Fluoro-bridged rare-earth metal–organic frameworks
Muhammad
Abbas†
 ,
Simin
Sheybani†
,
Marie L.
Mortensen†
and
Kenneth J.
Balkus
Jr.
,
Simin
Sheybani†
,
Marie L.
Mortensen†
and
Kenneth J.
Balkus
Jr.
 *
*
Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX 75080, USA. E-mail: balkus@utdallas.edu
First published on 22nd January 2024
Abstract
Rare-earth (RE) metal–organic frameworks (MOFs) offer unique optical, electronic, and magnetic properties. RE metals tend to make binuclear metal nodes resulting in dense nonporous coordination networks. Three dimensional porous RE-MOFs have been reported by preparing bigger metal nodes based on metal clusters often found as hexaclusters or nonaclusters. The formation of metal clusters (>2 metal ions) generally requires the use of fluorinated organic molecules reported as modulators. However, it was recently discovered that these molecules are not modulators, rather they act as reactants and leave fluorine in the metal clusters. The formation and types of fluorinated RE metal clusters have been discussed. These fluorinated clusters offer higher connectivity which results in porous MOFs. The presence of fluorine in these metal clusters offers unique properties, such as higher thermal stability and improved fluorescence. This frontier summarizes recent progress and gives future perspective on the fluorinated metal clusters in the RE-MOFs.
Introduction
Metal–organic frameworks (MOFs) are a class of hybrid materials consisting of inorganic metal nodes bridged by bidentate or polydentate organic linkers.1–9 Rare-earth MOFs (RE-MOFs) include metal nodes from scandium, yttrium, or any of the fifteen lanthanides.10,11 RE-MOFs offer a wide range of applications that take advantage of the magnetic, electrical, optical, and thermal properties in drug delivery, near infrared and white light emission, gas storage and separations.12–18 Rare-earth elements are well known for their high coordination numbers (CN: 7–12) which result in diverse structural arrangements in MOFs.19–22 A metal node with higher CNs generally connects to a larger number of organic linkers. For mononuclear or binuclear nodes, the coordination sites are often occupied by solvent molecules which can potentially result in two- or three-dimensional (2-3D) coordination polymers. Smaller metal nodes tend to form non-porous 2D and 3D coordination polymers.23–26 However, some RE-MOFs with smaller metal nodes also exhibit porous frameworks such as RE-MOF-76 [RE(btc), btc = benzene-1,3,5-tricarboxylat] and MIL-103 [RE(btb)(H2O), btb = 1,3,5-benzenetrisbenzoate].27–29 The introduction of modulators including organofluorine molecules has resulted in hexanuclear clusters, similar to the hexacluster nodes in Zr-UiO-66, [Zr6O4(OH)4(bdc)6, bdc = benzene-1,4-dicarboxylat].30,31 These hexaclusters generally offer higher connectivity and result in porous 3D MOFs. Due to the larger metal node, the coordinated solvent molecules do not significantly affect the porosity. In some cases, several sites are occupied by solvent molecules, yet the frameworks are porous and three dimensional due to the bigger metal cluster. For example, the Y-CU-45 [Y6(μ3-X)8(btc)2, X = F/OH] has a 6-connected hexacluster node, whereas the rest of the sites are coordinated by solvent molecules, and it is a highly porous three-dimensional framework.32 Thus, the discovery of hexaclusters in RE-MOFs created a pathway to make porous 3D RE-MOFs.33–35One of the earliest studies on the reactivity of lanthanides and aromatic organofluorine molecules was reported by Deacon et al. in 1984.37 Only redox active lanthanides were reported for the defluorination of aromatic organofluorine ligands.38,39 The fluorine was incorporated into metal complexes usually bridging two different lanthanide ions.40 Recently, lanthanides (non-redox active) were shown to form lanthanide fluorides as shown in Fig. 1. The synthesis of insoluble metal fluorides from 2-fluorobenzoic acid (2-FBA) was reported with redox inactive lanthanides such as holmium, gadolinium, and yttrium.41–43 The formation of the metal fluoride can be avoided by introducing ditopic or polytopic organic ligands to make fluorinated metal clusters in RE-MOFs.41 Fluorine bridges the metal ions forming clusters versus mononuclear or binuclear metal centers.24–26,44 A range of aromatic and aliphatic organofluorine molecules has been investigated to make fluorinated metal clusters as shown in Fig. 2.32,45 One of the earliest instances of breaking aliphatic C–F bonds using RE metals (RE = La–Lu, and Y) was reported by Sun et al.46 Metal fluorides and metal oxyfluorides were prepared by breaking down trifluoroacetate. These molecules were expanded to include the well-known forever chemicals with a longer carbon chain such as based on perfluoroalkyl and polyfluoroalkyl substances (PFAS) like perfluorohexanoic acid (PFHxA) and perfluorooctanoic acid (PFOA).42,43 Defluorination of these molecules is an advancement towards remediation of these pollutants by breaking highly stable carbon–fluorine bonds.
