 Open Access Article
Open Access ArticleMicroneedle (MN)-based sensing technology: an innovative solution for agriculture
Sonu
Kumari
a,
Neetu
Talreja
 *bc,
Divya
Chauhan
d and
Mohammad
Ashfaq
*bc,
Divya
Chauhan
d and
Mohammad
Ashfaq
 *e
*e
aDepartment of Bioscience and Biotechnology, Banasthali Vidyapith, Newai Rajasthan 304022, India
bFaculty of Science and Technology, Department of Science, Alliance University, Bengaluru, Karnataka 562106, India. E-mail: neetutalreja99@gmail.com
cCentre of Excellence For Advanced Material Synthesis and Centre of Excellence For Sophisticated Testing & Instrumentation Centre, Alliance University, Bengaluru, Karnataka 562106, India
dDepartment of Drinking Water and Sanitation, Ministry of Jal Shakti, 1208-A, Pandit Deendayal Antyodaya Bhawan, CGO Complex, Lodhi Road, New Delhi 110003, India
eDepartment of Biotechnology & University Centre for Research & Development (UCRD), Chandigarh University, Gharaun, Mohali, 140413, Punjab, India. E-mail: mohdashfaqbiotech@gmail.com
First published on 25th September 2024
Abstract
Agricultural health is one of the most important aspects of improving crop productivity, which can significantly decrease the demand for food. Plant diseases and nutritional value are among the crucial factors affecting food safety and quality, subsequently reducing the yield of the crops and increasing plant mortality. Therefore, continuous monitoring of plant health is of utmost importance to enhance the yield of crops. In this aspect, microneedle (MN)-based sensing technology is potentially able to monitor agricultural health. Borrowing a page from medicine, minimally invasive MNs have been effectively used to deliver drugs and biomolecules within the human body without any pain or tissue damage. Usually, MNs have been divided by researchers into four groups: solid microneedles (S-MNs), hollow microneedles (H-MNs), dissolving microneedles (D-MNs), and coated microneedles (C-MNs), which are effectively used according to requirements of delivery of biomolecules and sensing applications. The MN-based probe is directly attached to the relevant part of the plant tissue, thereby bypassing the cuticles. Interestingly, MN-based sensing technology offers newer insight into agriculture health by continuously monitoring plant health, including nutritional values and pathogens. This article opens newer avenues and provides knowledge about the fabrication of MN-based sensing technology for plant health that might benefit the food and agricultural industry.
1. Introduction
Food requirements relentlessly increase with the growing population and industrialization globally. Moreover, the productivity of crops is decreasing with the increasing stress of other things like salinity and pathogens, which exert more pressure on the agricultural and food industries.1–5 The loss of crops amounts to ∼220 billion dollars, and approximately 30–35% of the loss of crops globally is due to pathogens. Plant diseases and crop disasters adversely affect the yield of crops, which are some of the most common issues in the agricultural sectors.6–8 Therefore, it is necessary to develop newer, innovative technologies that improve the yield of crops and protect them from pathogens.Considerable research and development have been done so far to innovate next-generation technologies and apply them in agriculture for real-time monitoring. Such innovative technologies might be beneficial to retain sustainability, achieve food security, and control pathogenic infections by monitoring plants.9,10 Numerous diagnostic technologies, such as polymerase chain reaction (PCR), quantitative PCR (qPCR), digital PCR (dPCR), multiplex PCR, enzyme-linked immunosorbent assay (ELISA), fluorescent in situ hybridization (FISH), immunofluorescence, and flow cytometry, have been used for the detection of plant pathogen. However, these laboratory-based detection technologies require skilled workers to analyze the results, are costly, cannot be monitored continuously, and are inaccessible in remote locations.11–15 In this aspect, developing inexpensive, rapid, accurate, and early diagnostic sensing devices is necessary.
Early diagnostic sensing devices offer new avenues for monitoring and controlling plant pathogens by providing preliminary information on crop health in a non-destructive manner. The crop's health is important for crop productivity and protection against pathogens. Moreover, sensitivity, robustness, and accuracy are required to retain the sustainability and security of food.13,14,16–19 In this aspect, nanomaterials (NMs) might resolve issues associated with existing technologies.
NMs, including metals and their oxides (Cu, Ag, Fe, and Zn, etc.), and carbon-based nanomaterials (CB-NMs) such as carbon nanotubes (CNTs), carbon nanofibers (CNFs), graphene, and graphene oxide (GO), have been efficiently used as the electrode materials for the detection of various analytes of plants, including phytohormones, and plant pathogens due to their exceptional mechanical and electrical properties, as well as their high surface area and conductivity. Moreover, CB-NMs show remarkable triumphs in delivering micronutrients/biomolecules and detecting plant pathogens. However, real-time monitoring of the plants remains a concern.20–29 Therefore, we need to design electrode materials with specific shapes that can attach to the plants without any adverse effects or damaging plant tissues with the target analytes. In this aspect, microneedle (MN)-based technology enables innovative platforms to protect agricultural crops and monitor their productivity by reducing agricultural waste and improving nutritional values.
MNs are one of the growing technologies in medicine due to their painless and minimally invasive drug delivery systems. MNs are efficiently used to transdermally deliver numerous macromolecules, and biomolecules such as pharmaceutical compounds, DNA, proteins, and vaccines due to their cost-effectiveness, self-administration, and ease of handling.30–35 MN-based sensors easily analyze and monitor the release of drugs, biomarkers, metabolites, and other biological parameters. Undeniably, we have significant evidence suggesting that MNs are efficiently used for diagnostic and therapeutic purposes.36–38 With the immense success of MNs in biomedical applications, researchers are attracted to MNs for agricultural applications.39–41 A few articles based on MNs for agricultural applications, which focused on food, plant treatment, and sensing applications, have already been published. Moreover, specific attention is required to understand the role of MN-based sensing technology in the improvement of agriculture systems.39,40,42 MN-based technologies can bring innovation to farmers and assist them in managing the cause of crop failure, thereby enhancing the productivity of crops. MN-based technologies also deliver micronutrients, and biomolecules to the plants, controlling plant pathogens, sensing pathogens, and monitoring soil and plant health quality. Fig. 1 shows the graphical representation of the MNs and their application in agriculture, especially monitoring plant health.
 | ||
| Fig. 1 A schematic illustration of the MNs and their application in agriculture, especially monitoring plant health by insertion of MNs onto the surface of the leaf and stem. | ||
This perspective focused on the different types of MNs and their fabrication process. MN-based sensing technology has emerged as a next-generation tool for minimally invasive monitoring of plant health in real-time. We discuss the MN-based sensors for monitoring crops and detecting plant pathogens in real-time. Moreover, we also discuss the role of MN-based sensors in foods, such as determining quality and detecting pesticides/agrochemicals. Finally, we discuss how we can improve the sensitivity and accuracy of the MN-based sensors and their prospects. Moreover, MN-based sensing technology enables newer avenues for the detection of analytes even at lower concentrations. This perspective offers new insight for agricultural scientists to understand the newer MN-based technologies and their applications in agriculture.
2. MN-based technology for agriculture
The MN-based technology was proposed in 1976 and introduced as a transdermal delivery system in 1990, mainly due to the lack of fabrication technology. MNs are micron-size needles (25–2000 mm height) made of various materials, fabrication processes, and shapes. The MN-based technology is mainly applied in drug delivery systems.43–45 The tremendous success of MN-based technologies attracted us towards other research areas, including sensors and agriculture. The minimally invasive technology of MNs provides new insight into the delivery of drugs/biomolecules within the human body without damaging tissues, significantly attracting agricultural researchers.39,46,47 With the help of MNs, we can easily monitor plant health without damaging the plant tissue. Recently, MNs have been used in the agriculture industry to treat diseases, monitor health, and detect agrochemicals/pathogens within the plant, as traditional tools are invasive, relatively less effective, and unable to monitor plant health continuously. Moreover, researchers have focused on the efficient, accurate, and fast response of MNs in agricultural systems by modifying the materials, shape, size, and space between two MNs and their types.47,48 Interestingly, we can also develop an Ms-based micronutrient delivery system with the release behavior easily controlled by changing the polymeric matrix. In general, we can say that the fabrication process and selection of materials, and their architecture significantly affect the treatment and detection of plant diseases and their continuous health monitoring. We will further discuss the types of MNs and their importance in agriculture.2.1. Types of MNs
Fabrication of the MNs varies according to the types of MNs and their preferred applications. With the help of different fabrication processes, we can easily develop hollow MNs, honeycomb-like structures, angled MNs, and rapidly separating MNs. It is important to mention that the height of the MNs, aspect ratio, substrate diameter, and tensile strength should be considered while designing and manufacturing the MNs. Moreover, high-throughput manufacturing using various resources such as silicon, stainless steel, sugar, and polymers requires a high degree of uniformity, thereby effectively delivering drugs and biomolecules.49–51 Based on their structure and technique, MNs are divided into four groups: (i) solid MNs (S-MNs), which are made of silicone, glass, stainless steel, and aluminum are often utilized for transdermal testing. Usually, S-MNs create small pores in the epidermis and dermis to effectively deliver drugs, and biomolecules without damaging plant tissues.52,53 (ii) Coated MNs (C-MNs), where the exterior of the C-MNs is layered with a water-soluble medium so that the drugs/biomolecules mix quickly in the membrane after MN insertion. The covering process should make a thin covering on the exterior of the MNs and sustain adhesion when stored and placed on the membrane. To accomplish this, the covering must have appropriate viscosity. The quantity of drugs or biomolecules that can be loaded based on the film's width and the spike's extent is generally very small.54,55 (iii) Dissolvable MNs (D-MNs), which are made from biodegradable polymers (such as carbohydrates, polyvinyl alcohol (PVA), gelatin, chitosan, and hyaluronic acid (HA)) to encapsulate the drugs or biomolecules to effectively release the drugs/biomolecules,56,57 and (iv) hollow MNs (H-MNs), which have a hollow/hollow core/chamber design where liquid drug is injected/stored to be delivered. Interestingly, H-MNs can potentially deliver large amounts of drugs/biomolecules.39,40,58Fig. 2 shows the schematic illustration of the different types of MNs and their application in agriculture. | ||
| Fig. 2 Types of MNs and their application in agriculture: (a) solid, (b) hollow, (c) dissolving, and (d) coated-MNs. The image was reproduced with permission40 from Elsevier, copyright@2023. | ||
Unquestionably, there are enough reasons to choose MN-based technology in agricultural sciences. With the help of technological advancements in fabrication technology, material chemistry, and polymeric sciences it is possible to develop biocompatible, mechanically stable, and biodegradable MNs with numerous designs, which can monitor plant health and pathogens/agrochemicals.
3. There is plenty of possibility for MNs in agriculture
Incontrovertibly, the demands for food are endlessly increasing due to the growing population and loss of crops globally. The situation becomes more complicated by using grains to produce energy/biofuels in developing countries, which provides an extra-burden to producing crops.4,5,27 However, researchers have made substantial efforts towards the production of crops and protection against pathogens. The effective delivery system of agrochemicals, micronutrients and biomolecules, and continuous monitoring of the health of crops are the decisive factors for augmenting plant growth and crop protection. With the advancement of such innovative technologies, we can easily achieve high productivity of the crops and high nutritional values with insignificant wastage. Usually, two types of delivery systems are in practice: (1) foliar spray and (2) soil application. Delivery through the soil is most commonly used in agricultural practice, as foliar spray has significantly increased water and soil contamination. Despite the tremendous application of CB-NMs, especially CNTs, CNFs, and GO, for delivering micronutrients, biomolecules, and protection against pathogenic infection in agricultural crops due to their ability to translocate within the plants, continuous monitoring of the crop's health remains a concern.27,59–61 Therefore, there is a need for such devices that can connect with the plant without damaging their tissues. In this aspect, MNs can potentially transform the agriculture and food sectors.MNs are extensively used in medicine for the delivery of drug molecules. Unquestionably, we have enough evidence that suggests that MN patches effectively deliver the drugs, vaccine, DNA, and proteins to humans and animals without any adverse effects. Numerous studies suggested that inserting and removing MN patches does not affect/damage plant tissue because of their shape and size. Indeed, we can say that the remarkable discovery of MNs is for the painless delivery of chemical/biological molecules to humans. Researchers are attracted to the MNs for agricultural applications due to their enormous success in medicine. MNs offer plenty of possibilities for agriculture sectors, like micronutrients, agrochemicals, and biomolecule delivery, including genes within the plants. Moreover, MN-based sensing technology easily detects and discriminates the analytes based on the sensing mechanism. Usually, the detection process involves the generation/changes in the signal upon binding with analytes, and subsequent increase/decrease in signal intensity with the incorporation of analytes indicates the accuracy of the detection. Likewise, discrimination between various analytes is achieved through the specificity of the targeted analytes. It is important to mention here that MN-based sensing technology has the potential ability to accurately detect and discriminate analytes, thereby effectively used in real-time monitoring in agriculture.39,40,62,63 Additionally, we can make tiny patches of the MNs and attach them to the plants for continuous monitoring of crops. Although, we need to optimize the size and shape of the MNs according to the plants. Indeed, plant-specific MN-based devices create new wings for agriculture to improve plant health and make next-generation tools for diagnosing plant diseases and their development. MN-based technology can significantly transform the agriculture and food sectors by managing crop health and delivering micronutrients/biomolecules to the plant. Therefore, we can say that plenty of room is available for MNs in the agriculture sector.
4. MN-based sensors in crops
MNs are effectively used in food and agriculture areas due to the variety of materials used in their fabrication. Approximately 600 million people are affected by micro-organisms and foodborne diseases every year. In this aspect, there is a need to develop newer tools/techniques that effectively monitor plant diseases, agrifood issues, and plant growth. MNs can penetrate plants, foods, and soil, thereby easily reaching the food matrix and plant tissues. MNs can penetrate plants, soil, and food and reach the food matrix, packaging, or plant parts.64,65 This provides more accurate and precise information about these elements, enhancing quality control and process management.4.1. Crop monitoring
Monitoring crops is one of the important factors that helps improve crop production and protect against pathogens, thereby significantly increasing the research on sensing technologies, especially non-destructive sensors.17,66,67 Usually, the effective management of plant health depends on newer diagnostic assays that should be rapid, sensitive, accurate, and continuously monitored without damaging the plant tissue. However, traditional methods are used to observe plant health that destroys the plant tissues.4,5,16,27 Researchers developed a thermal probe to measure the movement of xylem juice in tomatoes. Daskalakis et al. developed a thermal sensing device using corn as a model. The MN-based sensing device measures the differential temperature between leaf and air related to the plant-water stress. Moreover, it can be measured for any plant, soil type, and relative humidity. This device is solar-powered and transmits data wirelessly via an antenna. The prepared backscatter wireless sensor network is a low-cost sensor to monitor agricultural environmental parameters.68 For more sensor accuracy, researchers think that if the probe is instead within the plants without damaging the tissue, that might revolutionize the sensing technologies in agriculture applications. In this aspect, advancement in sensing technologies is necessary despite the traditional methods used. MNs are a remarkable discovery and are continuously attracting attention in drug delivery systems and biomedical devices due to the pain-less delivery system without damaging the tissue. Moreover, MNs open new windows for diagnosing diseases in humans and plants. The advancement in sensing technologies based on MNs effectively analyzes and monitors plant health without damaging plant tissue. For instance, Jeon et al. fabricated an MN-based device with an impedance system to measure electrical conductivity within the stem of tomato plants. The data indicated that the MN-based real-time monitoring system measures the tomato plant's salinity. Moreover, understanding the response of nutrients is difficult until the growth of fruits. MN-based devices have been tested in both greenhouses and fields. The signal noise and electrical conductivity decrease in sensor measurements. The authors believed that the reduction problem could be solved by redesigning the electrical equipment electricity to be suitable for field use.69 Bukhamsin et al. fabricated MNs using a polydimethylsiloxane (PDMS) mold and developed an MN-based sensor to measure barley leaf epidermis bioimpedance. MNs were fabricated using PDMS as a substrate material and coated with metal (∼500 μm) to enhance mechanical strength. The prepared metal-coated MNs effectively measure bioimpedance in a controlled environment.70 Wang et al. fabricated an Au–SnO2-VG-MN-based sensor for the detection of abscisic acid (ABA) in plants. The data indicate that the ABA is detected by electrocatalytic oxidation due to the synergetic effects of Au–SnO2 and the exceptional conductivity of vertical graphene. Moreover, the prepared Au–SnO2-VG-MN-based sensor has high sensitivity, lower detection limit, and longer-term stability. Additionally, it is important to mention that MNs less than 700 μm are appropriate for insertion within the plant tissue.62Fig. 3 shows the graphical representation of the fabrication of an Au–SnO2-VG-MN-based sensor to detect ABA. Another research group fabricated a three-dimensional (3D) printed-MN (3D-P-MN)-based electrochemical sensor for real-time monitoring of phytohormone (salicylic acid (SA)) and the pH of the cabbage plant. The data indicate that the prepared 3D-P-MNs efficiently measure the SA and pH to understand water stress within the plant in real time. Interestingly, 3D-P-MN-based electrochemical sensors have a low detection limit of ∼37 μM. Additionally, the 3D-P-MN-based sensor precisely measures the live variation of SA through the stem of the plants, thereby easily understanding the unstressed and water-stressed plants.71 Baek et al. fabricated an MN-based Sap flow sensor to understand plant physiology via plant leaves. The data indicate that the MN-based Sap flow sensor effectively observed plant reactions under different environmental conditions, mainly humidity, sunlight, and soil–water content in tomato and bell pepper plants. With the help of this sensor, the authors believe that we can easily improve the productivity of the crops.72 Acanda et al. used Ti-MNs for GFP expression in citrus plants using agrobacterium infiltration methods. The data indicate that the MN-roller offers a simple and effective agroinfiltration process for gene expression in citrus plants.73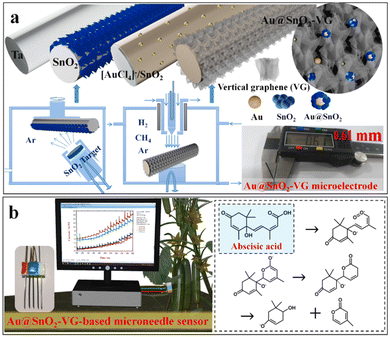 | ||
| Fig. 3 A schematic illustration of the (a) fabrication of Au–SnO2-VG-MN, and (b) detection mechanism of ABA. The image was reproduced with permission62 from Elsevier, copyright@2021. | ||
O'Flynn et al. fabricated Au–Cu-coated-MNs for the analysis of nitrate in soil. The data indicate that the Au–Cu-coated-MNs effectively evaluated nitrate concentration in soil that can analyze real-time growing conditions in agriculture.74 Cai et al. used SS-MNs to determine the vindoline and catharanthine in the Catharanthus roseus plant.75 Hegarthy et al. fabricated carbon-loaded polystyrene MNs (C–P-MNs) to monitor tomato leaves' pH continuously. The data indicate that the prepared C–P-MNs effectively detect pH from tomato skin.76 The aforementioned data and Table 1 suggest that the MN-based sensing device effectively monitors crop health. It is important to mention here that the diameter of the MN array should be less than 700 μm, easily inserted within the plant tissue without any damage. Furthermore, bioimpedance, pH, stress conditions, soil quality, and biomolecules are easily detected using MN-based sensing devices. With the help of MN-based sensing technologies, we can easily investigate continuous soil and plant monitoring that might improve their productivity.
| S. no. | MNs | Plants | Analytes | Remarks | Ref. |
|---|---|---|---|---|---|
| 1. | Au–SnO2-VG-MNs-based sensor | Cucumber | ABA | Effectively detect ABA within the plant, but little damage was observed during plug and unplug of the sensor. | 62 |
| 2. | MNs integrated with impedance system | Tomato stem | Salinity of the nutrients | Understanding the nutrients in plants is difficult until the growth of fruits. | 69 |
| 3. | Metal-coated-PDMS based MNs | Barley leaf | Bioimpedance | Effectively measure bioimpedance of barley leaves at controlled conditions. | 70 |
| 4. | 3D-P-MNs | Cucumber stem | SA and pH | Effectively measure the live variation of SA. | 71 |
| 5. | MNs-Sap flow sensor | Tomato and bell peppers leave | Sap flow through xylem | Effectively measure sap flow through to under-plant physiology | 72 |
| 6. | Ti-MNs | Citrus leave | GFP expression | Effectively express the gene in citrus plants. | 73 |
| 7. | Au–Cu-coated-MNs | Soil | Nitrate concentration | Effectively analyze the nitrate concentration in soil | 74 |
| 8. | SS-MNs | Catharanthus roseus (leaf, stem, and root) | Catharanthine and vindoline | Effectively detect anhydrovinblastine within the plant. | 75 |
| 9. | C–P-MNs | Tomato leaf | pH | Quinone group and carbon change the pH | 76 |
| 10. | PVA-MNs | Tomato leave | Pathogenic DNA extraction | 100% detection of late blight disease | 77 |
| 11. | MNs integrated device | Tomato leave | Nucleic acid | Virus and fungus infection | 63 |
4.2. Plant pathogen diagnostics
Food security is one global concern affecting the agricultural economy and sustainable goals. Food demand significantly increased with the growing population and simultaneously decreased agricultural land. Additionally, plant diseases significantly affect the productivity of crops and are one of the common reasons for the failure of crops, leading to extra pressure to improve agricultural crops. Numerous diagnostic technologies such as PCR, ELISA, FISH, IF, and FCM have been used to detect plant diseases. However, these assays require sophisticated instruments, skilled persons, and destructive processes.78 In this aspect, MN-based diagnostic devices have the potential ability to develop non-destructive sensors that can effectively be used to monitor plant pathogens continuously. Few studies have been done so far that indicate the effective use of MN-based sensors for detecting plant pathogens. For instance, Paul et al. fabricated PVA-based MNs using a vacuum-based molding process for the extraction of pathogenic DNA by applying tomato leaves. The data indicate that the PVA-MNs effectively extract the DNA within a min from different plant species. The DNA extraction ability of PVA-MNs helps to detect plant pathogens of late blight disease with a 100% detection rate. Therefore, the prepared PVA-MNs are simple and free from cellular lysis, and DNA purification might be revolutionized for molecular diagnosis in plants.77Fig. 4 shows the schematic illustration of the PVA-MNs for extracting pathogenic DNA. Paul et al. fabricated polymeric MNs integrated with a 3D imaging device for loop-mediated isothermal amplification (LAMP) reaction and plant disease diagnosis. The data indicate that the MN patch can effectively extract nucleic acids from plant leaves using compression and extraction methods within 1 min. The isolated nucleic acid was simultaneously amplified by LAMP using pre-loaded reagents. Next, a smartphone device reader can easily capture fluorescent images of the LAMP cassette, thereby easily detecting DNA/RNA with 1 pg sensitivity. Therefore, the prepared MN-based smartphone device has wider applicability in addition to plant health.63 Another study of a similar group focused on the fabrication of polymeric-MN patches for the extraction of nucleic acids from various tissues of plants. The data indicate that the MNs easily extract both DNA and RNA from plant leaves.79 The aforementioned study and Table 1 suggested that the MN-based sensor effectively detects plant pathogens. The MN-based in-vivo agricultural biosensor might offer on-site, non-destructive information on plant health to improve agricultural cultivation and prevent disease. MN integrated technology will support the rapid development of precision agriculture, improve food quality, and reduce crop losses. With integration of MN patches with molecular detection systems using amplification probes (biosensors), fast, cost-effective, simple, and delivery samples can be exposed, and controlled for plant diseases. The real-time detection of plant pathogens might be advantageous, allowing plants to be easily treated at the initial stage of infection, thereby improving the productivity of crops. | ||
| Fig. 4 A schematic representation of the PVA-MNs for extracting pathogenic DNA. (a) Schematic of conventional and PVA-MN-based DNA extraction, (b) SEM images of PVA-MNs, (c) photographic image of PVA-MNs patch, and (d) and (e) UV-viz spectra of DNA. The image was reproduced with permission77 from ACS, copyright@2019. | ||
In general, we can say that MN-based sensing technologies provide newer alternatives to non-destructive extraction of DNA/RNA, which are newer hallmarks of diagnosing diseases using MNs.
5. Application of MN-based biosensors in food
Food supply relentlessly faces the risk of emerging plant diseases caused by micro-organisms like fungi, viruses, and bacteria. Farmers continuously use agrochemicals and pesticides to protect their crops and improve the food's productivity, thereby significantly increasing the crops' average yield and reducing production costs.4,5,27 These agrichemicals and pesticides are commonly found in foods that can cause various diseases, like cancer, hormonal imbalance, allergy, asthma, etc. Monitoring the food during supply is essential to confirming food safety and controlling crop loss. Considering this, MN-based sensing devices have gained significantly increasing interest in monitoring plant and fruit health, which can allow continuous on-site monitoring of fruits and vegetables.5.1. Pesticide detection
Pesticides are commonly found in fruits and vegetables and are becoming a serious concern globally, as some pesticide residue is complicated to remove and can cause various diseases, including cancer. Therefore, there is a requirement to develop newer sensing technologies to monitor pesticides in foods. Although traditional processes such as gas chromatography (GC) and high-performance liquid chromatography (HPLC) have been used to detect pesticides, sophisticated instruments, a skilled person and a time-consuming process are required, and it is difficult to achieve on-site detection.80–82 In this aspect, MN-based sensing technologies have the potential ability to monitor pesticides from fruits and vegetables in real-time. A few studies have been done so far that suggest the MN-based innovative technology can be used to detect pesticides from foods. For instance, Mugo et al. fabricated a molecularly imprinted polymeric (MIP) MIP-MN-based sensor to detect imidacloprid pesticides from food samples. The MN-sensor was fabricated using layer-by-layer assembly of CNTs, cellulose nanocrystal (CNC), and imprinted polyaniline layer with co-polymerized imidazole-CNC based film. The data indicate that the IMP-MN-based sensor has excellent detection ability with a limit of detection of 0.06 μM and reusable ability.83 Chen et al. coated Au-nanoparticles onto the surface of adhesive tape similar to types of MN structures for detecting parathion-methyl, thiram, and chlorpyrifos pesticides from apple, orange, and cucumber. The data indicated that the surface-enhanced Raman spectroscopy (SERS) based sensor easily observed the SERS signal to detect pesticides from fruits and vegetables.84 Mishra et al. fabricated an MN-based electrochemical sensor to detect organophosphate pesticides. The data suggested that the fabricated MN-based sensor efficiently detects pesticides and might be used as a wearable sensor for continuous monitoring of toxicity.85 Yi et al. fabricated novel Ag-PVA-HA-based MNs (PVA-HA-MNs) using a PDMS mold to detect thiram and thiabendazole pesticides. Fig. 5 shows the graphical illustration of the pesticide residue in agricultural products using MNs. The data indicates that the prepared PVA-HA-MNs effectively detect thiram and thiabendazole inside and on the outer surfaces of the agriculture product with the detection limit of 10−7 and 10−8, respectively. The unique stepped structure of the MNs significantly increased the superficial area of the MNs, thereby providing more surface for Ag distribution and pesticide collection and subsequently high sensitivity of the sensor.86 The aforementioned data and Table 2 suggested that MN-based sensing technologies have emerged as newer technological solutions for pesticide detection in agriculture, especially real-time monitoring. Moreover, designing MNs significantly affects the sensitivity, selectivity, and stability towards pesticide detection due to increased active sites for sensing and collecting pesticide residue. In general, we can say that MN-based technology significantly influences the productivity of crops through real-time monitoring of toxic elements. | ||
| Fig. 5 A schematic representation of the pesticide residue in agricultural products using MNs. The image was reproduced with permission86 from ACS, copyright@2023. | ||
| S. no. | MNs-based sensor | Food/plant | Analytes | Remarks | Ref. |
|---|---|---|---|---|---|
| 1. | IMP-MNs | Honey | Imidacloprid | Effectively detect pesticides from food samples | 83 |
| 2. | PVA-HA-MNs | Leave | Thiram & thiabendazole | MN-based sensor detects thiram and thiabendazole with detection limits of 10−7 and 10−8 M, respectively. | 86 |
| 3. | Adhesive tape coated with Au-NPs | Apple, orange, cucumber | Parathion-methyl, thiram & chlorpyrifos | The paste and peel-off approach-based sensor effectively detects pesticides. | 84 |
| 4. | MNs | — | Organophosphates | The prepared sensor effectively detects pesticides as a wearable sensor. | 85 |
| 5. | MSS-MNs | Orange | GA and CA | MSS-MNs might be potential sensing devices to measure the total antioxidant capacity in fruits. | 87 |
| 6. | MSS-MNs-RMS | Fish spoilage, mushroom, and brew coffee samples. | ABA, H2O2, and putrescine | Effectively detects redox species and is easy to apply in real-time monitoring. | 88 |
| 7. | SF-MNs | Food supply | Pathogens | Effectively detects pathogens and spoilage by increasing the pH condition. | 89 |
| 8. | SERS-MNs | Fruits and vegetables | pH and redox species | Effectively detects pH and redox species with high sensitivity. | 90 |
5.2. Redox status of fruits and vegetables
The redox status of fruits and vegetables is an indicator of oxidative/reductive molecules like redox couples, free radicals, enzymes, and the thiols group of proteins. The balance of redox species includes reactive oxygen species (ROS) that maintain physiological function within the plants, whereas the imbalance of redox species leads to oxidative stress and cell death. Therefore, maintaining the balance of redox species is required to retain healthy plants. Unquestionably, fruits and vegetables are the main components in daily diets with numerous antioxidant molecules such as flavonoids, phenols, and vitamin C. Different fruits/vegetables have different antioxidant molecules, and the same fruits/vegetables also have different amounts of antioxidant molecules at different stages of growth/storage. However, antioxidant molecules are the main constraints for the estimation of fruits/vegetable's nutritional values and freshness.91–93 In this aspect, it is necessary to detect redox species to understand the nutritional values and freshness of the fruits/vegetables. A few studies have reported that MN-based sensors can be effectively used to detect redox species from fruits/vegetables. For instance, Dhanjai and Lu fabricated layer-by-layer assembly with CNT-cellulose nanocrystals and polyaniline conductive polymer modified stainless steel (MSS) MSS-MN-based electrodes for real-time monitoring of plant polyphenols such as chlorogenic acid (CA) and gallic acid (GA). The prepared MSS-MNs effectively detect GA and CA in orange. The data suggested that the MSS-MNs might be a potential sensing device to measure the total antioxidant capacity in fruits.87 In another study of the same research group, MSS-MNs integrated with a redox mediator system (MSS-MNs-RMS) were fabricated for detecting fish spoilage, antioxidant molecules in mushrooms, and brew coffee samples. The data indicated that the MSS-MNs-RMS effectively detect redox species, including ABA, H2O2, and putrescine, with the detection limit of ∼50, 80, and 263 ng mL−1, respectively. Interestingly, it was portable, rapid, sensitive, and easy to apply in real-time monitoring.88 Kim et al. fabricated bioink-incorporated silk fibroin-based porous MNs (BI-SF-MNs) to detect pathogens in the food supply. Fig. 6 shows the graphical representation of the fabrication of BI-SF-MNs and their application. The data indicate that the BI-SF-MNs effectively detect the pathogen in the food supply by changing the pH. Interestingly, the color of the bioink changes from blue to red, clearly indicating the unhealthy food.89 | ||
| Fig. 6 A schematic illustration of the fabrication of BI-SF-MNs and their application. The image was reproduced with permission89 from Wiley, copyright@2021. | ||
Li et al. fabricated MNs onto an Au-nano shell substrate to develop a SERS-MN-based sensor to detect the pH and redox status of the food sample. The data indicated that the SERS-MN-based sensor effectively detects the pH and redox status of the fruits and vegetables.90 The aforementioned studies and Table 1 suggested that the MN-based sensor effectively detects the redox status of the fruits and vegetables with high sensitivity and stability. Moreover, MN-based sensors prevent the extraction process and contact the atmospheric air. Furthermore, MN-based sensors can penetrate anywhere in fruits/vegetables (solid/liquid tissues) to detect pH and redox species. The interferent effect in the MN-based sensor might be minimized by employing several strategies including surface functional groups, selective coatings, and incorporating enzymes to ensure accurate detection of analytes. We can say that the MN-based sensing technology might offer a versatile platform to detect numerous indicators, including nutritional value, freshness, and redox species within the fruits/vegetables, without damaging the tissues.
6. Future perspectives
In recent years, the use of MNs and the interpretation of knowledge from human medicine have entered farming and flora wellbeing. To meet the challenges of the farming industry, innovation in planting and precision agriculture is necessary to increase crop production, rehabilitate plants against stress and disease, and improve crop stability. Recently, various MN-based biosensing platforms have been developed. Some ideas need to be pursued for simpler and cheaper microfabrication techniques to create MNs using simpler materials. Advances in ultra-rapid prototyping procedures will permit the production of MNs. In addition, due to the combination of liquids in plants, the steadiness of MN-based detectors after interaction with liquid should be evaluated. Thus, additional materials development for the synthesis of MNs is required in the upcoming period. This article presents the newest advances in MN technology for illness recognition and flora monitoring. The MN-based technology can potentially carry knowledge to agriculturalists and benefit them. This innovation allows many traditional chemical tests to be done more efficiently and quickly on crops, analyzing whole crops against pathogenic diseases that help in the prevention strategies. The upcoming era of smart farming biosensing depends on detectors' sensitivity, consistency, and specificity, with efficiency, low cost, and small components.7. Conclusion
MNs can be divided into solid, coating, separation, and hydrogel preparation. MNs are fabricated using silicon, metal, polymers, glass, and ceramics. The MN-based sensing device constantly monitors plant well-being and diagnoses of plant diseases. Although numerous MN-based sensing devices have been reported for human health, diagnosis, and treatment, using this knowledge in farming has only just begun. The in vivo and in vitro MN-based non-destructive sensing devices provide on-site information on plant health, thereby significantly improving crop productivity due to early treatment strategies to prevent disease. Moreover, MN-based innovative solutions will support agriculture in many ways: (1) rapid development of precision agriculture, (2) enhancement of the food quality by maintaining nutritional values, and (3) reduction of the loss of crops due to the early treatment strategy to protect against pathogens and control the nutrients within the plants. Therefore, real-time monitoring of the crops significantly aided the advantage of monitoring plants at the initial stage of infection, thereby increasing the productivity of crops. It is important to mention that the non-enzymatic sensors have several challenges like selectivity and specificity. It seems difficult to detect specific analytes accurately with non-enzymatic sensors due to the presence of interfering substances. We need to take care of this limitation of non-enzymatic sensors to design MN-based sensing technology in agriculture, especially in real-time monitoring of plant health. This perspective explores the latest developments in MN-based sensing technology in food and health of crops, including disease screening and diagnosis. Therefore, MN-based sensing technology might accelerate plant surveillance and disease detection progress.Author contributions
SK wrote the original draft of the manuscript. NT, DC, and MA conceptualized and revised the manuscript. All authors have read and agreed to the published version of the manuscript.Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.Conflicts of interest
The authors announce no competing financial interests.Acknowledgements
No funding was provided for the manuscript.References
- A. Raza, A. Razzaq, S. S. Mehmood, X. Zou, X. Zhang, Y. Lv and J. Xu, Plants, 2019, 8, 1–29 CrossRef.
- I. Egea, Y. Estrada, C. Faura, J. M. Egea-Fernández, M. C. Bolarin and F. B. Flores, Front. Plant Sci., 2023, 14, 1092885 CrossRef.
- M. G. Muluneh, Agric. Food Secur., 2021, 10, 36 CrossRef.
- S. C. Teixeira, N. O. Gomes, M. L. Calegaro, S. A. S. Machado, T. V. de Oliveira, N. de Fátima Ferreira Soares and P. A. Raymundo-Pereira, Biomaterials Advances, 2023, 155, 213676 CrossRef CAS.
- R. T. Paschoalin, N. O. Gomes, G. F. Almeida, S. Bilatto, C. S. Farinas, S. A. S. Machado, L. H. C. Mattoso, O. N. Oliveira and P. A. Raymundo-Pereira, Biosens. Bioelectron., 2022, 199, 113875 CrossRef CAS.
- M. Venbrux, S. Crauwels and H. Rediers, Front. Plant Sci., 2023, 14, 1120968 CrossRef.
- D. M. Rizzo, M. Lichtveld, J. A. K. Mazet, E. Togami and S. A. Miller, One Health Outlook, 2021, 3, 6 CrossRef.
- M. Tudi, H. Daniel Ruan, L. Wang, J. Lyu, R. Sadler, D. Connell, C. Chu and D. T. Phung, Int. J. Environ. Res. Public Health, 2021, 18, 1112 CrossRef CAS.
- B. K. Singh, M. Delgado-Baquerizo, E. Egidi, E. Guirado, J. E. Leach, H. Liu and P. Trivedi, Nat. Rev. Microbiol., 2023, 21, 640–656 CrossRef CAS PubMed.
- I. Buja, E. Sabella, A. G. Monteduro, M. S. Chiriacò, L. De Bellis, A. Luvisi and G. Maruccio, Sensors, 2021, 21, 2129 CrossRef CAS.
- M. J. Sue, S. K. Yeap, A. R. Omar and S. W. Tan, BioMed Res. Int., 2014, 2014, 653014 CrossRef PubMed.
- S. Liu, K. Zhao, M. Huang, M. Zeng, Y. Deng, S. Li, H. Chen, W. Li and Z. Chen, Front. Bioeng. Biotechnol., 2022, 10, 1056804 CrossRef.
- N. O. Gomes and P. A. Raymundo-Pereira, Small, 2023, 19, 2206753 CrossRef CAS PubMed.
- N. O. Gomes, S. C. Teixeira, M. L. Calegaro, S. A. S. Machado, N. de Fátima Ferreira Soares, T. V. de Oliveira and P. A. Raymundo-Pereira, Chem. Eng. J., 2023, 472, 144775 CrossRef CAS.
- S. C. Teixeira, N. O. Gomes, T. V. d Oliveira, P. Fortes-Da-Silva, N. d F. F. Soares and P. A. Raymundo-Pereira, Biosens. Bioelectron. X, 2023, 14, 100371 CAS.
- J. M. Roper, J. F. Garcia and H. Tsutsui, ACS Omega, 2021, 6, 5101–5107 CrossRef CAS PubMed.
- M. C. Ang and T. T. S. Lew, Front. Plant Sci., 2022, 13, 884454 CrossRef.
- K. Dyussembayev, P. Sambasivam, I. Bar, J. C. Brownlie, M. J. A. Shiddiky and R. Ford, Front. Chem., 2021, 9, 636245 CrossRef.
- D. Trippa, R. Scalenghe, M. F. Basso, S. Panno, S. Davino, C. Morone, A. Giovino, S. Oufensou, N. Luchi, S. Yousefi and F. Martinelli, Pest Manage. Sci., 2024, 80, 245–261 CrossRef CAS PubMed.
- M. Ashfaq, N. Verma and S. Khan, Mater. Chem. Phys., 2018, 217, 216–227 CrossRef CAS.
- R. A. Omar, S. Afreen, N. Talreja, D. Chauhan and M. Ashfaq, in Plant Nanobionics: Volume 1, Advances in the Understanding of Nanomaterials Research and Applications, ed. R. Prasad, Springer International Publishing, Cham, 2019, pp. 117–140 DOI:10.1007/978-3-030-12496-0_6.
- D. Chauhan, M. Ashfaq, R. V. Mangalaraja and N. Talreja, in Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications, ed. J. M. Al-Khayri, L. M. Alnaddaf and S. M. Jain, Springer International Publishing, Cham, 2023, pp. 299–316 DOI:10.1007/978-3-031-20878-2_11.
- M. A. Tahir, S. Hameed, A. Munawar, I. Amin, S. Mansoor, W. S. Khan and S. Z. Bajwa, J. Virol. Methods, 2017, 249, 130–136 CrossRef CAS.
- P. Zuo, X. Li, D. C. Dominguez and B. C. Ye, Lab Chip, 2013, 13, 3921–3928 RSC.
- F. Mahmood, S. S. Zehra, M. Hasan, A. Zafar, T. Tariq, H. U. Javed, X. Shu, H. Xue and M. Hatami, Plant Physiol. Biochem., 2023, 204, 108081 CrossRef CAS.
- Q. Zhan, A. Ahmad, H. Arshad, B. Yang, S. K. Chaudhari, S. Batool, M. Hasan, G. Feng, G. Mustafa and M. Hatami, Plant Physiol. Biochem., 2024, 211, 108719 CrossRef CAS.
- P. A. Raymundo-Pereira, N. O. Gomes, F. M. Shimizu, S. A. S. Machado and O. N. Oliveira, Chem. Eng. J., 2021, 408, 127279 CrossRef CAS.
- L. Tessaro, A. Aquino, P. Panzenhagen, A. C. Ochioni, Y. S. Mutz, P. A. Raymundo-Pereira, I. R. Vieira, N. K. Belem and C. A. Conte-Junior, Biosensors, 2022, 12, 1101 CrossRef CAS.
- A. Aquino, V. M. F. Paschoalin, L. L. G. Tessaro, P. A. Raymundo-Pereira and C. A. Conte-Junior, J. Pharm. Biomed. Anal., 2022, 211, 114608 CrossRef CAS PubMed.
- X. Jin, D. D. Zhu, B. Z. Chen, M. Ashfaq and X. D. Guo, Adv. Drug Delivery Rev., 2018, 127, 119–137 CrossRef CAS.
- V. Alimardani, S. S. Abolmaali, A. M. Tamaddon and M. Ashfaq, Drug Delivery Transl. Res., 2021, 11, 788–816 CrossRef.
- B. Z. Chen, M. Ashfaq, D. D. Zhu, X. P. Zhang and X. D. Guo, Macromol. Rapid Commun., 2018, 39, 1800075 CrossRef.
- J. N. Zhang, B. Z. Chen, M. Ashfaq, X. P. Zhang and X. D. Guo, J. Ind. Eng. Chem., 2018, 65, 363–369 CrossRef CAS.
- M. C. He, B. Z. Chen, M. Ashfaq and X. D. Guo, Drug Delivery Transl. Res., 2018, 8, 1034–1042 CrossRef CAS PubMed.
- B. Z. Chen, Y. Yang, B. B. Wang, M. Ashfaq and X. D. Guo, Int. J. Pharm., 2019, 556, 338–348 CrossRef CAS.
- P. Dardano, I. Rea and L. De Stefano, Curr. Opi. Electrochem., 2019, 17, 121–127 CrossRef CAS.
- J. J. García-Guzmán, C. Pérez-Ràfols, M. Cuartero and G. A. Crespo, TrAC, Trends Anal. Chem., 2021, 135, 116148 CrossRef.
- Y. Wu, F. Tehrani, H. Teymourian, J. Mack, A. Shaver, M. Reynoso, J. Kavner, N. Huang, A. Furmidge, A. Duvvuri, Y. Nie, L. M. Laffel, F. J. Doyle, III, M.-E. Patti, E. Dassau, J. Wang and N. Arroyo-Currás, Anal. Chem., 2022, 94, 8335–8345 CrossRef CAS.
- Z. Faraji Rad, Adv. Eng. Mater., 2023, 25, 2201194 CrossRef CAS.
- E. Ece, I. Eş and F. Inci, Mater. Today, 2023, 68, 275–297 CrossRef CAS.
- A. Bukhamsin, K. Moussi, R. Tao, G. Lubineau, I. Blilou, K. N. Salama and J. Kosel, Adv. Sci., 2021, 8, e2101261 CrossRef.
- Z. Li, T. Yu, R. Paul, J. Fan, Y. Yang and Q. Wei, Nanoscale Adv., 2020, 2, 3083–3094 RSC.
- R. F. Donnelly, T. R. Raj Singh and A. D. Woolfson, Drug Delivery, 2010, 17, 187–207 CrossRef CAS PubMed.
- T. M. Tuan-Mahmood, M. T. McCrudden, B. M. Torrisi, E. McAlister, M. J. Garland, T. R. Singh and R. F. Donnelly, Eur. J. Pharm. Sci., 2013, 50, 623–637 CrossRef CAS.
- D. Ramadon, M. T. C. McCrudden, A. J. Courtenay and R. F. Donnelly, Drug Delivery Transl. Res., 2022, 12, 758–791 CrossRef.
- J. Yang, J. Yang, X. Gong, Y. Zheng, S. Yi, Y. Cheng, Y. Li, B. Liu, X. Xie, C. Yi and L. Jiang, Adv. Healthcare Mater., 2022, 11, 2102547 CrossRef CAS PubMed.
- Y. Cao, S. S. Koh, Y. Han, J. J. Tan, D. Kim, N. H. Chua, D. Urano and B. Marelli, Adv. Mater., 2023, 35, e2205794 CrossRef PubMed.
- A. Kundu, M. G. Nogueira Campos, S. Santra and S. Rajaraman, Sci. Rep., 2019, 9, 14008 CrossRef.
- S. Dugam, R. Tade, R. Dhole and S. Nangare, Future J. Pharm. Sci., 2021, 7, 19 CrossRef.
- Ö. Erdem, I. Eş, G. A. Akceoglu, Y. Saylan and F. Inci, Biosensors, 2021, 11, 296 CrossRef PubMed.
- I. Eş, A. Kafadenk, M. B. Gormus and F. Inci, Small, 2023, 19, 2206510 CrossRef.
- N. Tariq, M. W. Ashraf and S. Tayyaba, J. Pharm. Innov., 2022, 17, 1464–1483 CrossRef.
- T. Waghule, G. Singhvi, S. K. Dubey, M. M. Pandey, G. Gupta, M. Singh and K. Dua, Biomed. Pharmacother., 2019, 109, 1249–1258 CrossRef CAS.
- R. S. J. Ingrole and H. S. Gill, J. Pharmacol. Exp. Ther., 2019, 370, 555–569 CrossRef CAS.
- H. S. Gill and M. R. Prausnitz, J. Controlled Release, 2007, 117, 227–237 CrossRef CAS PubMed.
- H. Du, P. Liu, J. Zhu, J. Lan, Y. Li, L. Zhang, J. Zhu and J. Tao, ACS Appl. Mater. Interfaces, 2019, 11, 43588–43598 CrossRef CAS.
- N. G. Oh, S. Y. Hwang and Y. H. Na, ACS Omega, 2022, 7, 25179–25185 CrossRef CAS PubMed.
- L. Van Hileghem, S. Kushwaha, A. Piovesan, P. Verboven, B. Nicolaï, D. Reynaerts, F. Dal Dosso and J. Lammertyn, Micromachines, 2023, 14, 615 CrossRef PubMed.
- M. Ashfaq, N. Verma and S. Khan, Environ. Sci.: Nano, 2017, 4, 138–148 RSC.
- R. Kumar, M. Ashfaq and N. Verma, J. Mater. Sci., 2018, 53, 7150–7164 CrossRef CAS.
- S. Afreen, R. A. Omar, N. Talreja, D. Chauhan, R. V. Mangalaraja and M. Ashfaq, in Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems, ed. K. A. Abd-Elsalam, Elsevier, 2022, pp. 367–391 DOI:10.1016/B978-0-12-823833-2.00004-0.
- Z. Wang, L. Xue, M. Li, C. Li, P. Li and H. Li, Mater. Sci. Eng. C, 2021, 127, 112237 CrossRef CAS PubMed.
- R. Paul, E. Ostermann, Y. Chen, A. C. Saville, Y. Yang, Z. Gu, A. E. Whitfield, J. B. Ristaino and Q. Wei, Biosens. Bioelectron., 2021, 187, 113312 CrossRef CAS PubMed.
- R. Omar, Y. Zheng, J. Wang and H. Haick, Adv. Sens. Res., 2023, 2, 2200032 CrossRef.
- S. Dutta, S. Pal, P. Panwar, R. K. Sharma and P. L. Bhutia, ACS Omega, 2022, 7, 25909–25920 CrossRef CAS PubMed.
- B. Kashyap and R. Kumar, Inventions, 2021, 6, 29 CrossRef.
- T. T. S. Lew, R. Sarojam, I.-C. Jang, B. S. Park, N. I. Naqvi, M. H. Wong, G. P. Singh, R. J. Ram, O. Shoseyov, K. Saito, N.-H. Chua and M. S. Strano, Nat. Plants, 2020, 6, 1408–1417 CrossRef.
- S. N. Daskalakis, G. Goussetis, S. D. Assimonis, M. M. Tentzeris and A. Georgiadis, IEEE Sens. J., 2018, 18, 7889–7898 Search PubMed.
- E. Jeon, S. Choi, K.-H. Yeo, K. S. Park, M. L. Rathod and J. Lee, J. Micromech. Microeng., 2017, 27, 085009 CrossRef.
- A. Bukhamsin, K. Moussi, N. Patel, A. Przybysz, Y. Wang, S. Krattinger and J. Kosel, Adv. Sci., 2021, 210161 Search PubMed.
- N. I. Hossain and S. Tabassum, Stem-FIT: a Microneedle-based Multi-parametric Sensor for In Situ Monitoring of Salicylic Acid and pH Levels in Live Plants, 2022 IEEE 17th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Taoyuan, Taiwan, 2022, pp. 312–316.
- S. Baek, E. Jeon, K. S. Park, K.-H. Yeo and J. Lee, J. Microelectromech. Syst., 2018, 27, 440–447 Search PubMed.
- Y. Acanda, S. Welker, V. Orbović and A. Levy, Plant Cell Rep., 2021, 40, 1171–1179 CrossRef CAS PubMed.
- B. O. Flynn, M. D. Donno, C. Barrett, C. Robinson and A. O. Riordan, 2017.
- S.-H. Cai, W. Chen, D. Di, Z.-C. Yuan, R. Jiang, W. Gao and B. Hu, Int. J. Mass Spectrom., 2022, 473, 116793 CrossRef CAS.
- C. Hegarty, A. McConville, R. J. McGlynn, D. Mariotti and J. Davis, Mater. Chem. Phys., 2019, 227, 340–346 CrossRef CAS.
- R. Paul, A. C. Saville, J. C. Hansel, Y. Ye, C. Ball, A. Williams, X. Chang, G. Chen, Z. Gu, J. B. Ristaino and Q. Wei, ACS Nano, 2019, 13, 6540–6549 CrossRef CAS.
- Y. Fang and R. P. Ramasamy, Biosensors, 2015, 5, 537–561 CrossRef CAS.
- R. Paul, E. Ostermann and Q. Wei, in Plant Pathology: Method and Protocols, ed. N. Luchi, Springer US, New York, NY, 2022 DOI:10.1007/978-1-0716-2517-0_4, pp. 77–90.
- F. Arduini, S. Cinti, V. Scognamiglio and D. Moscone, Microchim. Acta, 2016, 183, 2063–2083 CrossRef CAS.
- M.-L. Xu, Y. Gao, X. X. Han and B. Zhao, J. Agric. Food Chem., 2017, 65, 6719–6726 CrossRef CAS.
- S. Sindhu and A. Manickavasagan, Compr. Rev. Food Sci. Food Saf., 2023, 22, 1226–1256 CrossRef PubMed.
- S. M. Mugo, W. Lu and S. V. Robertson, Sensors, 2022, 22, 8492 CrossRef CAS PubMed.
- J. Chen, Y. Huang, P. Kannan, L. Zhang, Z. Lin, J. Zhang, T. Chen and L. Guo, Anal. Chem., 2016, 88, 2149–2155 CrossRef CAS PubMed.
- R. K. Mishra, A. M. Vinu Mohan, F. Soto, R. Chrostowski and J. Wang, Analyst, 2017, 142, 918–924 RSC.
- X. Yi, Z. Yuan, X. Yu, L. Zheng and C. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 4873–4882 CrossRef CAS.
- Dhanjai, S. M. Mugo and W. Lu, Anal. Bioanal. Chem., 2020, 412, 7063–7072 CrossRef CAS PubMed.
- S. M. Mugo, Dhanjai, W. Lu and S. Robertson, Biosensors, 2022, 12, 1001 CrossRef CAS PubMed.
- D. Kim, Y. Cao, D. Mariappan, M. S. Bono Jr, A. J. Hart and B. Marelli, Adv. Funct. Mater., 2021, 31, 2005370 CrossRef CAS.
- Z. Li, C. Pan, J. Sun, W. Qian and J. Dong, ACS Food Sci. Technol., 2021, 1, 1787–1791 CrossRef CAS.
- B. D'Autréaux and M. B. Toledano, Nat. Rev. Mol. Cell Biol., 2007, 8, 813–824 CrossRef PubMed.
- A. Mansouri, G. Embarek, E. Kokkalou and P. Kefalas, Food Chem., 2005, 89, 411–420 CrossRef CAS.
- K. Thaipong, U. Boonprakob, K. Crosby, L. Cisneros-Zevallos and D. Hawkins Byrne, J. Food Compos. Anal., 2006, 19, 669–675 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |
