Open Access Article
Open Access ArticleStructural predictions of three medium-sized thiolate-protected gold nanoclusters Au44(SR)30, Au56(SR)32, and Au60(SR)34†
Wenhua
Han‡
a,
Gang
Wang‡
a,
Pengye
Liu
b,
Wenliang
Li
*a and
Wen Wu
Xu
 *b
*b
aCollege of Energy Engineering, Xinjiang Institute of Engineering, Urumqi 830023, China. E-mail: wenliangli@vip.126.com
bDepartment of Physics, School of Physical Science and Technology, Ningbo University, Ningbo 315211, China. E-mail: xuwenwu@nbu.edu.cn
First published on 27th July 2023
Abstract
The knowledge of structural evolution among thiolate-protected gold nanoclusters is not only helpful for understanding their structure–property relationship but also provides scientific evidence to rule-guided structure predictions of gold nanoclusters. In this paper, three new atomic structures of medium-sized thiolate-protected gold nanoclusters, i.e. Au44(SR)30, Au56(SR)32, and Au60(SR)34, are predicted based on the grand unified model and ring model. Two structural evolution rules, i.e., Au44(SR)28 + [Au12(SR)4] → Au56(SR)32 + [Au12(SR)4] → Au68(SR)36 and Au44(SR)30 + [Au8(SR)2] → Au52(SR)32 + [Au8(SR)2] → Au60(SR)34 + [Au8(SR)2] → Au68(SR)36, are explored. The generic growth patterns underlying both sequences of nanoclusters can be viewed as sequential addition of four and three highly stable tetrahedral Au4 units on the cores, respectively. In addition, density functional theory calculations show that these three newly predicted gold nanoclusters have very close formation energies with their adjacent structures, large highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) gaps, and all-positive harmonic vibration frequencies, indicating their high stabilities.
Introduction
The knowledge of structural evolution among thiolate-protected gold nanoclusters is beneficial for better understanding their structure–property relationship.1–4 Thanks to the structural determination of Au28(TBBT)20 (TBBT = 4-tert-butyl-benzenethiolate), Au36(TBBT)24, Au44(TBBT)28, and Au52(TBBT)32, a “magic series” Au8n+4(TBBT)4n+8 (n = 3–6) with a uniform anisotropic growth pattern was identified as a typical case to investigate their size-dependent properties.5,6 For example, the size-dependent quantum confinement nature understood by the empirical scaling law and classical “particle in a box” model and the redshift of the onset of the optical absorption spectra with the increment of cluster size can be observed in this “magic series”. The femtonanosecond excited state dynamics of this “magic series” demonstrate that the relaxation of hot carriers and recombination of band-edge carriers will slow down as the size increases from Au28(TBBT)20 to Au52(TBBT)32. In addition, the evolution of the catalytic activity of the “magic series” Au8n+4(TBBT)4n+8 (n = 3–5) can potentially be attributed to the periodic evolution in the inner cores.Another important application for the understanding of structural evolution among thiolate-protected gold nanoclusters is to provide scientific evidence to rule-guided structure predictions of gold nanoclusters.7–9 To date, ten groups of “magic series” of thiolate-protected gold nanoclusters have been identified, as shown in Table 1, resulting in a large number of structural predictions of gold clusters.5,6,10–14 Particularly, among these predicted structures, two atomic structures of Au44(SR)28 and Au36(SR)24 were experimentally confirmed by X-ray crystallography.15,16 For each “magic series” of thiolate-protected gold nanoclusters, their structural evolution can be viewed as the addition of a common motif or addition of several highly stable triangular Au3 or tetrahedral Au4 units on the cores. Taking the “magic series” Au28+n(SR)20−n (n = 0–2) as an example, the structural evolution of Au28(SR)20, Au29(SR)19, and Au30(SR)18 can be viewed as sequential addition of a common motif [Au(SR)−1], namely Au28(SR)20 + [Au(SR)−1] → Au29(SR)19 + [Au(SR)−1] → Au30(SR)18, or sequential addition of one highly stable triangular Au3 unit on the cores.
| “Magic series” of gold nanoclusters | Common motif | Number of units added to the core | |
|---|---|---|---|
| Triangular Au3 | Tetrahedral Au4 | ||
| a (*) denotes the newly identified “magic series” of thiolate-protected gold nanoclusters in this work. | |||
| Au28+4n(SR)20+2n (n = 0–6) | [Au4(SR)2] | 0 | 1 |
| Au28+n(SR)20−n (n = 0–2) | [Au(SR)−1] | 1 | 0 |
| Au44+24n(SR)28+8n (n = 0–2) | [Au24(SR)8] | 0 | 8 |
| Au22+6n(SR)18+2n (n = 0–3) | [Au6(SR)2] | 0 | 2 |
| Au40+9n(SR)24+3n (n = 0–3) | [Au9(SR)3] | 0 | 3 |
| Au28+26n(SR)20+14n (n = 0–4) | [Au26(SR)14] | 0 | 6 |
| Au36+36n(SR)24+16n (n = 0–4) | [Au36(SR)16] | 0 | 10 |
| Au44+42n(SR)28+18n (n = 0–4) | [Au42(SR)18] | 0 | 12 |
| Au52+52n(SR)32+20n (n = 0–4) | [Au52(SR)20] | 0 | 16 |
| Au52+24n(SR)32+10n (n = 0–4) | [Au24(SR)10] | 0 | 7 |
| Au44+12n(SR)28+4n (n = 0–2)* | [Au12(SR)4] | 0 | 4 |
| Au44+8n(SR)30+2n (n = 0–3)* | [Au8(SR)2] | 0 | 3 |
With the development of theoretical models, i.e., the grand unified model (GUM) and ring model,17,18 structural insights into the better understanding of thiolate-protected gold nanoclusters can be obtained, thereby providing theoretical tools to identify “magic series” of thiolate-protected gold nanoclusters with different common motifs. In the GUM, the gold cores of ligand-protected gold nanoclusters can be viewed as several triangular Au3(2e) or tetrahedral Au4(2e) elementary blocks packing or fusing together.19 Meanwhile in the ring model, the interfacial interactions between SR [Au(SR)]x (x = 0, 1, 2, 3,…) protection motifs and gold cores in thiolate-protected gold nanoclusters can be understood by several [Aum(SR)n] (m = 4–8, 10, 12, and 0 ≤ n ≤ m) rings. Based on the GUM and ring model, some thiolate-protected gold nanoclusters, such as Au15(SR)13, Au22(SR)16, and Au30(SR)20, were predicted to have high stability.20–22 In this paper, based on the GUM and ring model, two new groups of “magic series” of thiolate-protected gold nanoclusters (Au44+8n(SR)30+2n (n = 0–3) and Au44+16n(SR)28+4n (n = 0–2)) were identified via structural predictions of three medium-sized nanoclusters (Au44(SR)30, Au56(SR)32, and Au60(SR)34).
Results and discussion
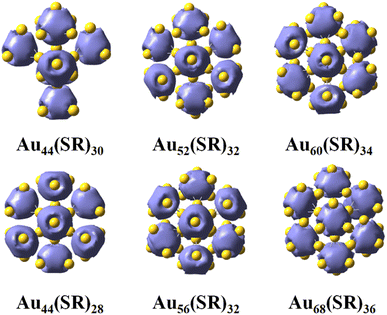
We focused on three thiolate-protected gold nanoclusters Au44(SR)28, Au52(SR)32, and Au68(SR)36, and found an interesting phenomenon: a top view of these structures reveals that they are hexagonal, while their gold cores are round (Fig. 1),23–25 suggesting that Au44(SR)28, Au52(SR)32, and Au68(SR)36 have very similar morphology. In addition, these thiolate-protected gold nanoclusters follow the grand unified model (GUM) and their gold cores can be regarded as several tetrahedral Au4 elementary blocks following the duet rule.17,18 This interesting phenomenon led us to ponder the question: Are there other new intermediate structures with similar morphology between these thiolate-protected gold nanoclusters?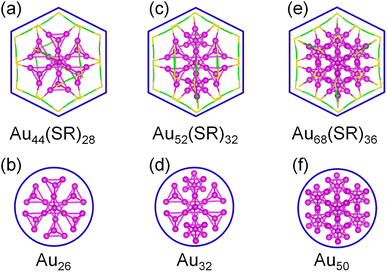 | ||
| Fig. 1 Structures and their gold cores of Au44(SR)28 (a and b), Au52(SR)32 (c and d), and Au68(SR)36 (e and f) nanoclusters. | ||
In Fig. 2, the Au26 core of Au44(SR)28 is formed by the face-to-face combination of two Au13 units, which can be regarded as four tetrahedral Au4 fusing together by sharing three Au atoms. The Au50 core of Au68(SR)36 nanoclusters is composed of two Au25 units, which can be decomposed into eight tetrahedral Au4 fusing together by sharing seven Au atoms. Thus, the Au26 core fused with four tetrahedral Au4 (blue atoms in Fig. 2a) or the Au50 core removed four tetrahedral Au4 (blue atoms in Fig. 2a) will form a new Au38 core. In the same way, a new Au41 and Au23 core can be formed by removing three tetrahedral Au4 (blue atoms in Fig. 2b) from the Au50 core of Au68(SR)36 nanoclusters or the Au32 core of Au52(SR)32 nanoclusters. It can be seen from the top view in Fig. 2 that these new cores are all round.
