Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoswitchable imines: aryliminopyrazoles quantitatively convert to long-lived Z-isomers with visible light†
Jiarong
Wu
 ab,
Lasse
Kreimendahl
a,
Suyuan
Tao
ab,
Olga
Anhalt
b and
Jake L.
Greenfield
ab,
Lasse
Kreimendahl
a,
Suyuan
Tao
ab,
Olga
Anhalt
b and
Jake L.
Greenfield
 *ab
*ab
aInstitut für Organische Chemie, Universität Würzburg, 97074 Würzburg, Germany. E-mail: Jake.Greenfield@uni-wuerzburg.de
bCenter for Nanosystems Chemistry (CNC), Universität Würzburg, 97074 Würzburg, Germany
First published on 14th February 2024
Abstract
Arylimines offer promise in dynamic-covalent materials due to their recyclability and ease of synthesis. However, their light-triggered E/Z isomerism has received little attention. This is attributed to challenges that include low thermal stability of their metastable state (<60 s at 20 °C), incomplete photoswitching (<50% to the metastable state), and the need for UV light (≤365 nm). We overcome these limitations with a novel class of imine photoswitch, the aryliminopyrazoles (AIPs). These AIPs can be switched using visible light (470 nm), attain photostationary states with over 95% of the Z-isomer, exhibit great resistance to fatigue, and have thermal half-lives up to 19.2 hours at room temperature. Additionally, they display T-type and negative photochromism under visible light irradiation—a useful property. The photochromic properties, quantitative assembly and accessibility of precursors set these photoswitches apart from their azo-based analogues. These findings open avenues for next-generation photoresponsive dynamic-covalent materials driven solely by these new photochromic linkages and further exploration of photocontrolled dynamic combinatorial chemistry.
Introduction
Molecular photoswitches, molecules that can reversibly switch between two or more states in response to light, are frequently used in the construction of light-responsive materials1–8 and the operation of functional molecular machines.9–13 In nature, the photoinduced E/Z isomerisation of a cis C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in retinal to the trans conformation is fundamental in the mechanism of vision, whilst commercial applications of photoswitches include photochromic lenses, energy storage materials,14,15 and volumetric 3D printers.16 Of the various classes of photoswitches,17,18 those that display a photoinduced trans–cis (E/Z) isomerism are arguably the most commonly studied.3,19–24 Examples of such switches include derivatives of stilbene (C
C bond in retinal to the trans conformation is fundamental in the mechanism of vision, whilst commercial applications of photoswitches include photochromic lenses, energy storage materials,14,15 and volumetric 3D printers.16 Of the various classes of photoswitches,17,18 those that display a photoinduced trans–cis (E/Z) isomerism are arguably the most commonly studied.3,19–24 Examples of such switches include derivatives of stilbene (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C),25,26 azobenzene and diazocine (N
C),25,26 azobenzene and diazocine (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N),19–21,27,28 and subsets of imines (C
N),19–21,27,28 and subsets of imines (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) that include hydrazones29 and acylhydrazones.22,30 Imines, specifically sterically hindered benzil-derived imines,31 ketimines32–34 and iminothioindoxyls3,35 have shown promise as photoswitches and light-activated molecular motors that display unidirectional rotation about the imine bond.12
N) that include hydrazones29 and acylhydrazones.22,30 Imines, specifically sterically hindered benzil-derived imines,31 ketimines32–34 and iminothioindoxyls3,35 have shown promise as photoswitches and light-activated molecular motors that display unidirectional rotation about the imine bond.12
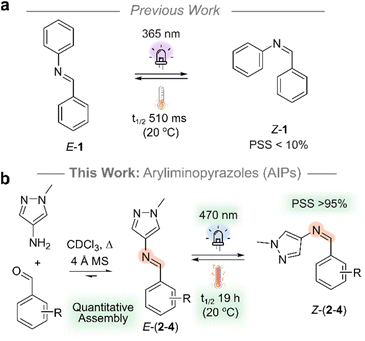
To date, photoswitches derived from arylimines have been seldomly explored despite their straightforward preparation and structural similarity to azobenzene and stilbene.30,36 This is attributed to their poor photoswitching properties, rendering them challenging systems to study (Fig. 1a). These properties include: (i) short thermal half-lives (t1/2)37,38 that typically range from 10−3 to 101 s at room temperature (Table S3†); (ii) low completeness of photoswitching to the metastable state at the photostationary state (PSS, <50%, Table S3†); (iii) the need for high energy UV light to induce photoisomerism, typically in the range of 254 to 365 nm.30 Overcoming these limitations would expand the toolkit of available photochromic compounds and enable the creation of new dynamic-covalent materials39,40 that responds to light directly at the imine bond.41 Such materials would be amenable to build photoresponsive polymers,42 2D and 3D architectures43 and act as more accessible azo-analogues in photopharmacology.44,45
 | ||
| Fig. 1 (a) Diphenylimine, 1, used as a reference compound for comparison. (b) AIPs derived from aminopyrazole and benzaldehyde precursors. Experimental details are provided in the ESI.† | ||
In the context of azo-based photoswitches, several strategies to enhance photoswitching properties have been identified and implemented in light-responsive applications. The substitution of one,46 or both,47,48 of the arene rings with a heteroarene has proved to be a reliable strategy for tuning the photoswitchable properties.17,49 Of the azoheteroarenes, the arylazopyrazoles are one of the most studied classes and have displayed quantitative bidirectional switching,46,50,51 as well as t1/2 reaching tens of years.52–54 Moreover, the ortho-amination of the phenyl rings in azobenzene-based55,56 and arylazopyrazole57 photoswitches achieved a bathochromic shift of the π–π* electronic absorption band with a concomitant reduction in t1/2.55,56 Inspired by these recent advancements in azo-based photoswitches and the timeliness of light-controlled systems, we turned our attention to the relatively over-looked imine photoswitches.
