Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interconversion between multicomponent slider-on-deck and palladium capsule: regulation of catalysis and encapsulation†
Isa
Valiyev
 ,
Indrajit
Paul
,
Yi-Fan
Li
,
Emad
Elramadi
,
Indrajit
Paul
,
Yi-Fan
Li
,
Emad
Elramadi
 and
Michael
Schmittel
and
Michael
Schmittel
 *
*
Center of Micro and Nanochemistry and (Bio)Technology, Organische Chemie I, School of Science and Engineering, Universität Siegen, Adolf-Reichwein-Str. 2, D-57068 Siegen, Germany. E-mail: schmittel@chemie.uni-siegen.de
First published on 2nd February 2024
Abstract
When the slider-on-deck [Cu3(1)(2)]3+ and guest G were treated with palladium(II) ions, the biped 2 was released from [Cu3(1)(2)]3+ generating the nanocage [Pd2(2)4(G)]4+ with guest G being encapsulated (NetState-II). This transformation that was reversed by the addition of DMAP enabled modulation of both the overall fluorescence and the activity of copper(I) catalyzing an aza Hopf cyclization.
Over the years, researchers have investigated methods to mimic complex multicomponent catalysts found in nature, such as ATP synthase,1 by manmade prototypes that bring together catalysis, host–guest chemistry,2 and dynamic molecular motion.3 All these elements are combined in slider-on-deck machinery4 with either on/off or up/down regulation of catalysis.5 Since nanoscale metallocages are well established in sensing,6 catalysis,7 and highly selective binding,8 interconnecting dynamic molecular machines and functional nanocages may offer new venues for functional applications.
Herein, we demonstrate (i) the fully reversible interconversion of a molecular slider-on-deck and a palladium(II) capsule9 enabling (ii) a copper(I)-based catalytic cyclization and (iii) parallel guest encapsulation. The design was based on two well-defined networked states (NetStates). NetState-I encompasses pentacene-6,13-dione as a prospective guest (G) and the three-component slider-on-deck DS (= [Cu3(1)(2)]3+), prepared from deck 1, the sliding biped 2, and copper(I) ions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (Scheme 1). Addition of the correct stoichiometric amount of Pd2+ ions afforded NetState-II involving the copper-loaded deck [Cu3(1)]3+ and the guest-filled palladium(II) capsule G@C (= [Pd2(2)4(G)]4+). Notably, both networked states were catalytically active, however, at distinct reaction rates.
3) (Scheme 1). Addition of the correct stoichiometric amount of Pd2+ ions afforded NetState-II involving the copper-loaded deck [Cu3(1)]3+ and the guest-filled palladium(II) capsule G@C (= [Pd2(2)4(G)]4+). Notably, both networked states were catalytically active, however, at distinct reaction rates.
The design of the slider-on-deck ⇄ cage interconversion has been inspired by recent progress in chemically networked slider-on-decks,10 nanorotors,3c and coordination capsules.9,11 The required deck 1 was synthesized via several successive Suzuki and Sonogashira coupling reactions. Ligand 2 is a literature-known compound.12 The stator's bipyridine stations and the biped's nitrogen-terminated feet were connected via copper(I) ion, i.e., by HETPYP complexation.10b The bipyridine unit in the deck was required instead of the frequently used phenanthroline core10,13 owing to a higher catalytic activity of the Cu+ bound bipyridine in the aza Hopf14 cyclization. Moreover, self-sorting between the analogous phenanthroline deck, Cu+, and Pd2+ was not clean. Ligand 2 has been selected because of its ability to form both a slider-on-deck system with the Cu+-loaded deck [Cu3(1)]3+ and a stable coordination capsule (C) with Pd2+ ions.
Upon addition of copper(I) ions to a mixture of ligands 1 and 2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), clean formation of the slider-on-deck [Cu3(1)(2)]3+ was observed. The multicomponent assembly was characterized by 1H-NMR (ESI,† page S5–6) and ESI-MS proving the presence of complex [Cu3(1)(2)]3+ by peaks at m/z = 611.4 (triply charged) and m/z = 989.4 (doubly charged) (ESI, Fig. S27†). In addition, a single set of 1H-DOSY signals (D = 5.69 × 10−10 m2 s−1, r = 9.28 Å, in CD2Cl2) corroborated the clean formation of the slider-on-deck (ESI, Fig. S14†).
1), clean formation of the slider-on-deck [Cu3(1)(2)]3+ was observed. The multicomponent assembly was characterized by 1H-NMR (ESI,† page S5–6) and ESI-MS proving the presence of complex [Cu3(1)(2)]3+ by peaks at m/z = 611.4 (triply charged) and m/z = 989.4 (doubly charged) (ESI, Fig. S27†). In addition, a single set of 1H-DOSY signals (D = 5.69 × 10−10 m2 s−1, r = 9.28 Å, in CD2Cl2) corroborated the clean formation of the slider-on-deck (ESI, Fig. S14†).
To investigate the dynamic behavior of the slider-on-deck, we analyzed the signal of proton 3-H by variable temperature (VT) 1H-NMR in the range from 25 to −75 °C in CD2Cl2 (Fig. 1a). At 25 °C the proton appeared as a sharp singlet at 2.47 ppm. At ca. −25 °C the signal started to broaden and with further lowering of the temperature, the proton signal started to split from a singlet into two singlets. At −75 °C it separated into two singlets (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 2.51 and 2.37 ppm. The singlet at 2.37 ppm was assigned to the two copper(I)-loaded bipyridine stations that are connected to the pyridine terminals of biped 2, whereas the unloaded [Cu(bipyAr2)]+ station showed up at 2.51 ppm. The exchange frequency was determined using WinD-NMR15 to k25 = 80 kHz at 25 °C. Activation data are displayed in Fig. 1b.
2) at 2.51 and 2.37 ppm. The singlet at 2.37 ppm was assigned to the two copper(I)-loaded bipyridine stations that are connected to the pyridine terminals of biped 2, whereas the unloaded [Cu(bipyAr2)]+ station showed up at 2.51 ppm. The exchange frequency was determined using WinD-NMR15 to k25 = 80 kHz at 25 °C. Activation data are displayed in Fig. 1b.
Upon addition of 0.5 equiv. of [Pd(CH3CN)4](BF4)2 to the slider-on-deck DS, the 1H NMR (Fig. 2) indicated complete dissociation of biped 2 from the copper-loaded deck and clean formation of C = [Pd2(2)4]4+, a Pd2L4-type coordination capsule reported earlier by Hooley and co-workers,12 and of [Cu3(1)]3+. The loss of biped 2 from the slider-on-deck was readily derived from the 1H-NMR data, as e.g. proton signals 11-H and 1-H of [Cu3(1)(2)]3+ shifted from 6.83 and 7.55 ppm to 6.97 and 7.73 ppm, respectively, as in independently prepared [Cu3(1)]3+. The association of Pd2+ to 2 was readily monitored by the appearance of new peaks for f′-H, and g′-H at 9.34 and 9.50 ppm as well as the disappearance of f-H and g-H from 6.46 and 7.71 ppm, respectively. The ESI-MS proved parallel presence of complex [Cu3(1)]3+ by peaks at m/z = 518.2 (triply charged) and m/z = 849.8 (doubly charged) (ESI, Fig. S26†) and of cage [Pd2(2)4]4+ by peaks at m/z = 333.5 (quadruply charged) and m/z = 473.9 (triply charged) (ESI, Fig. S28†).
Upon addition of two equiv. of DMAP to NetState-II (= cage C and copper(I)-loaded deck [Cu3(1)]3+), clean regeneration of the slider-on-deck [Cu3(1)(2)]3+ was achieved. Full reversibility of the interconversions over six cycles was proven by alternately adding Pd2+ and then DMAP by 1H NMR (ESI, Fig. S12†).
Encapsulation of pentacene-6,13-dione (G) into the cage C = [Pd2(2)4](BF4)4 was realized in CD2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN = 4
CD3CN = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
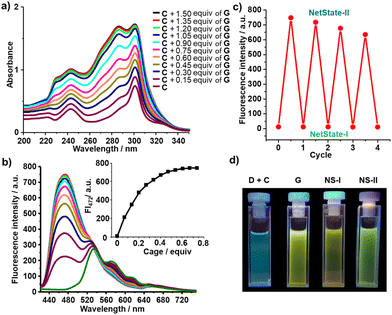
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, similar to the procedure by Lusby9et al. To achieve complete encapsulation, excess of G was required as shown in the UV-vis titration (peaks corresponding to cage C at 231, 243, 286, and 301 nm increased gradually saturating upon the addition of 1.5 equiv. of G, see Fig. 3a). Therefrom, the binding constant was determined as log
1, similar to the procedure by Lusby9et al. To achieve complete encapsulation, excess of G was required as shown in the UV-vis titration (peaks corresponding to cage C at 231, 243, 286, and 301 nm increased gradually saturating upon the addition of 1.5 equiv. of G, see Fig. 3a). Therefrom, the binding constant was determined as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 7.91 ± 0.29 (ESI, Fig. S29†). The encapsulation of G was also noted from drastic 1H NMR shifts, e.g., proton c′-H, of C shifted from 8.03 to 5.48 ppm and proton 1′-H of G shifted from 8.91 to 10.06 ppm. Parallel, the ESI-MS proved guest encapsulation into the cage by peaks at m/z = 411.0 (quadruply charged), m/z = 576.1 (triply charged), and m/z = 908.0 (doubly charged) (ESI, Fig. S28†). Cross-correlations between proton c′-H of the cage and protons 1′′-H, 2′′-H, and 3′′-H of G in the 1H–1H NOESY spectrum attested to the formation of the host–guest system (ESI, Fig. S13†). In fluorescence spectroscopy, the addition of the cage to the ligand showed a blue shift (Fig. 3b). Initially, the emission of G (excited at λ = 300 nm) revealed a bright yellow fluorescence at λ = 537 and 569 nm. In the ensuing titration with C, the initial peaks of G gradually disappeared, while simultaneously a new strong emission at λ = 472 nm emerged showing as a bright green fluorescence.
K = 7.91 ± 0.29 (ESI, Fig. S29†). The encapsulation of G was also noted from drastic 1H NMR shifts, e.g., proton c′-H, of C shifted from 8.03 to 5.48 ppm and proton 1′-H of G shifted from 8.91 to 10.06 ppm. Parallel, the ESI-MS proved guest encapsulation into the cage by peaks at m/z = 411.0 (quadruply charged), m/z = 576.1 (triply charged), and m/z = 908.0 (doubly charged) (ESI, Fig. S28†). Cross-correlations between proton c′-H of the cage and protons 1′′-H, 2′′-H, and 3′′-H of G in the 1H–1H NOESY spectrum attested to the formation of the host–guest system (ESI, Fig. S13†). In fluorescence spectroscopy, the addition of the cage to the ligand showed a blue shift (Fig. 3b). Initially, the emission of G (excited at λ = 300 nm) revealed a bright yellow fluorescence at λ = 537 and 569 nm. In the ensuing titration with C, the initial peaks of G gradually disappeared, while simultaneously a new strong emission at λ = 472 nm emerged showing as a bright green fluorescence.
To afford NetState-I = DS + G, deck 1, biped 2, [Cu(CH3CN)4]PF6, and G were mixed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5). After the clean formation of NetState-I, 0.5 equiv. of [Pd(CH3CN)4](BF4)2 was added which resulted in the formation of NetState-II = D + G@C + G. The binding constant of G@C in NetState-II was identical to that of the isolated system (ESI, Fig. S29b & S30†). The Pd(II) coordination to biped 2 happened quite rapidly (t½ = 26.5 ± 0.9 s) even at low concentration (c = 1.22 × 10−6 M) as used in a UV-Vis kinetic study (ESI, Fig. S37†). Equally in the reverse translocation of NetState-II to NetState-I, dissolution of cage G and coordination of Pd(II) to DMAP was rapid (t½ = 56.5 ± 1.4 s at c = 1.22 × 10−6 M; ESI, Fig. S38†). Both multi-component assemblies were identified by 1H-NMR (Fig. 2). Since there is no 1H-NMR peak shift in NetState-I regarding the separate entities, apparently there is no interaction between G and DS. In NetState-II though, the peaks of proton c-H of the DS and 1′-H of the G were shifted from 7.68 and 8.91 ppm to 5.49 and 10.06 ppm, respectively, due to cage formation along with guest encapsulation. In this regard, the shift of the proton signals f′-H and g′-H from 9.34 and 9.50 ppm (free C) to 9.42 and 9.66 ppm (after encapsulation of G), respectively, was diagnostic.
1.5). After the clean formation of NetState-I, 0.5 equiv. of [Pd(CH3CN)4](BF4)2 was added which resulted in the formation of NetState-II = D + G@C + G. The binding constant of G@C in NetState-II was identical to that of the isolated system (ESI, Fig. S29b & S30†). The Pd(II) coordination to biped 2 happened quite rapidly (t½ = 26.5 ± 0.9 s) even at low concentration (c = 1.22 × 10−6 M) as used in a UV-Vis kinetic study (ESI, Fig. S37†). Equally in the reverse translocation of NetState-II to NetState-I, dissolution of cage G and coordination of Pd(II) to DMAP was rapid (t½ = 56.5 ± 1.4 s at c = 1.22 × 10−6 M; ESI, Fig. S38†). Both multi-component assemblies were identified by 1H-NMR (Fig. 2). Since there is no 1H-NMR peak shift in NetState-I regarding the separate entities, apparently there is no interaction between G and DS. In NetState-II though, the peaks of proton c-H of the DS and 1′-H of the G were shifted from 7.68 and 8.91 ppm to 5.49 and 10.06 ppm, respectively, due to cage formation along with guest encapsulation. In this regard, the shift of the proton signals f′-H and g′-H from 9.34 and 9.50 ppm (free C) to 9.42 and 9.66 ppm (after encapsulation of G), respectively, was diagnostic.
Reversibility of the networked system upon alternate addition of Pd2+ and DMAP over four cycles was established by 1H NMR (Fig. 4). The interconversion of NetState-I ⇄ NetState-II was conveniently followed by fluorescence because the yellow emission of free G shifted to green after encapsulation of G. Upon addition of DMAP, the emission reversed to the initial wavelength. Changes between the two states were reproduced over four cycles (Fig. 3c) with a small decline in the emission intensity, most likely due to the formation of [Pd(DMAP)4]2+.
The catalytic cyclization of 4 to isoquinolinium imide 5 is known for Ag+,16 Fe3+,17 I2, Br2, I–Cl, NIS, NBS,18 and for Cu2+ ions.19 In the present work, we achieved cyclization with Cu+ ions (Scheme 2). The cyclization of 4 (c = 3.38 mM) was first studied in presence of [Cu(CH3CN)4]PF6 (15 mol%) at 40 °C in CD2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN = 4
CD3CN = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yielding 20 (±2)% of 5 after 7 h (ESI, Fig. S24†). Due to the facile oxidation of free [Cu(CH3CN)4]PF6 to copper(II), performing the second cycle of the reaction and simultaneously monitoring by 1H NMR was not achievable. In contrast, copper(I) in [Cu3(1)]3+ and [Cu3(1)(2)]3+ should be stabilized against oxidation because of coordination with the stator's bipyridine. As a result, we expected copper(I) to be catalytically active in the NetState-I/II system over multiple switching cycles and performed three reversible catalytic cycles (c = 3.38 mM, in CD2Cl2
1 yielding 20 (±2)% of 5 after 7 h (ESI, Fig. S24†). Due to the facile oxidation of free [Cu(CH3CN)4]PF6 to copper(II), performing the second cycle of the reaction and simultaneously monitoring by 1H NMR was not achievable. In contrast, copper(I) in [Cu3(1)]3+ and [Cu3(1)(2)]3+ should be stabilized against oxidation because of coordination with the stator's bipyridine. As a result, we expected copper(I) to be catalytically active in the NetState-I/II system over multiple switching cycles and performed three reversible catalytic cycles (c = 3.38 mM, in CD2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN = 4
CD3CN = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, at 40 °C, 7 h) alternatingly adding Pd2+ and DMAP (ESI, Fig. S19†). As determined by 1H NMR, the yield of product 5 was (43 ± 2)% in NetState-I and (14 ± 2)% in state-II, being reproducible over all three cycles (Scheme 2b and ESI, Fig. S20†). To exclude the possible involvement of Pd2+ as a catalyst, we tested the reaction of 4 (in CD2Cl2
1, at 40 °C, 7 h) alternatingly adding Pd2+ and DMAP (ESI, Fig. S19†). As determined by 1H NMR, the yield of product 5 was (43 ± 2)% in NetState-I and (14 ± 2)% in state-II, being reproducible over all three cycles (Scheme 2b and ESI, Fig. S20†). To exclude the possible involvement of Pd2+ as a catalyst, we tested the reaction of 4 (in CD2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN = 4
CD3CN = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 40 °C for 7 h) in the presence of either [Pd(DMAP)4]2+ or cage. Neither cage, free palladium ions nor [Pd(DMAP)4]2+ were active as catalysts (ESI, Fig. S21–23†).
1 at 40 °C for 7 h) in the presence of either [Pd(DMAP)4]2+ or cage. Neither cage, free palladium ions nor [Pd(DMAP)4]2+ were active as catalysts (ESI, Fig. S21–23†).
Although the higher yields in NetState-I vs. II are counterintuitive at first, the difference was expected based on previous studies.20 As outlined there,20 the motion in the slider-on-deck may reduce binding of 5 to copper(I) and thus product inhibition of DS as catalyst. Indeed, investigations show that 83% of 5 was liberated into solution from [Cu3(1)(2)(5)]3+ due to the sliding motion (ESI,† pages S33–35), whereas in a static reference, i.e., complex [Cu(6)(5)]+ representing D as catalyst, only 58% of 5 are freed (ESI, Fig. S35†).
In conclusion, we demonstrated a functional network, in which copper(I) catalysis was highly reproducibly up/down regulated by removal/addition of Pd2+. Parallel, the encapsulation of 6,13-pentacendione9 as a guest in NetState-II provided diagnostic on/off emission changes reporting the interconversion of the networked states. Fully reversible interconversion between NetState-I and II was realized by the addition of stoichiometric amounts of Pd2+ and DMAP over four cycles in the case of encapsulation and three in the case of catalysis. The present work demonstrates how new functions (catalysis with parallel fluorescence signalling) emerge from networked systems.
Author contributions
Isa Valiyev: conceptualization, investigation, writing – original draft. Indrajit Paul: investigation. Yi-Fan Li: investigation. Emad Elramadi: investigation. Michael Schmittel: project administration, funding acquisition, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are indebted to the Deutsche Forschungsgemeinschaft (Schm 647/22-1, No. 491092614) for financial support and Dr Thomas Paululat (Siegen) for help with the VT-1H NMR measurements.References
- P. Neupane, S. Bhuju, N. Thapa and H. K. Bhattarai, Biomol. Concepts, 2019, 10, 1–10, DOI:10.1515/bmc-2019-0001.
- (a) R. L. Spicer, A. D. Stergiou, T. A. Young, F. Duarte, M. D. Symes and P. J. Lusby, J. Am. Chem. Soc., 2020, 142, 2134–2139, DOI:10.1021/jacs.9b11273; (b) J. Tessarolo, H. Lee, E. Sakuda, K. Umakoshi and G. H. Clever, J. Am. Chem. Soc., 2021, 143(17), 6339–6344, DOI:10.1021/jacs.1c01931; (c) L. Escobar and P. Ballester, Chem. Rev., 2021, 121, 2445–2514, DOI:10.1021/acs.chemrev.0c00522; (d) R. Saha, B. Mondal and P. S. Mukherjee, Chem. Rev., 2022, 122, 12244–12307, DOI:10.1021/acs.chemrev.1c00811; (e) L. S. Lisboa, M. Riisom, H. J. Dunne, D. Preston, S. M. F. Jamieson, L. J. Wright, C. G. Hartinger and J. D. Crowley, Dalton Trans., 2022, 51, 18438–18445, 10.1039/D2DT02720H; (f) R.-J. Li, J. de Montmollin, F. Fadaei-Tirani, R. Scopelliti and K. Severin, Dalton Trans., 2023, 52, 6451–6456, 10.1039/D3DT00248A.
- (a) H. Ube, R. Yamada, J.-I. Ishida, H. Sato, M. Shiro and M. Shionoya, J. Am. Chem. Soc., 2017, 139, 16470–16473, DOI:10.1021/jacs.7b09439; (b) N. Mittal, S. Pramanik, I. Paul, S. De and M. Schmittel, J. Am. Chem. Soc., 2017, 139, 4270–4273, DOI:10.1021/jacs.6b12951; (c) P. K. Biswas, S. Saha, Y. Nanaji, A. Rana and M. Schmittel, Inorg. Chem., 2017, 56, 6662–6670, DOI:10.1021/acs.inorgchem.7b00740; (d) A. Goswami, S. Saha, P. K. Biswas and M. Schmittel, Chem. Rev., 2020, 120(1), 125–199, DOI:10.1021/acs.chemrev.9b00159.
- (a) I. Paul, A. Goswami, N. Mittal and M. Schmittel, Angew. Chem., Int. Ed., 2018, 57, 354–358, DOI:10.1002/anie.201709644; (b) S. Kundu, I. Valiyev, D. Mondal, V. V. Rajasekaran, A. Goswami and M. Schmittel, RSC Adv., 2023, 13, 5168–5171, 10.1039/d3ra00062a; (c) E. Elramadi, S. Kundu, D. Mondal and M. Schmittel, Chem. Commun., 2023, 59, 4915–4918, 10.1039/d3cc00637a.
- (a) L. van Dijk, M. J. Tilby, R. Szpera, O. A. Smith, H. A. P. Bunce and S. P. Fletcher, Nat. Rev. Chem., 2018, 2, 117, DOI:10.1038/s41570-018-0117; (b) A. Goswami, T. Paululat and M. Schmittel, J. Am. Chem. Soc., 2019, 141, 15656–15663, DOI:10.1021/jacs.9b07737.
- (a) L.-J. Chen, H.-B. Yang and M. Shionoya, Chem. Soc. Rev., 2017, 46, 2555–2576, 10.1039/c7cs00173h; (b) M. Pan, K. Wu, J.-H. Zhang and C.-Y. Su, Coord. Chem. Rev., 2019, 378, 333–349, DOI:10.1016/j.ccr.2017.10.031.
- (a) T. M. Bräuer, Q. Zhang and K. Tiefenbacher, J. Am. Chem. Soc., 2017, 139, 17500–17507, DOI:10.1021/jacs.7b08976; (b) Y. Zhu, J. Rebek Jr. and Y. Yu, Chem. Commun., 2019, 55, 3573–3577, 10.1039/c9cc01611b; (c) Y. Peng, Z. Su, M. Jin, L. Zhu, Z.-J. Guan and Y. Fang, Dalton Trans., 2023, 52, 15216–15232, 10.1039/D3DT01679J.
- (a) A. C. H. Jans, A. Gómez-Suárez, S. P. Nolan and J. N. H. Reek, Chem. – Eur. J., 2016, 22, 14836–14839, DOI:10.1002/chem.201603162; (b) Q. Zhang, L. Catti and K. Tiefenbacher, Acc. Chem. Res., 2018, 51, 2107–2114, DOI:10.1021/acs.accounts.8b00320.
- D. P. August, G. S. Nichol and P. J. Lusby, Angew. Chem., Int. Ed., 2016, 55, 15022–15026, DOI:10.1002/anie.201608229.
- (a) A. Ghosh, I. Paul, S. Saha, T. Paululat and M. Schmittel, Org. Lett., 2018, 20, 7973–7976, DOI:10.1021/acs.orglett.8b03541; (b) S. Saha, P. K. Biswas, I. Paul and M. Schmittel, Chem. Commun., 2019, 55, 14733–14736, 10.1039/c9cc07415e.
- (a) Q. Zhang and K. Tiefenbacher, Nat. Chem., 2015, 7, 197–202, DOI:10.1038/nchem.2181; (b) Q. Zhang and K. Tiefenbacher, Angew. Chem., Int. Ed., 2019, 58, 12688–12695, DOI:10.1002/anie.201906753.
- P. Liao, B. W. Langloss, A. M. Johnson, E. R. Knudsen, F. S. Tham, R. R. Juliana and R. J. Hooley, Chem. Commun., 2010, 46, 4932–4934, 10.1039/c0cc00234h.
- M. Schmittel, C. Michel, S.-X. Liu, D. Schildbach and D. Fenske, Eur. J. Inorg. Chem., 2001, 1155–1166, DOI:10.1002/1099-0682(200105)2001:5<1155::AID-EJIC1155>3.0.CO;2-C.
- S. Mandal and A. Basak, Tetrahedron Lett., 2009, 3641–3644, DOI:10.1016/j.tetlet.2009.03.168.
- H. J. Reich, NMR Spectrum Calculations: WinDNMR, Version 7.1.13, Department of Chemistry, University of Wisconsin Search PubMed.
- X. Cheng, X. Cao, J. Xuan and W.-J. Xiao, Org. Lett., 2018, 20, 52–55, DOI:10.1021/acs.orglett.7b03344.
- H.-F. Yao, D.-L. Wang, F.-H. Li, B. Wu, Z.-J. Cai and S.-J. Ji, Org. Biomol. Chem., 2020, 18, 7577–7584, 10.1039/d0ob01517b.
- Q. Dinga and J. Wu, Adv. Synth. Catal., 2008, 350, 1850–1854, DOI:10.1002/adsc.200800301.
- J. Chen, B. Liu, Y. Chen, Q. He and C. Yang, RSC Adv., 2014, 4, 11168–11175, 10.1039/c3ra47324d.
- P. K. Biswas, S. Saha, T. Paululat and M. Schmittel, J. Am. Chem. Soc., 2018, 140, 9038–9041, DOI:10.1021/jacs.8b04437.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, compound characterizations, spectral data, UV-vis titrations data. See DOI: https://doi.org/10.1039/d3dt04300b |
| This journal is © The Royal Society of Chemistry 2024 |