Anionic metal–organic framework hybrids: functionalization with lanthanide ions or cationic dyes and fluorescence sensing of small molecules†
Xiang Shen and
Bing Yan*
Department of Chemistry, Tongji University, Shanghai 200092, P. R. China. E-mail: byan@tongji.edu.cn; Fax: +86-21-65982287
First published on 11th March 2016
Abstract
A zinc-based anionic metal–organic framework, [HDMA]2[Zn2(BDC)3(DMA)]·6DMF (HDMA+: dimethylammonium, BDC2−: 1,4-benzenedicarboxilate, DMA: dimethylamine and DMF: N,N′-dimethylformamide) has been solvothermally synthesized. And its cations, HDMA+, are exchanged by various lanthanides to form the hybrid systems via a post-synthetic process. These lanthanide(III)-loaded materials have shown different luminescent behaviors, indicating that this kind of anionic MOF could effectively sensitive lanthanides and be used as a potential luminescent probe towards different lanthanide ions. Besides, this system exhibits selective adsorption ability to cationic dyes, such as Rhodamine B, Basic Red 2 and Methylene Blue. RhB@1 has a fastest selection and realizes the probing of various organic solvent molecules as well as volatile organic benzenes (VOBs), especially for sensing acetone and aniline.
Introduction
In the past decades, organic–inorganic hybrid materials have received tremendous attention. Metal–organic frameworks (MOFs) are a new family of this kind of materials and have been identified as an excellent platform for host–guest chemistry, which consist of metal ions (or clusters) as nodes and multiply organic ligands as linkers.1 Owing to their void space, MOFs could be used as potential materials in energy- and environment-related fields, notably for hydrogen and greenhouse gas storage.2 Besides, depending on their fascination structures, MOFs are unique luminescent platforms for luminescence,3 light-emitting4 and probe sensors.5 Generally, MOFs with specific functionalities or topologies are decided by their functionalized organic ligands or metal–organic fragments. The metal-cluster vertices generate in situ as secondary building units (SBUs) and further extend to multidimensional structures. What's more, the framework charge also plays a significant role on the performance of MOFs. The charged frameworks are capable to undergo post-synthetic chemical modification via ion exchange.6Ion exchange is a new strategy for the fabrication of ionic nanocrystals, including cation exchange and anionic exchange. Among ion exchange, cation exchange is a powerful post-synthetic tool for designing new materials in which the cations comprising an ionic solid can be exchanged with different cations. Cation exchange often occurs at the inorganic clusters which could be replaced by other metal ions without compromising the structure or counter-ions of the framework such as dimethylammonium cation which could be exchanged by external ions.7
Recently, it is still an attractive but challenging task for exploiting MOFs applications via cation exchange. Trivalent lanthanide ions with unique spectroscopic properties are the remarkable candidates as chromophore guest due to their sharp emission spectra for high color purity, broad emission bands covering ultraviolet-visible-near infrared region and a wide range of lifetimes.8 It is wisdom that encapsulating lanthanide cations into anioic frameworks could obtain primary color emission. For example, Rosi's group has reported a classic system of bio-MOF-1, which is an anionic framework doped lanthanide ions through cation exchange.9 Furthermore, when mixed, the two (blue and yellow) or three (blue, green, and red) primary colors together would generate white light emission.4b,c There also have been intensive studies on lanthanide encapsulated MOFs with promising applications of tunable luminescence materials,3b,10 PH probe,11 chemical sensors12 and so on.
Another fascinating chromophore guests are organic dyes because of excellent optical and electronic properties. Dyes are widely used in many industries such as paper, plastics, printing and so on. It is of great significance to research on the removal of dyes from effluents in an environmental point of view. Numerous studies have been reported on dye-loaded MOFs via ion exchange.13 However, the applications of dye-loaded MOFs as luminescent sensors still need to be further exploited.13a,c,14
Herein, [HDMA]2[Zn2(BDC)3(DMA)]·6DMF (1) (HDMA+: dimethylammonium, BDC2−: 1,4-benzenedicarboxilate, DMA: dimethylamine and DMF: N,N′-dimethylformamide) is selected as host material which is an anionic framework. The existence of HDMA+ in channels of compound 1 establishes the feasibility of its post-synthetic cation exchange with external ions, such as lanthanides and cationic organic dyes (Scheme 1). We show that compound 1 can not only serve as a host but also an antenna for protecting and sensitizing external lanthanides emitting in the visible and NIR that are encapsulated within the framework pores. Moreover, we investigate the capability of 1 to absorb cationic dyes and sense small molecules.
 | ||
| Scheme 1 The scheme for ZnII-MOF exchanging with lanthanide ions (including Eu3+, Tb3+, Sm3+, Dy3+ and near-infrared emitting ions, Nb3+ and Yb3+) and selectively adsorbing cationic dye molecules. | ||
Experimental section
Chemicals
All of the chemicals were purchased from commercial sources. Terephthalic acid (1,4-BDC), Rhodamine B, methylene blue and methyl orange were purchased from Adamas-beta. Safranine O was purchased by Alfa-Aesar. N,N′-Dimethylformamide (DMF, 99.9%) was from Aladdin. Lanthanide(III) nitrates were prepared by dissolving corresponding oxides (Eu2O3, Tb4O7, Sm2O3, Dy2O3, Y2O3 and Nd2O3) in nitric acid, evaporated and vacuum drying. Zinc acetate dehydrate, nitric acid were purchased from China National Medicine Group. Ultrapure water and ethanol were used throughout all experiment.Synthesis of ZnII-MOF (compound 1)15
Compound 1 was synthesized according to the previous reports. 1 was prepared from hydrothermal reaction of zinc acetate dehydrate (1 mmol), 1,4-BDC (1 mmol), DMF (15 mL) at 180 °C for 48 h, and then cooled to room temperature naturally. The material was collected with centrifugation and washed with DMF (5 mL × 3), and dried under vacuum (80 °C, 24 h).Encapsulation of Ln3+ cations in ZnII-MOF (Ln = Eu, Tb, Sm, Dy, Yb and Nd)16
A solution of Ln(NO3)3 in DMF (5 mmol L−1) was prepared. Ln3+ cations encapsulation was performed as follows: the as-synthesized ZnII-MOF was soaked in the Ln(NO3)3 solution and refreshed every 24 h for 3 days. After cation exchange completed, the materials were thoroughly washed with DMF and dried under vacuum (80 °C).Preparation of organic dye selective adsorption with various amount16
The freshly prepared ZnII-MOF (31 mg) was immersed in 5 mL DMF solutions of different organic dyes (Rhodamine B, methylene blue, Safranine O and Methyl orange, 0.1 mmol L−1) under stirring at room temperature for 12 h. The solution was centrifugated and the supernatant liquid was diluted to analyse by UV/Vis absorption spectroscopy.Luminescence sensing16
The fluorescence properties of 2%RhB@1 (2) were investigated in the solid state and in various solvents at room temperature. The mixtures were prepared by introducing 5 mg of 2%RhB@1 powder into 3 mL of pure small molecule solvents. Subsequently, the system was uniformly dispersed under ultrasound for 5 min. Then the luminescent properties were detected. All the solvents are listed as follows: 1,4-dioxane, acetone, isopropanol, methanol, ethanol, acetonitrile, ethyl acetate, CHCl3, CHCl2, THF, ethyl acetate, DMF, as well as volatile benzene series (benzene, toluene, o-xylene, m-xylene, p-xylene, ethylbenzene, chlorobenzene, aniline).Physical characterization
X-ray powder diffraction patterns (XRD) were recorded with a Bruker Focus D8 at 40 kV, 40 mA for Cu-Kα with a scan speed of 0.10 s per step and a step size of 0.02°; the data were collected within 2θ range of 3–50°. Fourier transform infrared spectra (FTIR) were measured with KBr slices from 4000 to 400 cm−1 using a Nexus 912AO446 infrared spectrum radiometer. UV-visible diffuse reflectance spectrum was taken by BWS003. Thermogravimetric analysis (TGA) was measured using a Netzsch STA 449C system at a heating rate of 15 K min−1 under nitrogen atmosphere. UV-Vis spectra were recorded on Agilent 8453.Luminescent measurements
The luminescence spectra were recorded on an Edinburgh FL920 phosphorimeter using a 450W xenon lamp as excitation source. Luminescence lifetime measurements are carried out on an Edinburgh FLS920 phosphorimeter using a microsecond pulse lamp as excitation source. The quantitative value of lifetime is calculated by linear fitting.Results and discussion
ZnII-MOF (compound 1) is an anionic MOF prepared from zinc acetate dehydrate and terephthalic acid in DMF solution under solvothermal conditions at 180 °C. This anionic framework is balanced by protonated [(CH3)2NH2]+ originating from the decomposition of DMF during the reaction. Based on previous research,15,17 1 is an anionic MOF with 1D rectangular shaped channel (with width and length of 0.77 nm and 1.25 nm, respectively) along the crystallographic b-axis which are occupied with guest DMF molecules and a HDMA+ cation with the ration of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S1†). Thermo gravimetric (TG) curve of 1 (Fig. S2†) displays mass loss in three steps between 30–550 °C. 25.51% of weight loss is related to removal of six guest DMF molecules and two coordinated DMA species from the room temperature to 300 °C. In the next step weight loss of 36.67% between 300 and 550 °C could be related to removal of BDC2− and HDMA+ species. Finally, 35.15% residue at 550 °C is attributed to formation of ZnO from thermolysis of 1. SEM image indicates the framework is in the large scale and shaped like a cube (Fig. S3†).
1 (Fig. S1†). Thermo gravimetric (TG) curve of 1 (Fig. S2†) displays mass loss in three steps between 30–550 °C. 25.51% of weight loss is related to removal of six guest DMF molecules and two coordinated DMA species from the room temperature to 300 °C. In the next step weight loss of 36.67% between 300 and 550 °C could be related to removal of BDC2− and HDMA+ species. Finally, 35.15% residue at 550 °C is attributed to formation of ZnO from thermolysis of 1. SEM image indicates the framework is in the large scale and shaped like a cube (Fig. S3†).
Subsequent post-synthetic cation exchange process leads to formation of Ln@1 (exchanged with Eu3+, Tb3+, Sm3+, Dy3+, Yb3+ and Nb3+) via soaked in DMF solutions of relative nitrate salts. Thanks to the inherent properties of the anionic framework and (CH3)2NH2+ in the 1D channel, 1 is suitable for encapsulation of lanthanides to the host framework to form novel Ln-doped luminescent materials through the antenna effect.8b Ln3+ loading does not impact the crystalline integrity of 1, as confirmed using X-ray powder diffraction (PXRD, Fig. 1) and FT-IR (Fig. S4†). Displayed in Fig. S4,† the FT-IR spectra of 1 and Ln@1 show the similar absorption bands. The absorption bands at 1603, 1575 and 1503 cm−1 belong to the stretching vibration of benzene ring while that at 1388 cm−1 is attributed to flexural vibration of carboxylate anion. This indicates that loading numerous lanthanides into the framework have not changed the chemical linking modes.
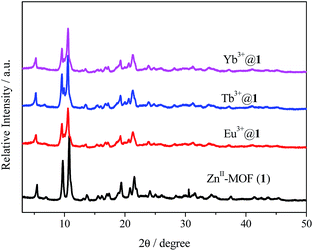 | ||
| Fig. 1 PXRD patterns of as-prepared ZnII-MOF (1) and lanthanide-doped materials (Eu3+@1, Tb3+@1 and Yb3+@1). | ||
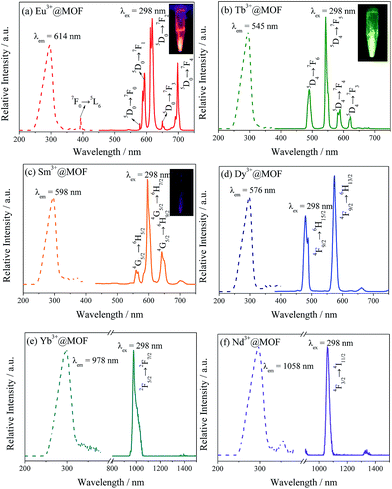
As expected, 1 has succeeded in sensitizing several visible and near-infrared emitting lanthanide cations. The excitation and emission spectra of the powder sample of 1,4-BDC are displayed in Fig. S5a.† 1,4-BDC ligand exhibits strong emission at 390 nm at the excitation at 332 nm due to the π–π* transitions. Fig. S5b† displays that 1 exhibits more enhanced blue light emission and shifts slightly to 440 nm with the excitation at 327 nm and its luminescent lifetime 7.27 μs. Under the excitation at 298 nm, Ln3+@1 emits the distinctive emissions both of visible and near-infrared lanthanide cations, shown in Fig. 2. The emission spectrum of Eu3+@1 emits its distinctive red colour with typical europium ion emissions at 578, 593, 614, 648 and 687 nm under the excitation of 298 nm, which are ascribed to the 5D0 → 7FJ (J = 0, 1, 2, 3 and 4) transitions. The characteristic terbium ion emission could be observed in 1 which is assigned to the 5D4 → 7FJ (J = 6, 5, 4 and 3) transitions at 490 nm, 544 nm, 583 nm and 622 nm. The unique samarium ion emission could also be observed in Sm3+@1 which is assigned to the 4G5/2 → 6F5/2, 4G5/2 → 6F7/2 and 4G5/2 → 6F9/2 transitions at 562 nm, 598 nm and 643 nm. Similarly, Dy3+@1 also displays its unique emission at 480 nm and 573 nm which are unique dysprosium emissions and correspond to 4F9/2 → 6H11/2 and 4F9/2 → 6H13/2 respectively. All the excitation spectra are obtained by monitoring the respective characteristic lanthanide ion emission at 614 nm of Eu3+, 545 nm of Tb3+, 598 nm of Sm3+ and 573 nm of Dy3+, which is dominated by a broad band centred at 298 nm. Besides, when it comes to excitation spectra of Eu3+@1, there is a sharp line centred at 394 nm which is assigned to Eu3+ characteristic excitation 7F0 → 5L6 as well as a broad band centred at 298 nm. Combined with the luminescent spectra of 1 in Fig. S5b,† its emission spectrum is a broad band from 350 to 500 nm which overlaps the excitation spectrum of Eu3+ characteristic excitation. It indicates the antenna effect occurs, which means that energy migration takes place upon ligand absorption, followed by intersystem crossing and antenna transfer, and then generating the emissions of Eu3+ cation. Luminescent lifetimes of the Ln3+-incorporated samples are measured at room temperature under the excitation wavelength that maximizes the emission intensity and monitored by the most intense emission at 614 (Eu3+), 545 (Tb3+), 598 (Sm3+) and 573 (Dy3+) nm and the results are shown in Table S1.† The best fit for each of the Ln3+@1 samples is systematically biexponential. Lifetime of each sample is 1542.82 μs, 1673.98 μs, 7.92 μs and 49.65 μs, respectively. Besides, the absolute quantum yields are determined with integrated sphere instruments as well (Table S1†). The luminescent quantum efficiencies of these four samples are separately 80.56%, 83.78%, 34.55% and 46.07%, respectively. The quantum efficiencies are all reasonably high that can be attributed to high-efficient energy transfer from the sensitizer embedded in the MOF to the incorporated Ln3+ cations. Furthermore, the CIE chromaticity diagrams are detected and shown in Fig. S6.† Eu3+@1 is located in the red (0.6442, 0.3521), Tb3+@1 is in the green (0.3123, 0.5744), Sm3+@1 is in the orange (0.5511, 0.4092) and Dy3+@1 is in the near yellow (0.3533, 0.403). Photographs in the insets of Fig. 2a–c show bright colours of corresponding samples under the excitation of 365 nm.
As there is a high-efficient antenna effect existing in the system, near-infrared emitting lanthanides are detected as well. Fig. 2e and f shows the spectra of Yb3+@1 and Nb3+@1. Yb3+@1 displays a strong typical Yb3+ emission at 980 nm assigned to the 2F5/2–2F7/2 transition under 298 nm excitation. While a dominant sharp emission peak at 1058 nm can be seen and this is contributed by the typical luminescent transition of 4F3/2 → 4F11/2. Thus, the near infrared luminescence for Yb3+ and Nd3+ can be obtained via the energy transfer from the framework.
With the development of the dyeing and finishing industry, dye effluent is emerging as an environmentally challenging issue all over the world. As is well-known, most of organic dyes are very stable against light and oxidation and are difficult to degrade, making them ideal for industrial applications. Unfortunately, various dyes are considered to be toxic and even carcinogenic. As a result, a rapid and effective method to remove toxic dyes from contaminated water is now a critical challenge and goal. Inspired by the successful loading lanthanides, we have tried to evaluate the adsorption ability towards dye molecules. Because many of them are charged (cationic, anionic and neutral), adsorption via ion exchange rises as one of the more feasible methods thanks to its efficiency and economic competitiveness.18
When immersed into DMF solutions of Rhodamine B (RhB), Methylene Blue (MB) and Basic Red 2 (BR2) and Methyl Orange (MO) at room temperature for about 24 h, 1 presents different adsorption behaviours simply observed by naked eyes (Fig. 3). What should be noteworthy is that 1 could rapidly and selectively adsorb cationic dyes such as RhB, MB and BR2 with white powders gradually becoming coloured, whereas 1 could hardly adsorb anionic MO with the colour unchanged. The phenomenon of the selective adsorption of cationic dyes is due to the interaction between cationic dye molecules and the anionic framework, which is supposed to change with DMA+. As shown in Fig. S7,† the successful exchange of cationic dyes does not influence the crystalline integrity of 1. Meanwhile, the SEM image in Fig. S11† could prove it as well. The morphology of the hybrid material in the presence of RhB is the same as that of matrix. It indicates that compound 1 could be a potential adsorbent for removal of cationic dyes.
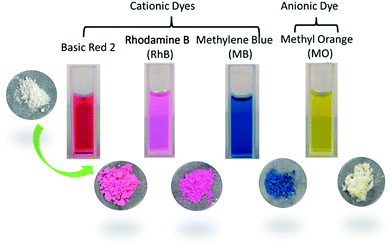 | ||
| Fig. 3 The dye molecules employed in the dye adsorption experiments and photographs of the corresponding powders after immersing in the dye-DMF for 24 h. | ||
Meanwhile, the abilities of 1 to absorb cationic dyes from solutions of DMF are determined through UV/Vis spectroscopic analysis (Fig. S8†). Compared with three kinds of cationic dyes, RhB could be adsorbed completely and immediately, nearly within 5 min while it would take about 12 h for MB to adsorb completely. The insert photographs on centrifugal supernatant fluid in Fig. S8† highlight the sorption effect. After centrifugation and RhB@1 powder collected, Fig. S9† displays spectroscopic study which indicates the more RhB adsorbed, the stronger intensity RhB emitted. Considering the immediately adsorption of RhB, we suppose there are two ways for RhB reacting with MOFs, cation exchange and adsorption. For one thing, MOF consists of a kind of aromatic compound and many hydrogen ions while there are conjugate ring and carboxy group in RhB. Therefore, there may exit many weak interactions (such as hydrogen bonding, π–π stacking and weak static effects). For another, RhB is a kind of cationic dye molecule which could be exchanged into MOFs via cation exchange.