In this work, we address the previously reported poor performance of the arylimine photoswitches: aryliminopyrazoles (AIPs) are introduced as an accessible class of photoswitch which exhibits significantly enhanced photoswitching properties compared to their non-heteroaryl analogues (Fig. 1). Specifically, we explore the impact of ortho-amination—both mono- and di-amination—of the phenyl ring with pyrrolidine on the photoswitching properties. We demonstrate the quantitative assembly of AIP photoswitches that can be switched with visible light (470 nm), achieve photostationary states in excess of 95% of the Z-isomer, and exhibit t1/2 of the Z-isomer that reach 19.2 hours at room temperature. Importantly, the AIPs can be assembled quantitatively, and the accessibility of precursors renders this class of photoswitch amenable to large scale application.
Results and discussion
To quantitatively prepare the AIPs in Fig. 2, the corresponding aldehyde and amine precursors were combined in CDCl3 and heated to reflux overnight with 4 Å molecular sieves (MS). Trace amounts of DCl in CDCl3 were sufficient to catalyze the condensation,58 and the resultant imines were neutralized before the characterization of their photoswitching properties. The isolated imines remained stable in the solid state for over two months and showed no signs of hydrolysis when dissolved in MeCN-d3 (ca. 2 mM) even after two weeks under ambient conditions. Alongside the AIPs, a reference compound, diphenylimine 1, was synthesized to underline the enhanced photoswitching properties of the AIPs, as summarized in Table 1.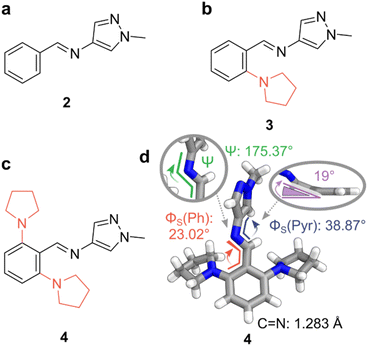 | ||
Fig. 2 Photoswitchable AIPs and X-ray crystal structure. (a) Replacement of a phenyl ring with a pyrazole unit. (b) Substitution of AIP 2 with a pyrrolidine at the ortho-position furnished photoswitch 3. (c) Substitution at both ortho-positions of the phenyl ring with pyrrolidine yields AIP 4. (d) The X-ray crystal structure of 4. (inset, right) Deviation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond from the plane containing the phenyl ring, with pyrrolidine units hidden for clarity. Color code: C, grey; N, blue; H, white. CCDC 2293811. N bond from the plane containing the phenyl ring, with pyrrolidine units hidden for clarity. Color code: C, grey; N, blue; H, white. CCDC 2293811. | ||
| % Z at PSS | t 1/2 | QY (%) | ||||
|---|---|---|---|---|---|---|
| 365 nm | 405 nm | 430 nm | 470 nm | (20 °C) | 365 nm | |
| a Note that the short t1/2 values preclude the measurement of the % Z at the PSS under these conditions (Fig. S15). b The maximum measurable % Z isomer recorded at 20 °C. | ||||||
| 1a | −(1)b | — | — | — | 0.5 s | — |
| 2a | −(21)b | — | — | — | 12.5 s | — |
| 3 | 66 | 95 | 90 | 55 | 22.1 min | 2 (2) |
| 4 | 68 | 95 | 99 | 94 | 19.2 h | 1 (1) |
The X-ray crystal structure of E-4 revealed a non-planar conformation. A twist of 23° was noted in the smaller dihedral angle between the phenyl and imine (Φs (Ph)), while a 38° twist is observed for Φs (Pyr). This twist results from steric clashes between the imine bond and the ortho-substituents the adjacent rings. Notably, the steric clash between the ortho-pyrrolidine units and the imine distorts the imine from planarity by ca. 19° (Fig. 2d).
Photoinduced E/Z isomerism was observed for the AIPs at room temperature using UV/vis spectroscopy, summarized in Table 1. The AIPs demonstrate T-type behavior, where the back reaction of the metastable state to the thermodynamically stable isomer occurs thermally. Notably, visible-light-induced negative photochromism was observed for 3 and 4 (Fig. 3a and S13†), whereby λmax(E) > λmax(Z). This specific type of photochromic behavior is advantageous in applications requiring large light penetration depths.48 The lowest energy absorption band in the UV/vis spectra is assigned to a π–π* transition, corroborated by TD-DFT studies (Section 5 of ESI†).
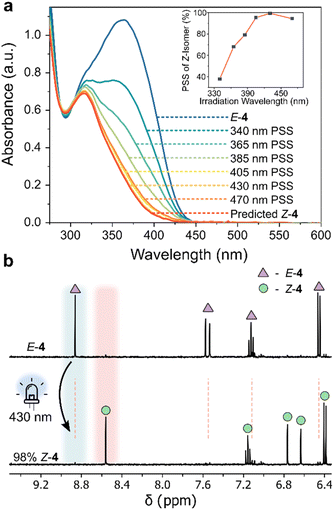 | ||
| Fig. 3 (a) UV/vis absorption spectra of 4 (170 μM, 20 °C, MeCN) at various photostationary states (PSS), and the spectrum of the Z-isomer obtained as described in Section 4.5 of the ESI.†59 (inset) Z-Isomer percentage generated at different irradiation wavelengths (Table S4†). (b) 1H NMR spectra (400 MHz, 298 K, CD3CN) of E-4 (triangle), highlighting the emergence of new signals attributed to Z-4 (circle) upon 430 nm photoirradiation. | ||
Examining the UV/vis absorption spectra revealed that ortho-aminated AIPs exhibit significant bathochromic shifts—up to 50 nm in the π–π* band—compared to the non-functionalized analogue 2 (Table S5†). Specifically, the pronounced 50 nm red-shift in the π–π* band of E-3 enabled E-to-Z photoisomerism with 470 nm light. Upon photoisomerization, we observed a hypsochromic shift in the π–π* transition, confirming negative photochromism. The largest separation between the π–π* bands of the two isomers occurred in 4, where both ortho-positions were aminated. This led to a 53 nm blue-shifted transition for the Z-isomer compared to its E-form. In contrast, compound 3, with a single ortho-aminated site, exhibited a smaller hypsochromic shift of 25 nm.
The AIPs demonstrated more complete E-to-Z photoswitching, evidenced by significantly larger compositions of the Z-isomer generated at the PSS compared to the maximum obtainable amount of the Z-isomer of 1 and the other previously reported arylimines (Table S3†). We quantified the Z-isomer composition at the PSS using UV/vis spectroscopy (Fig. 3a and S13†),59 and further corroborated these values with complementary NMR measurements (Fig. 3b and S14†). Near-complete E-to-Z switching (with ≥95% of Z-isomer at the PSS) was noted for 3 and 4 using visible light (Table 1, Fig. 3b, S13 and 14†). This is attributed to the bathochromically shifted π–π* transition, the large separation of this band between the E and Z isomers.54 Such quantitative E-to-Z photoswitching constitutes a significant achievement in the development of imine-based photoswitches.54 The measured quantum yields for both the forward and reverse photoswitching were found to be ≤10% (Section 4.2 and 4.7 of the ESI†).
To our surprise, the Z-AIPs displayed a markedly long thermal half-life, exemplified with 4 showing a t1/2 of 19.2 hours at 20 °C. This represents a four orders-of-magnitude increase in thermal stability compared to the most stable Z-arylimine reported so far and 105 times longer than 1 (Table 1 and S3†). It is intriguing that ortho-amination of the phenyl ring with pyrrolidine led to an increase in the t1/2. This behavior contrasts that of the azo-based photoswitches, where ortho-amination typically decreases the thermal stability of the Z-state.55,56 This deviation from established trends highlights unique properties of the AIPs, justifying further research into the structure–function relationships of these imine-based photoswitches.
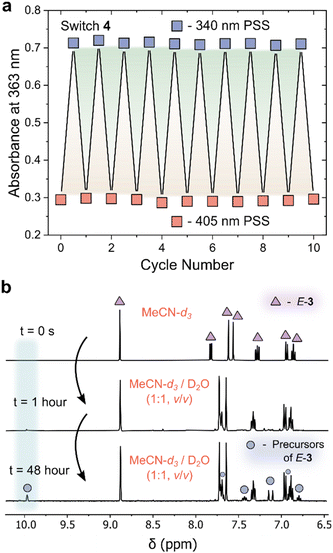
In addition to an enhanced thermal stability of the Z-isomer, AIPs 3 and 4 displayed excellent resistance to fatigue: no signs of degradation were observed in the UV/vis absorption spectra after 10 photoswitching cycles (Fig. 4a and S16†). Another important consideration is the hydrolytic stability of the imines towards water. The NMR spectra of AIP 3 (2 mM) was recorded in a MeCN-d3/D2O mixture after 1 hour and 48 hours (Fig. 4b). Even in this large excess of D2O, the imine remained surprisingly stable, showing only 16% of the imine hydrolyzing back to the aldehyde and amine precursors after 48 hours at 25 °C. The stability of these imines to the presence of water is attributed to the improved nucleophilicity of the aminopyrazole unit.
