Solution Chem., 1981, 10, 451 CrossRef CAS.
- Y. Wu, F. Zuo, Z. Zheng, X. Ding and Y. Peng, Nanoscale Res. Lett., 2009, 4, 738 CrossRef CAS.
- D. E. Rosenfeld, Z. Gengeliczki, B. J. Smith, T. D. P. Stack and M. D. Fayer, Science, 2011, 334, 634 CrossRef CAS.
- I. Noda, J. Am. Chem. Soc., 1989, 111, 8116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04882g |
| This journal is © The Royal Society of Chemistry 2018 |