A novel type of microscopic size chip based on double-stranded nucleic acids
Yu. M.
Yevdokimov
*,
V. I.
Salyanov
and
M. A.
Zakharov
Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Vavilova str. 32, 117984 Moscow, Russia. E-mail: yevdokim@genome.eimb.relarn.ru
First published on 9th August 2001
Abstract
The double-stranded molecules of nucleic acids (NA) of B- and A-families fixed in the structure of cholesteric liquid-crystalline dispersions, formed as a result of phase exclusion of these molecules from polymer-containing solution, have been used as ‘building blocks’ for the molecular design. Using the formation of polymeric chelate bridges between NA molecules, three-dimensional structures consisting of alternating NA, anthracycline and copper ions, were created. The formation of the polymeric chelate bridges allows one to stabilize the initial spatial mode of ordering of neighboring NA molecules in a form of so-called ‘molecular constructions’, immobilize these constructions onto supporting film and evaluate their sizes and shape. The creation of NA molecular constructions is accompanied by an ‘extra-increase’ in the amplitude of the bands in the CD spectra, despite the initial sense of cholesteric twisting characteristic of liquid-crystalline dispersions. Destroying of polymeric chelate bridges between NA molecules by action of biologically relevant compounds results in disintegration of NA liquid-crystalline molecular constructions. Three-dimensional NA molecular construction can be used as a microscopic size multifunctional chemical unit (chip) for biological or chemical needs.
Introduction
The creation of microscopic size structural elements (chips) with regulated spatial and chemical properties for the purposes of chemistry and biology as well as for advanced material engineering requires the use of new physicochemical and biotechnological approaches and techniques. These techniques include, in particular, the directed creation of complex supramolecular, three-dimentional structures, whose ‘building blocks’ are nucleic acid (NA) molecules, i.e. the formation of spatial molecular constructions from isolated, single- or double-stranded, linear or circular NA molecules.1–5Our approach6 to creating molecular constructions containing double-stranded (ds) NA molecules is based not on the use of isotropic NA solutions, but on the liquid-crystalline dispersions formed by phase separation of these molecules from polymeric water–salt solutions.
A characteristic feature of phase separation of ds NA molecules is that this process results in a sharp increase in the local concentration of these molecules, with their spontaneous spatial ordering, i.e. this process leads to formation of dispersed phase (dispersion) of NA consisting of the single condensed particles.
The very possibility of using ds NA for formation of molecular constructions with controlled parameters is based on a few properties characteristic only of particles of NA dispersions: (i) a single particle7 with a mean diameter of about 5000 Å of dispersed phase consists of about 104 NA molecules, whose diffusion degrees are, in part, ‘frozen’;8 (ii) within the particle, NA molecules are located at a fixed distance in an ordered manner, which depends on the osmotic pressure of the solvent. This distance can be changed9,10 over the range 25 to 50 Å; (iii) for each particle of dispersion the liquid-crystalline ordering of neighboring NA molecules is specific,11,12 hence, one can say that the phase separation results in formation of the NA liquid-crystalline dispersion; (iv) properties of rigid ds NA molecules could be easily predicted and the nature of their intermolecular interaction under different conditions can be programmed, making it possible to control the peculiarities of the resulting supramolecular structure. At fixed osmotic pressure of the solvent one can form the cholesteric liquid-crystalline dispersion with an intense band in the CD spectrum, located in the NA absorption region;13 (v) short, rigid molecules of ds NA with lengths of the order of 1000 Å in solvents with standard properties are molecules with high local rigidity, that allows such molecules to be used as ‘building blocks’ without change in their physical properties; (vi) chemical reactivity of the nitrogen bases of NA molecules does not change on phase separation. Nitrogen bases in the context of NA molecules within particles of liquid-crystalline dispersion, retain the ability not only to form complexes with a variety of chemical and biologically relevant compounds, but also the ability to take up specific orientation relative to the long axis of the NA molecules. These complexes add a new chemical reactivity to the formed particles, hence, theoretically, each particle of the liquid-crystalline NA dispersion can be used as a multifunctional (integral) chemical reactor or biosensing unit, whose physico-chemical properties can be changed in response to the action of a wide spectrum of chemical, biological or physical factors.
Consideration of the above points reveals the fundamental possibility for spatial fixation of the neighboring, closely-located and fairly low-mobile NA molecules by formation of polymeric bridges (crosslinks) between these molecules, i.e. it is possible to create a molecular construction, whose properties can be specified in advance by controlling the properties of NA molecules or the solvent used.
Recently, an attempt at formation of polymeric chelate bridges between DNA molecules was, in fact, described.14
It is of interest to form molecular constructions when ds DNA molecules are replaced by molecules of synthetic double- or triple-stranded polyribonucleotides, which belong to the A- rather than to B-structural family. The right-handed helices of the B- and the A- families differ, in particular, in the structural parameters of polynucleotide molecules. Therefore, these molecules are substantially different in the character of interaction with biologically relevant compounds. Various aromatic compounds cannot intercalate between the base pairs of polyribonucleotides in the A-form, although these compounds are able to form external complexes with molecules of polyribonucleotides.
We will focus our attention on the the properties of molecular constructions based on the particles of the cholesteric liquid-crystalline dispersions of ds DNA and ds RNA molecules, crosslinked by polymeric chelate bridges, and demonstrate the possibility of using the molecular constructions as multifunctional sensing units (chips).
Materials and methods
Ds DNA from chicken blood erythrocytes (Reanal, Hungary) was used. The molecular mass of DNA after ultrasonic depolymerization, estimated by gel electrophoresis in 1% agarose gel, was 0.5–0.8 × 106 Da.†Synthetic double-stranded polyribonucleotides poly(I)xpoly(C) (Sigma Co, USA, 500–1500 bp), poly(A)xpoly(U) (Sigma Co,USA, 500–1300 bp), designated below as ds RNA, were used without additional purification.
The anthracycline daunomycin (DAU) was purchased from Sigma Co, USA. The concentration of DAU in water–salt solutions was determined spectrophotometrically. Poly(ethylene glycol), PEG from Serva, molecular mass 4000 Da, was used.
The molecular constructions based on NA molecules were obtained as previously described.15,16 Initially, the liquid-crystalline dispersion (LCD) of NA was formed by mixing equal volumes of water–salt solutions (0.3 M NaCl; 2 mM Na-phosphate buffer; pH 6.67) of NA and PEG to induce the phase separation of NA molecules. Subsequently, to 2 mL of LCD of NA, small (1–10 μL) volumes of DAU stock solution (Ct ∼4 × 10−3 M) were added under vigorous stirring. Finally, adjacent NA molecules were crosslinked by polymeric chelate bridges, by addition of small (5–15 μL) volumes of CuCl2 solution (Ct = 1 × 10−3 M) to 2 mL of LCD of [NA–DAU] complex under stirring.
The molecular constructions based on NA molecules were disintegrated by means of the ‘extraction’ of the copper ions from the polymeric chelate crosslinks by various proteins such as BSA, insulin, pepsin, lysozyme, RNA-ase, γ-globulins, ‘total protein’ as well as polyaminoacids such as polyhistidine, polylysine, polyglutamic or polyaspartic acids.
The absorption spectra were recorded on a Specord-M 40 spectrophotometer (Germany) and the CD spectra were recorded by a portable device (produced by the Institute of Spectroscopy, RAS, Moscow, Russia). To isolate the particles of NA LCD dispersions, crosslinked by polymeric chelate bridges (molecular constructions), the solution in which they were formed was filtered through a poly(ethyleneterephthalate) nuclear membrane filter (diameter of pores 0.1–0.25 ; produced by the Institute of Crystallography, RAS), that allowed us to immobilize NA particles; filters were dried in air for no less than 1 h. A surface of the nuclear membrane filter with immobilized DNA particles was examined by a SOLVER-P47 scanning atomic force microscope (produced by NT-MDT, Zelenograd, Russia). The resonant mode was f = 546.4 kHz, the scanning area 13 × 14 μm, 512 × 512 pixels and the scanning rate 280 nm s−1.
Results
1 Molecular constructions based on the ds DNA molecules
The cholesteric packing of the DNA molecules induced by their phase separation in polymer containing solutions is characteristic of conditions7,10–12 at which the average distance between DNA molecules usually exceeds 30 Å. This causes the appearance of abnormal optical activity, i.e. an intense CD band in the absorption region of DNA chromophores (λmax ∼ 270 nm, Fig.1). Curve 1, specific for the initial linear B-form of double-stranded DNA, is eventually transformed into curve 2, specific to the cholesteric DNA liquid-crystalline dispersion (LCD). The molecular dichroism of this LCD, expressed as Δε, is substantially higher (Δε ∼ 140) than that of free DNA in water–salt solution (Δε ∼ 2.3).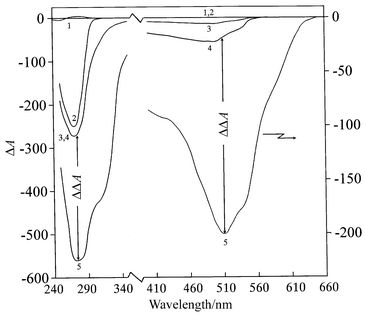 | ||
| Fig. 1 Circular dichroism spectra of LCD formed from DNA molecules (curve 2), treated with DAU (curves 3 and 4) and with addition of CuCl2 (curve 5). Curve 1: B-form of native DNA in water–salt solution (shown as a control); curve 3: 4.8 × 10−6 M DAU ; curve 4: 18.7 × 10−6 M DAU; curve 5 reflects the transformation of curve 4 after addition of 10 × 10−6 M CuCl2. 0.3 M NaCl; 0.002 M Na-phoshate buffer; pH 6.7; 170 mg mL−1 PEG; 5.5 mg mL−1 DNA. ΔA = AL − AR in mm; 1 mm = 1 × 10−5 optical units. | ||
The formation of the LCD is not accompanied by reactivity changes of the nitrogen bases, yet the neighboring nucleic acid molecules in the new structure represent ‘building blocks’ capable of chemical reactions. These can be used to generate molecular constructions with tailored properties. Indeed, the addition of a few antibiotics to the DNA LCD is accompanied by formation of an intercalation [DNA–antibiotic] complex, i.e. incorporation of antibiotic molecules into the content of the liquid-crystalline structure. This results in the appearance of an additional band in the CD spectrum,13 located in the antibiotic absorption region.
In fact, the treatment of the DNA LCD with low concentrations of anthracycline antibiotic–daunomycin, DAU, is accompanied by the appearance of an additional band in the DAU absorption region (λmax ∼ 505 nm, Fig. 1, curve 3). Taking into account the results of the theoretical calculations of the CD spectra for the NA LCD, this shows that the orientation of DAU molecules, with respect to the long DNA helical axis, coincides with the orientation of the nitrogen bases, i.e. DAU molecules are located anisotropically. This is possible only in the case where DAU molecules are inserted between the DNA base pairs. Subsequent treatment of the LCD of (DNA–DAU) intercalation complex, formed at low concentrations of DAU (up to CDAU ⩽ 6 × 10−6 M ) with copper salt (Cu2+ ions) is not accompanied by changes in the DNA LCD optical properties. This demonstrates that in the case of intercalation of DAU only its reactive groups are protected against chemical reaction, or more exactly, against chelate formation.
However, the treatment of the DNA LCD, complexed with higher concentrations of DAU (CDAU
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 6 × 10 −6 M, curve 4), with CuCl2 results in a manifold increase in the amplitudes of the bands, located both in the antibiotic (λmax
∼ 505 nm) and the DNA absorption region (λmax
∼ 270 nm) (Fig. 1, curve 5). Hence, there is ‘critical’ concentration of DAU that provides the ‘extra-increase’ (ΔΔA) of both bands.
6 × 10 −6 M, curve 4), with CuCl2 results in a manifold increase in the amplitudes of the bands, located both in the antibiotic (λmax
∼ 505 nm) and the DNA absorption region (λmax
∼ 270 nm) (Fig. 1, curve 5). Hence, there is ‘critical’ concentration of DAU that provides the ‘extra-increase’ (ΔΔA) of both bands.
Fig. 2 shows, as an example, the dependence of ΔΔA value upon CuCl2 concentration measured at λ = 505 nm (Fig. 2A) and λ = 270 nm (Fig. 2B). One can see that the ‘extra-increase’ in the amplitude, expressed as ΔΔA value, is observed after ‘critical’ concentration of CuCl2 is reached. In this case, the higher the DAU concentration in solution, the bigger the ‘extra-increase’ (curves 1–3). One can see as well that the ‘extra-increase’ measured at λ = 270 nm is about two-times higher in comparison to that measured at λ = 505 nm.
 | ||
| Fig. 2 The dependence of ‘extra-increase’ (ΔΔA) of amplitude of the bands at λ = 505 nm (A) and λ = 270 nm (B) in the CD spectra of LCD formed from DNA–DAU complexes upon CuCl2 concentration. Curve 1: 8.9 × 10−6 M DAU; curve 2: 15.7 × 10−6 M DAU; curve 3: 27.3 × 10−6 M DAU. 0.3 M NaCl; 0.002 M Na-phoshate buffer; pH 6.7; 170 mg mL−1 PEG; 5.5 mg mL−1 DNA. ΔΔA in arbitrary units; 1 unit = 1 × 10−6 optical units. | ||
2 Molecular constructions based on the ds RNA molecules
As opposed to the LCD of DNA, the addition of DAU to the LCD of poly(I)×poly(C) is not accompanied by an appearance of an intense band in the CD spectrum located in the DAU absorption region at any concentration of DAU. The CD spectrum shows here only a week band (Δεmax ∼ 5) at about 480 nm. Such a weak band may indicate isotropic location of DAU molecules both in water–salt solution and in the presumable external complex with poly(I)×poly(C).
However, in full agreement with data obtained for the ds DNA, the addition of copper salts (Cu2+ ions) to the LCD of poly(I)×poly(C) treated with DAU (CDAU
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 6 × 10−6 M) leads to the strong enhancement of the amplitude of band in the poly(I)×poly(C) absorption region (λmax
∼ 270 nm) and to the appearance of an intense band in the region of DAU absorption (λmax ∼ 505 nm; see, for instance, curve 5 in Fig. 1). Again, this increase is observed after the ‘critical concentrations’ of DAU or CuCl2 are reached.
6 × 10−6 M) leads to the strong enhancement of the amplitude of band in the poly(I)×poly(C) absorption region (λmax
∼ 270 nm) and to the appearance of an intense band in the region of DAU absorption (λmax ∼ 505 nm; see, for instance, curve 5 in Fig. 1). Again, this increase is observed after the ‘critical concentrations’ of DAU or CuCl2 are reached.
Similar to DNA (see above) the increase in abnormal optical activity in poly(I)×poly(C) in DAU absorption regions reflects both the anisotropic location of DAU molecules and the growth of their concentration in the content of the LCD particles.
Hence, a base sequence of short, rigid, ds nucleic acids and synthetic polynucleotides plays, if any, a minor role in the ‘extra-increase’ of intense bands in the CD spectrum of their liquid-crystalline dispersions as a result of DAU and CuCl2 treatment.
3 Shape and size of NA based molecular constructions
To prove that the particles of the ds NA LCD crosslinked by polymeric chelate bridges exist in the water–salt solution as a three-dimensional molecular construction even after removal of PEG, we have run experiments with atomic force microscopy (AFM) to measure the size of the particles of the DNA LCD, crosslinked by polymeric chelate bridges.Fig. 3 shows, as an example, the AFM images of the DNA particles. Similar results were obtained for poly(I)×poly(C) particles. The x–y particle size distributions, based on direct measurements of the visual shapes of these particles, are summarized in Fig. 4A and 4B. One can see that in the case of DNA the maximum of the mean size is about 4000–5000 Å, whereas in the case of poly(I)×poly(C) is about 4000 Å.
![AFM image of the DNA LCD crosslinked by polymeric chelate bridges and immobilized onto the surface of the nuclear membrane filter [poly(ethyleneterephthalate)]. The small dark spots correspond to pores in the membrane filter (D ∼ 0.2 μm).](/image/article/2001/LC/b103557f/b103557f-f3.gif) | ||
| Fig. 3 AFM image of the DNA LCD crosslinked by polymeric chelate bridges and immobilized onto the surface of the nuclear membrane filter [poly(ethyleneterephthalate)]. The small dark spots correspond to pores in the membrane filter (D ∼ 0.2 μm). | ||
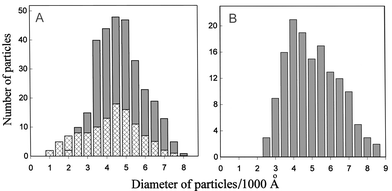 | ||
| Fig. 4 Size distribution for the DNA (two sets of experiments (A)) and poly(I)×poly(C) (B) LCD crosslinked by polymeric chelate bridges. | ||
Fig. 3 and 4 show unequivocally that particles of LCD of NA crosslinked by polymeric chelate bridges represent the stable spatial structures.
4 Destruction of polymeric chelate bridges in molecular constructions by proteins
Addition of different proteins to DNA LCD crosslinked by polymeric chelate bridges had the effect that the amplitude of the intense band in the CD spectra of molecular constructions based on NA of the B- or the A-families, reflecting the presence of polymeric chelate crosslinks between these molecules, decreases quickly. This optical effect is primary evidence of the presence of protein in solution. The decrease in the amplitude of the intense band in the CD spectrum does not depend upon the initial sense of cholesteric twisting NA LCD.As an example, Fig. 5 shows the realtionship between changes in the relative amplitude of the CD band at λ = 505 nm and the time of treatment of molecular construction by BSA. These data show that the change in the amplitude of the band in the CD spectrum of the molecular construction depends on the protein concentration in solution (curves 1–3). One can see as well that the DNA LCD is destabilized at rather low concentrations of this compound.
 | ||
| Fig. 5 The relative change in the amplitude of the band at λ = 505 nm (ΔArel = 1 − ΔΔA/ΔΔAmax) in the CD spectrum versus time of treatment of the DNA molecular construction with BSA. Curve 1: 1.1 μg mL−1; curve 2: 3.4 μg mL−1 and curve 3: 5.7 μg mL−1 of BSA. 0.037 M NaCl; 0.00025 M Na-phoshate buffer; pH 6.7; 21.25 mg mL−1 PEG; 2.05 × 10−6 M DAU; 0.74 × 10−6 M CuCl2; 0.69 μg mL−1 DNA. | ||
Similar data were obtained not only at treatment of the molecular construction by BSA, but also using individual proteins such as insulin, pepsin, lysozyme, RNA-ase, γ-globulins or their mixture in a form of ‘total protein’, as well as polyaminoacids such as polyhistidine, polylysine, polyglutamic or polyaspartic acids. Fig. 6 shows, as an example, the relationship between changes in the relative amplitude (ΔArel) of the CD band at λ = 505 nm and the time of treatment of molecular construction by polyaspartic acid. These curve were taken for different concentrations (1 and 0.1 μg mL−1) of polyaspartic acid.
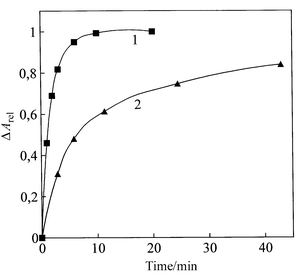 | ||
| Fig. 6 The relative change in the amplitude of the band at λ = 505 nm in the CD spectrum versus time of treatment of the DNA molecular construction with poly-L-aspartic acid. Curve 1: 1 μg mL−1; curve 2: 0.1 μg mL−1 of poly-L-aspartic acid. 0.3 M NaCl; 0.002 M Na-phoshate buffer; pH 6.7; 42.5 mg mL−1 PEG; 21.06 × 10−6 M DAU; 2.42 × 10−6 M CuCl2; 1.42 μg mL−1 DNA. | ||
The addition of these compounds to the DNA-based molecular construction results in a quick decrease in the amplitude of the negative band in the CD spectrum. The existence of a directly proportional relationship between the relative amplitude (ΔArel) and the protein concentration (over the range 0–10 μg mL−1) allows this realationship to be used as a calibration curve for detection of low (up to 0.5 μg mL−1) protein concentration in various liquids.
Thus, use of a molecular construction based on NA liquid-crystalline particles allows the presence of proteins to be detected in tested liquids, as well as to estimate them at quite low concentration.
Discussion
1 Polymeric chelate bridges between the neighboring NA molecules
Our results show that the right-handed ds DNA molecules as a result of phase separation form cholesteric LCD, which exhibits an intense negative CD band in the absorption region of nitrogen bases, whereas the right-handed ds RNA molecules under the same conditions form cholesteric LCD with an intense positive CD band. The difference in the signs of intense bands of cholesteric LCD of DNA and RNA shows that the energies of interaction of neighboring DNA and RNA molecules are different. In accordance with theoretical estimations,13 this difference results in formation of left-handed cholesterics from the right-handed ds DNA molecules and right-handed cholesterics from the right-handed ds RNA molecules. Hence, the different intense bands in the region of nitrogen bases absorption could appear in the CD spectrum without any change in properties of nitrogen bases.Treatment of the DNA LCD by DAU results in intercalation of antibiotics molecules between nitrogen bases. The formation of intercalation [DNA–DAU] complex results in anisotropic ordering of DAU molecules and it is accompanied by the appearance of an easily detected CD band in the antibiotic absorption region (Fig. 1, curve 3). It is necessary to add that the amplitude of this new band exceeds many times the amplitude of the band specific for DAU molecular dichroism and is unequivocally determined by the amount of DAU molecules intercalated between the DNA base pairs.
No substantial alterations in the CD spectrum in the DAU absorption region were observed, when cholesteric LCD composed of ds RNA molecules were treated with DAU. The lack of a strong CD band in the region of DAU absorption indicates, that in this case, no intercalation complex is formed between DAU and ds RNA. Hence, in contrast to DNA, DAU molecules are located isotropically near the RNA surface (they can form only an external complex).
However, very big alterations in the CD spectra of the DNA and the RNA cholesteric LCD, added with a concentration of DAU higher than ‘critical’, are observed when these LCD are treated with Cu2+ ions (see Fig. 1, curve 5), namely, intense bands (despite their inital signs!) in both cases at λ = 270 nm and λ = 505 nm appeared. According to theoretical calculations,13 the emergence of an intense band in the DAU absorption region reflects the anisotropic location of DAU molecules both in DNA and RNA LCD particles.
The optical properties of 10 different anthracycline antibiotics added to DNA LCD and treated by Cu2+ ions have been compared recently.14–16 All studied anthracycline antibiotics form intercalation complexes with DNA LCD, the latter being easily detected by the appearance of the corresponding bands in the CD spectrum. The sign of the band in the antibiotic region is the same as in the DNA nitrogen base absorption region. Taking into account the results of the theoretical calculations of the CD spectra for the NA LCD, this testifies that the orientation of antibiotic molecules, with respect to the long DNA helical axis, coincides with the orientation of the nitrogen bases. However, the extra-increase in the amplitude of the band upon addition of Cu2+ ions is observed only for those antibiotics that contain four reactive oxygen atoms at positions 5, 6 and 11, 12. This means, the keto-oxygen and the peri-hydroxy groups of DAU molecules are available for chemical reactions, particularly for chelate formation.17–19 Hence, the crosslinking of neighboring NA molecules is possible only at formation of external DAU complexes with NA molecules. It is relevant to note that, under the test conditions, only minor changes in the anthracycline absorption spectra are observed upon addition of magnesium, zinc, cadmium or manganese salts. Instead, in the case of copper, nickel, iron, palladium or aluminium salts the mentioned changes in the spectra, indicating the formation of complexes of these metal ions with anthracyclines, are marked. However, the amplitude of the CD band is intensified upon the addition of Cu2+ ions only.
Here we stress again that DAU molecules located isotropically along neighboring NA molecules are not visible in the CD spectrum. In addition, anisotropic location of DAU molecules, due to their intercalation between DNA nitrogen base pairs, does not induce the ‘extra-increase’ of the band in the CD spectrum after addition of CuCl2. Hence, one can suppose that there is a quite different mechanism, that provides another mode of anisotropic location of DAU molecules and explains the ‘extra-increase’ of the CD band after addition of CuCl2. This mechanism can be realized without intercalation of DAU molecules between NA base pairs.
The unique fact that after CuCl2 treatment the intense band appears for the RNA LCD added with DAU, despite the inability of DAU molecules to intercalate between the RNA base pairs,20–23 allows us to consider two models of spatial location of DAU molecules responsible for ‘extra-increase’ of optical activity for both the LCD RNA and DNA.
First, it not excluded that ‘stacks’24 from externally bound DAU and DAU molecules from solution are formed on the NA surfaces in the presense of Cu2+ ions. The resulting ‘stacks’ of DAU molecules are stabilized due to both the formation of dimeric complexes [DAU–Cu2+–DAU] and stacking interaction between neighboring DAU molecules inside the ‘stacks’. The axes of stacks of DAU molecules coincide in this case with the long axes of adjacent NA molecules.24
Second, it is not excluded that in the presence of Cu2+ ions, ‘horizontal’ clelate complexes17–19 are formed, which include both externallly bound DAU and DAU molecules from solution. The appropriate spatial arrangement of DAU molecules on neighboring NA molecules in LCD may result in their crosslinking through flat polymeric chelate complexes (bridges) (DAU–Cu2+–DAU–Cu2+ –⋯– Cu2+ –DAU–Cu2+ –DAU) located perpendicular to the axes of adjacent NA molecules.
In any of the models above, the sharp increase in the amplitude of the CD band resulting from addition of DAU and copper ions indicates the large rise in the number of DAU molecules located anisotropically in the structure of the cholesteric NA LCD particles.
2 Hypothetical scheme of molecular construction
Obviously, the stability of the crosslinked LCD of NA will depend on the number of polymeric chelate bridges between neighboring NA molecules. In the presence of a given number of these crosslinks, the factor that can influence the structure and the properties of a molecular construction, is no longer the osmotic pressure of the solvent, but the enthalpy provided by the crosslinks.Hence, having a large number of chelate bridges, there is a chance that PEG is not needed to stabilize the LCD. Under these conditions, one can expect to preserve the abnormal optical properties of the molecular construction, despite a major change in the solvent osmotic pressure.
If this is the case, one can use a rather simple experimental technique to make a choice between two suggested models for the ‘stacked’ or ‘horizontal’ orientation of (DAU–Cu2+–DAU) chelate complexes. The technique is based on the fact that, on the decrease of PEG concentration below a ‘critical’ value,9 the liquid-crystalline packing of neighboring NA molecules due to osmotic pressure of PEG solution will disappear and, hence, NA molecules should be in the isotropic state. This transition will be accompanied by disintegtation of LCD particles and, hence, by disappearance of intense bands in the CD spectra. Such a transition is possible only if there are no crosslinks between adjacent NA molecules, which fix these molecules in an ordered state and which are stable even at low PEG concentration. This means that in the case of the first model above, the particles of LCD should disappear, whereas, in the case of the second model, particles crosslinked by polymeric chelate bridges should be retained.
From this point of view, it is of interest to look at the data on the shape of particles of LCD of NA molecules crosslinked by polymeric chelate bridges (Fig. 3). These particles were detected in PEG solution after its concentration was decreased from 170 to 10 mg mL−1. It should be taken into account that the ionic strength of solution also decreased with dilution and the liquid-crystalline cholesteric structure of ds NA molecules could not exist under these conditions of dilution.10 The results presented in Fig. 3 deserve additional comment. First, they demonstrate that the NA LCD crosslinked by polymeric chelate bridges are stable in water–salt solutions, as predicted. Hence, under these conditions one can fix a single particle of the NA LCD and investigate its properties. Second, despite possible tip-induced flattening of particles, the mean diameter of about 4500 Å corresponds to that obtained by parallel transmission electron microscopy (data not shown). Moreover, the particle diameter of the DNA LCD is similar to that of the particles prepared in PEG-containing solutions without polymeric chelate bridges, as determined by a variety of other techniques.7 This means, that the three-dimensional structure of the NA cholesteric LCD is not altered by crosslinking.
Hence, the obtained results show an unambiguous conclusion in favor of the second model of formation of the polymeric chelate bridges between neighboring NA molecules. This, in turn, means that the amplification of the intense (negative or positive) bands in the CD spectrum of the cholesteric LCD of NA’s by adding anthracyclines and Cu2+ ions, reflects the formation of flat polymeric chelate bridges of the type (DAU–Cu2+ –DAU–Cu2+ –⋯– Cu2+ –DAU–Cu2+ –DAU) between neighboring NA molecules.17,19 These bridges reflect the well-known stereochemical and electronic properties of complexes in which Cu2+ ions interact with oxygen atoms of anthracyclines forming an ‘external’, rather than intercalation, complexes with the NA molecules. Taking into account the planar geometry of the polymeric chelate (DAU–Cu2+–DAU–Cu2+ –⋯–Cu2+ –DAU–Cu2+–DAU) bridges,17–19 it is possible to prove that the increase in the amplitude of the abnormal band in the CD spectrum upon addition of Cu2+ is related to the rise in concentration of anisotropically oriented DAU molecules in the dispersion. Since polymeric chelate bridges can be formed in any direction starting from any NA molecule fixed in the LCD, it is possible to infer that, as a result of interaction of NA molecules with anthracyclines and subsequent addition of Cu2+ ions, a three-dimensional structure is formed, in which the neighboring NA molecules are crosslinked by polymeric chelate bridges containing Cu2+ ions. One can call this structure a ‘molecular construction’. In Fig. 7 a hypothetical scheme of this molecular construction is shown.
 | ||
| Fig. 7 Scheme of the molecular construction based on the double-stranded nucleic acid molecules fixed in a liquid-crystalline structure and crosslinked by polymeric chelate bridges. Nucleic acid molecules are shown as rods (the left part of the figure). Circular insert (the right part of the figure) shows the flat polymeric chelate bridge (DAU–Cu2+–⋯–Cu2+–DAU) between two adjacent nucleic acid molecules in a particle of the liquid-crystalline dispersion (a view alongside the molecules axes). | ||
In addition, one can say that Fig. 3 theoretically shows that there is a possibility to functionalize a supporting film by a monomolecular layer of the NA liquid-crystalline particles. Such a procedure can attract much attention in the field of supramolecular engineering, because it offers a novel way for the fabrication of advanced materials. In addition, these structures, containing metal ions in their content, would be relevant for nanoelectronics.25
3 Destruction of polymeric chelate bridges by proteins
The general scheme shown in Fig. 7 allows the following statement to be made: any factor affecting the stability of polymeric chelate bridges will cause dramatic changes in the CD spectra of NA molecular constructions. In particular, redox systems changing the valence of the metal ion17 or compounds able to compete with DAU for complex formation with Cu2+ ions will strongly reduce the amount of polymeric chelate bridges and, consequently, change the extra-increase in optical activity to a value characteristic for the [DAU–NA] complex in the absence of copper ions. This will enable one to test this property of the molecular construction as a biosensing unit (chip).In fact, treatment of the DNA molecular construction by various biologically relevant compounds (see Fig. 5 and Fig. 6) is accompanied by the diminishing amplitude of the intense band. These compounds are capable of interacting with copper ions18,26,27 by forming stable complexes. This means that the treatment of the molecular construction with proteins can lead to ‘withdrawal’ of copper ions from the content of polymeric chelate bridges and to degradation of the polymeric chelate crosslinks. Hence, the ‘withdrawal’ of metal ions from bridged LCD structures leads to their collapse with consequent decrease in the amplitude of the CD band.
Preliminary comparison of kinetic curves corresponding, for instance, to action of various proteins on molecular constructions characterized by different spatial twisting shows that this property can influence the protein disruption efficiency. This means that the chemical structures of ds NA can, in principle, effect the formation and the stability of polymeric chelate bridges.
As far as proteins and other Cu2+ chelating agents are concerned, the minimal concentrations of BSA, or other proteins which are necessary to affect the optical properties of the molecular construction, are in the micromolar range (Figs. 5 and 6) . However, one can expect that under properly chosen conditions (concentration of NA, DAU and copper ions, etc.), the twisted structure of molecular conctruction could be sensitive to lower concentrations of the compound to be detected. In favour of this suggestion, the concentration of BSA, which is necessary to demolish the polymeric chelate bridge network between DNA molecules at low salt and PEG concentration, can reach up to ∼10−8 M ( 0.6 mg mL−1). This concentration is quite comparable with the detection limit obtained by modern techniques,28 which is about 1–10 μg mL−1.
The other example of destruction is the teatment of bridged NA LCD by ascorbic acid (data not shown). The addition of ascorbic acid results in practically complete annihilation of molecular construction. The higher the concentration of polymeric chelate bridges, the slower is the change in the amplitude of the CD band in the presence of ascorbic acid. It is noteworthy that subsequent oxidation processes (Cu1+ → Cu2+) favoured by the action of oxygen in solution, tends to restore the initial polymeric chelate bridges, and, hence, the original optical activity as well.6
It should be noted that the minimal concentration of ascorbic acid, which is able to affect the optical properties of NA LCD is about 10−7 M. This concentration compares favourably with the ascorbic acid detection limits reported for other techniques which are in the range 0.1–10 μM.29
Hence, the ds NA molecules fixed at a certain distance in the cholesteric structure of LCD can be used for the design of a molecular construction (chip) sensitive to the presence of chemical compounds with specific properties.
In conclusion, our results show that the cholesteric structure of double-stranded NA molecules is compatible with complexation to anthracycline drugs and with formation of polymeric chelate bridges connecting NA helices. Therefore, NA molecules fixed in the LCD structure represent useful tools for a superstructural ‘molecular design’. This approach, correlating the sterical and chemical properties of NA and anthracycline molecules as the ‘building elements’, allows us to design appropriate links between NA molecules in the liquid-crystalline state. Such bridges enhance the stability of the spatial structure of LCD. Indeed, under bridging conditions, PEG could be completely removed from the solution, without affecting the cholesteric structure of LCD to an appreciable extent. In addition to allowing a detailed investigation of the physico–chemical properties of the small size, stable particles formed by bridging together ds NA molecules, our results open a gate for the application of the crosslinked particles of LCD of ds NA as a chip (biosensing unit) for the detection of numerous compounds able to interfere with the formation of bridged structure. Since even a few analyte molecules strongly affect the liquid-crystalline state of the ordered structure, very high sensitivity is to be expected for the proposed application. These molecular constructions could be used as well as microscopic size chemical reactors. Finally, the possibility can be envisaged that LCD particles formed from NA molecules crosslinked by anthracyclines in the presence of Cu2+ can be used as a drug ‘reservoir’ in molecular pharmacology applications.
Acknowledgements
This study was supported by a grant from the Russian Program ‘New Trends in Bioengineering’.References
- D. Bethell and D. J. Schiffrin, Nature, 1996, 382, 581 CrossRef CAS.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607 CrossRef CAS.
- R. F. Service, Science, 1997, 277, 1036 CrossRef CAS.
- N. C. Seeman, Acc. Chem. Res., 1997, 30, 357 CrossRef CAS.
- N. C. Seeman, Annu. Rev. Biophys. Biomol. Struct., 1998, 27, 225 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif)
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This review demonstrates the use of DNA molecules to scaffold assembly of other molecules in periodic arrays for nanotechnology needs.
This review demonstrates the use of DNA molecules to scaffold assembly of other molecules in periodic arrays for nanotechnology needs. - Yu. M. Yevdokimov, V. I. Salyanov, E. Gedig and F. Spener, FEBS Lett., 1996, 392, 269 CrossRef CAS.
- Yu. M. Yevdokimov, S. G. Skuridin and G. B. Lortkipanidze, Liq. Cryst., 1996, 12, 1.
- A. A. Kornyshev and S. Leikin, Phys. Rev. E, 2000, 62, 2567 CrossRef .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper describes the electrostatic interaction between long, rigid helical macromolecules that may have arbitrary surface charge distribution, and, in addition, the spatial properties of the liquid crystals formed.
This paper describes the electrostatic interaction between long, rigid helical macromolecules that may have arbitrary surface charge distribution, and, in addition, the spatial properties of the liquid crystals formed. - Yu. M. Yevdokimov, S. G. Skuridin, S. V. Semenov, V. I. Salyanov and G. B. Lortkipanidze, Biofisika (Russian), 1998, 43, 240 Search PubMed.
- W. M. Gelbart, R. F. Bruinsma, P. A. Pincus and V. A. Parsegian, Phys. Today, 2000,(September), 38 CAS.
- D. H . Van Winkle, M. W. Davidson, W.-X. Chen and R. L. Rill, Macromolecules, 1990, 23, 4140 CrossRef CAS.
- F. Livolant, Prog. Polym., Sci., 1996, 21, 1115 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif)
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This review describes the properties of multiple liquid-crystalline phases formed from double-stranded DNA under different conditions obtained by authors from various laboratories during the last 40 years.
This review describes the properties of multiple liquid-crystalline phases formed from double-stranded DNA under different conditions obtained by authors from various laboratories during the last 40 years. - V. A. Belyakov, V. P. Orlov, S. V. Semenov, S. G. Skuridin and Y. M. Yevdokimov, Liq. Cryst., 1996, 20, 777 CAS.
- Yu. M. Yevdokimov, V. I. Salyanov, F. Spener and M. Palumbo, Int. J. Biol. Macromol., 1996, 19, 247 CrossRef CAS.
- Yu. M. Yevdokimov, V. I. Salyanov, L. V. Buligin, A. T. Dembo, E. Gedig, F. Spener and M. Palumbo, J. Biomol. Struct. Dynam., 1997, 15, 97 Search PubMed.
- Yu. M. Yevdokimov, V. I. Salyanov, B. V. Mchedlischvili, V. A. Bykov, A. V. Belyaev, S. A Saunin, F. Spener and M. Palumbo, Nucleosides Nucleotides Nucleic Acids, 2000, 19, 1355 Search PubMed.
- F. T. Greenaway and J. C. Dabrowiak, J. Inorg. Biochem., 1982, 16, 91 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper shows that daunomycin and adriamycin form two water soluble complexes with Cu-ions having Cu-drug stoichiometries of 1∶1 and 1∶2. The structure of complexes have been interpreted in terms of binding of the deprotonated hydroquinone portion of the drug to copper ions.
This paper shows that daunomycin and adriamycin form two water soluble complexes with Cu-ions having Cu-drug stoichiometries of 1∶1 and 1∶2. The structure of complexes have been interpreted in terms of binding of the deprotonated hydroquinone portion of the drug to copper ions. - A. Garnier-Suillerot, in Anthracycline and anthracenedione-based anticancer agents, ed. J. W. Lown, Elsevier, Amsterdam, 1988, pp. 129–161 Search PubMed.
- H. D. Coble and H. F. Holtzclaw, J. Inorg. Nucl. Chem., 1974, 36, 1049 CrossRef CAS.
- V. Barthelemy-Clavey, J.-C. Maurizot and P. J. Sicard, Biochimie, 1973, 55, 859 CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif)
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper shows that two types of complexes are formed between daunorubicin and native DNA; for the first one the ‘strong’ binding is specific, whereas for the second one the ‘weak’ binding is specific.
This paper shows that two types of complexes are formed between daunorubicin and native DNA; for the first one the ‘strong’ binding is specific, whereas for the second one the ‘weak’ binding is specific. - J. Doskocil and I. Fric, FEBS Lett., 1973, 37, 55 CrossRef CAS .
![[*]](https://www.rsc.org/images/entities/char_e103.gif) It was shown that in contrast to double-stranded DNA , double-stranded RNA is incapable of forming an intercalation complex with daunomycin.
It was shown that in contrast to double-stranded DNA , double-stranded RNA is incapable of forming an intercalation complex with daunomycin. - T. W. Plumberg and J. R. Brown, Biochim. Biophys. Acta, 1977, 479, 441.
- T. W. Plumberg and J. R. Brown, Biochim. Biophys. Acta, 1979, 563, 181.
- V. Malatesta, A. Gervasini and F. Morazzoni, Inorg. Chim. Acta, 1987, 136, 81 CrossRef CAS.
- J. J. Storhoff, R. Elghanian, R. C. Mucic, C. A. Mirkin and R. L. Letsinger, J. Am. Chem. Soc., 1998, 120, 1959 CrossRef CAS.
- E. J. Baran, B. S. Parajoin-Costa, T. Rajo, R. Saez-Puche, F. Fernandes, R. M. Torano, M. C. Apella, S. B. Etcheverry and M. H. Torre, J. Inorg. Chem., 1995, 58, 279 Search PubMed.
- L. Show-Jy and B. Sarkar, J. Biol. Chem., 1971, 246, 5938.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248 CrossRef CAS.
- I. Kobashiishi and T. Imanari, Anal. Chem., 1997, 69, 216 CrossRef CAS.
Footnote |
| † 1 Da (dalton) = 1 u. |
| This journal is © The Royal Society of Chemistry 2001 |