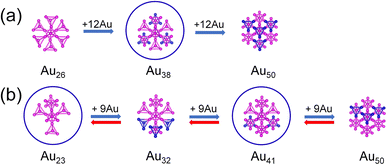 | ||
| Fig. 2 The construction of new Au38, Au23, and Au41 cores (a and b). Au: magenta and blue, respectively. The blue atoms are the added gold atoms. | ||
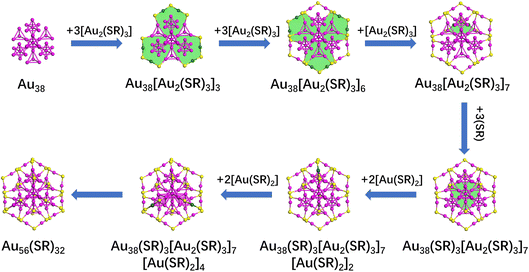
Employing the newly constructed Au38, Au23, and Au41 cores, new structures of thiolate-protected gold nanoclusters can be predicted by the GUM and ring model. In Fig. 3, the process for the Au38 core involves several steps. Initially, three [Au2(SR)3] protection motifs are added around the Au38 core, resulting in the formation of three [Au6(SR)3] rings and an Au38[Au2(SR)3]3 structure. Subsequently, three [Au2(SR)3] protection motifs are further added around the structure Au38[Au2(SR)3]3, forming three [Au6(SR)3] rings and an Au38[Au2(SR)3]6 structure. In the third step, [Au2(SR)3] protection motifs are incorporated behind the structure Au38[Au2(SR)3]6, resulting in the formation of a single [Au6(SR)3] ring and Au38[Au2(SR)3]7 structure. Then, in the fourth step, three [SR] molecules are added to the middle of the structure Au38[Au2(SR)3]7, forming another [Au6(SR)3] ring and Au38(SR)3[Au2(SR)3]7 structure. Finally, the structure Au38(SR)3[Au2(SR)3]7 undergoes the addition of four [Au(SR)2] protection motifs, leading to the prediction of a new structure, Au56(SR)32. Since the structure is the intermediate structure between Au44(SR)28 and Au68(SR)36, a new evolutionary path Au44+8n(SR)30+2n (n = 0–3) was identified.
