Open Access Article
Open Access ArticleSO2 capture and detection with carbon microfibers (CMFs) synthesised from polyacrylonitrile†
Ana
Yañez-Aulestia
 a,
Valeria B.
López-Cervantes
a,
Valeria B.
López-Cervantes
 b,
J. Marcos
Esparza-Schulz
a,
Diego
Solis-Ibarra
b,
J. Marcos
Esparza-Schulz
a,
Diego
Solis-Ibarra
 b,
Ilich A.
Ibarra
b,
Ilich A.
Ibarra
 bc,
Salomón
Cordero-Sánchez
*a,
Elí
Sánchez-González
bc,
Salomón
Cordero-Sánchez
*a,
Elí
Sánchez-González
 *b and
Reyna
Ojeda-López
*a
*b and
Reyna
Ojeda-López
*a
aLaboratorio de Fisicoquímica de Superficies, Departamento de Química, Universidad Autónoma Metropolitana-Iztapalapa (UAM-I), Mexico City, CDMX 09310, Mexico. E-mail: scs@xanum.uam.mx; rol@xanum.uam.mx
bLaboratorio de Fisicoquímica y Reactividad de Superficies (LaFReS), Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior s/n, CU, Del Coyoacán, 04510, Ciudad de México, Mexico. E-mail: elisg@materiales.unam.mx
cOn sabbatical as “Catedra Dr Douglas Hugh Everett” at Departamento de Química, Universidad Autónoma Metropolitana-Iztapalapa, San Rafael Atlixco 186, Col. Leyes de Reforma 1ra Seccion, Iztapalapa, C.P. 09310, Ciudad de México, Mexico
First published on 18th March 2024
Abstract
SO2 emissions not only affect local air quality but can also contribute to other environmental issues. Developing low-cost and robust adsorbents with high uptake and selectivity is needed to reduce SO2 emissions. Here, we show the SO2 adsorption–desorption capacity of carbon microfibers (CMFs) at 298 K. CMFs showed a reversible SO2 uptake capacity (5 mmol g−1), cyclability over ten adsorption cycles with fast kinetics and good selectivity towards SO2/CO2 at low-pressure values. Additionally, CMFs’ photoluminescence response to SO2 and CO2 was evaluated.
Sulphur dioxide (SO2) is a highly toxic gas that is accountable for severe respiratory illnesses, even at very low concentrations. For example, exposure to small amounts of SO2 (as low as 1.5 ppm) for only a few minutes can cause momentary incapacity to breathe, and at higher concentrations (above 100 ppm) can cause death.1
Different strategies to remove SO2 (flue gas desulphurisation processes FGD) have been typically used with acceptable results. These include limestone scrubbers (producing calcium sulphite)2 and even SO2 fixation (disulfitomercurate).3 However, these procedures exhibited drawbacks associated with large amounts of wastewater, high toxicity, corrosion of pipelines, and high recuperation fees. Other SO2 capture alternatives, such as silicas, zeolites, metal oxides, and activated carbons, have exhibited low SO2 efficiency.4,5 Although metal–organic frameworks (MOFs) have demonstrated promising SO2 capture results, for example, MOF-177 and MIL-101(Cr) showing high SO2 capture values, the crystal structure of these materials collapsed after being in contact with SO2.6
Most of the current research on SO2 has been narrowly focused on capturing this corrosive gas. However, the SO2 capture is not the only relevant; SO2 detection is as suitable as the capture and conversion of SO2.7 Efficient materials for SO2 detection are required to comply with the following characteristics: (i) high chemical stability towards SO2 under more realistic conditions (60% of relative humidity), (ii) non-dependency on relatively high surface areas, and (iii) high processability.6 In addition to remarkable chemical and structural stability, such “detector materials” are characterised for showing high SO2 uptake at low pressure, providing feasible applicability in SO2 detection devices.8 Cooper et al. demonstrated outstanding SO2 capture in porous organic cages (POCs) at low pressure.9,10 Therefore, new porous platforms have appeared as exciting alternatives to capture and detect corrosive and explosive gases. For example, Hiraoka and co-workers reported a functionalised organic nanotube with optimal selective fluorescence properties to detect liquefied petroleum gas.11