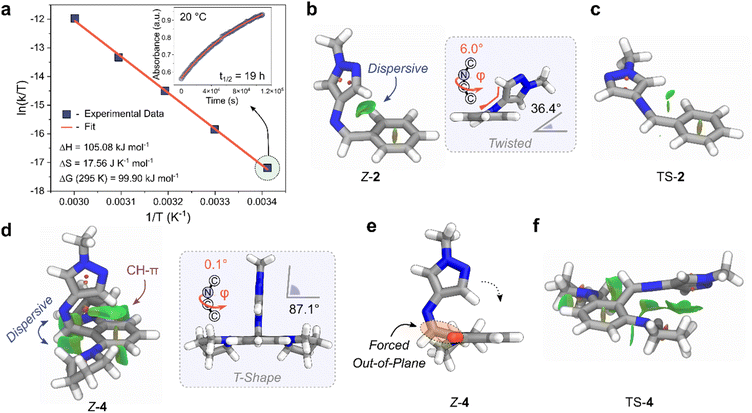
The influence of different solvents on the photoswitching behaviour of AIP 4 was examined (Section 4.8 of ESI†). UV/vis absorption spectra and the proportion of Z-isomer at the PSS showed minimal variation across the solvents studied (which included toluene, 1,4-dioxane, tetrahydrofuran, dichloromethane, dimethyl sulfoxide, and acetonitrile, see Table S8 and Fig. S23†). For instance, the maximum absorption wavelength of the E-isomer's lowest energy band shifted by less than 10 nm among the solvents tested. However, the choice of solvent markedly influenced the thermal half-life of the Z-to-E isomerization (Fig. S24 and Table S8†). Dichloromethane resulted in the shortest t1/2 (3.2 h), while acetonitrile showed the longest (19.2 h), indicating a 4 kJ mol−1 difference in the apparent thermal barrier to reversion. Generally, we observed that aprotic solvents with higher empirical solvent parameter, ENT values,60 correlated with a longer t1/2, suggesting that the Z-to-E isomerization likely proceeds through an inversion transition state.61
We investigated the mechanism behind the significant stabilization of the Z-isomer using DFT, adopting the ωB97X-D4 functional in combination with a def2-TZVPP basis set, and a CPCM solvation model for acetonitrile (Section 5 of the ESI†).62,63 Ground-state optimized structures revealed a twisted conformation for Z-2 and Z-3, while Z-4 adopted a T-shaped structure (Fig. 5b and d). Significant stabilizing non-covalent interactions (NCIs) were observed for the Z-isomer, illustrated graphically by the NCI surfaces in Fig. 5.64 The impact of dispersive interactions was quantified by evaluating the difference in the dispersive energy between the Z and E isomers (referred to as ED4Z–E) with Grimmes DFT-D4 approach.62
The NCI interactions are particularly pronounced for ortho-aminated derivatives with ED4Z–E values as high as +17.45 kJ mol−1 for 3 compared to +6.97 kJ mol−1 for 2. In the case of 4, the additional steric bulk of the two flanking pyrrolidine units forces the structure to adopt a T-shape conformation by minimizing the steric repulsion between the imine hydrogen and the pyrrolidine units (Fig. 5d and e).54 Closer inspection of the structure revealed that the carbon atom of the imine bond in Z-4 is also forced out of plane relative to the phenyl ring due to the steric requirements of the ortho-pyrrolidine units, similar to the crystal structure of E-4 (Fig. 2d and 5e). Importantly, this distortion brings the CH of the pyrazole closer to the centroid of the phenyl ring (distance of 2.3 Å). The resulting conformation enhances the NCI stabilization, favoring the formation CH–π interactions between the pyrazole and the phenyl ring, while maximizing the dispersive interactions with the flanking pyrrolidine motifs.
For the Z-to-E thermal reversion process, the AIPs follow an inversion trajectory with linearization of the C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (Fig. S26†).65 Stabilizing NCIs are also observed in the TS structures, and are more pronounced for the ortho-functionalized derivatives (Fig. 5c and f and Section 5 ESI†). Given this, we infer that the increase in t1/2 predominantly originates from an increased stabilization of the Z-isomer due to NCI interactions as opposed to a destabilization of the TS. Specifically, the near 90° arrangement (T-Shape) of the pyrazole ring with the phenyl ring of Z-4 is positioned to conformations of 2 and 3. Additionally, unlike azo-based photoswitches, the absence of adjacent non-bonding lone pairs in the imine bond prevents any additional destabilizing electron repulsion in the HOMO when incorporating the electron-donating pyrrolidine motifs.
C bond (Fig. S26†).65 Stabilizing NCIs are also observed in the TS structures, and are more pronounced for the ortho-functionalized derivatives (Fig. 5c and f and Section 5 ESI†). Given this, we infer that the increase in t1/2 predominantly originates from an increased stabilization of the Z-isomer due to NCI interactions as opposed to a destabilization of the TS. Specifically, the near 90° arrangement (T-Shape) of the pyrazole ring with the phenyl ring of Z-4 is positioned to conformations of 2 and 3. Additionally, unlike azo-based photoswitches, the absence of adjacent non-bonding lone pairs in the imine bond prevents any additional destabilizing electron repulsion in the HOMO when incorporating the electron-donating pyrrolidine motifs.
Taken together, the key observations reported here for this new class of dynamic-covalent photoswitch include: (i) the π–π* absorption transition can be red-shifted by ortho-aminating the phenyl ring. This bathochromic shift allows for visible light operation, widening the scope of practical applications. (ii) Maximizing NCIs through ortho-functionalization of the phenyl ring markedly stabilizes the Z-isomer, resulting in enhanced t1/2. This contrasts with azo-based systems, where ortho-amination typically reduces Z-isomer stability. (iii) Unlike azo-based switches, the absence of lone pair repulsion in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the Z-AIPs facilitates the inclusion of electron donating units without significantly destabilizing the HOMO.
N bond of the Z-AIPs facilitates the inclusion of electron donating units without significantly destabilizing the HOMO.
Setting this work in context with other photoswitches that exhibit E/Z isomerism, the AIPs display a distinct combination of properties. While the geometric change from the relatively planar E-isomer to the twisted or T-shaped Z-isomer in AIPs resembles azo-based photoswitches, the effects of incorporating a pyrazole ring and ortho-amination are different. Notably, in contrast to arylazopyrazoles,46,54 which show markedly extended half-lives, near-quantitative PSSs, and positive photochromism compared to azobenzene, AIPs do not exhibit a significant improvement by the sole incorporation of the pyrazole ring, as highlighted for 2. However, the introduction of ortho-pyrrolidine units, typically reducing the t1/2 in azo-based switches,55,56 stabilizes the metastable state of the AIPs for the reasons presented above, and imparts negative photochromism to the switch. These properties, paired with the ease of synthesis, accessibility of precursors, and reversibility of the imine bond, set the AIPs apart from the azo-based photoswitches.
The arylhydrazones also contain a dynamic-covalent motif (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH),66 albeit less dynamic than an imine bond, and can undergo E/Z photoisomerism. Unlike the AIPs however, the photochromic motif of the arylhydrazones is comprised of a six-membered H-bonded ring system with an acidic NH site,66 thus different strategies are used for tuning the stability of their metastable state. Regarding the geometric change associated with E/Z isomerism, the arylhydrazones are more planar compared to the larger three-dimensional change observed for the AIPs.66 Thus, AIPs are arguably more suited for applications requiring a greater dynamicity or a larger geometric change upon photoisomerism, such use in self-assembled photoresponsive organic capsules.67,68 Other recently reported classes of photoswitch that operate around a C
N–NH),66 albeit less dynamic than an imine bond, and can undergo E/Z photoisomerism. Unlike the AIPs however, the photochromic motif of the arylhydrazones is comprised of a six-membered H-bonded ring system with an acidic NH site,66 thus different strategies are used for tuning the stability of their metastable state. Regarding the geometric change associated with E/Z isomerism, the arylhydrazones are more planar compared to the larger three-dimensional change observed for the AIPs.66 Thus, AIPs are arguably more suited for applications requiring a greater dynamicity or a larger geometric change upon photoisomerism, such use in self-assembled photoresponsive organic capsules.67,68 Other recently reported classes of photoswitch that operate around a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond include the iminothioindoxyl35 and phenylimino indolinone3 photoswitches, though these systems have not exhibited dynamic-covalent chemistry. These imino-based switches display negative photochromism and a t1/2 on the μs to ms time scale.3,35
N bond include the iminothioindoxyl35 and phenylimino indolinone3 photoswitches, though these systems have not exhibited dynamic-covalent chemistry. These imino-based switches display negative photochromism and a t1/2 on the μs to ms time scale.3,35
Collectively, the AIPs stand out as dynamic-covalent photoswitches that exhibit large geometric changes upon isomerization.69 Moreover, the simplicity and availability of their precursors, paired with quantitative imine-formation and useful photoswitching properties makes them enticing photoswitches in their own right.
Conclusions
In conclusion, we have successfully addressed the key challenges that previously hindered arylimines from being utilized as effective photoswitchable molecules. Notably, we have identified structural motifs that exhibit: (i) negative photochromism; (ii) photoisomerization with visible light (470 nm); (iii) display near-quantitative E-to-Z photoisomerism (>95% Z-isomer); (iv) excellent fatigue resistance (v) and prolonged thermal half-lives up to 19.2 hours at room temperature. Key to these improved properties is the use of an aminopyrazole subcomponent, combined with ortho-amination of the phenyl ring, which synergistically extended the thermal stability of the metastable state and red-shift the UV/vis absorption. The increased t1/2 is attributed to the strong NCI stabilization in the Z-isomer. The negative photochromism distinguishes AIPs from azo-analogues as the former exhibits no competing Z-to-E photoisomerism at the longest wavelengths of electronic absorption. Moreover, the negative photochromic behavior of these switches, in particular, presents advantages in applications requiring large light penetration depths,48,53 such as molecular solar-thermal fuels (MOST).48 It is also important to consider the scalability and accessibility of the material, and its precursors, when evaluating suitability for an application. Thus, the quantitative assembly and availability of precursors render AIPs as attractive candidates. To this end, our laboratory is currently investigating new imine-based photoswitches and working towards the utilization of these photochromic linkages in larger organic architectures and adaptive dynamic-covalent networks, these results will be reported in due course.Data availability
The experimental procedures, analytical data and computational details are available within the manuscript and its ESI.† CCDC number 2293811 contains the supplementary crystallographic data for AIP E-4. Data are available upon request from the authors.Author contributions
J. W. synthesized the subcomponents and imines, characterized the imines by NMR, MS, and UV/vis, and performed the photoswitching experiments. S. T. assisted in the synthesis of the subcomponents and imines, and their molecular characterization. L. K. conducted the computational investigations. O. N. collected and solved the crystallographic data. J. L. G. conceived the project, supervised the research, and wrote the manuscript, with input from all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Fonds der Chemischen Industrie (FCI, Liebig Fellowship for J. L. G., PhD Fellowship for J. W.) and the Alexander von Humboldt-Stiftung (Post-Doctoral Fellowship for J. L. G.). We sincerely thank Prof. Frank Würthner for his mentorship, support and for providing infrastructure. We thank Dr Matthias Stolte for valuable discussions and assistance regarding the photoswitching setup.Notes and references
- M. Regehly, Y. Garmshausen, M. Reuter, N. F. König, E. Israel, D. P. Kelly, C.-Y. Chou, K. Koch, B. Asfari and S. Hecht, Nature, 2020, 588, 620–624 CrossRef CAS PubMed.
- G. C. Thaggard, J. Haimerl, K. C. Park, J. Lim, R. A. Fischer, B. K. P. Maldeni Kankanamalage, B. J. Yarbrough, G. R. Wilson and N. B. Shustova, J. Am. Chem. Soc., 2022, 144, 23249–23263 CrossRef CAS PubMed.
- S. Crespi, N. A. Simeth, M. Di Donato, S. Doria, C. N. Stindt, M. F. Hilbers, F. L. Kiss, R. Toyoda, S. Wesseling, W. J. Buma, B. L. Feringa and W. Szymański, Angew. Chem., Int. Ed., 2021, 60, 25290–25295 CrossRef CAS PubMed.
- D. Hugenbusch, M. Lehr, J. von Glasenapp, A. J. McConnell and R. Herges, Angew. Chem., Int. Ed., 2023, 62, e202212571 CrossRef CAS PubMed.
- L. A. Tatum, X. Su and I. Aprahamian, Acc. Chem. Res., 2014, 47, 2141–2149 CrossRef CAS PubMed.
- B. E. Jones, E. A. Kelly, N. Cowieson, G. Divitini and R. C. Evans, J. Am. Chem. Soc., 2022, 144, 19532–19541 CrossRef CAS PubMed.
- E. A. Kelly, J. E. Houston and R. C. Evans, Soft Matter, 2019, 15, 1253–1259 RSC.
- M. C. Brand, N. Rankin, A. I. Cooper and R. L. Greenaway, Chem.–Eur. J., 2023, 29, 5770–5781 CrossRef PubMed.
- A. Goulet-Hanssens, F. Eisenreich and S. Hecht, Adv. Mater., 2020, 32, 1905966 CrossRef CAS PubMed.
- S. Corra, M. T. Bakić, J. Groppi, M. Baroncini, S. Silvi, E. Penocchio, M. Esposito and A. Credi, Nat. Nanotechnol., 2022, 17, 746–751 CrossRef CAS PubMed.
- A. Gerwien, P. Mayer and H. Dube, Nat. Commun., 2019, 10, 4449 CrossRef PubMed.
- D. R. S. Pooler, A. S. Lubbe, S. Crespi and B. L. Feringa, Chem. Sci., 2021, 12, 14964–14986 RSC.
- N. Duindam, M. van Dongen, M. A. Siegler and S. J. Wezenberg, J. Am. Chem. Soc., 2023, 145, 21020–21026 CrossRef CAS PubMed.
- J. Orrego-Hernández, A. Dreos and K. Moth-Poulsen, Acc. Chem. Res., 2020, 53, 1478–1487 CrossRef PubMed.
- Z.-Y. Zhang, Y. He, Z. Wang, J. Xu, M. Xie, P. Tao, D. Ji, K. Moth-Poulsen and T. Li, J. Am. Chem. Soc., 2020, 142, 12256–12264 CrossRef CAS PubMed.
- M. Regehly, Y. Garmshausen, M. Reuter, N. F. König, E. Israel, D. P. Kelly, C.-Y. Chou, K. Koch, B. Asfari and S. Hecht, Nature, 2020, 588, 620–624 CrossRef CAS PubMed.