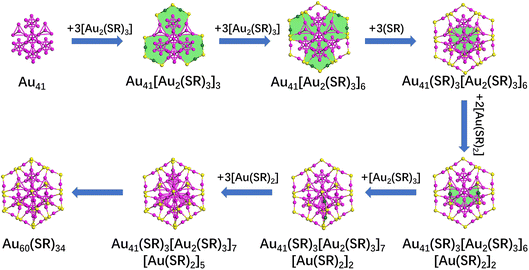
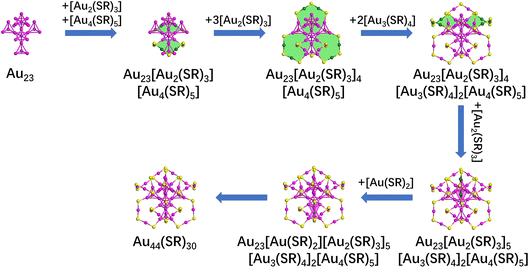
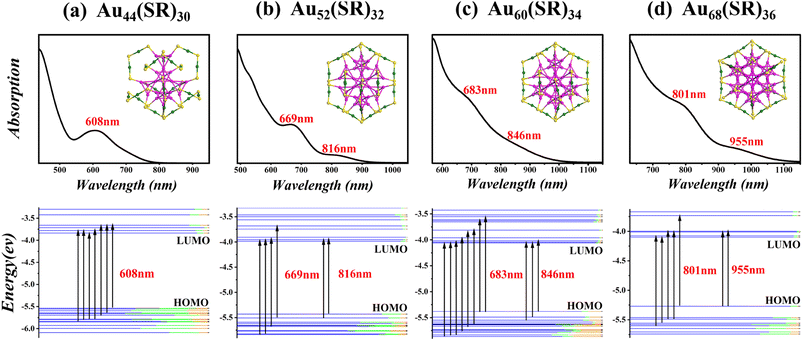
In Fig. 4, we illustrate the structure prediction of another gold nanocluster starting from an Au41 core. Initially, six [Au2(SR)3] protective motifs were introduced, resulting in the formation of six [Au6(SR)3] rings and an Au41[Au2(SR)3]6 structure. Subsequently, three [SR] ligands were added, leading to the creation of a single [Au6(SR)3] ring and Au41[Au2(SR)3]6[SR]3 structure. Next, two [Au(SR)2] ligands were further added to Au41[Au2(SR)3]6[SR]3 to form two [Au6(SR)2] rings and an Au41[SR]3[Au2(SR)3]6[Au(SR)2]2 structure. Finally, one [Au2(SR)3] and three [Au(SR)2] ligands were bonded to the Au41[SR]3[Au2(SR)3]6[Au(SR)2]2 structure to achieve the full structure prediction of Au60(SR)34. Since the structure is the intermediate structure between Au52(SR)32 and Au68(SR)36, a new evolutionary path Au44+8n(SR)30+2n (n = 1–3) was identified. In addition, the structure prediction of Au44(SR)30 nanoclusters starting from the Au23 core is presented in Fig. 5 following the same way as the structure prediction of Au56(SR)32 and Au60(SR)34. Interestedly, the structure of Au44(SR)30 nanoclusters can be brought into the evolutionary path of Au44+8n(SR)30+2n (n = 0–3).
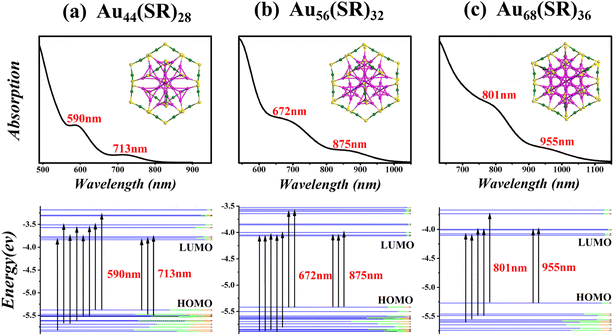
Employing Gaussian 09 software,26 density general function theory (DFT) calculations for Au44+8n(SR)30+2n (n = 0–3) and Au44+12n(SR)28+4n (n = 0–2) were performed to obtain the electronic properties of Au44(SR)30, Au56(SR)32, and Au60(SR)34 structures. In all calculations, the Perdew–Burke–Ernzerhof (PBE) and the all-electron basis set 6-31G* for H and S, and effective-core basis set LANL2DZ pseudopotential groups for Au were adopted.27,28 In addition, the R groups were simplified with H atoms in order to save computational costs. In Table 2, the computed harmonic vibrational frequencies of three new structures as well as other two reported structures are all positive, which indicates that these structures are located at the local minima of potential energy surfaces. The highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) gaps of the predicted nanoclusters are 1.71, 1.36, and 1.33 eV, respectively. The large HOMO–LUMO gaps and all-positive harmonic vibrational frequencies suggest that the predicted structures have high chemical stability. Furthermore, the formation energy E = E(n−1) + E(n+1) − E(n) of these newly predicted structures in two magic series Au44+8n(SR)30+2n (n = 0–3) and Au44+12n(SR)28+4n (n = 0–2) was calculated. The results show that the formation energies of Au44(SR)30, Au56(SR)32, and Au60(SR)34 structures are 0.50, 0.26, and 0.04 eV, respectively, suggesting their very close stability to their adjacent nanoclusters.
| Structures | HOMO–LUMO gaps/eV | Lowest vibrational frequency/cm−1 |
|---|---|---|
| Au44(SR)28 | 1.660 | 11.120 |
| Au44(SR)30 | 1.714 | 7.491 |
| Au52(SR)32 | 1.470 | 6.737 |
| Au56(SR)32 | 1.361 | 10.680 |
| Au60(SR)34 | 1.333 | 8.162 |
| Au68(SR)36 | 1.170 | — |
The superatom network model with natural density zoning (AdNDP) analysis was always employed to describe thiolate-protected gold nanoclusters.29 Taking the Au239+ core of Au44(SR)30 in Fig. 6 as an example, the 14 valence electrons of Au44(SR)30 can be viewed to be equally distributed on seven Au4 units. Therefore, the Au239+ core of Au44(SR)30 can be regarded as a network of seven 4c–2e (4c and 2e represent 4 centers and 2 electrons, respectively) units. A similar behaviour can also be seen in the Au(23+9n)(9+3n)+ core of Au44+8n(SR)30+2n (n = 0–3) and the Au(26+12n)(10+4n)+ core of Au44+12n(SR)28+4n (n = 0–2) nanoclusters (Fig. 6).
In Fig. 7 and 8, the optical absorption spectra and corresponding Kohn–Sham (KS) orbital energy levels of six nanoclusters computed by the time-dependent DFT (TD-DFT) method at the PBE/LANL2DZ level are shown. In addition, 300 singlet states were included in all TD-DFT calculations. Taking Au56(SR)32 in Fig. 7 as an example, Au56(SR)32 shows two broad peaks at 672 and 875 nm. The absorption peak at 875 nm mainly stems from the HOMO to LUMO, LUMO+1, and LUMO+2, and the absorption peak at 672 nm mainly stems from HOMO to LUMO+7 and LUMO+8, HOMO−7 and HOMO−8 to LUMO+2, HOMO−10 to LUMO+1, and HOMO−9 and HOMO−10 to LUMO. In addition, the absorption peaks of all the nanoclusters in Fig. 7 and 8 mainly involve the Au(sp) → Au(sp) transitions, which may be attributed to their similar morphology.
Conclusions
In summary, we have predicted the theoretical structures of three medium-sized nanoclusters Au44(SR)30, Au56(SR)32, and Au60(SR)34 based on the GUM and ring model, resulting in two new magic series Au44+8n(SR)30+2n (n = 0–3) and Au44+16n(SR)28+4n (n = 0–2). DFT calculations show that the predicted structures have high chemical stability and are likely to be synthesized in the laboratory. Our work further proves the high reliability and practicality of the GUM and ring model in designing the structures of thiolate-protected gold nanoclusters.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors are supported by the Natural Science Foundation of China (Grant No. 11974195) and Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant No. 2022D01B137).References
- D. Yang and Y. Zhu, Chin. J. Catal., 2021, 42, 245–250 CrossRef CAS.
- Y. Pei, P. Wang, Z. Ma and L. Xiong, Acc. Chem. Res., 2019, 52, 23–33 CrossRef CAS PubMed.
- W. W. Xu, Y. Li, Y. Gao and X. C. Zeng, Nanoscale, 2016, 8, 7396–7401 RSC.
- S. Chen, L. Xiong, S. Wang, Z. Ma, S. Jin, H. Sheng, Y. Pei and M. Zhu, J. Am. Chem. Soc., 2016, 138, 10754–10757 CrossRef CAS PubMed.
- C. Zeng, Y. Chen, K. Iida, K. Nobusada, K. Kirschbaum, K. J. Lambright and R. Jin, J. Am. Chem. Soc., 2016, 138, 3950–3953 CrossRef CAS PubMed.
- M. Zhou, C. Zeng, M. Y. Sfeir, M. Cotlet, K. Iida, K. Nobusada and R. Jin, J. Chem. Phys., 2017, 8, 4023–4030 CAS.
- W. W. Xu, X. Duan and X. C. Zeng, J. Phys. Chem. Lett., 2020, 11, 536–540 CrossRef CAS PubMed.
- X. Sun, P. Wang, L. Xiong and Y. Pei, Chem. Phys. Lett., 2018, 704, 68–75 CrossRef CAS.
- P. Liu, W. Han, M. Zheng and W. W. Xu, Nanoscale, 2020, 12, 20677–20683 RSC.
- M. Zhou, R. Jin, M. Y. Sfeir, Y. Chen, Y. Song and R. Jin, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E4697–E4705 CrossRef CAS PubMed.
- L. Yang, P. Wang, Z. Yang and Y. Pei, Nanoscale, 2020, 12, 5554–5566 RSC.
- Y. Sun, E. Wang, Y. Ren, K. Xiao, X. Liu, D. Yang, Y. Gao, W. Ding and Y. Zhu, Adv. Funct. Mater., 2019, 29, 1904242 CrossRef.
- Q. Li, M. Zhou, W. Y. So, J. Huang, M. Li, D. R. Kauffman, M. Cotlet, T. Higaki, L. A. Peteanu, Z. Shao and R. Jin, J. Am. Chem. Soc., 2019, 141, 5314–5325 CrossRef CAS PubMed.
- X. Du, J. Chai, S. Yang, Y. Li, T. Higaki, S. Li and R. Jin, Nanoscale, 2019, 11, 19158–19165 RSC.
- X. Liu, W. W. Xu, X. Huang, E. Wang, X. Cai, Y. Zhao, J. Li, M. Xiao, C. Zhang, Y. Gao, W. Ding and Y. Zhu, Nat. Commun., 2020, 11, 3349 CrossRef CAS PubMed.
- C. Zeng, Y. Chen, G. Li and R. Jin, Chem. Commun., 2014, 50, 55–57 RSC.
- W. Han, P. Liu, M. Zheng, X. C. Zeng and W. W. Xu, J. Phys. Chem. Lett., 2021, 12, 3006–3013 CrossRef CAS PubMed.
- W. W. Xu, B. Zhu, X. C. Zeng and Y. Gao, Nat. Commun., 2016, 7, 13574 CrossRef CAS PubMed.
- W. W. Xu, X. C. Zeng and Y. Gao, Acc. Chem. Res., 2018, 51, 2739–2747 CrossRef CAS PubMed.
- Y. Liu, W. Han, Z. Hong, W. W. Xu and E. Wang, J. Phys. Chem. Lett., 2022, 13, 5387–5393 CrossRef CAS PubMed.
- W. Han, E. Wang and W. W. Xu, Phys. Chem. Chem. Phys., 2022, 24, 15920–15924 RSC.
- P. Liu, W. Han, M. Zheng, W. Li, J. Ren, A. Tlahuice-Flores and W. W. Xu, J. Phys. Chem. A, 2021, 125, 5933–5938 CrossRef CAS PubMed.
- Y. Pei, S. Lin, J. Su and C. Liu, J. Am. Chem. Soc., 2013, 135, 19060–19063 CrossRef CAS PubMed.
- P. Wang, X. Sun, X. Liu, L. Xiong, Z. Ma and Y. Pei, J. Phys. Chem. Lett., 2017, 8, 1248–1252 CrossRef CAS PubMed.
- S. Zhuang, L. Liao, M.-B. Li, C. Yao, Y. Zhao, H. Dong, J. Li, H. Deng, L. Li and Z. Wu, Nanoscale, 2017, 9, 14809–14813 RSC.
- G. W. T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, revision D, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
- M. Caricato, B. Mennucci, G. Scalmani, G. W. Trucks and M. J. Frisch, J. Chem. Phys., 2010, 132, 084102 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- L. Cheng, Y. Yuan, X. Zhang and J. Yang, Angew. Chem., Int. Ed. Engl., 2013, 52, 9035–9039 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The cartesian coordinates of the three predicted thiolate-protected gold nanoclusters. See DOI: https://doi.org/10.1039/d3na00372h |
| ‡ W. Han and G. Wang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |