Enhancing multiphoton upconversion emissions through confined energy migration in lanthanide-doped Cs2NaYF6 nanoplatelets†
Chang
Zhou
ab,
Datao
Tu
 *abc,
Siyuan
Han
a,
Peng
Zhang
ab,
Luping
Wang
a,
Shaohua
Yu
a,
Jin
Xu
*abc,
Siyuan
Han
a,
Peng
Zhang
ab,
Luping
Wang
a,
Shaohua
Yu
a,
Jin
Xu
 ac,
Renfu
Li
ac,
Renfu
Li
 ac and
Xueyuan
Chen
ac and
Xueyuan
Chen
 *abc
*abc
aCAS Key Laboratory of Design and Assembly of Functional Nanostructures, Fujian Key Laboratory of Nanomaterials, and State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: dttu@fjirsm.ac.cn; xchen@fjirsm.ac.cn
bCollege of Chemistry, Fuzhou University, Fuzhou, Fujian 350108, China
cFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian 350108, China
First published on 29th April 2021
Abstract
Lanthanide (Ln3+)-doped upconversion (UC) nanocrystals have drawn tremendous attention because of their intriguing optical properties. Currently, it is highly desired but remains challenging to achieve efficient multiphoton UC emissions. Herein, we report the controlled synthesis of a new class of UC nanocrystals based on Cs2NaYF6:Yb/Tm nanoplatelets (NPs), which can effectively convert the 980 nm light to five-photon and four-photon UC emissions of Tm3+ without the fabrication of a complicated core/multishell structure required in traditional nanocrystals. Particularly, the as-prepared Cs2NaYF6:Yb/Tm NPs exhibit a maximal UV-to-NIR emission intensity ratio of 1.2, which is the highest among Tm3+-doped core-only UC nanocrystals. We reveal that the enhanced multiphoton UC emissions may benefit from the confined energy migration of Ln3+ dopants in the unique two-dimensional-like structure of Cs2NaYF6 NPs. As such, intense red and green UC emissions of Eu3+ and Tb3+ can further be generated via the cascade sensitization of Tm3+ and Gd3+ in Cs2NaYF6:Yb/Tm/Gd/Eu and Cs2NaYF6:Yb/Tm/Gd/Tb NPs, respectively. These results validate the superiority of Cs2NaYF6 for the future design of efficient UC nanocrystals towards versatile applications.
Introduction
Lanthanide (Ln3+)-doped upconversion (UC) nanocrystals can convert low-energy excitation to high-energy emission, which makes them attractive for applications in photocatalysis, anti-counterfeiting and biosensing.1–8 Based on the unique ladder-like 4f energy configurations of Ln3+ ions, UC emission from high energy levels is generated through successive absorption of low-energy photons with sensitizer ions (e.g., Yb3+, Nd3+) and transfer of the energy to activator ions (e.g., Er3+, Tm3+, Ho3+).9–14 Theoretically, elevating the concentration of Ln3+ dopants may improve the energy transfer efficiency and enhance the UC luminescence (UCL).15,16 Unfortunately, a high concentration of dopants may cause concentration quenching of UCL due to the deleterious energy migration or cross-relaxation processes between the neighboring Ln3+ ions.17,18 Moreover, the long-distance migration of excitation energy via highly concentrated Ln3+ dopants to lattice/surface defects may severely deteriorate the multiphoton UC emission in the ultraviolet (UV) spectral region.19Hitherto, substantial efforts have been made to overcome such obstacles and to enhance the multiphoton UV emission in Ln3+-doped UC nanocrystals.20–22 Typically, a core/multishell structure has to be constructed to suppress the energy migration in traditional nanocrystals. For example, Zhou et al. designed NaYF4:Er@NaYbF4@NaYF4 core/shell/shell nanocrystals to alleviate the energy back-transfer from Er3+ to Yb3+, which promoted the three-photon UC emission of Er3+.23 Chen et al. synthesized NaYF4@NaYbF4:Tm@NaYF4 core/shell/shell nanocrystals, where efficient five-photon UCL of Tm3+ was realized without suffering from concentration quenching of Yb3+.24 To circumvent the complicated synthesis procedures of core/multishell nanocrystals, it is of utmost importance to explore special host lattices to confine energy migration between Ln3+ dopants for producing intense multiphoton UC emissions.25,26
Cs2NaYF6, a face-centered cubic crystal with the space-group symmetry of Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, has been reported as an excellent laser or scintillation host.27,28 In the Cs2NaYF6 crystal, Na+ and Y3+ ions are surrounded by six halogen ions that form an octahedron. Particularly, the unit cell parameters a = b = c = 9.08 Å are much larger than those of widely reported α-NaYF4 with the same space-group symmetry (unit cell parameters a = b = c = 5.47 Å). As such, the energy migration between Ln3+ dopants is expected to be effectively confined.29,30 So far, all the previous research studies of Ln3+-doped Cs2NaYF6 have been carried out via hydrothermal and solid-state reaction methods, which resulted in bulk materials.31,32 Nowadays, it remains a great challenge to synthesize monodisperse Ln3+-doped Cs2NaYF6 nanocrystals, whose UCL properties and the related UC mechanism deserve to be investigated.
m, has been reported as an excellent laser or scintillation host.27,28 In the Cs2NaYF6 crystal, Na+ and Y3+ ions are surrounded by six halogen ions that form an octahedron. Particularly, the unit cell parameters a = b = c = 9.08 Å are much larger than those of widely reported α-NaYF4 with the same space-group symmetry (unit cell parameters a = b = c = 5.47 Å). As such, the energy migration between Ln3+ dopants is expected to be effectively confined.29,30 So far, all the previous research studies of Ln3+-doped Cs2NaYF6 have been carried out via hydrothermal and solid-state reaction methods, which resulted in bulk materials.31,32 Nowadays, it remains a great challenge to synthesize monodisperse Ln3+-doped Cs2NaYF6 nanocrystals, whose UCL properties and the related UC mechanism deserve to be investigated.
Herein, we propose a facile strategy for the controlled synthesis of Ln3+-doped Cs2NaYF6 nanoplatelets (NPs). Upon excitation at 980 nm, an integrated UV-to-NIR emission intensity ratio of up to 1.2 can be achieved based on the as-prepared Cs2NaYF6:Yb/Tm NPs, which is ∼40 times higher than that of α-NaYF4:Yb/Tm counterparts. By means of structural analysis, the enhanced multiphoton UCL mechanism is attributed to the inhibition of the energy migration between Ln3+ dopants in Cs2NaYF6. Furthermore, we demonstrate that intense UC emissions of Eu3+ and Tb3+ can be generated via the sensitization of Tm3+ and Gd3+ in Cs2NaYF6:Yb/Tm/Gd/Eu and Cs2NaYF6:Yb/Tm/Gd/Tb NPs, respectively (Scheme 1).
 | ||
| Scheme 1 Schematic illustration of Cs2NaYF6:Ln3+ NPs with highly efficient multiphoton UC emission upon 980 nm excitation. | ||
Results and discussion
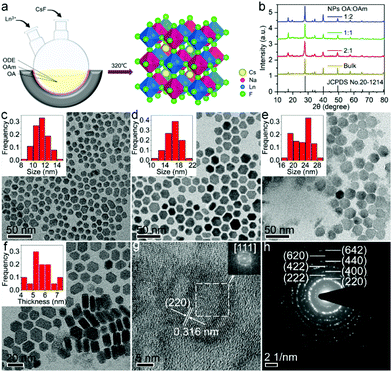
Cs2NaYF6:Yb/Tm NPs were synthesized via a facile high-temperature co-precipitation method, where CsF reacted with metal precursors in the mixed solvents containing oleic acid (OA), oleylamine (OAm) and octadecene (ODE) (Fig. 1a). X-ray diffraction (XRD) patterns show that all the diffraction peaks of the as-prepared NPs match well with the cubic Cs2NaYF6 (JCPDS no. 20-1214). Compared with those of the bulk counterparts synthesized via the solid-state reaction method (Fig. S1†), the XRD peaks of these NPs are broadened, indicating the smaller size of the as-prepared NPs (Fig. 1b). The growth rate of the NPs can be controlled with the reaction solvent because the ion diffusion is faster at a lower OA/OAm ratio.33 As a result, the size of NPs can be tuned by adjusting the OA/OAm ratio. When the OA/OAm ratio decreased from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the average size increased from 11.4 ± 1.1 nm, 16.4 ± 1.9 nm to 22.9 ± 2.9 nm (Fig. 1c–e). The thickness of the Cs2NaYF6:Yb/Tm NPs was determined to be 5.7 ± 0.8 nm (Fig. 1f). The high-resolution transmission electron microscopy (HRTEM) image of Cs2NaYF6 NPs shows clear lattice fringes (Fig. 1g). The fast Fourier transform (FFT) image of the NPs from the HRTEM image indicates that there are six nearest scattering points around each white scattering point, and the distance between them is 0.316 nm, verifying that the dominant arrangement of the synthesized Ln3+ doped Cs2NaYF6 NPs is the [111] orientation.34 The selected-area electron diffraction (SAED) pattern of these NPs displays intense diffraction rings of cubic Cs2NaYF6 (Fig. 1h), confirming the pure phase and high crystallinity of the obtained NPs. In addition, the energy-dispersive X-ray (EDX) spectrum confirms the successful doping of Yb3+ and Tm3+ in Cs2NaYF6 NPs (Fig. S2†).
2, the average size increased from 11.4 ± 1.1 nm, 16.4 ± 1.9 nm to 22.9 ± 2.9 nm (Fig. 1c–e). The thickness of the Cs2NaYF6:Yb/Tm NPs was determined to be 5.7 ± 0.8 nm (Fig. 1f). The high-resolution transmission electron microscopy (HRTEM) image of Cs2NaYF6 NPs shows clear lattice fringes (Fig. 1g). The fast Fourier transform (FFT) image of the NPs from the HRTEM image indicates that there are six nearest scattering points around each white scattering point, and the distance between them is 0.316 nm, verifying that the dominant arrangement of the synthesized Ln3+ doped Cs2NaYF6 NPs is the [111] orientation.34 The selected-area electron diffraction (SAED) pattern of these NPs displays intense diffraction rings of cubic Cs2NaYF6 (Fig. 1h), confirming the pure phase and high crystallinity of the obtained NPs. In addition, the energy-dispersive X-ray (EDX) spectrum confirms the successful doping of Yb3+ and Tm3+ in Cs2NaYF6 NPs (Fig. S2†).
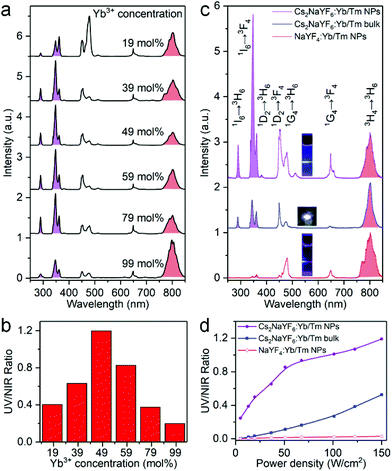
To examine the effect of Ln3+ dopants on UC emission, we synthesized a series of Cs2NaYF6:Yb/Tm NPs with different Tm3+ and Yb3+ concentrations. The optimal Tm3+ concentration was 1 mol%, which resulted in the maximal UCL emission upon excitation with a 980 nm laser (Fig. S3†). For Cs2NaYF6:x mol% Yb, 1 mol% Tm NPs with different Yb3+ concentrations (Fig. S4†), their UCL spectra consist of characteristic sharp emission peaks which can be assigned to the five-photon 1I6 → 3H6 and 3F4 (290 and 348 nm), four-photon 1D2 → 3H6 and 3F4 (362 and 450 nm), three-photon 1G4 → 3H6 and 3F4 (478 and 649 nm) and two-photon 3H4 → 3H6 (803 nm) transitions of Tm3+ (Fig. 2a). Interestingly, the five-photon and four-photon UCL emissions of Cs2NaYF6:Yb/Tm NPs in the UV region were observed to be strikingly strong. With the Yb3+ concentration of 49 mol%, a maximum integrated UV-to-NIR emission intensity ratio can be achieved (Fig. 2b).
We then compared the UCL spectra of Cs2NaYF6:Yb/Tm NPs and bulk materials with that of typical Tm3+ doped fluoride nanocrystals like α-NaYF4 (Fig. 2c). Bright blue UCL can be observed for these samples upon 980 nm excitation at a power density of 150 W cm−2. The absolute upconversion quantum yields for the visible and NIR regions (400–850 nm) of Cs2NaYF6:Yb/Tm NPs and bulk have been determined to be 0.10 ± 0.02% and 1.27 ± 0.10%, respectively, which are higher than that of α-NaYF4:Yb/Tm nanocrystals (0.05 ± 0.02%). Moreover, the five-photon and four-photon UCL emissions of Cs2NaYF6:Yb/Tm NPs in the UV region were observed to be much stronger than those of Tm3+ doped α-NaYF4 nanocrystals (Fig. S5†). The integrated UV-to-NIR emission intensity ratio of these samples increased with an increase in the excitation power density. With a power density of 150 W cm−2, the UV-to-NIR ratios for Cs2NaYF6:49 mol%Yb, 1 mol%Tm NPs and bulk materials were calculated to be 1.20 and 0.51, respectively, which are ∼40 and ∼17 times higher than that of α-NaYF4:49 mol%Yb, 1 mol%Tm nanocrystals (Fig. 2d). Note that the integrated UV-to-NIR emission intensity ratio of 1.20 is also the highest among Tm3+-activated core-only UC nanocrystals (Table S1†).
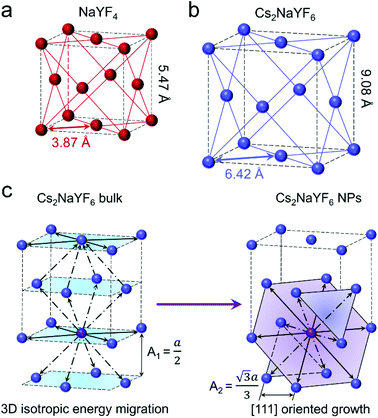
As shown in Fig. 3a and b, both α-NaYF4 and Cs2NaYF6 are face-centered cubic crystals with the space-group symmetry of Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. In α-NaYF4 and Cs2NaYF6, Ln3+ dopants like Yb3+ and Tm3+ ions are doped to substitute for Y3+ ions. The nearest distance between Yb3+ and Tm3+ ions is 3.87 Å in α-NaYF4 (Fig. 3a), which is much smaller relative to that in Cs2NaYF6 (6.42 Å, Fig. 3b). As such, the excitation energy in α-NaYF4 may be easily depleted owing to the increased probability of energy migration, which does not favor the multiphoton UCL emission.35
m. In α-NaYF4 and Cs2NaYF6, Ln3+ dopants like Yb3+ and Tm3+ ions are doped to substitute for Y3+ ions. The nearest distance between Yb3+ and Tm3+ ions is 3.87 Å in α-NaYF4 (Fig. 3a), which is much smaller relative to that in Cs2NaYF6 (6.42 Å, Fig. 3b). As such, the excitation energy in α-NaYF4 may be easily depleted owing to the increased probability of energy migration, which does not favor the multiphoton UCL emission.35
We investigated the microscopic models of Cs2NaYF6:Yb/Tm bulk materials and NPs (Fig. 3c). For each Tm3+ ion in bulk Cs2NaYF6:Yb/Tm, there are twelve nearest neighboring Ln3+ ions, four of which are situated at the top, middle or bottom [001] layer, respectively. The existence of isotropically substituted Ln3+ ions may promote the dissipation of the excitation energy in such a three-dimensional (3D) structured crystal sublattice.25,36,37 By contrast, the Cs2NaYF6:Yb/Tm nanocrystalline materials experience an anisotropic growth along the [111] orientation (Fig. 1g), resulting in two-dimensional (2D)-like nanoplatelets. For each Tm3+, six nearest neighboring Ln3+ ions are situated at the middle [111] layer and the other six nearest neighboring Ln3+ ions are situated at the top and bottom [111] layer, respectively. Moreover, the distance between two [111] layers (A2) is calculated to be 5.24 Å, which is longer than that of two [100] layers (A1, 4.54 Å).38 Thus, such a 2D-like structure can effectively minimize the migration of excitation energy compared with the 3D structure,25,39 which resulted in enhanced multiphoton UCL emission in Cs2NaYF6:Yb/Tm NPs relative to that in the bulk counterparts.
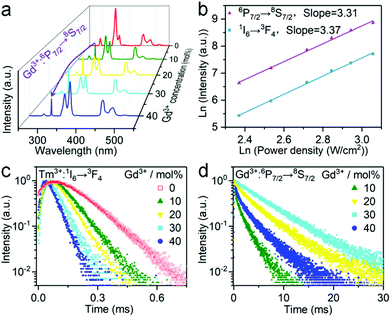
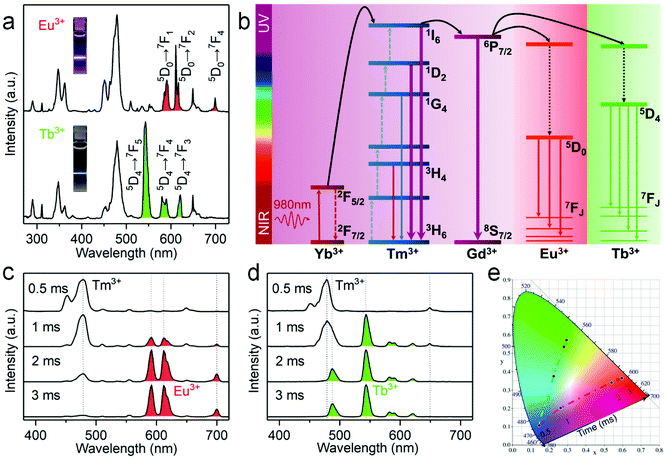
Besides Tm3+, another Ln3+ ion like Gd3+ is often doped into inorganic nanocrystals to produce UV emission.40 In this regard, we synthesized Cs2NaYF6:Yb/Tm/Gd NPs with different Gd3+ concentrations. EDX element mapping images indicate that the Ln3+ dopants are homogeneously distributed among the as-prepared NPs (Fig. S6†). Upon 980 nm excitation, a sharp UV emission peak at 311 nm can be observed, which originated from the 6P7/2 → 8S7/2 transition of the Gd3+ ion (Fig. 4a). The luminescence intensity of such UV emission increased with increasing Gd3+ content from 10 mol% to 30 mol%, and then decreased at higher concentrations. Meanwhile, the five-photon 1I6 → 3H6 and 3F4 transitions of Tm3+ peaking at 290 and 348 nm continuously decreased with an increase in the Gd3+ concentrations. Moreover, in a control experiment by replacing Cs2NaYF6:Yb/Tm/Gd with Cs2NaYF6:Yb/Gd NPs under otherwise identical conditions, negligible UCL was detected, which indicates that the luminescence of Gd3+ was mainly sensitized by the nearby Tm3+ ions.
To shed more light on the mechanism of the UV emission of Gd3+, we obtained the UC spectra of Cs2NaYF6:Yb/Tm/Gd NPs excited with a 980 nm laser with different power densities. For a typical UC emission process, the value of pump photons (n) required to populate a certain emission can be determined by the relationship of I ∝ Pn, where I and P are the UCL intensity and the pump power density, respectively. The n value for 1I6 → 3F4 (348 nm) of Tm3+ was determined to be essentially identical to that of 6P7/2 → 8S7/2 (311 nm) of Gd3+ (Fig. 4b), which indicates that the UCL of Gd3+ may occur through the sensitization of the 1I6 state of Tm3+.
To further verify the process of energy transfer from Tm3+ to Gd3+, we measured the UCL decays from 1I6 of Tm3+ and 6P7/2 of Gd3+ in Cs2NaYF6:Yb/Tm/Gd NPs. The effective lifetime of the 6P7/2 level of Gd3+ increased from 1.61 ms to 7.03 ms with increasing Gd3+ content from 10 mol% to 30 mol%, and then decreased to 2.97 ms at higher concentrations (Fig. 4c and Table S2†). Meanwhile, the effective lifetime of the 1I6 level of the Tm3+ ion was determined to be shortened from 163.96 μs to 50.47 μs with the increase of Gd3+ concentration from 0 to 40 mol% (Fig. 4d and Table S2†). All these results verified that the UCL of the 6P7/2 state of Gd3+ is sensitized by the 1I6 state of Tm3+ ions.
It has been reported that Gd3+ can be used as a bridge ion to sensitize a variety of Ln3+ ions (e.g., Eu3+ or Tb3+) without long-lived intermediate energy states to produce UC emission.41,42 For Cs2NaYF6:Yb/Tm/Gd/Eu and Cs2NaYF6:Yb/Tm/Gd/Tb NPs, a set of new UC emission peaks appeared besides those of Tm3+ and Gd3+ upon excitation at 980 nm (Fig. 5a). These newly appeared UC emission peaks can be assigned to the 5D0 → 7FJ transitions of Eu3+ and 5D4 → 7FJ transitions of Tb3+, respectively. Correspondingly, the overall UC color output turned from blue to pink or cyan, respectively (insets of Fig. 5a).
The UC mechanism responsible for the intense UCL of Eu3+ and Tb3+ in Cs2NaYF6 is illustrated in Fig. 5b. Upon excitation with a 980 nm laser, sensitizer ions (Yb3+) absorb the pumped photons, followed by energy transfer to the neighboring Tm3+ to populate the 3F4, 3H4, 1G4, 1D2, and 1I6 states of Tm3+ in sequence. Note that the energy of the 6P7/2 level of the Gd3+ ion matches well with that of the 1I6 level of the Tm3+ ion. Once the 1I6 level of the Tm3+ ion is populated, its excitation energy will be partially transferred to the Gd3+ ion. The excitation energy will then be transferred to the Eu3+ or Tb3+ ion, because the 6P7/2 level of the Gd3+ ion matches well with the 5HJ level of Eu3+ or the 5DJ level of Tb3+. As a result, red UCL of Eu3+ or green UCL of Tb3+ can be observed. The proposed energy transfer mechanism was further confirmed by the observed UCL decays. It was determined that the photoluminescence lifetime of 6P7/2 of Gd3+ was shortened from 7.03 ms to 2.83 ms, and the photoluminescence lifetime of 1I6 of Tm3+ was shortened from 59.89 μs to 40.93 μs after the doping of Eu3+ (Fig. S7†).
It is worth emphasizing that two key issues contribute to the generation of efficient UCL of Eu3+ and Tb3+ in Cs2NaYF6:Yb/Tm/Gd/Eu and Cs2NaYF6:Yb/Tm/Gd/Tb NPs, respectively. Firstly, Gd3+ plays an important role as the energy transfer bridge. Without the doping of Gd3+, the UCL of Eu3+ in Cs2NaYF6:Yb/Tm/Eu NPs was observed to be negligibly weak because of the large energy mismatch between the 2F5/2 → 2F7/2 transition of Yb3+ and the 5DJ → 7FJ transition of Eu3+. As such, the UCL of Eu3+ cannot be efficiently sensitized by the direct energy transfer between Tm3+ and Eu3+. By contrast, additional doping of Gd3+ resulted in much enhanced UCL of Eu3+ by ∼25 times (Fig. S8†). In addition, the confined energy migration of Ln3+ dopants in the Cs2NaYF6 lattice is also critical. Note that in the previous reports regarding the generation of UCL from Eu3+ and Tb3+, the core–shell or core–multishell structure is a prerequisite for space separation of Tm3+ and Eu3+/Tb3+ so as to restrain the deleterious cross-relaxations between them.5,41 In this work, the UCL of Eu3+ in Cs2NaYF6:Yb/Tm/Gd/Eu NPs can be markedly enhanced by ∼38 times that of traditional fluorides like α-NaYF4:Yb/Tm/Gd/Eu nanocrystals (Fig. S8†). To the best of our knowledge, such an efficient UCL of Eu3+ or Tb3+ in Cs2NaYF6 NPs has not been realized in other Eu3+ or Tb3+-doped core-only nanocrystals before.
As shown in Fig. 5a, the pure UCL of Eu3+ or Tb3+ cannot be obtained directly using the steady-state luminescence mode. The effective lifetimes of the 5D0 level of Eu3+ and the 5D4 level of Tb3+ in Cs2NaYF6 NPs have been determined to be 2.60 ms and 2.25 ms, respectively (Fig. S9†), which are about two orders of magnitude longer than those of Tm3+ in these NPs (Fig. S7†). By virtue of the large difference in the UC luminescence lifetime between Eu3+/Tb3+ and Tm3+ ions, it is easy to separate their UCL based on the time-resolved detection technique by setting an appropriate delay time and gate time. Fig. 5c and d show the time-resolved UCL spectra of Cs2NaYF6:Yb/Tm/Gd/Eu and Cs2NaYF6:Yb/Tm/Gd/Tb NPs, respectively. Their UCL spectra were dominated by the emission of Tm3+ when the delay time was within 1 ms. When the delay time is longer than 3 ms, pure red UCL attributed to the 5D0 → 7FJ (J = 1, 2, 3, and 4) transition of the Eu3+ ion and green UCL attributed to the 5D4 → 7FJ (J = 3, 4, 5, and 6) transition of Tb3+ were observed, respectively. As revealed by their corresponding CIE chromaticity coordinates (Fig. 5e), the UC color changed from blue to red or green for Cs2NaYF6:Yb/Tm/Gd/Eu or Cs2NaYF6:Yb/Tm/Gd/Tb NPs, respectively. Such an intriguing time-dependent UCL color output in Ln3+-doped Cs2NaYF6 NPs is favorable for potential applications such as in time-resolved bioimaging and multilevel anti-counterfeiting.
Conclusions
In summary, we have developed a new class of Ln3+-doped Cs2NaYF6 NPs without a core/shell structure to achieve highly efficient multiphoton UCL emissions. Upon excitation at 980 nm, intense UV emissions of Tm3+ were realized in Cs2NaYF6:Yb/Tm NPs by virtue of the large distance between the adjacent Ln3+ dopants in the host lattice. The optimal UV-to-NIR emission intensity ratio of Cs2NaYF6:Yb/Tm NPs has been determined to be 1.2, which is the highest among Tm3+-activated core-only UC nanocrystals. Moreover, intense UV emission of Gd3+ has been realized in Cs2NaYF6:Yb/Tm/Gd NPs via the energy transfer from Tm3+ to Gd3+. Particularly, we have demonstrated that bright red and green UC emissions of Eu3+ and Tb3+ can be generated via the cascade sensitization of Tm3+ and Gd3+ in Cs2NaYF6:Yb/Tm/Gd/Eu and Cs2NaYF6:Yb/Tm/Gd/Tb NPs, respectively. These findings reveal the great potential of tuning UCL emissions in Ln3+-doped Cs2NaYF6 NPs, which may open up a promising avenue for the exploitation of Ln3+-doped nanocrystals with excellent optical properties for versatile applications.Experimental
Detailed experimental procedures are reported in the ESI.†Author contributions
C. Z. prepared samples and collected data for the original draft. S.Y. H. and P. Z. assisted in PL measurements. L.P. W., S.H. Y., J. X. and R.F. L. contributed to conceptualization and investigation. C. Z., D.T. T. and X.Y. C. prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Science and Technology Cooperation Fund between Chinese and Australian Governments (2017YFE0132300), the Strategic Priority Research Program of the CAS (XDB20000000), the NSFC (no. U1805252, 21975257, 21771185, 12074380, and 11704380), and the CAS/SAFEA International Partnership Program for Creative Research Teams, NSF of Fujian Province (no. 2019I0029).Notes and references
- M. L. Deng, Y. X. Ma, S. Huang, G. F. Hu and L. Y. Wang, Nano Res., 2011, 4, 685–694 CrossRef CAS.
- B. Zhou, B. Shi, D. Jin and X. Liu, Nat. Nanotechnol., 2015, 10, 924–936 CrossRef CAS PubMed.
- D. Li, S. H. Yu and H. L. Jiang, Adv. Mater., 2018, 30, 1707377 CrossRef PubMed.
- W. J. Yao, Q. Y. Tian, B. Tian, M. X. Li, H. J. Wang, P. Zeng, L. Liu, H. Zheng and W. Wu, Sci. China Mater., 2019, 62, 368–378 CrossRef CAS.
- M. Ding, B. Dong, Y. Lu, X. Yang, Y. Yuan, W. Bai, S. Wu, Z. Ji, C. Lu and K. Zhang, Adv. Mater., 2020, 32, 2002121 CrossRef CAS PubMed.
- N. Xin, D. Wei, Y. Zhu, M. Yang, S. Ramakrishna, O. Lee, H. Luo and H. Fan, Mater. Today Chem., 2020, 17, 100329 CrossRef CAS.
- J. K. Chen, S. X. Wang, J. D. Lin and D. Q. Chen, Nanoscale, 2019, 11, 22359–22368 RSC.
- H. Huang, J. K. Chen, Y. T. Liu, J. D. Lin, S. X. Wang, F. Huang and D. Q. Chen, Small, 2020, 16, 12 Search PubMed.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061–1065 CrossRef CAS PubMed.
- C. Zhang, L. D. Sun, Y. W. Zhang and C. H. Yan, J. Rare Earths, 2010, 28, 807–819 CrossRef CAS.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed.
- W. Luo, Y. Liu and X. Chen, Sci. China Mater., 2015, 58, 819–850 CrossRef CAS.
- X. W. Zhang, Z. Zhao, X. Zhang, D. B. Cordes, B. Weeks, B. S. Qiu, K. Madanan, D. Sardar and J. Chaudhuri, Nano Res., 2015, 8, 636–648 CrossRef CAS.
- Q. Zou, P. Huang, W. Zheng, W. You, R. Li, D. Tu, J. Xu and X. Chen, Nanoscale, 2017, 9, 6521–6528 RSC.
- J. Shen, G. Chen, T. Y. Ohulchanskyy, S. J. Kesseli, S. Buchholz, Z. Li, P. N. Prasad and G. Han, Small, 2013, 9, 3213–3217 CrossRef CAS PubMed.
- A. Punjabi, X. Wu, A. Tokatli-Apollon, M. El-Rifai, H. Lee, Y. Zhang, C. Wang, Z. Liu, E. M. Chan and C. Duan, ACS Nano, 2014, 8, 10621–10630 CrossRef CAS PubMed.
- S. Wen, J. Zhou, K. Zheng, A. Bednarkiewicz, X. Liu and D. Jin, Nat. Commun., 2018, 9, 2415 CrossRef PubMed.
- B. Chen and F. Wang, Acc. Chem. Res., 2019, 53, 358–367 CrossRef PubMed.
- L. Tu, X. Liu, F. Wu and H. Zhang, Chem. Soc. Rev., 2015, 44, 1331–1345 RSC.
- L. Zhao, J. Peng, Q. Huang, C. Li, M. Chen, Y. Sun, Q. Lin, L. Zhu and F. Li, Adv. Funct. Mater., 2014, 24, 363–371 CrossRef CAS.
- Q. S. Chen, X. J. Xie, B. L. Huang, L. L. Liang, S. Y. Han, Z. G. Yi, Y. Wang, Y. Li, D. Y. Fan, L. Huang and X. G. Liu, Angew. Chem., Int. Ed., 2017, 56, 7605–7609 CrossRef CAS PubMed.
- S. Mei, J. Zhou, H. T. Sun, Y. Cai, L. D. Sun, D. Jin and C. H. Yan, Adv. Sci., 2021, 2003325 CrossRef CAS PubMed.
- B. Zhou, B. Tang, C. Zhang, C. Y. Qin, Z. J. Gu, Y. Ma, T. Y. Zhai and J. N. Yao, Nat. Commun., 2020, 11, 1174 CrossRef CAS PubMed.
- X. Chen, L. M. Jin, W. Kong, T. Y. Sun, W. F. Zhang, X. H. Liu, J. Fan, S. F. Yu and F. Wang, Nat. Commun., 2016, 7, 10304 CrossRef CAS PubMed.
- J. Wang, R. Deng, M. A. MacDonald, B. Chen, J. Yuan, F. Wang, D. Chi, T. S. A. Hor, P. Zhang and G. Liu, Nat. Mater., 2014, 13, 157–162 CrossRef CAS PubMed.
- L. Marciniak, W. Strek, A. Bednarkiewicz, A. Lukowiak and D. Hreniak, Opt. Mater., 2011, 33, 1492–1494 CrossRef CAS.
- T. Pawlik and J. M. Spaeth, J. Appl. Phys., 1997, 82, 4236–4240 CrossRef CAS.
- V. N. Makhov, N. M. Khaidukov, D. Lo, J. C. Krupa, M. Kirm and E. Negodin, Opt. Mater., 2005, 27, 1131–1137 CrossRef CAS.
- P. A. Loiko, N. M. Khaidukov, J. Mendez-Ramos, E. V. Vilejshikova, N. A. Skoptsov and K. V. Yumashev, J. Lumin., 2016, 175, 260–266 CrossRef CAS.
- P. A. Loiko, E. V. Vilejshikova, N. M. Khaidukov, J. Mendez-Ramos, X. Mateos and K. V. Yumashev, J. Lumin., 2017, 185, 279–285 CrossRef CAS.
- P. A. Tanner, Y. L. Liu, N. M. Edelstein, K. M. Murdoch and N. M. Khaidukov, J. Phys.: Condens. Matter, 1997, 9, 7817–7836 CrossRef CAS.
- Y. M. Yang, Z. Y. Li, J. Y. Zhang, Y. Lu, S. Q. Guo, Q. Zhao, X. Wang, Z. J. Yong, H. Li, J. P. Ma, Y. Kuroiwa, C. Moriyoshi, L. L. Hu, L. Y. Zhang, L. R. Zheng and H. T. Sun, Light: Sci. Appl., 2018, 7, 11 CrossRef PubMed.
- J. Gu, Z. Q. Zhao, Y. Ding, H. L. Chen, Y. W. Zhang and C. H. Yan, J. Am. Chem. Soc., 2013, 135, 8363–8371 CrossRef CAS PubMed.
- W. Lee, D. Choi and S. Kim, Chem. Mater., 2020, 32, 6864–6874 CrossRef CAS.
- O. Malta, J. Non-Cryst. Solids, 2008, 354, 4770–4776 CrossRef CAS.
- H.-X. Mai, Y.-W. Zhang, R. Si, Z.-G. Yan, L.-d. Sun, L.-P. You and C.-H. Yan, J. Am. Chem. Soc., 2006, 128, 6426–6436 CrossRef CAS PubMed.
- G. Wang, Q. Peng and Y. Li, J. Am. Chem. Soc., 2009, 131, 14200–14201 CrossRef CAS PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- T. C. Ozawa, K. Fukuda, K. Akatsuka, Y. Ebina and T. Sasaki, Chem. Mater., 2007, 19, 6575–6580 CrossRef CAS.
- V. Mahalingam, R. Naccache, F. Vetrone and J. A. Capobianco, Chem. – Eur. J., 2009, 15, 9660–9663 CrossRef CAS PubMed.
- F. Wang, R. Deng, J. Wang, Q. Wang, Y. Han, H. Zhu, X. Chen and X. Liu, Nat. Mater., 2011, 10, 968–973 CrossRef CAS PubMed.
- X. Chen, D. Peng, Q. Ju and F. Wang, Chem. Soc. Rev., 2015, 44, 1318–1330 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nr01745d |
| This journal is © The Royal Society of Chemistry 2021 |