Miniaturized flow-through PCR with different template types in a silicon chip thermocycler
Ivonne Schneegaß*a, Reiner Bräutigamb and Johann Michael Köhlera
aInstitute for Physical High Technology (IPHT), Micro System Division, Department of Biotechnical Micro Systems, Winzerlaer Str. 10, D-07745, Jena, Germany. E-mail: schneegass@ipht-jena.de; Fax: +49 3641 206 399
bCLONDIAG chip technologies GmbH, Loebstedter Str. 105, D-07743, Jena, Germany
First published on 9th August 2001
Abstract
Flow-through chip thermocyclers can be used in miniaturized rapid polymerase chain reaction (PCR) despite their high surface to volume ratio of samples. We demonstrated that a thermocycler made of silicon and glass chips and containing thin film transducers for heating and temperature control can be adapted to the amplification of various DNA templates of different sources and properties. Therefore, the concept of serial flow in a liquid/liquid two-phase system was combined with a surface management of inner side walls of the microchannel and an adaptation of PCR mixture composition. In addition, the process temperatures and the flow rates were optimized. Thus, a synthetic template originating from investigations on nucleic acid evolution with 106 base pairs [cooperative amplification of templates by cross hybridization (CATCH)], a house keeping gene with 379 base pairs [glutaraldehyde 3-phosphate dehydrogenase (GAPDH)] and a zinc finger protein relevant in human pathogenesis with 700 base pairs [Myc-interacting zinc finger protein-1, knock-out (Miz1-KO)] were amplified successfully. In all three cases the selectivity of priming and amplification could be shown by gel electrophoresis. The typical amplification time was 1 min per temperature cycle. So, the typical residence time of a sample volume inside the 25 cycle device amounts to less then half an hour. The energy consumption of the PCR chip for a 35 min PCR process amounts to less than 0.012 kW h.
1. Introduction
The polymerase chain reaction (PCR) is a well-established nucleotide amplification technique, which became one of the most important techniques for genetic identification and gene diagnostics during the last decade.1–3 The method uses a repeated temperature cycling involving three different temperatures. This process is performed in thermocycler devices. A selective doubling of concentration of a certain segment of double stranded DNA during a single amplification cycle can be realized within about 4 min using a conventional thermocycler device. This time can be further reduced by rapid thermocycling leading to cycle times of about 1 min or less. Most of the conventional cyclers require roughly 90 min to complete an amplification. For application of thermocyclers in high-throughput amplifications, e.g. of medical diagnostic, shorter amplification times are required. Therefore, faster thermocycler devices are desirable.Chip thermocyclers might fulfill this demand. They are being developed in order to minimize the process time as well as power consumption and required space.4–7 In addition, micro-system technology opens the way to miniaturized complete DNA analysis systems.8,9 Chip thermocyclers are realized in several types in the laboratory. Single chamber thermocyclers5,10–14 were prepared as well as array thermocyclers and flow-through devices.15-17 With respect to the high temperature during denaturation (typically 94 °C), free surfaces of the PCR sample liquid must be avoided in order to prevent evaporation. The transport of sample liquid volumes into the reaction site, the thermocycling and the transport of products away from the reaction site favors sealed arrangements for the handling of small liquid volumes. This fact is an important reason for the use of flow-through devices instead of stationary devices. A second argument for a flow-through chip reactor is its independence from the need of fast temperature changes of the device as usually required for conventional thermocyclers. In such flow-through devices only the temperature of the streaming liquid is changed, whereas the temperature of the walls remains constant in contrast to PCR chips working in a stationary mode.
The temperature regime in chip thermocyclers is controlled by well defined thermal transport between heating elements, walls, sample and outer sphere heat sinks. Chip thermocyclers made of silicon and glass are of interest, because of the very different heat conductivity of both materials. Regions made of silicon ensure a homogeneous local temperature distribution, due to the very high thermal conductivity, while gaps in the silicon and glass as wall material are used for thermal insulation and for building-up high temperature gradients.
However, the application of chip thermocyclers and particularly of flow-through thermocyclers is difficult due to the low compatibility of glass and silicon surfaces with the PCR process.18 Although several different types of thermocycler chips were prepared during the last few years, there are only a few realized protocols. The application of chip thermocyclers suffered from handling problems and particularly from the chemical management of the surfaces. The properties of the surfaces are particularly important in the application of chip thermocyclers because the ratio of inner wall surfaces of micro-chambers and channels to sample volume is much higher than in conventional systems. The situation concerning the compatibility of surfaces is still more critical in case of microfluid channels.
Beside the problem of surface compatibility, the dispersion of concentration peaks, e.g. the templates of a sample, is a serious problem for the application of PCR processes in microfluid arrangements with the character of a flow-injection analysis system. Therefore, we focused on a thermocycler construction and experimental setup, which is capable of being used for a liquid/liquid two-phase system, in which the interface tension compensates dispersion effects of liquid transport in the micro-channel. Here, the development of adapted PCR protocols and the experimental arrangement for the application of a flow-through Si/glass chip thermocycler for miniaturized rapid amplification is reported. The high quality of amplification of very different template types will be demonstrated for the three amplification systems: CATCH, a synthetic system developed for investigation of cooperative amplification of templates by cross-hybridization; GAPDH, a gene encoding the glutaraldehyde 3-phosphate dehydrogenase; and Miz1-KO, encoding a human zinc finger protein.
2. Experimental
2.1. Construction of chip thermocycler
The chip thermocycler consists of the reaction channel, etched into a glass chip, and a cover chip made of silicon and equipped with thin film platinum heaters and sensors for temperature control. The glass channel with a meandering design guides the reaction mixture through the three temperature zones. The chip was designed with channel loops for 25 amplification cycles. The extension zone was arranged in the chip center, the denaturation and the annealing zone at the opposite sides (Fig. 1). The outer dimensions of the chip thermocycler are 26 mm × 50 mm. Syringe pumps were used for the fluid transport through the chip. | ||
| Fig. 1 Schematic sketch of the fluid channel through the individual temperature zones (A, B, C) of the flow-through thermocycler chip with inlet and outlet. After the inlet, the channel crosses the denaturation zone for complete initial denaturation of the DNA template. After the final cycle, an additional extension zone is incorporated for complete synthesis of amplification products. (schematically: 7 amplification cycles). | ||
The thermocycler device was fabricated using standard micromachining techniques as described previously.16,17 The flow channel was isotropically etched into the glass chip (Fig. 2) using a HF etchant and a micropatterned chromium film as etch mask. The fluid channel has a width of 0.25 mm, a depth of 0.10 mm, a length of 1512 mm, and a total volume of 33 μl. Etched channels into glass were preferred to channels in silicon, because they show smooth and rounded edges and curves as well as constant cross-sections. A rounded shape of channel loops should support not only a continuous flow PCR, but also a homogeneous flow behavior of a serial flow regime in a two-phase-system with low sensitivity against disturbance such as gas bubbles (Fig. 3).
 | ||
| Fig. 2 Top-view of the flow-through thermocycler with the fluid channel layout for 25 PCR-cycles and the individual temperature zones insulated by thermal gap. | ||
 | ||
| Fig. 3 Magnification of a glass channel section with dimensions as followed: channel width: 250 μm, channel depth: 100 μm. | ||
Silicon wafers (100) were micropatterned using a Si3N4 mask, whereas thermal gaps were etched anisotropically using an orientation-dependent NaOH etchant (Fig. 4). Thin platinum films (0.2 μm thick) were deposited by sputtering and microlithographically etched for the preparation of thermal transducers, acting as resistive heaters and sensors. Three sensor elements were integrated in each temperature zone for accurate temperature control. The primer annealing zone was connected to a cooling block to avoid overheating from the adjacent temperature zones. No additional active heating elements were necessary for the annealing zone. Contact pads for wire bonding were made of sputtered thin aluminium films (Fig. 5).
 | ||
| Fig. 4 Part of the top view of the thermocycler chip with fluid channels and inserted thermal gaps for insulation of the individual temperature zones from each other. | ||
 | ||
| Fig. 5 Back-side view of the silicon chip of the flow-through thermocycler with the integrated Pt thin-film heater (wide structures) and thermal sensors (fine structures). | ||
The prepared chips were assembled by anodic bonding. Fluid connections were prefabricated mechanically and attached by glue bonding on top of the glass chip, where holes were drilled by ultrasonic drilling.
2.2. Sample handling and instrumentation arrangement
The PCR mixtures were split into portions and loaded in parallel onto the flow-through amplification chip and the commercial thermocycler device (Mastercycler gradient, Eppendorf-Netheler-Hinz, Germany). In the commercial device, 0.2 ml thin-wall reaction tubes were used for fast temperature distribution.In both devices, we subjected the reactions to a unique initial cycle of denaturation at 94 °C, followed by 25 cycles of template denaturation at 94 °C, primer annealing at the appropriate temperature depending on the PCR system, e.g. 58 °C, and extension at 72 °C. An additional final extension at 72 °C was also included. After completion of the amplification, the samples were stored on ice or frozen at –20 °C until further analysis.
For amplification of a single sample, the mixture was injected and transported across the flow-through chip by a syringe pump (P-200 module, World Precision Instruments). The serial flow mode was achieved by periodical injection of small sample volumes into a continuous carrier flow, which was generated by a syringe pump. The sample injection was performed by a precision syringe drive module (PSD/2, Hamilton) with integrated valve for automatic sample uptake and a connected three-way selector valve with a sample loop (PR-750, Rheodyne). In our arrangement a sample loop was incorporated instead of the often used T-injectors. This loop holds sample volumes, loaded by a separate syringe pump (PSD/2) and transfers them into the carrier flow without disturbing it by switching the selector valve and leading the flow through the sample loop. The usage of a computer control for precise sample intervals supports the development of an automated sample loading. Our instrumental arrangement of the different modules for sample amplification in a serial flow mode is shown in Fig. 6. This module arrangement allowed a sample injection free of air bubbles.
 | ||
| Fig. 6 Schematic diagram of instrumentation for a serial flow mode of the flow-through PCR chip from the sample application to a manual analysis by gel electrophoresis. | ||
2.3. Process control
The fluid movement was observed by a stereo microscope. Temperature accuracy of the chip device was achieved by calibration of the transducers using the interdependence of resistance and the temperature, which was determined in a precision thermal oven. The stability of temperature of the individual temperature zones was ±0.1 K during the amplification process. The process temperature was controlled by an analog electronic controller. We developed and wrote a specially adapted software package, which communicates with the syringe pumps for sample injection and transport of carrier flow and checks the temperature progression and stability of the temperature zones by using the thin film transducers. (Figs. 6 and 7). | ||
| Fig. 7 Schematic of the individual modules with electronic control and the temperature control circuit of the PCR chip device (exemplary for one temperature zone). | ||
2.4. Chemicals and materials
All chemicals for PCR amplification were purchased from Qiagen (Taq PCR Core Kit). Tween 20, Trizma base, ethidium bromide, and bovine serum albumin (BSA) were purchased from Sigma. All syntheses of primers and of the template of the synthetic PCR system CATCH were performed by MWG (Germany).2.5. Templates
In addition to the appropriate quantity of DNA template and primer of each PCR system, the PCR amplification mixtures contained 200 μM of each deoxynucleoside triphosphate, 1.5 mM MgCl2 for CATCH and GAPDH, and 2.5 mM MgCl2 for Miz1-KO (Myc-interacting zinc finger protein-1, knock-out), respectively, Tween 20 within the range of 1 to 40% (w/v), BSA within the range of 0.1 to 20 μg, 20% (v/v) of Q-Solution (Qiagen), and 5 U Taq polymerase in Tris buffer, pH 8.0 in a final volume of 50 μl. The optimal concentration of Tween 20 and BSA was investigated and determined for the used amplification systems. The PCR mixtures were prepared prior to the sample application. For each amplification a maximum volume of 10 μl was applied.The amplification mixture with a final volume of 50 μl contained 25 ng template DNA and 50 μg of each primer. An optimal annealing temperature of 59 °C was determined.
3. Results and discussion
3.1. Composition of amplification mixture
The CATCH system was selected first for amplification experiments in the flow-through thermocycler. Then the other two PCR systems were tested in order to prove a universal applicability of the flow-through thermocycler chip. These studies included variations of reactant composition and temperatures, which were not all described here in detail.The amplification products of the three PCR systems, which were successfully amplified in the chip thermocycler, are represented in Fig. 8. The product of CATCH corresponds to a DNA fragment with a length of 106 bp, the GAPDH of 379 bp and the Miz1-KO of 700 bp. We selected these amplification systems, because the length of their products represents the range of medically important and clinically relevant applications of PCR.
 | ||
| Fig. 8 PCR products of three different PCR systems amplified in the flow-through PCR chip. Product detection was performed in agarose gel (3%); M—marker, lane 1—CATCH, lane 2—GAPDH, lane 3—Miz1-KO. | ||
3.2. Optimization of the chemical state of channel surfaces and the effects of additives
The advantage of silicon as bottom material of the flow channel is its very high thermal conductivity in comparison to the plastics usually used as material of reaction tubes in conventional thermocycler devices. On the other side, the biocompatibility of native silicon is comparably low.Therefore, in order to perform PCR in a silicon micro-channel, special attention must be paid to the conditions of the internal surfaces. For microreactors, surface effects are generally pronounced because the surface to volume ratio increases upon miniaturization.12
We examined possible surface treatments to passivate the inner surface of the flow channel of the silicon-glass chip to find an inert surface compatible with PCR. Initial amplification tests using silicon-glass powder indicated the inhibitory effect of the untreated material. In Fig. 9 the influence of the addition of silicon-glass powder in different quantities to a PCR mixture, amplified in the commercial thermocycler, is shown. The DNA amplification, here shown for the example of the CATCH system, is strongly affected by increasing quantities of untreated powder. The bands of amplification products disappeared by increasing the material addition, therefore we assumed a possible adsorptive effect of the material on the basis of hydroxyl groups formed at the surface of the oxidized material. A treatment of the material with a silanizing agent such as hexamethyldisilazane (HMDS) before the addition to the amplification mixture indicated the positive effect of a hydrophobic material surface to the amplification. In spite of increasing the added amount of material powder, the product signal did not decline significantly. This result agrees with results obtained by Lao et al., who found an enhanced amplification by silanizing the reactor.24
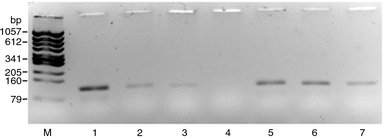 | ||
| Fig. 9 PCR amplification of the CATCH system in a commercial thermocycler, affected by the addition of glass-silicon powder in different quantities, untreated (lane 2 to 4) and treated with hexamethyldisilazane (HMDS) (lane 5 to 7) to a final volume of 25 μl in comparison to a control without material addition (lane 1). The effect of the addition is demonstrated in the following order: 0.8 mg (lane 2), 1.9 mg (lane 3), 3.3 mg (lane 4), 0.9 mg (lane 5), 1.9 mg (lane 6), 3.2 mg (lane 7). M—marker. | ||
Beside this material treatment of the inner channel surface, several additives were included into the amplification mixture to minimize negative surface effects. We found BSA to be very effective in preventing surface denaturation of polymerase on glass capillary surfaces, in agreement with previous work.25 The final concentration of 5 μg μl−1 BSA was determined to be optimal for amplification of the investigated PCR systems. Smaller amounts were found to be less effective and have resulted in lower product yields and weaker product bands respectively, as well as larger amounts of BSA which also led to decreased signals, probably on the basis of reduced mobility of the enzyme and limitations of diffusion. The surfactant Tween 20, which acts in relaxing the surface-tension of solutions and is often used in protocols of protein and nucleic acid handling, served as a dispersant, emulsifier and solubilizer in protecting the enzyme. A final concentration of up to 10% Tween in the amplification mixtures showed an enhanced amplification efficiency in comparison to the addition of smaller or larger quantities, where weaker signals were found.
The obvious effect of the surface on the enzyme was demonstrated by amplification of the PCR mixture in an untreated thermocycler chip. No amplification was observed, but the subsequent addition of polymerase to this sample resulted in a strong product signal after amplification in the commercial thermocycler. The resulting signal intensity was adequate to the product yield of the sample, which was directly amplified as a control in the commercial thermocycler . This is shown for the example of GAPDH in Fig. 10. Several tests revealed that no significant effect of the surface appeared on the nucleotides, but on the protein component of the mixture, e.g. by adsorption. So, the absence of a signal after amplification in the untreated chip and the reappearance of the signal after polymerase addition provided strong evidence for the influence of the surface on the polymerase activity and the requirement of thorough surface treatment for efficient amplification in silicon-glass chip devices. Therefore, all chips were silanized after assembly prior to the first usage. The fluid channel was filled with the silanizing agent and incubated for 30 min at room temperature, followed by a drying step with nitrogen. Then the chip was flushed for 10 min with a flow rate of 3 μl min−1 with a solution consisting of the corresponding concentration of the additives BSA and Tween 20 and the amplification mixture, without DNA template, primer and polymerase enzyme. After that treatment the chips were ready to use and showed reproducible amplification results as proved by the application of a sample divided into small volumes, which were processed successively and analyzed by electrophoresis.
 | ||
| Fig. 10 Influence of the flow channel surface on the Taq polymerase. PCR amplification of the GAPDH system in an untreated flow-through thermocycler (lane 2) in comparison to the commercial thermocycler (lane 1). After supplement of additional polymerase to the sample from the chip device, the signal yield of the commercial cycler was achieved with this sample, amplified in the commercial cycler (lane 3). M—marker. | ||
3.3. The temperature protocol and the optimization of flow rates
The flow-through thermocycler chip depends no longer on the temperature to time protocol required of most of the commercial cyclers, but only on a temperature to channel length protocol. The residence time of the PCR reaction mixture within the appropriate reaction zone only depends on the length of the channel, the cross-section of the channel and the applied flow rate of the reaction mixture within the reactor channel.The influence of the flow rate on the amplification efficiency was investigated in order to minimize the demand of time for the whole amplification procedure. In Fig. 11 the effect of different flow rates on the signal intensity, i.e. the amplification yield, is shown. For the investigated systems, an optimal flow rate of 1 μl min−1 was determined, which represented a total procedure time of about 35 min for 25 cycles. The decrease of the flow rate down to 0.5 μml min−1 led to a decrease of the signal intensity. We attribute this to the extended stay of the polymerase enzyme at high temperatures such as 94 °C in the denaturation zone, which may inactivate the enzyme resulting in lower amplification yields over time. Increasing the flow rate up to 2.0 μl min−1, the signal intensity continuously declined indicating that the amplification process requires a certain time for its completion. The period of one PCR process depends on the synthesis rate of the polymerase enzyme and the diffusion time.25 Due to the small channel cross section, the diffusion time is almost negligible, therefore the flow rate can theoretically be adjusted up to the synthesis rate of the enzyme. Wittwer et al.25 optimized PCR temperature and cycle times of their microchamber device and found that they could improve product specificity significantly, while decreasing the required amplification time in comparison to conventional standard heat block instruments. The amplification of our 106 bp CATCH fragment was performed only slightly slower than the fastest amplification reported by Kopp et al.,15 who were the first to refer to the successful rapid amplification in a continuous flow chip thermocycler. They described the 20-cycle amplification of a 176 bp DNA fragment at various flow rates, resulting in reaction times of 1.5 to 18.8 min, i.e. 4.2 s up to about 56 s per cycle, whereas only the longer cycling times resulted in product yields comparable to those of a conventional cycler device. We performed in our flow-through thermocycler a 25-cycle amplification of a 379 bp GAPDH fragment from genomic DNA in a total reaction time of 35 min using the optimal flow rate of 1 μl ml−1, i.e. 84 s per cycle, and found significant product amplification using a flow rate of 2 μl min−1, i.e. 42 s per cycle. We suppose that a further reduction of the total procedure time is possible by careful adaptation of the flow rate to appropriate amplification product lengths.
 | ||
| Fig. 11 Influence of the flow rate of the amplification mixture within the fluid channel on the amplification efficiency, illustrated by the example of GAPDH. The yield of the amplified products at 0.5 μl min−1 (lane 1), 1.0 μl min−1 (lane 2), 1.5 μl min−1 (lane 3), and 2.0 μl min−1 (lane 4) is demonstrated in an agarose gel (3%). M—marker. | ||
3.4. Liquid/liquid two-phase system
Mineral oil, which was used sometimes in thermocyclers for overlaying of samples to prevent evaporation, was applied to the flow-through thermocycler to generate a liquid two-phase system. For generating a continuous carrier stream, the oil emerged as optimal because of its compatibility with the PCR process. For this purpose a syringe pump was used to create a precise flow of mineral oil through the cycling chip (P200 pump, see Fig. 6). In addition to the surface treatment of the chip device and the supplement of BSA and Tween, the usage of mineral oil presented a good combination for efficient amplification of DNA in the chip device. As shown in Fig. 12 for the example of GAPDH, the flow-through thermocycler chip generated a product signal with adequate yield and specificity compared to the sample of the commercial thermocycler. | ||
| Fig. 12 PCR products of GAPDH. Comparison of amplification efficiency (signal intensity) and specificity of the amplified product of the investigated system between a conventional thermocycler (lane 1) and the flow-through chip device (lane 2). M—marker. | ||
3.5. Realization and optimization of serial flow
For realization and optimization of the serial flow, a specific template pair of the GAPDH amplification system was utilized. One of the templates was the wildtype with a longer amplified product (460 bp). The other template was modified and the amplification resulted in a shorter product (379 bp) due to an excision. The application of the different templates, but the same primer pairs and amplification condition resulted in products with different length, which can be clearly distinguished in the gel. On this basis, both systems were used to investigate cross-contaminations between samples in serial flow. For this aim both templates were applied to the amplification in the chip device, whereas the template for the longer product (460 bp) was injected first followed by the shorter (379 bp) and again by the longer product. In the case of cross-contaminations the second sample should show an additional band of 460 bp besides its main product band of 379 bp, and the third sample should also have an addition signal. As an interesting result, by using the liquid/liquid two-phase system of sample and mineral oil no amplification attributed to cross-contaminations have been detected so far, although the reactions were carried out one after another without additional rinsing meanwhile (Fig. 13). Thus, establishing the more sophisticated two-phase system, we upgraded and improved our earlier experimental arrangements, which were created on the basis of an unsegmented flow of the different samples in an one-phase system, where we could not prevent cross-contamination between the samples.17 The volume ratio of sample to mineral oil as carrier medium which enables an amplification without cross-contamination could be determined to be 1∶0.8. Thus in 1 h about 50 samples with a volume of 1 μl can be amplified. | ||
| Fig. 13 Products of the GAPDH amplification system in agarose gel (3%). In lane 1 the product of the amplification with a modified template is shown, and in lane 2 the amplification using the wildtype template. The products are clearly distinguished in the gel from each other. Because the same primer pairs and amplification condition are used for both templates, this system was well suited for investigations of cross-contaminations between samples in serial flow. M—marker. | ||
3.6. Product evaluation and perspectives of product analysis system
Comparing the amplification results of the conventional thermocycler and the flow-through thermocycler chip in more profounded analyses, we observed, that the product bands of the chip thermocycler often appeared more clear and without the blurring of the product bands due to a small quantity of unspecific by-products as observed in samples of the conventional cycler (Fig. 12). Therefore we conclude that the flow-through thermocycler has the capacity for higher temperature-dependent product specificity than the conventional thermocycler, due to its faster temperature transitions between the individual temperature zones.For more convenient product detection, the direct coupling of the device to sensitive analytical systems is feasible, and conventional parts for e.g. fluorescence detection of DNA are available.26–29 The use of other detection systems than agarose gel electrophoresis, e.g. online fluorescence detection by photodiodes, may eliminate the need to remove the samples from the chip. The integration of such a detector with an additional module for chip-based separation in our chip design is currently under investigation. As a result, the creation of a complete lab-on-the-chip analytical chip device for DNA amplification is expected.
4. Conclusions
In conclusion, we have demonstrated successful DNA amplification in a microfabricated silicon-glass chip thermocycler. Optimal thermal and chip design, surface treatment and PCR processing resulted in equivalent performance in yield and specificity for the silicon-glass flow-through thermocycler chip as compared to reactions in polypropylene tubes of a conventional thermocycler.We have shown by the example of three different amplification systems that the described flow-through thermocycler chip was suited for μ-PCR and is applicable to very different templates. The amplification was efficient and highly selective for all templates; even higher product selectivity of the chip thermocycler was detected for some amplification systems by generation of faster temperature transitions between the temperature zones in comparison to the commercial thermal block devices. The time demand for amplification was low corresponding to the miniaturization of the device, the choice of material and the optimized layout of the device.
We found the mineral oil to be well suited as separation liquid for serial flow; no inhibition effects were observed in the microsystem despite the high surface to volume ratio. A continuous flow PCR was performed as well as a serial flow. The application of the serial flow modus enables a high-throughput of samples and establishes the feasibility of performing regular screening assays for clinically relevant sequences. In the case of 1 μl samples, 50 samples h−1 in one microchannel were available for amplification. The electrical power consumption of the microchip is small, and therefore, the device could be integrated in battery supplied portable compact analysis or diagnostic systems.
The described kind of chip design provides an excellent set of possibilities for practical application. The flow-through thermocyclers with constant ratio of residence times in the different temperature zones can be applied for different analytical problems. The demonstrated layout allows in principle approaches in the various field of biochemical thermoregulated processes for a new generation of microstructured chip devices for automated lab-on-a-chip strategies.
Acknowledgements
In cooperation with Bruker Daltonik GmbH (assign. No. 0311419/UA2) and Biometra Biomedizinische Analytik GmbH (proj. no. 03110111), this work has been supported by the German Ministry of Education and Research (BMBF).We are grateful to R. Ehricht (Clondiag Chip Technologies, Jena, Germany) for kindly providing the CATCH system, to K. Peukert (Institute of Molecular Biology and Tumor Research, Philipps University Marburg, Germany) for the Miz1-KO system, and to T. Henkel (IPHT, Jena) for the GAPDH system. Many thanks to M. Sossna, M. Urban, H. Fischer, H. Porwol, R. Stöpel, and M. Zieren (IPHT) for technical support and helpful discussions.
References
- K. B. Mullis, F. Ferré and R. A. Gibbs, The polymerase chain reaction, Birkhäuser, Boston, 1994 Search PubMed
.
- G. R. Taylor and P. Robinson, Curr. Opin. Biotechnol., 1998, 9, 35 CrossRef CAS
.
- D. Whitcombe, C. R. Newton and S. Little, Curr. Opin. Biotechnol., 1998, 9, 602 CrossRef CAS
.
- M. A. Northrup, B. Benett, D. Hadley, P. Stratton and P. Landre, Advantages afforded by miniatuization and integration of DNA analysis instrumentation. 1st International Conference on Microreaction Technology, ed. W. Ehrfeld, Frankfurt a.M., 1997, pp. 278–287 Search PubMed
.
- S. Poser, T. Schulz, U. Dillner, V. Baier and J. M. Köhler, Chip elements for fast thermocycling. Eurosensors X—The 10th European Conference on Solid-State Transducers, Leuven, Belgium, 1996, 4, pp. 1197–1199 Search PubMed
.
- S. Poser, T. Schulz, U. Dillner, V. Baier, J. M. Köhler, D. Schimkat, G. Mayer and A. Siebert, Sens. Actuators A, 1997, 62, 672 CrossRef
.
- S. Poser, T. Schulz, U. Dillner, V. Baier, J. M. Köhler, G. Mayer, A. Siebert and D. Schimkat, Temperature controlled chip reactor for rapid PCR.. 1st International Conference on Microreaction Technology, ed. W. Ehrfeld, Frankfurt a.M., 1997, pp. 294–301 Search PubMed
.
- C. H. Mastrangelo, M. A. Burns and D.
T. Burke, IEEE, 1998, 86, 1769 Search PubMed
.
- M. Freemantle, Chem. Eng. Newsring, 1999, 77, 27
.
- J. Cheng, M. A. Shoffner, G. E. Hvichia, L. J. Kricka and P. Wilding, Nucleic Acids Res., 1996, 24, 380 CrossRef CAS
.
- A. T. Woolley, D. Hadley, P. Landre, A. J. Demello, R. A. Mathies and M. A. Northrup, Anal. Chem., 1996, 68, 4081 CrossRef CAS
.
- J. H. Daniel, S. Iqbal, R. B. Milligton, D. F. Moore, C. R. Lowe, D. L. Leslie, M. A. Lee and M. A. Pearce, Sens. Actuators A, 1998, 71, 81 CrossRef
.
- M. A. Northrup, C. Fonzalez, D. Hadley, R. F. Hills, P. Landre, S. Lehew, R. Saiki, J. J. Sninsky, R. Watson and R. J. Watson, A MEMS-based miniaturized DNA analysis system. TRANSDUCER ′95. EUROSENSOR IX—The 8th International Conference on Solid-State Sensors and Actuators and Eurosensors IX, Stockholm, Sweden, 1995, 193, pp.
764–767 Search PubMed
.
- P. Wilding, M. A. Shoffner and L. J. Kricka, Clin. Chem., 1994, 40, 1815 CAS
.
- M. U. Kopp, A. J. DeMello and A. Manz, Science, 1998, 280, 1046 CrossRef CAS
.
![[*]](https://www.rsc.org/images/entities/char_e103.gif)
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper was the first to refer to the successful rapid amplification in a continuous flow chip thermocycler.
This paper was the first to refer to the successful rapid amplification in a continuous flow chip thermocycler. - J. M. Köhler, U. Dillner, A. Mokansky, S. Poser and T. Schulz, Micro channel reactors for fast thermocycling. 2nd International Conference on Microreaction Technology, ed. W. Ehrfeld, New Orleans, LA, USA, 1998, pp. 241–247 Search PubMed
.
- S. Poser, R. Ehricht, T. Schulz, S. Uebel, U. Dillner and J. M. Köhler, Rapid PCR in flow-through Si chip thermocyclers. 3th International
Conference on Microreaction Technology, ed. W. Ehrfeld, Frankfurt a.M., 1999, pp. 294–301 Search PubMed
.
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This paper describes the different designs of continuous flow chip thermocyclers, presenting results of FEM simulations and demonstrating successful DNA amplification in these devices.
This paper describes the different designs of continuous flow chip thermocyclers, presenting results of FEM simulations and demonstrating successful DNA amplification in these devices. - M. A. Shoffner, J. Cheng, G. E. Hvichia, L. J. Kricka and P. Wilding, Nucleic Acids Res., 1996, 24, 375 CrossRef CAS
.
![[*]](https://www.rsc.org/images/entities/char_e103.gif) This is one of the first papers in which the authors descibe in detail basic methods for the treatment
of silicon-glass chips, in order to achieve a surface passivation for the PCR compatibility.
This is one of the first papers in which the authors descibe in detail basic methods for the treatment
of silicon-glass chips, in order to achieve a surface passivation for the PCR compatibility. - R. Ehricht, T. Ellinger and J. S. McCaskill, Eur. J. Biochem., 1997, 243, 358 CrossRef CAS
.
- T. Ellinger, R. Ehricht and J. S. McCaskill, Chem. Biol., 1998, 5, 729 CrossRef CAS
.
- M. J. Apostolakos, W. H. Schuermann, M.
W. Framton, M. J. Utell and J. C. Willey, Anal. Biochem., 1993, 213, 277 CrossRef CAS
.
- K. Peukert, P. Staller, A. Schneider, G. Carmichael, F. Hanel and M. Eilers, EMBO J., 1997, 16, 5672 CrossRef CAS
.
- J. Sambrook, E. F. Fritsch
and T. Maniatis, Molecular cloning: a laboratory manual, Cold Spring Habor, NY, 1989 Search PubMed
.
- A. I. K. Lao, T. M. H. Lee, M. C. Carles and I.-M. Hsing, Thermal management and surface passivation of a miniaturized PCR device for traditional chinese medicine. Micro Total Analysis Systems 2000, ed. A. van den Berg, Enschede, The Nederlands, 2000, pp. 139–142 Search PubMed
.
- C. T. Wittwer, G. B. Reed
and K. M. Ririe, in The polymerase chain reaction, ed. K. B. Mullis, F. Ferré and R. A. Gibbs, Birkhauser, Boston, 1994, pp. 174–181 Search PubMed
.
- E. T. Lagally, P. C. Simpson and R. A. Mathies, Sens. Actuators B, 2000, 63, 138 CrossRef
.
- M. A. Burns, B. N. Johnson, S. N. Brahmasandra, K. Handique, J. R. Webster, M. Krishnan, T. S. Sammarco, P. M. Man, D. Jones, D. Heldsinger, C. H. Mastrangelo and D. T. Burke, Science, 1998, 282, 484 CrossRef CAS
.
- C. L. Colyer, T. Tang, N. Chiem and D. J. Harrison, Electrophoresis, 1997, 18, 1733 CAS
.
- D. J. Harrison, K. Fluri, K. Seiler, Z. Fan, C. S. Effenhauser and A. Manz, Science, 1993, 261, 895 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2001 |