Carbon materials have been explored for SO2 capture. Yi et al., tested coconut shell-based activated carbon (SAC) and coal-based activated carbon (CAC), where SAC was the best adsorbent for SO2.12 Muñiz et al. performed thermal and chemical treatments to enhance the SO2 uptake on activated carbon fibres, and they concluded that the superficial functionalities with a basic character seem to be the most important characteristic concerning SO2 capture.13 Wang et al., developed a series of N-doped coal-based porous carbons (NCPCs) by calcining a mixture of anthracite, MgO, KOH and carbamide at 1073 K; their results showed that the balance between nitrogen doping content and specific surface area (microporosity) improved the number of active adsorption sites of SO2.14 In this context, the carbon microfibers (CMFs) obtained by calcination of polyacrylonitrile microfibers (PANMFs)15 present an opportunity for SO2 detection due to the following aspects: (i) chemical composition based on nitrogen and oxygen functional groups resulting from the polymer precursor (PAN), (ii) high microporosity controllable depending on calcination temperature, (iii) good thermal stability, and (iv) reversible gas adsorption (e.g., CO2 or CH4). Concerning nitrogen functional groups, there have been identified four groups in the CMFs: N-6 (pyridine-N), N-5 (pyrrolic-N), N-X (pyridine-N-oxide) and N-Q (quaternary-N or graphitic-N).15 Some of these groups have improved the performance of CMFs in oxygen reduction reactions (ORR) in fuel cells16 and their gas adsorption properties (CO2 and CH4).15
Textural, chemical, and structural characterisation of the CMFs have been reported previously (Fig. S1, ESI†).17 CMFs were obtained by calcination of PANMFs at 1173 K; this material has a specific surface area of 731 m2 g−1, a total pore volume of 0.348 cm3 g−1, and a microporosity above 70%. An average pore size of 0.78 nm was calculated from the N2 adsorption isotherm at 77 K, and 0.5 nm was estimated using the CO2 adsorption isotherm at 273 K.17 The CMFs average chemical composition is C: 89%, N: 6%, and O: 5%. It is important to mention that the fibrous structure of PANMFs is preserved after calcination with fibre diameters between 200 and 400 nm.
Since CMFs contain several nitrogen sites, which can be potential SO2-adsorption sites, we measured the SO2 adsorption at 298, 303 and 308 K (Fig. 1a). SO2 isotherms showed a type-I profile based on IUPAC18 with a small hysteresis. CMFs showed a maximum uptake of 5.2, 4.9 and 4.6 mmol g−1 at 1 bar for 298, 303 and 308 K, respectively. This value is higher than several reported in the literature compared to other carbonaceous or inorganic materials in the function of superficial area BET (Fig. S4, ESI†). The three SO2 adsorption isotherms were used to calculate the isosteric enthalpy of adsorption, obtaining values around −30 kJ mol−1 (see Fig. S3, ESI†), consistent with a physisorption process and mild regeneration conditions.
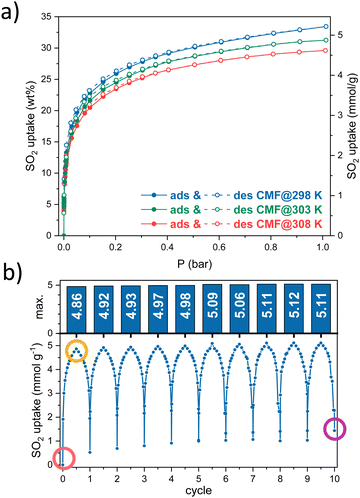 | ||
| Fig. 1 (a) SO2 adsorption–desorption isotherms at 298, 303, and 308 K, (b) ten SO2 adsorption–desorption cycles on CMFs. | ||
Then, cyclability tests were carried out to evaluate the reusability of the material at the conditions where the highest SO2 capture was obtained. Ten SO2 adsorption–desorption cycles were performed at 298 K until 1 bar. The amount of SO2 captured in each cycle is stable, around 5 mmol g−1 (Fig. 1b). Between each cycle, a vacuum activation process was enough to desorb almost all the SO2 adsorbed, leading to the slight increase in the baseline and thus, the maximum SO2 uptake in each cycle.
FTIR-ATR and SEM measurements were performed to characterise the CMFs in the SO2 capture process (Fig. 2). In the three different stages during the SO2 uptake (before and after the first adsorption cycle and after ten desorption cycles indicated by pink, yellow and purple colours, respectively, in Fig. 1 and 2), the IR-ATR spectra showed the presence of ester groups between 2250 to 2000 cm−1, and coupling C–N stretching and N–H deformation modes of C–N–H groups (amide) around 1522 cm−1. For the SO2-saturated CMFs sample, a characteristic band in 1050 cm−1 was identified, indicating the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group was present (Fig. 2a).19,20 These results are in good agreement with the SEM micrographs and EDX analyses. Carbon microfibres morphology only changes when the SO2 capture process occurs; the surface showed small globularities protruding from the fibre channels, and the EDX results showed sulphur presence of around 7 wt% and an increment of the oxygen percentage as well (Fig. 2d). The EDX mapping displayed a homogeneous distribution of C, N, O and S on the surface in the area shown inside the 5 μm scale micrograph in Fig. 2d. The sample showed the same morphology and composition before starting the cycles (Fig. 2b), with the sample pristine and after the last desorption cycle (Fig. 2c). These results indicate that the CMFs are stable against SO2 for ten adsorption–desorption cycles.
O group was present (Fig. 2a).19,20 These results are in good agreement with the SEM micrographs and EDX analyses. Carbon microfibres morphology only changes when the SO2 capture process occurs; the surface showed small globularities protruding from the fibre channels, and the EDX results showed sulphur presence of around 7 wt% and an increment of the oxygen percentage as well (Fig. 2d). The EDX mapping displayed a homogeneous distribution of C, N, O and S on the surface in the area shown inside the 5 μm scale micrograph in Fig. 2d. The sample showed the same morphology and composition before starting the cycles (Fig. 2b), with the sample pristine and after the last desorption cycle (Fig. 2c). These results indicate that the CMFs are stable against SO2 for ten adsorption–desorption cycles.
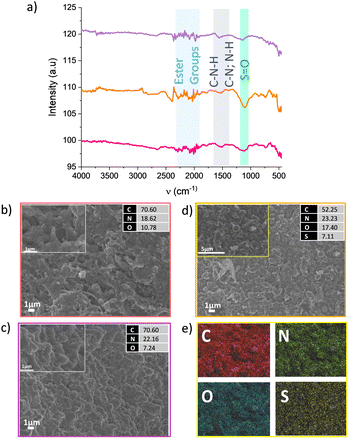 | ||
| Fig. 2 (a) IR-ATR spectra at different stages according to the points marked in Fig. 1b, pristine sample (pink), after SO2 uptake (yellow), and after 10 desorption cycles (violet). SEM micrographs with their EDX results on (b) pristine sample, (c) after 10 desorption cycles, (d) after SO2 uptake, and (e) the EDX mapping results of the rectangular area inside panel (d). | ||
Considering that SO2 is often found as a minor component of flue gas mixtures (diluted in CO2 and/or N2), the separation selectivity is a crucial factor to consider. The SO2/CO2 separation selectivity was determined employing the ideal adsorbed solution theory (IAST) using two monocomponent isotherms of SO2 and CO2 at 298 K (Fig. 3a, details on ESI†). The CMFs exhibited good selectivity values for the binary mixtures SO2/CO2 in the low-pressure domain, 122, 118 and 110 for 1%, 5% and 10% of SO2 at 0.05 bar, respectively (Fig. 3b). The IAST selectivity result is comparable with similar superficial area BET adsorbents such as zeolite Y (180, 930 m2 g−1),21 Mg-gallate (321, 576 m2 g−1),22 Co-gallate (143, 494 m2 g−1),22 DMOF-TM (169, 900 m2 g−1),23 MIL-160 (128, 1170 m2 g−1),24 Cu-ATC (114, 600 m2 g−1),25 NbOFFIVE-Cu-TPA (78, 1179 m2 g−1).26 Granted, the SO2 uptake of CMFs falls short in front of benchmark materials. However, these results invite us to explore another application of the CMFs, SO2 detection, where the reversible adsorption and selectivity are relevant.
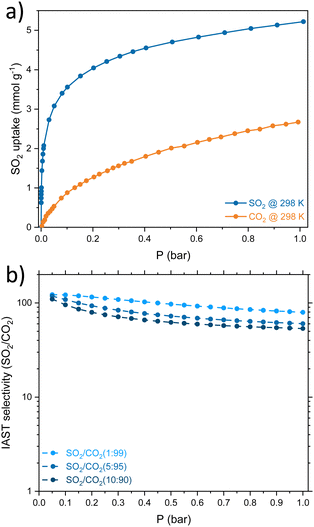 | ||
| Fig. 3 (a) Comparison of the SO2 and CO2 adsorption isotherms at 298 K on CMFs. (b) IAST selectivity of SO2/CO2 on MCFs for different concentrations of the binary mixture. | ||
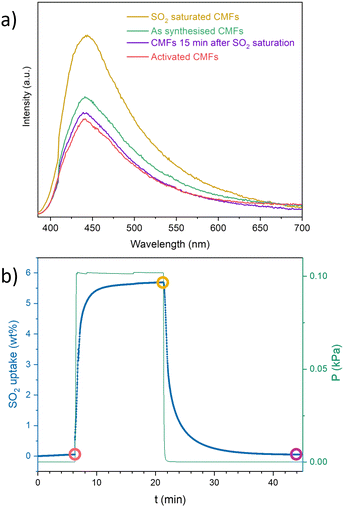
Photoluminescence experiments were carried out on the CMFs using a λex = 370 nm after exposure to an SO2-saturated atmosphere (details on ESI†). The PL intensity increased by about 50% after the SO2 exposure, compared to the activated sample (Fig. 4a). This switch-on emission decreased over time: after 15 min of exposure, the emission returned to the value of the reference sample. However, when the sample is not activated and has been left in contact with the environment, the signal increases, indicating that it detects other molecules, such as H2O or CO2. To evaluate this hypothesis, PL measurements were performed by saturating the CMFs with CO2 and H2O separately (Fig. S6, ESI†). The results showed a positive response for carbon dioxide but not for water. The presence of specific functional groups on carbon materials as the nitrogen-bearing active sites in the CMFs (in the form of NH2, for example, where nitrogen acts as an electron donor) may enhance the interaction with SO2 and the resulting photoluminescent response. The interaction between these gases with free electron pairs favours light absorption and subsequent emission for detection.27 For the SO2 interaction, the observed reversibility agrees with the observed adsorption–desorption kinetics of SO2 obtained by gravimetric experiments (Fig. 4b). However, even though a similar PL intensity was observed for CO2 exposed sample compared to SO2, the PL emission of the CO2 exposed sample remained after several hours, indicating a slow desorption of this gas molecule (Fig. S7, ESI†).
In summary, SO2 adsorption–desorption capacity at room temperature and 1 bar of CMFs was around 5 g mol−1. It maintained good chemical and morphological stability during 10 adsorption–desorption cycles of SO2 and a good SO2/CO2 selectivity, achieving a reasonable degree of reuse. When evaluating the photoluminescence of the material, it was determined that it can detect SO2 and CO2 but not H2O and that SO2 desorption is faster than CO2. CMFs may be functionalised to improve their textural properties, SO2 uptake and selectivity overall.
I. A. I. thanks PAPIIT UNAM (IN201123), México, for financial support, U. Winnberg (Euro Health) for scientific discussions and G. Ibarra-Winnberg for scientific encouragement. R. O.-L. thanks are also given to the project “Medios porosos y superficies: preparación y caracterización” (UAM-I, DCBI).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Schwartz and D. W. Dockery, Am. Rev. Respir. Dis., 1992, 145, 600–604 CrossRef CAS PubMed.
- F. J. Gutiérrez Ortiz, F. Vidal, P. Ollero, L. Salvador, V. Cortés and A. Giménez, Ind. Eng. Chem. Res., 2006, 45, 1466–1477 CrossRef.
- P. W. West and G. C. Gaeke, Anal. Chem., 1956, 28, 1816–1819 CrossRef CAS.
- M. A. Hanif, N. Ibrahim and A. Abdul Jalil, Environ. Sci. Pollut. Res., 2020, 27, 27515–27540 CrossRef CAS PubMed.
- R. K. Srivastava, Controlling SO2 Emissions – A Review of Technologies, DIANE Publishing, Washington, D. C., 2000 Search PubMed.
- E. Martínez-Ahumada, M. L. Díaz-Ramírez, M. de, J. Velásquez-Hernández, V. Jancik and I. A. Ibarra, Chem. Sci., 2021, 12, 6772–6799 RSC.
- S. J. Rowland and J. A. F. Rook, Int. J. Dairy Technol., 1961, 14, 112–114 CrossRef.
- V. Chernikova, O. Yassine, O. Shekhah, M. Eddaoudi and K. N. Salama, J. Mater. Chem. A, 2018, 6, 5550–5554 RSC.
- E. Martínez-Ahumada, D. He, V. Berryman, A. López-Olvera, M. Hernandez, V. Jancik, V. Martis, M. A. Vera, E. Lima, D. J. Parker, A. I. Cooper, I. A. Ibarra and M. Liu, Angew. Chem., Int. Ed., 2021, 60, 17556–17563 CrossRef PubMed.
- N. K. Gupta, A. López-Olvera, E. González-Zamora, E. Martínez-Ahumada and I. A. Ibarra, ChemPlusChem, 2022, 87, e202200006 CrossRef CAS PubMed.
- Y.-Y. Zhan, J. Liao, M. Kajita, T. Kojima, S. Takahashi, T. Takaya, K. Iwata and S. Hiraoka, Commun. Chem., 2019, 2, 107 CrossRef.
- H. Yi, Z. Wang, H. Liu, X. Tang, D. Ma, S. Zhao, B. Zhang, F. Gao and Y. Zuo, J. Chem. Eng. Data, 2014, 59, 1556–1563 CrossRef CAS.
- J. Muñiz, J. E. Herrero and A. B. Fuertes, Appl. Catal., B, 1998, 18, 171–179 CrossRef.
- Q. Wang, L. Han, Y. Wang, Z. He, Q. Meng, S. Wang, P. Xiao and X. Jia, RSC Adv., 2022, 12, 20640–20648 RSC.
- R. Ojeda-López, J. M. Esparza-Schulz, I. J. Pérez-Hermosillo, A. Hernández-Gordillo and A. Domínguez-Ortiz, Fibers, 2019, 7, 81 CrossRef.
- R. Ojeda-López, G. Ramos-Sánchez, J. M. Esparza-Schulz, L. Lartundo and A. Domínguez-Ortiz, Int. J. Hydrogen Energy, 2017, 42, 30339–30348 CrossRef.
- R. Ojeda-López, E. Vilarrasa-García, D. C. S. Azevedo, C. Felipe, J. A. Cecilia and E. Rodríguez-Castellón, Fuel, 2022, 324, 124242 CrossRef.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- P. Larkin, Infrared and Raman Spectroscopy, Elsevier, 2011 Search PubMed.
- M. R. Derrick, D. Stulik and J. M. Landry, Infrared Spectroscopy in Conservation Science, Getty Conservation Institute, 2000 Search PubMed.
- P. Brandt, A. Nuhnen, S. Öztürk, G. Kurt, J. Liang and C. Janiak, Adv. Sustainable Syst., 2021, 5, 2000285 CrossRef CAS.
- F. Chen, D. Lai, L. Guo, J. Wang, P. Zhang, K. Wu, Z. Zhang, Q. Yang, Y. Yang, B. Chen, Q. Ren and Z. Bao, J. Am. Chem. Soc., 2021, 143, 9040–9047 CrossRef CAS PubMed.
- S. Xing, J. Liang, P. Brandt, F. Schäfer, A. Nuhnen, T. Heinen, I. Boldog, J. Möllmer, M. Lange, O. Weingart and C. Janiak, Angew. Chem., Int. Ed., 2021, 60, 17998–18005 CrossRef CAS PubMed.
- P. Brandt, A. Nuhnen, M. Lange, J. Möllmer, O. Weingart and C. Janiak, ACS Appl. Mater. Interfaces, 2019, 11, 17350–17358 CrossRef CAS PubMed.
- Z. Zhu, K. Wu, X. Liu, P. Zhang, S. Chen, J. Chen, Q. Deng, Z. Zeng, S. Deng and J. Wang, AIChE J., 2022, 68, e17811 CrossRef CAS.
- W. Xu, L. Li, M. Guo, F. Zhang, P. Dai, X. Gu, D. Liu, T. Liu, K. Zhang, T. Xing, M. Wang, Z. Li and M. Wu, Angew. Chem., Int. Ed., 2023, 62, e202312029 CrossRef CAS PubMed.
- X. Zhang, B. Yang, X. Wang and C. Luo, Sensors, 2012, 12, 9375–9385 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CMFs synthesis, characterisation details, and additional photoluminescence experiments. See DOI: https://doi.org/10.1039/d3cc04437h |
| This journal is © The Royal Society of Chemistry 2024 |