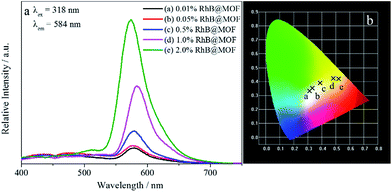
The luminescent spectra of RhB@1 with various amounts are displayed in Fig. 4a. Compound 1 is soaked in RhB/DMF solution for 24 h in purpose of undergoing a complete cation exchange. Because of the high-efficient antenna effect within the framework, the dye encapsulated sample shows strong characteristic emission of RhB in the solid state at room temperature. Upon increasing the RhB included in 1, the system exhibits increasing emission intensity in coincidence with the feed ratio. The correspondent excitation spectra are displayed in Fig. S10,† which are detected at the emission band of Rhodamine B at 584 nm. The excitation spectra show two kinds of exciting band, centred at 318 nm and 355 nm respectively. The band at 318 nm is attributed to the matrix (namely compound 1) and that at 355 nm is corresponding to the RhB. Calculated by the CIE chromaticity diagram (Fig. 4b), the observed emission colours of RhB@1 match well with the calculated chromaticity coordinates, changing from white (x = 0.3336, y = 0.3515) to orange (x = 0.5208, y = 0.4195).
The results clearly demonstrate that the photoluminescent properties of 1 are variable, depending on the amounts of doped RhB. Furthermore, as RhB is bulky, there remains enough vacant space to accommodate small solvent molecule. We use 2.0%RhB@1 to probe different VOMs. The powder samples are exposed to different solvents for 5 min, as is shown in Fig. 5 (also see Fig. S12 and S13†), such as volatile organic benzenes (VOBs) and organic solvent molecules. Fig. 5a displays the luminescent response for sensing organic solvent molecules, including 1,4-dioxane, acetone, methanol, ethanol, acetonitrile, diethyl ether, CHCl3, CHCl2, THF, ethyl acetate and DMF. The bar chart in Fig. 5a is strongly dependent on the solvents, as different solvent molecules exhibiting different degrees of quenching effects. Among these small solvent molecules, acetone has the strongest quench effect that indicates 2%RhB@1 could only selectively sense acetone solvent. When it comes to several volatile organic benzenes with very similar structural motifs (see in Fig. 5b and S13†), these benzene series could be unambiguously differentiable, such as isomers of o-, m- and p-xylene, homologues of benzene, toluene and ethyl-benzene, as well as aniline. Quenching effect is observed in aniline with its luminescent intensity has sharply decreased. The PXRD patterns are detected after samples are dispersed in several organic solvents, especially in acetone and aniline. From Fig. S15,† the materials crystallizations haven't changed although some peaks become weaker or stronger.
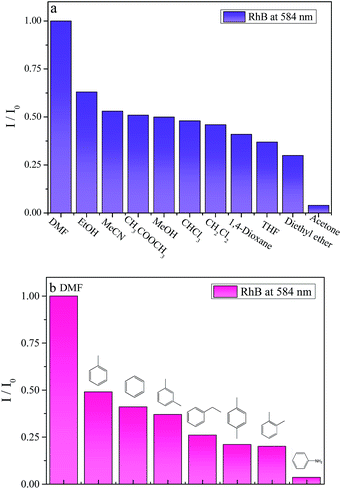 | ||
| Fig. 5 The solvent-dependent luminescent intensity of 2%RhB@1 when dispersed into several organic small molecules (a) as well as volatile organic benzenes (b). | ||
The mechanism of quenching effect has been discussed. However, the quenching effect of solvents molecules is still not very clear in previous research. We suppose the quenching behaviour can be rationalized by considering that acetone may interact with the framework. In other words, there is a negative influence on the energy transfer. To deeply understand the luminescent quenching effect by acetone and aniline, the UV-Vis spectra are measured in Fig. S14.† The luminescent signal induced by organic small molecules could be partially attributed to the UV-Vis absorption. The result indicates that the absorption band of acetone partially overlaps with the absorption band of RhB, which would reduce the absorption of light by organic ligand and affected the energy transformation from the host to RhB.
Conclusions
In summary, compared to previous work, the superiority of this system is that a comprehensive work have been done, not only in fluorescence properties after encapsulated lanthanides and dyes but also in chemical sensing. Thanks to the porous and anionic frameworks, it can encapsulate numerous lanthanide ions emitting in the visible and NIR via cation exchange but also can be used for adsorption cationic dyes, especially for Rhodamine B selectively and immediately within 5 min, the shortest time that have ever reported. As the amount of RhB varying, the color of the material can be relatively tuning. Furthermore, 2%RhB@1 can be employed as potential luminescent probe, since it shows significantly quenching effect in acetone among small solvent molecules as well as aniline among volatile organic benzenes.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21571142) and Developing Science Funds of Tongji University.Notes and references
- (a) H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed; (b) J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213 RSC; (c) Z. J. Zhang and M. J. Zaworotko, Chem. Soc. Rev., 2014, 43, 5444 RSC; (d) H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. O. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424 CrossRef CAS PubMed.
- (a) M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2012, 112, 782 CrossRef CAS PubMed; (b) Y. X. Zhao, M. Seredych, Q. Zhong and T. J. Bandosz, ACS Appl. Mater. Interfaces, 2013, 5, 4951 CrossRef CAS PubMed; (c) C. Wang, L. Li, S. Tang and X. Zhao, ACS Appl. Mater. Interfaces, 2014, 6, 16932 CrossRef CAS PubMed; (d) H. H. Wu, C. G. Thibault, H. Wang, K. A. Cychosz, M. Thommes and J. Li, Microporous Mesoporous Mater., 2016, 219, 186 CrossRef CAS.
- (a) X. Lian and B. Yan, New J. Chem., 2015, 39, 5898 RSC; (b) Y. Zhou and B. Yan, Inorg. Chem., 2014, 53, 3456 CrossRef CAS PubMed; (c) D. F. He, Q. Tang, S. M. Liu, F. Luo, Y. W. Liu, N. Li, J. Miao, X. Q. Wang, X. G. Chen, F. J. Ma and S. X. Liu, Dyes Pigm., 2015, 122, 317 CrossRef CAS.
- (a) Y. J. Cui, T. Song, J. C. Yu, Y. Yang, Z. Y. Wang and G. D. Qian, Adv. Funct. Mater., 2015, 25, 4796 CrossRef CAS; (b) Y. Lu and B. Yan, Chem. Commun., 2014, 50, 15443 RSC; (c) C. Y. Sun, X. L. Wang, X. Zhang, C. Qin, P. Li, Z. M. Su, D. X. Zhu, G. G. Shan, K. Z. Shao, H. Wu and J. Li, Nat. Commun., 2013, 4 Search PubMed.
- (a) D. W. Huang, Z. P. Gao, H. Yi, Y. X. Bing, C. G. Niu, Q. W. Guo and C. Lai, Anal. Methods, 2015, 7, 353 RSC; (b) X. Y. Xu and B. Yan, Sens. Actuators, B, 2016, 222, 347 CrossRef CAS; (c) Y. Lu, B. Yan and J.-L. Liu, Chem. Commun., 2014, 50, 9969 RSC; (d) J. N. Hao and B. Yan, Chem. Commun., 2015, 51, 14509 RSC.
- (a) Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315 RSC; (b) Z.-J. Zhang, W. Shi, Z. Niu, H.-H. Li, B. Zhao, P. Cheng, D.-Z. Liao and S.-P. Yan, Chem. Commun., 2011, 47, 6425 RSC.
- (a) J. B. Rivest and P. K. Jain, Chem. Soc. Rev., 2013, 42, 89 RSC; (b) C. K. Brozek and M. Dinca, Chem. Soc. Rev., 2014, 43, 5456 RSC.
- (a) B. Yan, RSC Adv., 2012, 2, 9304 RSC; (b) J. Feng and H. J. Zhang, Chem. Soc. Rev., 2013, 42, 387 RSC; (c) L. D. Carlos, R. A. S. Ferreira, V. D. Bermudez and S. J. L. Ribeiro, Adv. Mater., 2009, 21, 509 CrossRef CAS PubMed; (d) M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC.
- (a) J. An and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 5578 CrossRef CAS PubMed; (b) J. Y. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2009, 131, 8376 CrossRef CAS PubMed; (c) J. Y. An, C. M. Shade, D. A. Chengelis-Czegan, S. Petoud and N. L. Rosi, J. Am. Chem. Soc., 2011, 133, 1220 CrossRef CAS PubMed.
- (a) T.-W. Duan and B. Yan, J. Mater. Chem. C, 2015, 3, 2823 RSC; (b) X.-Y. Xu and B. Yan, Dalton Trans., 2015, 44, 1178 RSC.
- Y. Lu and B. Yan, Chem. Commun., 2014, 50, 13323 RSC.
- (a) Y. Zhou, H.-H. Chen and B. Yan, J. Mater. Chem. A, 2014, 2, 13691 RSC; (b) J.-N. Hao and B. Yan, J. Mater. Chem. A, 2015, 3, 4788 RSC; (c) X. Shen and B. Yan, J. Mater. Chem. C, 2015, 3, 7038 RSC.
- (a) C.-Y. Sun, X.-L. Wang, C. Qin, J.-L. Jin, Z.-M. Su, P. Huang and K.-Z. Shao, Chem.–Eur. J., 2013, 19, 3639 CrossRef CAS PubMed; (b) J.-S. Qin, S.-R. Zhang, D.-Y. Du, P. Shen, S.-J. Bao, Y.-Q. Lan and Z.-M. Su, Chem.–Eur. J., 2014, 20, 5625 CrossRef CAS PubMed; (c) M.-J. Dong, M. Zhao, S. Ou, C. Zou and C.-D. Wu, Angew. Chem., Int. Ed., 2014, 53, 1575 CrossRef CAS PubMed; (d) Z. Zhu, Y.-L. Bai, L. Zhang, D. Sun, J. Fang and S. Zhu, Chem. Commun., 2014, 50, 14674 RSC; (e) L. Liu, X.-N. Zhang, Z.-B. Han, M.-L. Gao, X.-M. Cao and S.-M. Wang, J. Mater. Chem. A, 2015, 3, 14157 RSC.
- L. L. Wen, X. Y. Xu, K. L. Lv, Y. M. Huang, X. F. Zheng, L. Zhou, R. Q. Sun and D. F. Li, ACS Appl. Mater. Interfaces, 2015, 7, 4449 CAS.
- K. Akhbari and A. Morsali, Dalton Trans., 2013, 42, 4786 RSC.
- S.-N. Zhao, X.-Z. Song, M. Zhu, X. Meng, L.-L. Wu, J. Feng, S.-Y. Song and H.-J. Zhang, Chem.–Eur. J., 2015, 21, 9748 CrossRef CAS PubMed.
- (a) S. Beheshti and A. Morsali, RSC Adv., 2014, 4, 41825 RSC; (b) B. Cheng, F. Z. Karizi, M. L. Hu and A. Morsali, Mater. Lett., 2014, 137, 88 CrossRef CAS; (c) Z. J. Li, S. K. Khani, K. Akhbari, A. Morsali and P. Retailleau, Microporous Mesoporous Mater., 2014, 199, 93 CrossRef CAS.
- (a) A. Mittal, A. Malviya, D. Kaur, J. Mittal and L. Kurup, J. Hazard. Mater., 2007, 148, 229 CrossRef CAS PubMed; (b) M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70 CrossRef CAS PubMed; (c) Z. Zhu, Y.-L. Bai, L. Zhang, D. Sun, J. Fang and S. Zhu, Chem. Commun., 2014, 50, 14674 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02043g |
| This journal is © The Royal Society of Chemistry 2016 |