- S. Crespi, N. A. Simeth and B. König, Nat. Rev. Chem, 2019, 3, 133–146 CrossRef CAS.
- Molecular Photoswitches, ed. Z. L. Pianowski, Wiley, 2022 Search PubMed.
- D. Bléger, J. Schwarz, A. M. Brouwer and S. Hecht, J. Am. Chem. Soc., 2012, 134, 20597–20600 CrossRef PubMed.
- S. Samanta, C. Qin, A. J. Lough and G. A. Woolley, Angew. Chem., Int. Ed., 2012, 51, 6452–6455 CrossRef CAS PubMed.
- M. Blanco-Lomas, S. Samanta, P. J. Campos, G. A. Woolley and D. Sampedro, J. Am. Chem. Soc., 2012, 134, 6960–6963 CrossRef CAS PubMed.
- H. Qian, S. Pramanik and I. Aprahamian, J. Am. Chem. Soc., 2017, 139, 9140–9143 CrossRef CAS PubMed.
- L. Greb, H. Mutlu, C. Barner-Kowollik and J. M. Lehn, J. Am. Chem. Soc., 2016, 138, 1142–1145 CrossRef CAS PubMed.
- X. Su and I. Aprahamian, Chem. Soc. Rev., 2014, 43, 1963–1981 RSC.
- M. Kathan and S. Hecht, Chem. Soc. Rev., 2017, 46, 5536–5550 RSC.
- D. Villarón and S. J. Wezenberg, Angew. Chem., Int. Ed., 2020, 59, 13192–13202 CrossRef PubMed.
- J. Isokuortti, T. Griebenow, J.-S. von Glasenapp, T. Raeker, M. A. Filatov, T. Laaksonen, R. Herges and N. A. Durandin, Chem. Sci., 2023, 14, 9161–9166 RSC.
- R. Falkenburg, M. J. Notheis, G. Schnakenburg and L. K. S. von Krbek, Org. Biomol. Chem., 2023, 21, 4993–4998 RSC.
- S. Yang, J. D. Harris, A. Lambai, L. L. Jeliazkov, G. Mohanty, H. Zeng, A. Priimagi and I. Aprahamian, J. Am. Chem. Soc., 2021, 143, 16348–16353 CrossRef CAS PubMed.
- L. Greb, G. Vantomme and J. Lehn, in Molecular Photoswitches, Wiley, 2022, pp. 325–349 Search PubMed.
- L. Greb, A. Eichhöfer and J. Lehn, Eur. J. Org Chem., 2016, 1243–1246 CrossRef CAS.
- L. Greb and J.-M. Lehn, J. Am. Chem. Soc., 2014, 136, 13114–13117 CrossRef CAS PubMed.
- L. Greb, A. Eichhöfer and J. Lehn, Angew. Chem., Int. Ed., 2015, 54, 14345–14348 CrossRef CAS PubMed.
- D. Roke, S. J. Wezenberg and B. L. Feringa, Proc. Natl. Acad. Sci. U.S.A., 2018, 115, 9423–9431 CrossRef CAS PubMed.
- M. W. H. Hoorens, M. Medved, A. D. Laurent, M. Di Donato, S. Fanetti, L. Slappendel, M. Hilbers, B. L. Feringa, W. Jan Buma and W. Szymanski, Nat. Commun., 2019, 10, 2390 CrossRef PubMed.
- E. Fischer and Y. Frei, J. Chem. Phys., 1957, 27, 808–809 CrossRef CAS.
- M. Hammerich, T. Rusch, N. R. Krekiehn, A. Bloedorn, O. M. Magnussen and R. Herges, ChemPhysChem, 2016, 17, 1870–1874 CrossRef CAS PubMed.
- D. Veselý, J. Jančík, M. Weiter, D. Blasi, N. Ivanova, J. Krajčovič and A. Georgiev, J. Photochem. Photobiol., A, 2022, 430, 113994 CrossRef.
- R. L. Greenaway, V. Santolini, M. J. Bennison, B. M. Alston, C. J. Pugh, M. A. Little, M. Miklitz, E. G. B. Eden-Rump, R. Clowes, A. Shakil, H. J. Cuthbertson, H. Armstrong, M. E. Briggs, K. E. Jelfs and A. I. Cooper, Nat. Commun., 2018, 9, 2849 CrossRef CAS PubMed.
- R. J. Kearsey, A. Tarzia, M. A. Little, M. C. Brand, R. Clowes, K. E. Jelfs, A. I. Cooper and R. L. Greenaway, Chem. Commun., 2023, 59, 3731–3734 RSC.
- N. F. König, D. Mutruc and S. Hecht, J. Am. Chem. Soc., 2021, 143, 9162–9168 CrossRef PubMed.
- C. Kulkarni, R. H. N. Curvers, G. Vantomme, D. J. Broer, A. R. A. Palmans, S. C. J. Meskers and E. W. Meijer, Adv. Mater., 2021, 33, 2005720 CrossRef CAS PubMed.
- C. L. Jones, A. J. Tansell and T. L. Easun, J. Mater. Chem. A, 2016, 4, 6714–6723 RSC.
- K. Hüll, J. Morstein and D. Trauner, Chem. Rev., 2018, 118, 10710–10747 CrossRef PubMed.
- W. A. Velema, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 2178–2191 CrossRef CAS PubMed.
- C. E. Weston, R. D. Richardson, P. R. Haycock, A. J. P. White and M. J. Fuchter, J. Am. Chem. Soc., 2014, 136, 11878–11881 CrossRef CAS PubMed.
- Y. He, Z. Shangguan, Z. Zhang, M. Xie, C. Yu and T. Li, Angew. Chem., Int. Ed., 2021, 60, 16539–16546 CrossRef CAS PubMed.
- A. Gonzalez, M. Odaybat, M. Le, J. L. Greenfield, A. J. P. White, X. Li, M. J. Fuchter and G. G. D. Han, J. Am. Chem. Soc., 2022, 144, 19430–19436 CrossRef CAS PubMed.
- J. L. Greenfield, A. R. Thawani, M. Odaybat, R. S. L. Gibson, T. B. Jackson and M. J. Fuchter, in Molecular Photoswitches, Wiley, 2022, pp. 83–112 Search PubMed.
- L. Stricker, E.-C. Fritz, M. Peterlechner, N. L. Doltsinis and B. J. Ravoo, J. Am. Chem. Soc., 2016, 138, 4547–4554 CrossRef CAS PubMed.
- Z. Zhang, Y. He, Y. Zhou, C. Yu, L. Han and T. Li, Chem.–Eur. J., 2019, 25, 13402–13410 CrossRef CAS PubMed.
- J. Calbo, A. R. Thawani, R. S. L. Gibson, A. J. P. White and M. J. Fuchter, Beilstein J. Org. Chem., 2019, 15, 2753–2764 CrossRef CAS PubMed.
- J. L. Greenfield, M. A. Gerkman, R. S. L. Gibson, G. G. D. Han and M. J. Fuchter, J. Am. Chem. Soc., 2021, 143, 15250–15257 CrossRef CAS PubMed.
- J. Calbo, C. E. Weston, A. J. P. P. White, H. S. Rzepa, J. Contreras-García and M. J. Fuchter, J. Am. Chem. Soc., 2017, 139, 1261–1274 CrossRef CAS PubMed.
- O. Sadovski, A. A. Beharry, F. Zhang and G. A. Woolley, Angew. Chem., Int. Ed., 2009, 48, 1484–1486 CrossRef CAS PubMed.
- Z. Ahmed, A. Siiskonen, M. Virkki and A. Priimagi, Chem. Commun., 2017, 53, 12520–12523 RSC.
- J. Simke, T. Bösking and B. J. Ravoo, Org. Lett., 2021, 23, 7635–7639 CrossRef CAS PubMed.
- R. S. L. Gibson, J. Calbo and M. J. Fuchter, ChemPhotoChem, 2019, 3, 372–377 CrossRef CAS.
- E. Fischer, J. Phys. Chem., 1967, 71, 3704–3706 CrossRef CAS.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- J. Volarić, W. Szymanski, N. A. Simeth and B. L. Feringa, Chem. Soc. Rev., 2021, 50, 12377–12449 RSC.
- E. Caldeweyher, J.-M. Mewes, S. Ehlert and S. Grimme, Phys. Chem. Chem. Phys., 2020, 22, 8499–8512 RSC.
- A. Najibi and L. Goerigk, J. Comput. Chem., 2020, 41, 2562–2572 CrossRef CAS PubMed.
- R. A. Boto, F. Peccati, R. Laplaza, C. Quan, A. Carbone, J.-P. Piquemal, Y. Maday and J. Contreras-García, J. Chem. Theory Comput., 2020, 16, 4150–4158 CrossRef CAS PubMed.
- Y. Luo, M. Utecht, J. Dokić, S. Korchak, H.-M. M. Vieth, R. Haag and P. Saalfrank, ChemPhysChem, 2011, 12, 2311–2321 CrossRef CAS PubMed.
- B. Shao and I. Aprahamian, Chem, 2020, 6, 2162–2173 CAS.
- M. Ovalle, M. Kathan, R. Toyoda, C. N. Stindt, S. Crespi and B. L. Feringa, Angew. Chem., Int. Ed., 2023, 62, e202214495 CrossRef CAS PubMed.
- R. G. DiNardi, A. O. Douglas, R. Tian, J. R. Price, M. Tajik, W. A. Donald and J. E. Beves, Angew. Chem., Int. Ed., 2022, 61, e202205701 CrossRef CAS.
- J.-M. Lehn, Chem.–Eur. J., 2006, 12, 5910–5915 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: A materials and methods description and additional data on the characterization of precursor and product (ESI-MS, NMR, UV/vis), a description of the photoswitching experiments (PSS, t1/2, QY) and the full data and description of the computational investigations. CCDC 2293811. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc05841g |
| This journal is © The Royal Society of Chemistry 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: