Open Access Article
Open Access ArticleSensing a Bacillis anthracis biomarker with well-known OLED emitter EuTta3Phen†‡
M. A.
Shipman
,
K. J.
Ramhit
and
B. A.
Blight
*
School of Physical Sciences, University of Kent, Ingram Building, Canterbury, CT2 7NH, UK. E-mail: b.blight@kent.ac.uk
First published on 2nd February 2016
Abstract
Dipicolinic acid (DPA) is a distinctive biomarker for bacterial spores. Here, we present the successful demonstration of dramatic Switch-OFF sensing of DPA using an easily synthesised Eu(III) phosphor applied primarily in light-emitting devices. The sensor in the presence of water and phosphate is also demonstrated to be effective.
Luminescent lanthanide (Ln) complexes are an important class of emitter with applications in organic light-emitting diodes (OLEDs),1 sensors,2 and cellular imaging3 due to their distinct and narrow f–f emission bands, long lifetimes and broad stokes shifts.4 Quite often, however, the emission intensity of lanthanide compounds can be weak due to their poor molar absorptivity, due to Laporte forbidden f–f transitions. This is especially true with weakly coordinated Ln aqua cations.5 Introduction of appropriate ligands (usually with extended π-systems) to the Ln3+ trication can activate highly efficient emission profiles by sensitization of the Ln center with a more absorptive ligand ‘antenna’. This process has been termed Absorption-Energy Transfer-Emission (AETE).6,7 One distinct advantage to employing sensitised Ln3+ emitters is their low sensitivity to molecular oxygen, which can easily quench transition metal-centred phosphorescence.8 This is a crucial design consideration as it relates to practical small molecule sensors. Of particular interest, is the detection of 2,6-pyridine-dicarboxylic acid or dipicolinic acid (DPA; Fig. 1).9–12 DPA is a unique bio-marker for bacterial spore detection, with bacterial endospores comprising up to 10% by mass of DPA;13 the Bacillus anthracis spore, for instance, is the endospore vessel used in the transmission of the anthrax bioweapon.14
 | ||
| Fig. 1 Chemical structures of 2,6-pyridine dicarboxylic acid (dipicolinic acid or DPA; analyte) and EuTta3Phen (1; sensor). | ||
A very nice example of such a Ln3+-based sensor was first introduced in 2007 by Ponce an co-workers, outlining a simple synthetic route to a coordinated terbium(III)-based Turn-ON sensor (using cyclen derivative DO2A),9 where the detection of DPA sensitises the Ln-centre, activating the very efficient AETE pathway. The authors later demonstrated that europium(III) was a much less effective Turn-ON sensor than Tb3+, likely due to a larger energy gap (thus weakly coupled) between the DPA triplet state and the Eu3+ 5D0 excited state.12 With this consideration in mind, we set out to exploit this gap by alternatively identifying a strongly emissive Eu(III) complex that could act as a dramatic and sensitive Turn-OFF sensor.
In this communication we outline the use of well known Eu3+ complex as a potential DPA sensing material. As noted above, Eu3+ is not effectively sensitised by DPA, therefore, our approach is to use an easily synthesised emitter (one-pot) that is highly sensitised with the expectation that emission will be quenched in the presence of DPA. Europium3+(thenoyltrifluoroacetonato)3(1,10-phenanthroline), or EuTta3Phen (1; first reported in 1995; Fig. 1),15,16 is still one of the brightest red emitters, and has been widely used in red-emitting OLED materials.1 We postulated that with a quantum yield of approximately Φ = 0.48 in dichoromethane (Φ = 0.72 in the solid-state),17 interference by a competitive tridentate ligand such as DPA would have a visually noticeable effect on the emission intensity.
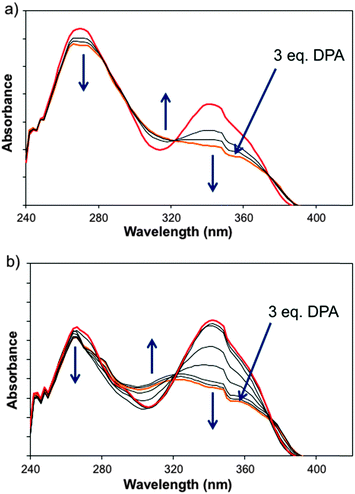
Given the surrounding environment associated with bacterial spore analysis, the effects of water, phosphate, and DPA were assessed against 1 separately and also together. Respective UV-Vis titrations with both water and N,N,N,N-tetrabutylammonium phosphate monobasic (TBA·H2PO4) into solutions of THF containing Eu(Tta)3Phen 1 resulted in diminished absorbance with little indication of any distinct reactive process or ligand exchange (refer to Fig. S1 and S2 of ESI‡). However, titration with DPA exhibited a clear interaction with the appearance of an isosbestic point at 326 nm, altering the absorbance of the parent species (Fig. 2a). This was further reinforced by titration of 1 in 2% H2O and phosphate (1 × 10−4 M) with DPA, exhibiting a near identical profile with additional H2O/H2PO4− equilibria (Fig. 2b), indicating a change in the substrate composition.
As eluded above, we then performed emission studies to further test the efficacy of this system by observing how the introduction of DPA alters the fluorescence spectra of lanthanide complex 1. In its unperturbed state, 1 exhibits characteristic Eu(III) 5D0–7Fn (n = 0–4) transitions. Upon addition of water (0–20% v/v; Fig. S4, ESI‡) complete quenching of 1 is exhibited above 10% water content in THF. Emission loss is likely a result of a combination of proton transfer and vibrational decay processes (through the ninth coordination site). This is supported by minimal perturbations in the corresponding UV-Vis titration displaying little evidence of any ligand exchange. Surprisingly, titration of 1 with TBA·H2PO4 (Fig. S5, ESI‡) showed an increase in emission intensity by approximately 20%. Repeating the experiment with a solvent mixture of 2% water in THF, however, exhibited a modest decrease in emission intensity (Fig. S6, ESI‡), though far from a complete emission quench.
Introduction of DPA to a THF solution of 1 showed a remarkable decrease in emission (Fig. 3a), particularly the strong 5D0–7F2 transition. From the reaction profile (Fig. S7, ESI‡) it is evident that emission is fully quenched upon full saturation of the Eu3+ coordination sphere with three molar equivalents of DPA. The robustness of the system was also successfully assessed with titrations of 1 with DPA in a 2% water/THF mixture (Fig. S8, ESI‡), and moreover in a 2% water/THF mixture with a background concentration of TBA·H2PO4 (5 × 10−6 M; Fig. 3b). These experiments demonstrate that with modest water and phosphate concentrations present, sensitivity and selectivity of 1 as a DPA sensor remain intact, and that this small allowance for water and phosphate content should translate to 1 performing as an effective practical in-the-field sensor. Fig. 3c illustrates how 1 performs as visual sensor for DPA detection in the absence and presence of water and phosphate. In order to probe the selective utility of 1 we further undertook a small selectivity study that included to amino acids (glycine and L-aspartic acid) as well as a representative selection of various competitive protic/bidentate substrates (benzoic acid, catechol, nicotinic acid, 2-picolinic acid) and evaluated these results against those of DPA (Fig. 4). This analysis demonstrated that a number of biologically or structurally relevant small molecule analytes have is little to no effect on the emission profile of 1 compared to the extent of quenching imparted by DPA. Lastly, we assessed the sensitivity of the system, and can demonstrate that DPA does enact a spectroscopically measurable emission quench on 1 at nanomolar concentrations (Fig. S9 (ESI‡); titrating 1 (10 nM) with small aliquots of DPA (2 × 102 nM)). It has been established that there is approximately 1 × 108 molecules of DPA per bacterial spore,10 and thus based on crude mathematics, this corresponds to the detection of as few as 100 spores by employing 1 as a sensor.
 | ||
| Fig. 3 Fluorescence emission titration experiments where 1 (1 × 10−5 M; THF) is titrated with (a) DPA (2 × 10−4 M; THF) and (b) DPA (2 × 10−4 M; THF) in the presence of 2% water and 0.5 equivalents of TBA·H2PO4 (5 × 10−6 M) at 298 K (λex = 340 nm). Reaction profiles are illustrated in ESI.‡ (c) Visual comparison of emission properties of 1 upon exposure to DPA, H2O, and/or TBA·H2PO4. Insets in (a) and (b): emission response as a function of increasing DPA concentration. | ||
Mechanistically, there are two initial pathways for ligand exchange: displacement of 1,10-phenanthroline (Phen) for DPA; displacement of thenoyltrifluoroacetone (TtaH) for DPA. Exchange of the ET ligand, Phen, for DPA would exhibit a complete loss of emission after one molar equivalent of added DPA,1,18 destroying the AETE process. The non-equimolar stoichiometry observed in the fluorescence emission spectra leads one to believe that the ligand acting as the ‘antenna’ is being displaced first, reducing the efficiency of AETE, but not eliminating it until higher concentrations of DPA are reached. Assessment of the quenching mechanism, which will enable future improvements to the sensor, led to a series of 1H NMR studies. Initially, we wanted to see if a ligand exchange process could be identified from THF-d8 solutions of 1 titrated with DPA, however due to the paramagnetic characteristics of Eu3+, broadening of the signals made the protons indistinguishable (Fig. S10, ESI‡). A diamagnetic lanthanum(III) analogue was then synthesised, LaTta3Phen (2),19 and treated with DPA. 1H NMR analysis confirmed that upon addition of DPA to a sample of 2 (the La3+–DPA product precipitates from solution due to higher NMR concentrations), unreacted 2 and freshly displaced TtaH are observed in the spectrum (Fig. S11, ESI‡). This study gives us insight into the behaviour of 1 when exposed to DPA, it does confirm the reaction stoichiometry and why the loss of emission is not immediate.
In summary, we have identified that the easily synthesised 1 can act as an effective Turn-OFF sensor in the detection of 2,6-pyridinedicarboxylic acid (DPA), a prevalent biomarker in bacterial spores. We further demonstrated that this lanthanide-based emitter can continue to function effectively in the presence of small quantities of both water and phosphate, is selective for DPA, and is detectable down to nanomolar concentrations. The mechanism for emission quenching was also revealed to proceed first via ligand displacement of the thenoyltrifluoroacetonato-ligand by DPA. We are currently developing analogues of 1 to improve stability for incorporation into various polymer matrices.
We would like to gratefully acknowledge the Royal Society (RG130043), Edinburgh Instruments Ltd. (http://www.edinst.com) and the University of Kent, School of Physical Sciences for supporting this work.
Notes and references
- J. Wang, R. Wang, J. Yang, Z. Zheng, M. D. Carducci, T. Cayou, N. Peyghambarian and G. E. Jabbour, J. Am. Chem. Soc., 2001, 123, 11378 Search PubMed.
- L. F. Smith, B. A. Blight, H.-J. Park and S. Wang, Inorg. Chem., 2014, 53, 8036 CrossRef CAS PubMed.
- A. Thibon and V. C. Pierre, Anal. Bioanal. Chem., 2009, 394, 107 CrossRef CAS PubMed.
- H. C. Aspinall, Chemistry of the f-Block Elements, Gordon and Breach Science Publishers, Amsterdam, 2001 Search PubMed.
- J. Georges, Analyst, 1993, 118, 1481 RSC.
- M. L. Cable, D. J. Levine, J. P. Kirby, H. B. Gray and A. Ponce, Advances in Inorganic Chemistry, Elsevier, San Diego, 2011, vol. 63, pp. 1–45 Search PubMed.
- K. Binnemans, Chem. Rev., 2009, 109, 4283 CrossRef CAS PubMed.
- N. Kaltsoyannis and P. Scott, The f-elements, Oxford University Press Inc., New York, 1999 Search PubMed.
- M. L. Cable, J. P. Kirby, K. Sorasaenee, H. B. Gray and A. Ponce, J. Am. Chem. Soc., 2007, 129, 1474 CrossRef CAS PubMed.
- M. L. Cable, J. P. Kirby, D. J. Levine, M. J. Manary, H. B. Gray and A. Ponce, J. Am. Chem. Soc., 2009, 131, 9562 CrossRef CAS PubMed.
- I. Lee, W.-K. Oh and J. Jang, J. Hazard. Mater., 2103, 252–253, 186 Search PubMed.
- H. Tan, C. Ma, L. Chen, F. Xu, S. Chen and L. Wang, Sens. Actuators, B, 2014, 190, 621 CrossRef CAS.
- W. G. Murrel, The Bacterial Spore, Academic Press, New York, 1969 Search PubMed.
- J. A. Jernigan, D. S. Stephens, D. A. Ashford, C. Omenaca, M. S. Topiel, M. Galbraith, M. Tapper, T. L. Fisk, S. Zaki, T. Popovic, R. F. Meyer, C. P. Quinn, S. A. Harper, S. K. Fridkin, J. J. Sejvar and C. W. Shepard, Emerging Infect. Dis., 2001, 7, 933 CrossRef CAS PubMed.
- T. Sano, M. Fujita, T. Fujii, Y. Hamada, K. Shibata and K. Kuroki, Jpn. J. Appl. Phys., Part 1, 1995, 34, 1883 CAS.
- L. Liu, Z. Hong, J. Peng, X. Liu, C. Liang, Z. Liu, J. Yu and D. Zhao, Synth. Met., 1997, 91, 267 CrossRef CAS.
- C. Freund, W. Porzio, L. Giovanella, F. Vignali, M. Pasini and S. Destri, Inorg. Chem., 2011, 50, 5417 CrossRef CAS PubMed.
- J.-C. G. Bünzli and C. Piquet, Chem. Soc. Rev., 2005, 34, 1048 RSC.
- G. Zhong, Y. Feng, K. Yang and G. Zhu, Chem. Lett., 1996, 775 CrossRef CAS.
Footnotes |
| † This submission is dedicated to the late Prof. Richard F. Langler for his contributions to undergraduate research. Fittingly, the experimental work in this account was fully carried out by final year undergraduate project students. |
| ‡ Electronic supplementary information (ESI) available: Supporting UV-Vis, fluorescence emission, and NMR spectra. See DOI: 10.1039/c5tb02469b |
| This journal is © The Royal Society of Chemistry 2016 |