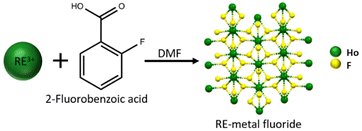 | ||
| Fig. 1 Preparation of RE-metal fluoride from the 2-FBA. Crystal structure simulated from mercury and single crystal data from ref. 36. | ||
Significance of fluoro-bridged clusters
Incorporation of fluoro-bridged clusters in RE-MOFs can affect the material's properties. Fluoroclusters are known to have interesting magnetic and fluorescence properties.47–49 For example, the fluoro-bridged RE (Dy, Tb) oxidiacetate complexes exhibit ferromagnetic interactions, while hydroxo-bridged analogs of the same complexes showed antiferromagnetic behavior.47 The unique magnetic exchange interactions and relaxation dynamics of these complexes were assigned to the difference in bridging ligand.48 While this phenomenon has been reported for small RE complexes, these have yet to be studied for RE-MOFs. Similarly, it is known that optical or luminescent properties are also influenced by fluoro-bridging.49 A fluoro-bridged terbium complex with pivalic acid was reported having Tb6(μ3-F)8 clusters. It was found that the presence of bridging fluorines promotes non-radiative multi-phonon relaxation processes in the Tb complex. These observations were further validated by theoretical calculations. It was shown for an isostructural hydroxo-bridged complex, Tb6(μ3-OH)8, non-radiative relaxations occur. Similarly, in Ln28F68 (Ln = Nd/Pr) clusters highly emissive behavior was assigned to the presence of bridging fluorines.50 Hence, the development and investigation of fluoro-bridged RE-MOFs holds significant importance for the development of functional materials.In the absence of organofluorine molecules, RE metal ions are well known to make mononuclear or binuclear nodes in MOFs.51 For example, a holmium benzene-1,4-dicarboxylate (bdc) MOF shows 8-coordinated dodecahedron metal nodes to make [Ho2(bdc)3(H2O)4]n. Each metal node is coordinated to six bdc linkers and two water molecules and propagates along the b-axis to make one-dimensional (1D) chains as shown in Fig. 3a.24 The addition of 2-FBA molecules resulted in MOFs with previously reported oxo/hydroxo bridged clusters.31 However, the discovery that 2-FBA reacts with RE ions to make fluorinated metal clusters raises the question of whether a significant number of the RE-MOFs previously reported actually contain bridging fluorine ions.41 Since 2013, 2-FBA has been used to form RE clusters ranging from trinuclear clusters to nonaclusters as the metal nodes shown in Fig. 4. For example, a series of UiO-66 analogs was reported with RE metals from Eu to Yb.52,53 The holmium version of UiO-66 [Ho6F8(bdc)6] shown in Fig. 3b was shown to have only fluorine bridges. Single crystal data is not definitive proof of fluorine bridges because of the similarity of size in electron density between O and F. In contrast, X-ray photoelectron spectroscopy (XPS) provides definitive proof of M–F bonding. For example, the binding energy for the M–F bond found in Ho-UiO-66 was determined to be 685.7 eV, a distinct shift from the organic fluorine bond in 2-fba determined to be 688.5 eV.41
 | ||
| Fig. 3 Crystal structure of (a) [Ho2(BDC)3(H2O)4]n showing coordination environment and metal chains propagating along the b-axis, and (b) Ho-UiO-66 [Ho6F8(bdc)6] along the a-axis. | ||
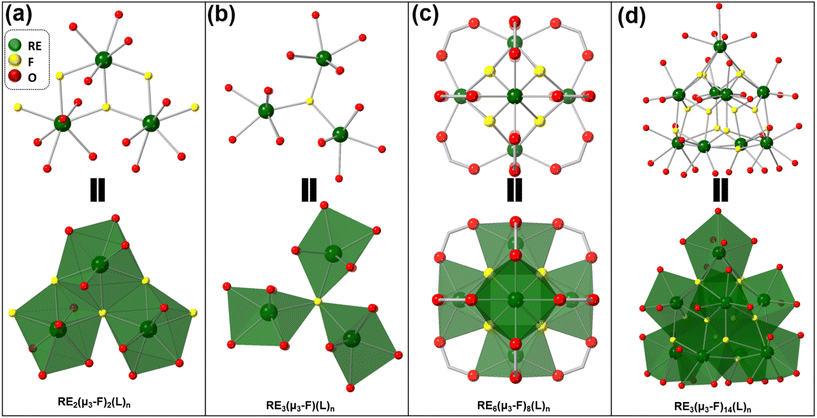 | ||
| Fig. 4 Fluorinated metal clusters found in the RE-MOFs are shown as follows (a) an asymmetric tricluster, (b) a symmetric tricluster, (c) a hexacluster, and (d) a nonacluster. | ||
Choice of fluorinated molecules
Some of the common organofluorine molecules used in the synthesis of RE-MOFs are shown in Fig. 2. The choice of modulators depends on the combination of metal and the linker. For example, in the case of yttrium and bicinchoninic acid (BCA), 2-FBA was shown to be the least reactive and required an excess amount of 2-FBA.43 2,6-DFBA is a stronger fluorinating molecule, while the PFHxA is the strongest. The ideal mole ratios for linker to modulator were found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18, 1
18, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, and 1
14, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 for 2-FBA, 2,6-DFBA, and PFHxA, respectively. The addition of fluorine to the clusters has implications for future applications of these MOFs. Some reports have determined that the fluorine within these clusters has greater thermal stability than the oxo/hydroxo versions.42,54 Christian et al. predicted an improvement in the stability of rare-earth metal–organic frameworks (MOFs) through fluorination of metal clusters. It was found that fluorinated clusters are thermodynamically more stable than hydroxylated clusters by up to 8–16 kJ per mol per atom for a 100% fluorination of the clusters.54 When fluorine is incorporated in the cluster, the framework can become neutral or gain a charge.42 This affects the available pore volume which can be partially obstructed by a counter ion, and its affinity for charged pollutants. This is also the case when comparing the zirconium analog of these MOFs to the RE analog. The Zr ion has a 4+ charge, such that the Zr 4+ hexanuclear cluster MOFs are generally neutral. While the RE 3+ analogs are normally charged, the same differences in adsorption as discussed above should be present between these MOFs.30,41 Fluorine is a common motif used to increase a MOF's affinity for CO2. While fluorine is normally incorporated within the linker, there have been studies that indicate an increased CO2 selectivity when fluorine was incorporated into the metal cluster.55 Computationally, the addition of fluorine within the cluster and its effects on the MOF's gas adsorption properties has also been studied.54,56 It was predicted that the increased electronegativity from the fluorine would create a beneficial interaction between fluorine and hydrogen gas.56 However, when adsorbing acidic gases (H2O, SO2, NO2) it was found by density-functional theory calculations and fluorine calculations through 19F-NMR that the addition of fluorine within the cluster did not have any effect on these gases.54
7 for 2-FBA, 2,6-DFBA, and PFHxA, respectively. The addition of fluorine to the clusters has implications for future applications of these MOFs. Some reports have determined that the fluorine within these clusters has greater thermal stability than the oxo/hydroxo versions.42,54 Christian et al. predicted an improvement in the stability of rare-earth metal–organic frameworks (MOFs) through fluorination of metal clusters. It was found that fluorinated clusters are thermodynamically more stable than hydroxylated clusters by up to 8–16 kJ per mol per atom for a 100% fluorination of the clusters.54 When fluorine is incorporated in the cluster, the framework can become neutral or gain a charge.42 This affects the available pore volume which can be partially obstructed by a counter ion, and its affinity for charged pollutants. This is also the case when comparing the zirconium analog of these MOFs to the RE analog. The Zr ion has a 4+ charge, such that the Zr 4+ hexanuclear cluster MOFs are generally neutral. While the RE 3+ analogs are normally charged, the same differences in adsorption as discussed above should be present between these MOFs.30,41 Fluorine is a common motif used to increase a MOF's affinity for CO2. While fluorine is normally incorporated within the linker, there have been studies that indicate an increased CO2 selectivity when fluorine was incorporated into the metal cluster.55 Computationally, the addition of fluorine within the cluster and its effects on the MOF's gas adsorption properties has also been studied.54,56 It was predicted that the increased electronegativity from the fluorine would create a beneficial interaction between fluorine and hydrogen gas.56 However, when adsorbing acidic gases (H2O, SO2, NO2) it was found by density-functional theory calculations and fluorine calculations through 19F-NMR that the addition of fluorine within the cluster did not have any effect on these gases.54
One of the smallest fluorinated clusters created with the use of 2-FBA are triclusters (Fig. 4). Two different arrangements have been observed where one arrangement consists of a uniform cluster (b) with three lanthanides forming a bond around a central fluoride. This cluster was reported in a mixed cluster MOF also containing a nonacluster (d) as well as a tricluster.42 The second arrangement (a) consists of a chain of lanthanides extending the tricluster infinitely out in a repeating fashion. The hexacluster (c) is the most commonly found motif. The effects of the metal nodes connectivity can be related to the gas adsorption/gas separation of fluorinated rare-earth MOFs (8–18). Bicalho et al. synthesized the Y(III) version of the Zr(IV) MOF-808, (Y-CU-45), using the BTC linker (Fig. 5).32 In this case, the fluorinated rare-earth MOF exhibited one of the lowest reported connectivity with just 6-coordinated nodes. This was accomplished by using trifluoroacetic acid and formic acid as caps on the fluorinated clusters, like MOF-808. These capping agents can be removed by acid wash to open metal sites in the cluster. These open metal sites create binding sites for guest molecules improving the MOF's potential for gas separation and catalysis.32
Linkers used in fluorinated RE-MOFs
The linkers shown in Fig. 5 have been used to form RE-MOFs containing fluorinated clusters. All the linkers have carboxylic functional groups to bind to the RE ions. The functional groups in the linker backbone such as nitrogen and sulphur are used to interact with guest molecules and do not bind with the rare-earth ions. Most linkers have at least two different carboxylic groups on opposite ends of the linker, except for the linker CPT which has a triazole across from the carboxylic group. It can also be noted that even though all these linkers have different geometries, a hexanuclear cluster can easily form. Meanwhile some linkers with similar geometry such as BTB can make tri- and nonaclusters. However, TCPB, and TBAPy are reported to make only nonaclusters.57 A complete list of these linkers are provided in Table 1 along with their respective metal clusters. There are indications that the presence of bridging fluorides will enhance the luminescent intensity of the fluorescing lanthanides.49 Several fluorinated MOFs have focused on the creation of white light emission.58 For example, for RE-UiO-66, the combination of three lanthanides (RE = Eu(III), Tb(III) and Gd(III)) produced white light. The mono- bi- and tri-metal nodes of RE-UiO-66 were synthesized, and the elemental analysis of the bi- and tri-analogues, determined by ICP-MS and SEM-EDS, showed an even distribution of Eu(III), Tb(III) and Gd(III) ions. The highly desirable white light emission applicable in solid state lighting and display applications was found for the optimised Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd
Gd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu-UiO-66.58 Another study reported fluorinated 2-dimensional metal–organic frameworks (MOFs) using rare-earth metal ions to selectively detect vapours of volatile organic compounds and nitroaromatic explosives.59 The photoluminescence of hexanuclear UCY-15 [RE6(μ3-OH/F)8(SDBA)4(NO3)2(H2O)6]n (RE = Y, Eu, Gd, Tb) used 4,4′-sulfonyldibenzoic acid (H2SDBA) linker (Fig. 5) to sensitize the Eu(III) and Tb(III) nodes. The trimetallic fluoro-bridged analogue of UCY-15 showed the best white light emission and the selective detection of vapors.
Eu-UiO-66.58 Another study reported fluorinated 2-dimensional metal–organic frameworks (MOFs) using rare-earth metal ions to selectively detect vapours of volatile organic compounds and nitroaromatic explosives.59 The photoluminescence of hexanuclear UCY-15 [RE6(μ3-OH/F)8(SDBA)4(NO3)2(H2O)6]n (RE = Y, Eu, Gd, Tb) used 4,4′-sulfonyldibenzoic acid (H2SDBA) linker (Fig. 5) to sensitize the Eu(III) and Tb(III) nodes. The trimetallic fluoro-bridged analogue of UCY-15 showed the best white light emission and the selective detection of vapors.
| Organic linker | Resulting MOFs | Modulator | Metal cluster | Application | Ref. |
|---|---|---|---|---|---|
| 2,2′-Bipyridine-4,4′-dicarboxylate (BPDC) | RE2F2(BPDC)2(DMF)2 and REF(BPDC)(H2O) | 2-FBA | Tricluster | Extraction of fluorine/ magnetism | 41 |
| 2,6-DFBA | 64 | ||||
| Benzene dicarboxylate (BDC) | RE6(μ3-X)8(bdc)6 | 2-FBA | Hexacluster | Extraction of fluorine | 41 |
| X = F/OH | 2,6-DFBA | White light emission | 58 | ||
| Radioluminescence | 57 | ||||
| Gas separations | 54 | ||||
| 4,4′-Sulfonyldibenzoate (SDBA) | [RE6(μ3-X)8(SDBA)4(NO3)2(H2O)6] | 2-FBA | Hexacluster | Sensor | 59 |
| 4-(p-Carboxyphenyl)-1,2,4-triazole (CPT) | [RE6(μ3-F)x(μ3-OH)8-x(CPT)6(EtOH)6](NO3)4 | 2-FBA | Hexacluster | Gas storage | 61 |
| 2,6-Naphthalenedicarboxylate (NDC) | [DMA]2[RE6(μ3-F)8(NDC)6(H2O)6] | 2-FBA | Hexacluster | Sensor | 41 |
| 60 | |||||
| Fumaric acid (H2fum) | RE6(μ3-X)6(fum)6 | 2-FBA | Hexacluster | Hydrocarbons separation | 41 |
| X = μ3-F/OH | 65 | ||||
| 5′,5′′′′-(Benzo[c][1,2,5]thiadiazole-4,7-diyl)bis(([1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylate)) (BTTC) | RE6(μ3-F)8(L)12 L = tetracarboxylate linkers | 2-FBA | Hexacluster | Near-IR emission | 45 |
| Bicinchoninic acid (H2BCA) | [RE6(μ3-F)8(BCA)6] | 2-FBA | Hexacluster | Gas separation | 43 |
| 2,6-DFBA | PFAS fluorine extraction | ||||
| PFHxA | |||||
| Benzenetricarboxylate (BTC) | RE6X8(BTC)2(2,6-DFBA)2(TFA)(HCOO−), X = μ3-F/OH | 2-FBA | Hexacluster | Topology study | 32 |
| 2,6-DFBA | |||||
| TFA | |||||
| 4,4′,4′′-(Benzene-1,3,5-triyl-tris(benzene-4,1-diyl))tribenzoate (BBC) | Y3(μ3-X)4(BBC)2 | 2-FBA | Hexacluster | Gas separations | 62 |
| X = F/OH | |||||
| 5-(2,6-Bis(4-carboxyphenyl)pyridin-4-yl)isophthalate (hcpia) | [DMA]2[RE6(μ3-X)8(hcpia)2(2-FBA)4(DMF)4], X = F/OH | 2-FBA | Hexacluster | Gas/vapors sorption | 66 |
| 4,4′,4′′-Nitrilotribenzoate (NTB) | (DMA)2[RE6(μ3-F)8(NTB)2(BDC)3] | 2-FBA | Hexacluster | Gas separation/gas storage | 63 |
| Dithieno[3,2-b:2′,3′-d] thiophene-2,6-dicarboxylate (DTTDC) | [DMA]2[Tb6(μ3-F)8(NTB)2(DTTDC)3(H2O)6] | 2-FBA | Hexacluster | GAS separation/gas storage | 63 |
| 1,4-Naphthalenedicarboxylate (NDC) | [DMA]2[Tb6(μ3-F)8(NTB)2(OBDC)3(H2O)6] | 2-FBA | Hexacluster | Gas separation/gas storage | 63 |
| 4,4′-(Oxalylbis(azanediyl))dibenzoate (OBDC) | |||||
| Anthracene-9,10-dicarboxylate (ADC) | Y6(μ3-F)8(L)6, L = dicarboxylate linkers | 2-FBA | Hexacluster | Gas separation | 67 |
| 2,5-Dimethoxyterephthalate (MOBDC) | |||||
| 2,5-Diaminoterephthalate (ABDC) | |||||
| 2,5-Bis(methylamino)terephthalate (MABDC) | |||||
| 2,5-Bis(trifluoromethyl)terephthalate (FMBDC) | |||||
| 2-(Trifluoromethoxy)terephthalate (FMOBDC) | |||||
| 10-(4-Carboxyphenyl)anthracene-9-carboxylate (PADC) | |||||
| [1,1′-Binaphthalene]-4,4′-dicarboxylate (BDA) | |||||
| 1,2,4,5-Tetrakis(4-carboxyphenyl)benzene (H4TCPB) | DMA[Tb9(μ3-X)12(μ3-O)2(TCPB)3], X = F/OH | 2-FBA | Nonacluster | Radioluminescence | 57 |
| Gas separation | 54 | ||||
| 4,4′-(4H-1,2,4-Triazole-3,5-diyl)dibenzoate (TZDB) | [DMA]4[RE9(μ3-F)12(μ2-F)3(μ3-O)2(TZDB)6(H2O)6] and [DMA]7[Tb9(μ3-F)12(μ3-O)2(H2O)9][(Tb6(μ3-F)8(OH)4(H2O)6)3(TZDB)18] | 2-FBA | Nonacluster/hexacluster | Gas storage | 68 |
| 69 | |||||
| 1,3,6,8-Tetrakis(4-carboxyphenyl)pyrene (H4TBAP) | [DMA]3[RE9(μ3-X)12(μ3-O)2(TBAP)3(2-FBA)2], X = F/OH | 2-FBA | Nonacluster | Radioluminescence | 57 |
| Photoluminescent tags | 70 | ||||
| Catalysis | 71 | ||||
| 1,3,5-Tris(4-carboxyphenyl)benzene (H3BTB) | [RE9(μ3-F)14(H2O)6][RE3(μ3-F)(H2O)3](HCO2)3(BTB)6 | 2-FBA | Tri/nonaclusters | PFAS fluorine extraction | 42 |
| PFHxA | Gas storage | 72 | |||
| PFOA | Sensor | 73 |
The photoluminescence (PL) and radioluminescence (RL) properties of metal–organic frameworks containing fluorinated terbium (Tb) clusters have also been studied.57 Terbium-based MOFs are known for their luminescent properties, and in this study, the various factors such as ligand design and crystal structure were shown to control and enhance the photoluminescent and radioluminescent characteristics of these materials. Three different RE-MOFs of Tb-UiO-66, Tb-CU-27 DMA[RE9(μ3-X)12(μ3-O)2(TCPB)3], X = OH or F, DMA = dimethyl ammonium, and Tb-CU-10 DMA3[Tb9(μ3-X)12(μ3-O)2(TBAPy)3(2-FBA)2] were synthesized. Each MOF's photoluminescence and radioluminescence characteristics show how the polynuclear cluster and corresponding organic linker interact to influence these characteristics.57
Another application for fluorinated MOFs includes the production of near infrared emission. A series of anionic RE hexacluster MOFs (Y3+, Eu3+, Gd3+, Tb3+, Dy3+, Ho3+, Er3+, Tm3+, Yb3+, Lu3+) with the fcu topology achieved near-infrared (NIR) excitation and emission.60 This was achieved by the encapsulation of organic dyes which facilitate energy transfer to the rare-earth ions. The anionic RE(III)-NDC, RE(III)-BPDC, and RE(III)-ABDC undergo a cationic exchange of the dimethylammonium ion from pores by five cationic dyes (Rhodamine 590, Rhodamine 610, Rhodamine 640, LDS 698, LDS 750).60
Xia et al. reported a series of fluoro-bridged Y(III) hexanuclear cluster MOFs with the ftw topology using H4DTTC, H4BTTC, H4BSTC, H4NTTC and H4NSTC (Fig. 5) as the organic linkers to obtain near-infrared emission. These MOFs used the different linkers and functional groups to engineer a broad range of emission wavelengths that may have applications in photocatalysis or bioimaging.45
Fluorinated MOFs could be used to separate a range of gases. For example, Zwanziger et al. reported a cationic fluoro-bridged cluster-based fcu-lanthanide MOF, UOTT-4 [RE6(μ3-F)x(μ3-OH)8−x(cpt)6(EtOH)6](NO3)4, for the adsorption of iodine vapor at room temperature using the linker, 4-(4′-carboxyphenyl)-1,2,4-triazole (CPT). They also compared the iodine adsorption of the anionic RE-UiO-66 with the cationic RE-UOTT-4. Their comparison demonstrates the impact of the framework's charge on the confinement of molecules like iodine.61
Loukopoulos et al., discusses how the Zr/Hf flu MOF and RE ken MOF were formed with the linker BBC, and how the effects of the addition of the polar group, SO2 to the linker, and change in topology enhances the adsorption of CO2.62 Li et al. reported a series of pillared MOFs using a kgd supramolecular building block and a mixture of linkers including, NTB, DTTDC, NDC, and OBDC. These formed five fluoro-bridged hexanuclear cluster Tb MOFs with three different topologies of trk, zma, and tpk. Taking advantage of the small changes in the pore size of these five MOFs, the separations of C2H6/C2H4 and C2H2/CO2 were studied. Abbas et al., explored the effect of introducing a fluorinated hexanuclear cluster into a MOF which was created with the linker, BCA, to separate CO2 and N2.43,63
The 1,3,5-tris(4-carboxyphenyl)benzene (BTB) linker (Fig. 5) and RE ions (Gd3+, Tb3+, Er3+) form nonanuclear and trinuclear clusters in JXNU-3.72 These nonaclusters are 15 connected SBUs (15-c) and are connected through 12 BTB and three formate carboxylates. The highly stable Tb-JXNU-3 MOF shows selective CO2 sorption over methane. Moreover, the same MOF was used as a luminescent pH sensor over a range of pH 3–7.72 The BTB linker is also reported to exhibit only nonaclusters, e.g., yttrium based gea-MOF-1 containing 18-c fluorinated nonaclusters. The framework consisting of only nonanuclear clusters is negatively charged which is balanced by two dimethylammonium cations.73 However, in the case of JXNU-3, the charge was balanced by three cations.72 A highly fluorinated and neutral version of JXNU-3 was obtained by using perfluorohexanoic acid (PFHxA) or perfluorooctanoic acid (PFOA) as fluorine sources. The charge balance occurred due to the replacement of oxo bridges with fluoride ions which was verified using 19F-NMR and XPS.42
Nonaclusters are generally formed when polytopic linkers are used. However, the ditopic linker of 4,4′-(4H-1,2,4-triazole-3,5-diyl)dibenzoic acid (TZDB) (Fig. 5) was found to form nonaclusters, as well.68,69 The carboxylate groups in the linkers are not linear, but rather at an angle of θ ≈ 145°, giving a bent geometry. The RE (Y/Yb) resulted in a zeolite-like MOF (ZMOF) with gme topology. The Yb and Y-ZMOFs exhibit BET surface area of 2107 and 2902 m2 g−1 respectively. These fluorinated RE-ZMOFs are mesoporous with pore sizes up to 21.95 Å and possess high gravimetric methane storage capacities, 339 and 432 cm3 g−1, respectively. The same TZDB linker also produced a combination of hexaclusters and nonaclusters in a zeolite like Tb-MOF. Similarly, nonaclusters were also observed with the linker 1,3,6,8-tetrakis(p-benzoic acid)pyrene (TBAPy) (Fig. 5) in MOFs.57,70,71 A series of RE metals (RE = Y(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Tm(III), Yb(III), and Lu(III)) with TBAPy resulted in shp-topology MOFs (RE-CU-10). An unusual RE11 cluster (not shown) was found in the case of Yb(III) and Lu(III) with 2 metal ions caping the nonaclusters axially. However, the overall connectivity of these RE11 clusters (not shown) and nonaclusters was the same 12-c, resulting in the same topology. Another tetratopic linker, 1,2,4,5-tetrakis(4-carboxyphenyl)benzene (TCPB) (Fig. 5) resulted in 12-c nonaclusters in a Tb-MOF (Tb-CU-27) with shp topology.
A range of fluoro-bridging and hydroxo-bridging mixtures have been discovered in these clusters. This ranges from 100% fluoro-bridging found in Ho-UiO-66 within the cluster,41 to almost 100% hydroxo-bridging found in Ho-TCPB-MOF with H2O2.42 In these clusters, the ratio of hydroxo-bridging vs. fluoro-bridging changes as observed through XPS and TEM/EDS.42,62 The presence of this mixture of F/OH bridging was determined for the DOBDC linker found in Fig. 5 through the computational analysis of these structures compared to their solid state 19F-NMR.54
The transformation of a rare-earth based dimeric complex with 2-FBA bound to the rare-earth metal ions in N,N-dimethylformamide (DMF) to a fluoro-bridged MOF suggests that the dimeric complex is a model for the first step in the defluorination of the 2-FBA.42 It was proposed that the 2-FBA must first bind to the metal ions before the fluoride ions can be removed with a nucleophile such as dimethylamine (DMA) found in solvents such as N,N-dimethylformamide (DMF), N,N-diethylformamide (DEF) or dimethylacetamide (DMAc). However, when solvents like water or ethanol were used, the product is a dimeric complex instead of metal fluorides or fluoro-bridged cluster MOFs.42,74
The use of forever chemicals, per- and polyfluoroalkyl substances (PFAS) (Fig. 2) as fluorinated modulators for the first time in the synthesis of a highly fluorinated tri- and nonacluster MOF was reported.42 The extent of fluorine in the cluster depends on the nature of the organofluorine which may result in different ratios of F-bridged clusters versus OH-bridges. The tri- and nonacluster MOF was reported to have >96% F in the structure when PFAS was used as the modulator. It can be explained by the higher fluorine content of PFAS molecules. The use of hydrogen peroxide as a promoter for the formation of oxo/hydroxo bridged rare-earth clusters was recently reported.42 The oxo-bridged MOF prepared with hydrogen peroxide showed no fluorine bridges in the cluster and was used to compare the thermal stability between the two MOFs. Interestingly, the thermogravimetric analyses of the fluoro-bridged MOF showed higher stability compared to the oxo-bridged one. The competition between linker and organic modulator for the metal determines the level of fluorination. The pKa of the modulators may be a good predictor for fluorine extraction. More acidic modulators like PFAS and trifluoroacetic acid with a pKa of −0.16 and 0.1, respectively, will protonate the linkers in solution more readily.75,76 This can allow for the modulators to react faster with the metals allowing for a greater amount of fluorine in the cluster. As shown with the synthesis of Ho-TCPB-MOF, the use of PFAS increased the percentage of fluoro-bridging within the MOF.39
Conclusions and future directions
For close to a decade now, 2-FBA has been used for the synthesis of RE-MOFs containing metal clusters to improve stability and direct the topology. These MOFs were synthesized for various applications, ranging from gas separations and storage to catalysts and sensors. The discovery of the extraction of fluorine from a range of organofluorine molecules such as 2-FBA and PFAS resulting in fluoro-bridged RE clusters is an advancement in MOF synthesis and lanthanide chemistry. With the expanding pool of organofluorine molecules that can be used to form RE-MOFs, there is a wide range of molecules to investigate. A better understanding of what drives the formation of different sizes of clusters (tri-, hexa-, and nona-), would allow for more directed synthesis of the desired MOFs and clusters as well as the formation of higher nuclearity clusters. Knowledge of the properties and applications of fluoro-bridged RE-MOFs is just starting to emerge. Many of these studies focus on the effects that linkers have on the various properties of these MOFs. If the effects of the fluorine in the fluoro-bridged MOFs are to be analyzed, then RE-MOFs with lower connectivity and more open metal sites must be designed. Fluoro-bridged clusters can have a charge difference from the oxo/hydroxo analogs which can affect the properties of MOFs. Thus, the preparation of the oxo/hydroxo analog of fluoro-bridged MOFs will help delineate the effect of fluorine.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Robert A. Welch Foundation (AT-1153) for supporting this work.References
- S. L. James, Chem. Soc. Rev., 2003, 32, 276 RSC.
- H.-C. “Joe” Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- A. J. Howarth, A. W. Peters, N. A. Vermeulen, T. C. Wang, J. T. Hupp and O. K. Farha, Chem. Mater., 2017, 29, 26–39 CrossRef CAS.
- C. Doonan, R. Riccò, K. Liang, D. Bradshaw and P. Falcaro, Acc. Chem. Res., 2017, 50, 1423–1432 CrossRef CAS PubMed.
- N. L. Rosi, M. Eddaoudi, J. Kim, M. O'Keeffe and O. M. Yaghi, CrystEngComm, 2002, 4, 401–404 RSC.
- M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319–330 CrossRef CAS PubMed.
- M. Bosch, S. Yuan, W. Rutledge and H.-C. Zhou, Acc. Chem. Res., 2017, 50, 857–865 CrossRef CAS PubMed.
- S. Barthel, E. V. Alexandrov, D. M. Proserpio and B. Smit, Cryst. Growth Des., 2018, 18, 1738–1747 CrossRef CAS PubMed.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546–1554 CrossRef CAS.
- F. Saraci, V. Quezada-Novoa, P. R. Donnarumma and A. J. Howarth, Chem. Soc. Rev., 2020, 49, 7949–7977 RSC.
- J. Chen, H. Gao, Z. Tao, L. Wang, R. Li and G. Wang, Coord. Chem. Rev., 2023, 485, 215121 CrossRef CAS.
- J. Della Rocca, D. Liu and W. Lin, Acc. Chem. Res., 2011, 44, 957–968 CrossRef CAS PubMed.
- V. Quezada-Novoa, H. M. Titi, A. A. Sarjeant and A. J. Howarth, Chem. Mater., 2021, 33, 4163–4169 CrossRef CAS.
- J. M. Rimsza, S. E. Henkelis, L. E. S. Rohwer, D. F. Sava Gallis and T. M. Nenoff, Adv. Opt. Mater., 2022, 2200058 CrossRef CAS.
- X. Sun, K. Yuan and Y. Zhang, J. Rare Earths, 2020, 38, 801–818 CrossRef CAS.
- J. Luo, H. Xu, Y. Liu, Y. Zhao, L. L. Daemen, C. Brown, T. V. Timofeeva, S. Ma and H.-C. Zhou, J. Am. Chem. Soc., 2008, 130, 9626–9627 CrossRef CAS PubMed.
- P. M. Bhatt, Y. Belmabkhout, A. H. Assen, Ł. J. Weseliński, H. Jiang, A. Cadiau, D. X. Xue and M. Eddaoudi, Chem. Eng. J., 2017, 324, 392–396 CrossRef CAS.
- K. M. L. Taylor, A. Jin and W. Lin, Angew. Chem., Int. Ed., 2008, 47, 7722–7725 CrossRef CAS PubMed.
- T. M. Reineke, M. Eddaoudi, M. Fehr, D. Kelley and O. M. Yaghi, J. Am. Chem. Soc., 1999, 121, 1651–1657 CrossRef CAS.
- T. Xia, W. Cao, L. Guan, J. Zhang, F. Jiang, L. Yu and Y. Wan, Dalton Trans., 2022, 51, 5426–5433 RSC.
- G. B. Deacon, C. M. Forsyth, P. C. Junk, R. P. Kelly, A. Urbatsch and J. Wang, Dalton Trans., 2012, 41, 8624 RSC.
- B. Ugale, S. S. Dhankhar and C. M. Nagaraja, Cryst. Growth Des., 2018, 18, 2432–2440 CrossRef CAS.
- C. Pagis, M. Ferbinteanu, G. Rothenberg and S. Tanase, ACS Catal., 2016, 6, 6063–6072 CrossRef CAS.
- J. P. Vizuet, A. L. Lewis, G. T. Mccandless and K. J. Balkus, Polyhedron, 2021, 205, 115283 CrossRef CAS.
- J. P. Vizuet, A. L. Lewis, G. T. Mccandless and K. J. Balkus, J. Nanosci. Nanotechnol., 2020, 20, 3019–3024 CrossRef CAS PubMed.
- M. L. Mortensen, A. L. Lewis, G. Mccandless and K. J. Balkus, Crystals, 2021, 11, 1547 CrossRef CAS.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed.
- T.-Y. Luo, C. Liu, S. V. Eliseeva, P. F. Muldoon, S. Petoud and N. L. Rosi, J. Am. Chem. Soc., 2017, 139, 9333–9340 CrossRef CAS PubMed.
- T. Devic, C. Serre, N. Audebrand, J. Marrot and G. Férey, J. Am. Chem. Soc., 2005, 127, 12788–12789 CrossRef CAS PubMed.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- D.-X. Xue, A. J. Cairns, Y. Belmabkhout, L. Wojtas, Y. Liu, M. H. Alkordi and M. Eddaoudi, J. Am. Chem. Soc., 2013, 135, 7660–7667 CrossRef CAS PubMed.
- H. A. Bicalho, F. Saraci, J. de J. Velazquez-Garcia, H. M. Titi and A. J. Howarth, Chem. Commun., 2022, 58, 10925–10928 RSC.
- Z. Chen, Ł. J. Weseliński, K. Adil, Y. Belmabkhout, A. Shkurenko, H. Jiang, P. M. Bhatt, V. Guillerm, E. Dauzon, D. X. Xue, M. O'Keeffe and M. Eddaoudi, J. Am. Chem. Soc., 2017, 139, 3265–3274 CrossRef CAS PubMed.
- H. Jiang, J. Jia, A. Shkurenko, Z. Chen, K. Adil, Y. Belmabkhout, L. J. Weselinski, A. H. Assen, D. X. Xue, M. O'Keeffe and M. Eddaoudi, J. Am. Chem. Soc., 2018, 140, 8858–8867 CrossRef CAS PubMed.
- D. Alezi, A. M. P. Peedikakkal, Ł. J. Weseliński, V. Guillerm, Y. Belmabkhout, A. J. Cairns, Z. Chen, Ł. Wojtas and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 5421–5430 CrossRef CAS PubMed.
- M. Piotrowski, H. Ptasiewicz-Bąk and A. Murasik, Phys. Status Solidi, 1979, 55, K163–K166 CrossRef CAS.
- G. B. Deacon and P. I. Mackinnon, Tetrahedron Lett., 1984, 25, 783–784 CrossRef CAS.
- G. B. Deacon, P. C. Junk, R. P. Kelly and J. Wang, Dalton Trans., 2016, 45, 1422–1435 RSC.
- G. B. Deacon, C. M. Forsyth and J. Sun, Tetrahedron Lett., 1994, 35, 1095–1098 CrossRef CAS.
- G. B. Deacon, G. D. Fallon, C. M. Forsyth, S. C. Harris, P. C. Junk, B. W. Skelton and A. H. White, Dalton Trans., 2006, 802–812 RSC.
- J. P. Vizuet, M. L. Mortensen, A. L. Lewis, M. A. Wunch, H. R. Firouzi, G. T. Mccandless and K. J. Balkus, J. Am. Chem. Soc., 2021, 143, 17995–18000 CrossRef CAS PubMed.
- S. Sheybani, M. Abbas, H. R. Firouzi, Z. Xiao, H.-C. Zhou and K. J. Balkus, Inorg. Chem., 2023, 62, 4314–4321 CrossRef CAS PubMed.
- M. Abbas, A. M. Maceda, H. R. Firouzi, Z. Xiao, H. D. Arman, Y. Shi, H.-C. Zhou and K. J. Balkus, Chem. Sci., 2022, 13, 14285–14291 RSC.
- J. P. Vizuet, A. L. Lewis, G. T. McCandless and K. J. Balkus, Polyhedron, 2019, 159, 12–17 CrossRef CAS.
- H. L. Xia, K. Zhou, J. Guo, J. Zhang, X. Huang, D. Luo, X. Y. Liu and J. Li, Chem. Sci., 2022, 13, 9321–9328 RSC.
- X. Sun, Y. W. Zhang, Y. P. Du, Z. G. Yan, R. Si, L. P. You and C. H. Yan, Chem. – Eur. J., 2007, 13, 2320–2332 CrossRef CAS PubMed.
- G. Brunet, F. Habib, I. Korobkov and M. Murugesu, Inorg. Chem., 2015, 54, 6195–6202 CrossRef CAS PubMed.
- Q. Zhou, F. Yang, D. Liu, Y. Peng, G. Li, Z. Shi and S. Feng, Inorg. Chem., 2012, 51, 7529–7536 CrossRef CAS PubMed.
- B.-K. Ling, J. Li, Y.-Q. Zhai, H.-K. Hsu, Y.-T. Chan, W.-P. Chen, T. Han and Y.-Z. Zheng, Chem. Commun., 2020, 56, 9130–9133 RSC.
- M. Romanelli, G. A. Kumar, T. J. Emge, R. E. Riman and J. G. Brennan, Angew. Chem., Int. Ed., 2008, 47, 6049–6051 CrossRef CAS PubMed.
- G. Bousrez, O. Renier, V. Paterlini, V. Smetana and A.-V. Mudring, Inorg. Chem., 2021, 60, 17487–17497 CrossRef CAS PubMed.
- P. R. Donnarumma, S. Frojmovic, P. Marino, H. A. Bicalho, H. M. Titi and A. J. Howarth, Chem. Commun., 2021, 57, 6121–6124 RSC.
- M.-L. Gao, W.-J. Wang, L. Liu, Z.-B. Han, N. Wei, X.-M. Cao and D.-Q. Yuan, Inorg. Chem., 2017, 56, 511–517 CrossRef CAS PubMed.
- M. S. Christian, K. J. Fritzsching, J. A. Harvey, D. F. Sava Gallis, T. M. Nenoff and J. M. Rimsza, JACS Au, 2022, 2, 1889–1898 CrossRef CAS PubMed.
- P. M. Bhatt, Y. Belmabkhout, A. Cadiau, K. Adil, O. Shekhah, A. Shkurenko, L. J. Barbour and M. Eddaoudi, J. Am. Chem. Soc., 2016, 138, 9301–9307 CrossRef CAS PubMed.
- N. Prasetyo and F. I. Pambudi, Int. J. Hydrogen Energy, 2021, 46, 4222–4228 CrossRef CAS.
- Z. Ajoyan, G. A. Mandl, P. R. Donnarumma, V. Quezada-Novoa, H. A. Bicalho, H. M. Titi, J. A. Capobianco and A. J. Howarth, ACS Mater. Lett., 2022, 4, 1025–1031 CrossRef CAS.
- Z. Ajoyan, H. A. Bicalho, P. R. Donnarumma, A. Antanovich and A. J. Howarth, J. Mater. Chem. C, 2023, 11, 8929–8934 RSC.
- N. Panagiotou, F. G. Moscoso, T. Lopes-Costa, J. M. Pedrosa and A. J. Tasiopoulos, Inorg. Chem. Front., 2022, 9, 4850–4863 RSC.
- C. Liu, S. V. Eliseeva, T.-Y. Luo, P. F. Muldoon, S. Petoud and N. L. Rosi, Chem. Sci., 2018, 9, 8099–8102 RSC.
- C. Zwanziger, W. D. do Pim, A. A. Kitos, J. S. Ovens, P. J. Pallister and M. Murugesu, Chem. Commun., 2022, 58, 12700–12703 RSC.
- E. Loukopoulos, G. K. Angeli, K. Kouvidis, C. Tsangarakis and P. N. Trikalitis, ACS Appl. Mater. Interfaces, 2022, 14, 22242–22251 CrossRef CAS PubMed.
- H.-X. Li, H. Fang, Y.-F. Zhang, Z.-H. Zhang and D.-X. Xue, Inorg. Chem. Front., 2023, 10, 1795–1802 RSC.
- M. L. Mortensen, S. Bisht, M. Abbas, H. Firouzi, G. T. McCandless, M. Shatruk and K. J. Balkus, Inorg. Chem., 2024, 63, 219–228 CrossRef CAS PubMed.
- A. H. Assen, Y. Belmabkhout, K. Adil, P. M. Bhatt, D. Xue, H. Jiang and M. Eddaoudi, Angew. Chem., Int. Ed., 2015, 54, 14353–14358 CrossRef CAS PubMed.
- E. Loukopoulos, G. K. Angeli, C. Tsangarakis, E. Traka, K. G. Froudas and P. N. Trikalitis, Chem. – Eur. J., 2023, e202302709 Search PubMed.
- J. Liu, K. Zhou, S. Ullah, J. Miao, H. Wang, T. Thonhauser and J. Li, Small, 2023, 19, 2304460 CrossRef CAS PubMed.
- H.-X. Li, Z.-H. Zhang, H. Fang, D.-X. Xue and J. Bai, J. Mater. Chem. A, 2022, 10, 14795–14798 RSC.
- H.-X. Li, H. Fang, G.-T. Du and D.-X. Xue, Inorg. Chem., 2023, 62, 18014–18019 CrossRef CAS PubMed.
- J. I. Deneff, L. E. S. Rohwer, K. S. Butler, N. R. Valdez, M. A. Rodriguez, T. S. Luk and D. F. Sava Gallis, ACS Appl. Mater. Interfaces, 2022, 14, 3038–3047 CrossRef CAS PubMed.
- V. Quezada-Novoa, H. M. Titi, F. Y. Villanueva, M. W. B. Wilson and A. J. Howarth, Small, 2023, 19, 1–9 Search PubMed.
- Y.-J. Li, Y.-L. Wang and Q.-Y. Liu, Inorg. Chem., 2017, 56, 2159–2164 CrossRef CAS PubMed.
- V. Guillerm, Ł. J. Weseliński, Y. Belmabkhout, A. J. Cairns, V. D'Elia, Ł. Wojtas, K. Adil and M. Eddaoudi, Nat. Chem., 2014, 6, 673–680 CrossRef CAS PubMed.
- X. Li and Y.-Q. Zou, Z. Kristallogr. – New Cryst. Struct., 2004, 219, 451–452 Search PubMed.
- S. Rayne and K. Forest, J. Mol. Struct.: THEOCHEM, 2010, 949, 60–69 CrossRef CAS.
- M. Namazian, M. Zakery, M. R. Noorbala and M. L. Coote, Chem. Phys. Lett., 2008, 451, 163–168 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |


