Open Access Article
Open Access ArticleFabrication of porous polymeric microneedles: a concise overview
Eyman M. Eltayib
 *
*
Department of Pharmaceutics, College of Pharmacy, Jouf University, Sakaka 72388, Saudi Arabia. E-mail: emeahmed@ju.edu.sa
First published on 4th June 2025
Abstract
Recent advancements in microneedle (MN) technology have increasingly focused on porous polymeric microneedles (PPMNs), which are, among various types of MN, emerging as a promising platform for diverse biomedical applications, including transdermal drug delivery, interstitial fluid (ISF) extraction, and biosensing. This growing interest stems from their distinctive internal architecture, characterized by continuous nano- or micro-scale pores that enable the efficient transport of drugs and biofluids, primarily through capillary action. The optimal selection of polymeric materials, combined with appropriate fabrication techniques, plays a critical role in enhancing the functional performance of PPMNs while ensuring sufficient mechanical strength. This concise review summarizes recent research progress in the fabrication methods of PPMNs, emphasizing the interplay between polymer(s) choice, manufacturing technique, intended biomedical application, and the resulting structural and functional properties of the microneedles. It also addresses key challenges in the fabrication field and discusses future development.
Introduction
By definition, the microneedle (MN) system consists of integrally formed micron-scale needles on a patch substrate.1–3 These tiny needles, which are 25 to 2000 μm long,4,5 can go through the outerlayer of skin to effectively transport a plethora of diverse bioactive materials while avoiding skin injury.1,5,6 This system neatly integrates the benefits of skin injection with the safety of a transdermal patch.1 Because of their promising clinical results, tissue tolerability, patient acceptance, and capacity to self-administer, MN systems offer easy-to-use tool for controlled transdermal drug release in a variety of settings.1MNs are usually fabricated from metal, silicon, glass, ceramic,7,8 or polymer (e.g., carbohydrates and hydrogels).9 These materials have been used to fabricate microneedles for diverse purposes, based on their mechanical and degrading qualities.9 Silicon, ceramics, and metals have stiffness values over 10 GPa and are nondegradable under typical circumstances.9 Metal MNs are cost-effective to produce and exhibit superior mechanical and physical properties; yet, they are non-degradable and not flexible.10 There are currently six main categories of MNs: solid, coated, dissolving, hollow, hydrogel,11–15 and porous.11,13,16 Porous microneedles (PMNs) are channel-based devices1 made up of arrays with a network of linked channels or pores capable of delivering medications17–19 or capturing biological fluids via the epidermis or other tissues. Furthermore, PMNs facilitate therapeutic monitoring11 or biosensing applications by periodically and selectively capturing (when functionalized) and detecting biological molecules11,17via capillary action11,18,19 (Fig. 1). They are also attracting interest for their capacity to encapsulate larger volumes of fluid and for providing superior isotropic fluid directionality compared to hollow MNs, enabling both fluid injection and extraction at significantly greater volumes.20 PMNs are usually fabricated using inorganic substances, biocompatible metals, or polymers.11,19,21
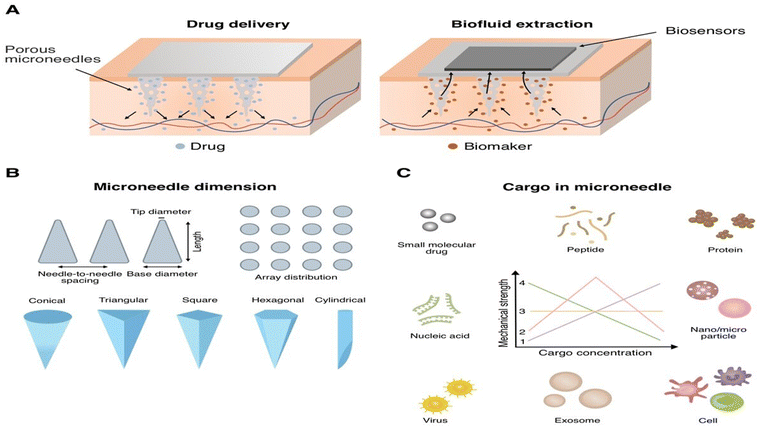 | ||
| Fig. 1 (A) Microneedle structures and the delivery mechanisms, and microneedle structures and the sampling mechanisms. (B) Microneedle dimensions. Reproduced from26. (C) Cargos including the small molecular drug, peptide, protein, nucleic acid, nanoparticle, microparticle, virus, exosome, and cell could be loaded in microneedle for delivery. The cargo in microneedle maybe linear increase,1 first increase and then decrease,2 not affect,3 or linear decrease4 the mechanical strength of microneedle in response to the increase of cargo concentration. Reproduced with permission from15 [Copyright© 2023, Elsevier]. | ||
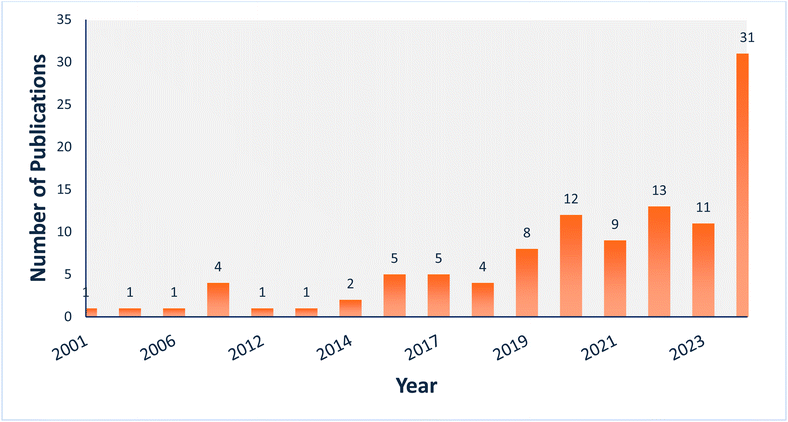
Over the past twenty years, great progress has been made in developing MN-based drug delivery systems.3 Among the various types of MNs, polymeric MNs have garnered significant interest in drug delivery research due to limitations associated with other materials—such as high cost of raw materials, complex fabrication techniques, fragility, limited biocompatibility, low drug-loading capacity, and the risk of MN facture in the skin upon insertion.22 Polymeric porous microneedles (PPMNs), in particular, have drawn substantial attention for their unique features, as they are fabricated using biocompatible and biodegradable polymers, which can be customized to control the release profile of the encapsulated active compounds.23 Fig. 2 depicts the research trends for each type of PPMN over the last two decades (2001–2024) based on data from the Web of Science (∼109 articles), excluding review articles (https://www.webofscience.com/wos/woscc/basic-search).
The use of PPMNs for transdermal drug delivery (TDD) offers several advantages. First, the minimally invasive nature of PPMNs can considerably reduce pain and discomfort experienced by patients during application, making them more patient-friendly. Additionally, the ability to engineer the MN geometry and porosity allows for tailored drug release profiles, enabling both burst and sustained release, depending on the specific therapeutic needs.24,25 Furthermore, PPMNs exhibit greater flexibility in applications compared to other MN types due to their distinctive drug-loading method, which enables the separation of the MN preparation process from the drug loading process, therefore minimizing drug loss and inactivation during preparation, especially for large-molecule drugs like vaccines, and improving mass production of MNs. Moreover, PPMNs offer excellent detection capabilities because of their large specific surface area and their flexibility to be integrated with other detection platforms, making detection easier and faster. This type is an adaptable and useful choice when designing and manufacturing wearable products and point-of-care (POC) testing devices.11
In this review paper, we considered recent studies involving the fabrication of PPMNs. These strategies were discussed in detail, together with examples, challenges, limitations, and factors to be considered.
Consideration and insights on the formulation aspects of PPMNs
The formulation and composition of MNs depend on the type of MN and its intended use. For example, drug-loaded PPMN comprises a polymer, drug (e.g., protein, vaccine, or nanocarrier), and other excipients such as solvent, plasticizer, and/or permeability enhancer.22 To further enhance a drug's solubility in the polymer or in water, PPMNs may also contain a copolymer or cosolvent in their formulation. Moreover, PPMNs can be formulated using advanced materials to obtain stimuli-responsive systems that respond to both internal and external stimuli.22In general, PPMN fabrication involves two main steps. First, polymer blends are prepared, with or without various other excipients. Then, a pore-forming step is performed to obtain the interconnected porous architecture. Therefore, there are three main focal points in the preparation of PPMNs.
1-Polymers
Over the past decade, polymers have been extensively researched and developed as microneedle materials for a range of biomedical applications, particularly transdermal drug delivery (TDD) and biofluid extraction.26 Polymeric materials have sparked attention in the medical field due to their ease of manufacture, low cost, and beneficial biological and mechanical properties.27 Polymers offer multiple advantages, including biodegradability, favorable biocompatibility, adjustable molecular weight and hydrophilicity,28 nontoxicity and inexpensive29 as well as straightforward fabrication processes.28Polymers are known to have the ability to withstand large bending forces without being fractured.26 Although they possess lower tensile strength than metals or silicon; yet, they are tougher than most other materials used for MN fabrication.30,31 Fabricating MNs from polymeric materials offers substantial benefits in terms of structure controllability,1 biocompatibility, biodegradability, solubility, the ability to accommodate both small and large molecules, extended drug release characteristics, mechanical properties, as well as functionality modulation via physicochemical modifications.12 In addition, polymer biocompatibility and biodegradability ensure that even if the needle breaks, it naturally degrades inside the body,32,33 which is immensely important in the case of PPMNs. Polymers are also preferred for their cost-effectiveness, hygiene, and safety, in addition to their swelling and dissolving capabilities. The in vivo enzymatic degradation of a polymer yields harmless products. Therefore, the likehood of infection within the body is reduced.34 In addition, the polymeric nature of PPMNs allows for the incorporation of various functional groups that can enhance drug specificity and targeting capabilities. This approach can potentially improve the therapeutic efficacy of the delivered drugs while minimizing off-target effects.1 More importantly, PMNs can be fabricated without complicated micromachining processes, equipment, or the requirement for a clean room environment.26 However, it is important to note that biodegradable polymers may have different particle size distributions and pharmacokinetics that are difficult to recreate due to unanticipated hydrolytic or enzymatic degradation of the drug carrier.35 Additionally, if made of nonbiodegradable materials, PPMNs array debris stays beneath the skin after shattering, potentially causing discomfort and inflammation.28
Polymers like polylactic acid (PLA), poly(lactic-co-glycolic) acid (PLGA), cellulose acetate (CA), polyglycolic acid (PGA), poly (glycidyl methacrylate), polydimethylsiloxane (PDMS), polyethersulfone (PES), and polysulfone (PSF) have all been utilized in the fabrication of PPMNs.19
2-Fabrication methods
The fabrication process is a key aspect of developing PPMNs. The choice of fabrication method depends on various factors, including the manufacturing material, access to specific technologies, and the intended application.27 The fabrication approach allows for precise control of the MN geometry, dimensions, and porosity, enabling the tailored design of the drug delivery system to meet specific therapeutic requirements.The geometry of porous microarray structures is an important consideration in the design of MN-based drug delivery systems because the porosity and surface area can considerably affect the loading capacity, drug release kinetics, and mechanical properties of the MNs.36 However, advances in manufacturing techniques have enabled the production of more complex MN geometries.27
A range of techniques have been developed for the fabrication of PPMNs. Micromolding,21 leaching, phase separation, hot embossing, freeze-drying, ultrasonic welding,11 two-photon polymerization (TPP),20 wet etching,21 and emulsion and bolding are the main fabrication processes to achieve porous MNs.26 The current methods for producing PPMNs, which are complicated and only applicable to limited types of materials,26 are introduced here.
 | ||
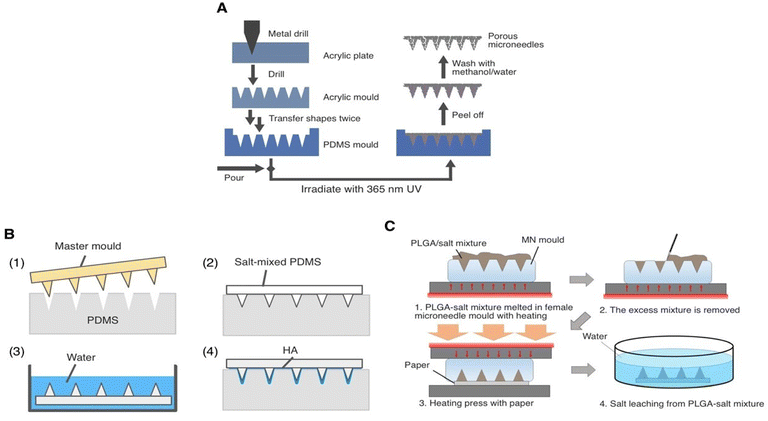
| Fig. 3 Polymeric microneedle production with micromolding (A) pouring the liquid polymeric formulation mixture, (B) vacuum degasification, (C) drying and (D) removal of MNs from the mould [Reproduced from37 under a Creative Commons CC BY 4.0 license]. | ||
This technology has been developed to fabricate microstructures that are cheap and simple to process, and the potential for mass production using injection molding, embossing, and other methods.38
Notwithstanding the numerous advantages of polymer molding, traditional micromolding fails to fabricate sophisticated microdevices or ones that include several materials. Microstructures with high aspect ratios or complicated geometries are difficult to manufacture using injection molding or embossing molds techniques, as the high viscosity of the thermoplastic polymer melt often causes premature cooling before filling the mold cavity.38
Centrifugation micromolding and vacuum micromolding are the most commonly employed technologies to introduce the needle solution or suspension into the microholes of male molds for MN preparation. Yet, centrifugation micromolding may lead to separation of the polymer matrix and microparticles due to the higher-density microparticles settling into the mold microholes during centrifugation. Furthermore, asynchronous centrifugation creates an inhomogeneous distribution of needle compositions, resulting in needle fracture because of the poor mechanical properties of the microparticles. On the other hand, the vacuum micromolding technique may be an improved option to centrifugation micromolding, since it uses a moderate pressure gradient to allow synchronous settling of needle components into the microholes of the mold, potentially enhancing MNs' formability.39
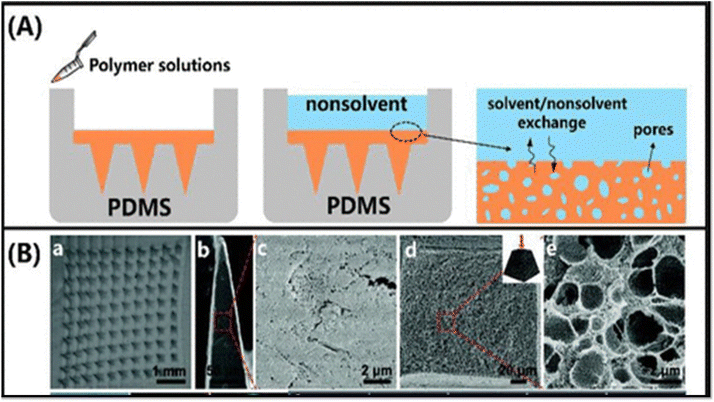
 | ||
| Fig. 4 (A–C) Illustration of porogen leaching process to produce porous microneedles: a polydimethylsiloxane (PDMS) blended with salt eliminated by deionized (DI) water after curing reproduced with permission; c poly (lactic-co-glycolic acid) (PLGA)-mixed salt removed by DI water after PLGA solidification [Reproduced from26 under a Creative Commons CC BY 4.0 license]. | ||
(i) Thermal-induced phase separation (TIPS): TIPS is an effective method for producing porous membranes.44 Phase separation can be induced by the removal of thermal energy. This involves dissolving the polymer in a solvent at an elevated temperature and then cooling the solution to induce phase separation, resulting in the formation of a porous structure. The diluent is removed via extraction, evaporation, or freeze-drying to obtain the porous membrane.45 In TIPS, thermodynamic properties, such as those employed in phase diagrams, can greatly affect the pore size and porosity.44
(ii) Nonsolvent-induced phase separation (NIPS): The NIPS technique can be applied using three distinct approaches: air-casting of a polymer solution, precipitation from a vapor phase, and immersion precipitation.45 In the first approach of air-casting, the polymer is first dissolved in a mixture of a volatile solvent and a nonvolatile nonsolvent. As the volatile solvent evaporates, phase separation is induced.45 During vapor phase-precipitation, nonsolvent vapor infiltrates the polymer solution, promoting phase separation and solidification of the polymer matrix.42,45 During the immersion precipitation process, the homogeneous polymer solution is cast into a film or molded into an MN shape, briefly exposed to air, and then immersed in a bath of a nonsolvent medium to form the membrane or polymer matrix solution. The nonsolvent causes the polymer to precipitate and solidify, forming a porous structure26,42 (Fig. 5).
 | ||
| Fig. 5 Process and pictures of PPMNs prepared by the nonsolvent induced phase separation method. (A): The process of preparing PPMNs by nonsolvent induced phase separation. (B): Structural characterization of porous microneedles made of CA (a–e): optical microscopy images of the microneedle arrays (a) and surface (b–c) and cross-sectional (d and e) SEM images of microneedles at different magnifications. Reproduced with permission from11 [Copyright© 2023, ACS]. | ||
 | ||
| Fig. 6 Schematic illustration of modified hot embossing process setup for the GMPA fabrication. Reproduced with permission from16 [Copyright ©2019, Elsevier]. | ||
To improve the mechanical strength of the produced MN, hot embossing is usually combined with a coating.18 Hot embossing is simple and can be introduced in industrialization. Nevertheless, it is challenging to precisely control the temperature, pressure, and time during the process. The high embossing temperature may limit its application because it can affect the pharmacological activity of protein peptides, vaccines, gene therapy pharmaceuticals, nano-formulations, etc.46
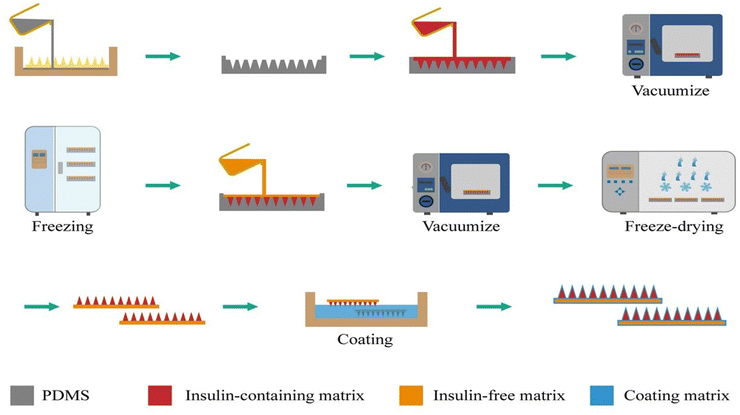 | ||
| Fig. 7 Schematic illustration of freeze-drying method for fabrication of microneedle loaded with insulin. Reproduced with permission from49 [Copyright ©2024, Springer Nature]. | ||
Freeze-drying is a scalable fabrication process in which the process parameters can be adjusted and product quality can be controlled, making it highly suitable for large-scale industrial production.49 However, it is often reported to be unsuitable because it results in mechanically weak, porous structures caused by water sublimation from a stiff, frozen structure. Therefore, the MN patch production process requires considerable development of specialized formulation and manufacturing processes for each type of medication, particularly vaccines, to preserve stability during manufacture and storage.46 The use of freeze-drying technology to prepare MNs can potentially improve the transdermal delivery of biotechnology formulations, advancing clinical research and commercialization of MNs in drug delivery.49
In general, there are two types of CBAs: endothermic CBAs absorb heat continuously during decomposition, whereas exothermic CBAs release heat during decomposition.52 Gaseous products include nitrogen (N2), carbon dioxide (CO2), carbon monoxide, ammonia, and other by-products, with most CBAs releasing N2 or CO2 during decomposition. Azodicarbonamide is the most representative exothermic CBA, often having a high gas yield, whereas sodium bicarbonate and zinc bicarbonate are the most common endothermic CBAs.53 Other CBAs include isocyanate and water (for polyurethanes, PU), azo-hydrazine and other nitrogen-based materials (used in thermoplastic and elastomeric foams), as well as sodium bicarbonate (for thermoplastic foams). Organic and inorganic CBAs are usually used for the production of closed- and open-cell products, respectively.54
The gas foaming method typically yields a structure characterized by high porosity and efficiency. The entire process generates minimal pollution, particularly when utilizing an inert gas directly. Tuning viscosity is essential because gas foaming is associated with gelation, which presents challenges for flexible fabrication, particularly in laboratory settings. Low-viscosity blends are detrimental to pore maintenance, whereas a substantial increase in viscosity during gelation inhibits further gel expansion.57
 | ||
| Fig. 8 (a) Scheme of preparation process of porous MN arrays with etching method. (b) Solid MN array without SiO2 glass spheres etching and (c) local enlarged image; scale bars both are 400 μm. (d) Porous MN array after etching image and (e) local enlarged image; both scale bars are 400 μm. SEM images of (f) side view and (g) cross-sectional view of a needle; scale bar in (f) is 400 μm and in (g) is 50 μm (h) the preparation of porous MNs loaded with ADSCs Reproduced with permission from77 [Copyright ©2024, John Wiley and Sons]. | ||
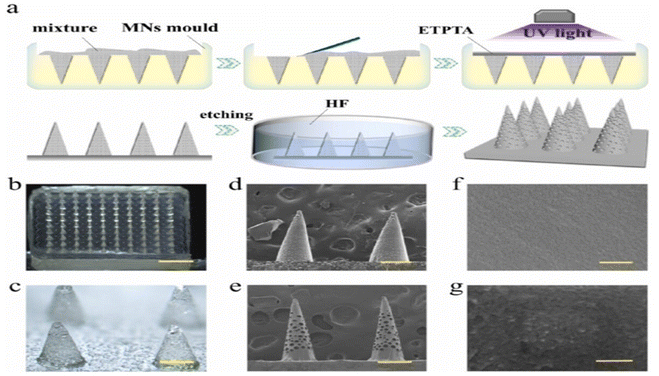 | ||
| Fig. 9 Fabrication and characterization of the porous MNs. (a) Schematic of the manufacture of the porous MNs array, by replicating glass microspheres filled negative molds with ETPTA. The optical image of (b) the porous MNs array and (c) the magnified MN tips; (d) SEM photo of MNs embedded with glass microspheres; (e) SEM image of the porous MNs after etching by hydrofluoric acid; (f) the pore wall of the porous MNs before PDA coating; (g) surface of the MNs coated with PDA. The scale bars are 2 mm in (b), 350 μm in (c), 300 μm in (d) and (e), 300 nm in (f) and (g). Reproduced with permission from75 [Copyright ©2021, Elsevier]. | ||
The etching process yields needles of different lengths and shapes depending on the intended purpose. Common forms include hollow or solid cylinders, triangular tips, and conical tips. In addition, the etchant can treat the needle surfaces, resulting in a surface suitable for a variety of applications. The etching method is a cost-effective and efficient way to create MNs.12
TPP initiates resin polymerization via multiphoton absorption, facilitated by the excitation of the photoinitiator. A near infrared (NIR) wavelength laser, such as a titanium–sapphire laser, is used in place of UV light. The TPP method facilitates the curing reaction exclusively at the focal point, rather than along the entire illumination path of the laser beam. Thus, the manufacturing of intricate and complex 3D structures is feasible.7 The unpolymerized material is then removed using a suitable solvent or solution. One advantage of the TPP process is that it enables 3D processing of photosensitive resins, which are transparent to NIR light.59 However, TPP is limited by its slow printing speed, often necessitating extended periods to produce an MN array.58,59 Despite this limitation, TPP offers significant advantages, including the ability to be implemented in a conventional clinical setting, such as an outpatient medical office, to create patient-specific medication delivery systems that are tailored to individual anatomical and medical needs. In contrast, many standard MN production procedures demand cleanroom settings and exhibit high energy consumption, reaching up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 kW m−2. Notably, the processing capabilities of TPP are compatible with scaling for high-throughput commercial production.59
200 kW m−2. Notably, the processing capabilities of TPP are compatible with scaling for high-throughput commercial production.59
Fundamentally, ice crystallization squeezes out other components upon cooling. Following cryogelation and melting of the ice crystals, the sites occupied by ice become pores, thus acquiring a porous structure. Unlike conventional templating, ice templating does not require organic solvents for washing, presenting a green procedure. Because the water is constantly distributed, the formed ice crystals create interconnected porous structures with high porosities for cryogels. The crosslinked polymer possesses good mechanical properties, and the modulus and mechanical strength tend to improve with repeated freeze–thaw cycles. Intriguingly, the polymer crystals can be melted upon heating; thus, the physically crosslinked polymer can thermo-reversibly convert into a polymer solution, showing a repeatable gelation characteristic.57 The PPMN patch thickness is controlled by molding the solution.60 This technique is recommended for drug loading in insoluble, soluble, and nano systems. This MN preparation procedure requires less time and is therefore simpler than other methods.18
 | ||
| Fig. 10 Process and PPMNs images for the preparation of PPMNs by ultrasonic welding. (A): Ultrasonic welding process for preparing of PPMNs. (B): Porous microstructure fabricated by ultrasonic welding imaged by scanning electron microscopy. (a) A portion of an array of porous, beveled MNs measuring 600 μm in height and 100 μm in base diameter. (b) A magnified view showing the individual PLA microparticles welded together to form the microstructure. Reproduced with permission from11 [Copyright© 2023, ACS]. | ||
| Material | Method of fabrication | Suggested application | Reference |
|---|---|---|---|
| PLA as a polymer and PEG as a surfactant | Emulsion and bonding | This work created a patch sensor (PMNIA) that uses porous microneedles and an immunochromatographic test to quickly detect anti-SARS-CoV-2 IgM/IgG in cutaneous interstitial fluid | 62 |
| PGMA (a mixture of glycidyl methacrylate), TRIM, TEGDMA, and PEG in methoxy ethanol was poured in a PDMS mold | Leaching followed by UV light curing | Integrated to electrode for electrochemical glucose sensing | 63 |
| Polydimethylsiloxane (PDMS) precursor and curing agent, and NaHCO3 | Chemical blowing | Physiological signal monitoring | 64 |
| Poly(vinyl alcohol) (PVA) and poly(vinyl pyrrolidone) (PVP) | Freeze drying | For treatment of ocular diseases such as keratitis or glaucoma | 65 |
| PGMA (GMA, monomer), and porogen solution of 10 kDa PEG. | Leaching | Dual-mode delivery of molecules. (Methylene blue, rhodamine B, and fluorescein isothiocyanate–dextran) | 66 |
| Biocompatible/photo-curable resin and a colloidal mixture of the solid drug powder | Molding and UV light curing | Large area transdermal delivery of solid drug formulations of lidocaine and ibuprofen | 67 |
| PLGA and CMC. | Molding and freeze-drying | The electrical monitoring of the skin condition and iontophoresis for drug delivery and medical diagnosis | 68 |
| TRIM and TEGDMA were used as a crosslinker for PGMA as a polymer, and PEG solution in 2- methoxyethanol is used as (as porogen) | Photopolymerization followed by leaching | For rapid fluid transport from and into a body through the skin | 69 |
| PLGA and NaCL | Salt leaching and molding | The formed MN was integrated to paper-based bio-sensors for the purpose painless, disposal and fast screening and diagnostic testing for patient, as well as those with prediabetes | 70 |
| A nanoscribe polymer (IP–S) was used to create the MNs | Two-photon polymerization (2 PP). The MNs were printed using a 2 PP printer (photonic professional GT2) with a 780 nm laser | Drug delivery and biological sampling | 20 |
| Polymer solution made of different polymers (PSF, PLA, and PVDF). PDA and PEG for hydrophilic and anti-adhesive coating | Phase inversion method followed by freeze-drying | Which provides a new way to prepare porous MNs suitable for dermal ISF extraction for POCT. | 71 |
| CA and DMSO | Casting and phase separation | Noninvasive quantification of topically applied pharmaceutical products | 8 |
| PLGA powders | Hot embossing method | Transdermal insulin delivery | 16 |
| CSA solution | Ice templating and freeze-drying | Suitable for insoluble, soluble, and nano system drug loading | 60 |
| CA and DSMO | Phase separation method followed by a deacetylation process | Rapid fluid transport suitable drug delivery and ISF sampling | 32 |
| PGMA and PEG was used as the porogen | Photopolymerization followed by leaching | Microneedle-based sensing with minimally invasive sample has significant implications for point-of-care diagnosis and diabetic health management | 72 |
| Then the enzymes were introduced into the porous matrix by immersion to improve reaction efficacy | |||
| CA polymer solution, and silica nanoparticles | Direct ink drawing technique | Transdermal collection of ISF for transdermal diagnosis and therapy | 73 |
| Mixing the monomer solution GMA, TEGDMA, as cross-linker, and TRIM as a co-cross-linker and 10 kDa PEG solution as a pore forming agent | Leaching process | Dual delivery of a variety of molecules (methylene blue, rhodamine B, and fluorescein isothiocyanate–dextran) | 74 |
| 1% HMPP (v/v) was dissolved in ETPTA (monomer) to achieve a uniform solution, serving as a photo initiator. Glass microspheres were incorporated into the solution with complete mixing to achieve suspension solution | Molding, UV curing, and then chemical etching | Extraction and detection of skin interstitial fluid biomarkers | 75 |
| MNA is a mixed product of polydimethylsiloxane and NaHCO3 | Chemical blowing | Integrated to electrodes. Porous MNA-based pressure sensor flexible pressure sensors have many potential applications in the monitoring of physiological signals | 76 |
| PLGA powders | Hot embossing | Transdermal delivery of rhodamine B (in vitro) in the rabbit skin dermis. The GPMA can efficiently administer an insulin solution (in vivo) in diabetic rats | 16 |
| Salt-mixed liquid PDMS | Salt leaching | ISF is extracted both in vitro and in vivo by repetitive compressions rather than capillary action. Flexible MNs are effective for continuous blood glucose monitoring, according to the study's findings | 21 |
| Polymer microparticles of 1 to 30 μm in size were made from PLA, PGA and PLGA. | Ultrasonically welding | To assess possible applications, microstructures were designed as microneedles for minimally invasive drug delivery | 38 |
| The porous MN arrays were fabricated by simply using UV-curable GelMA and PEGDA hybrid mixed with glass microspheres | Template filling, and particle etching method | Porous MNs can act as excellent stem cell scaffolds and will find many practical values in clinic wound healing | 77 |
| 5% (w/v) solution of either traditional GelMA or porous GelMA at 37° | Molding and blue light curing (405 nm, 30 mW/cm2) for 30 s | PPMN patch with sustained delivery of extracellular vesicles for treatment of severe spinal cord injury | 78 |
| A monomer stock solution of (GMA), crosslinker (TRIM), and crosslinker (TEGDMA) | Molding process and porogen method | For local monitoring of intercellular swelling, edema | 79 |
| A porogen stock (PEG, 10 kDa) | |||
| A photoinitiator irgacure 184 was added | |||
| PGMA and negatively charged hydrogel | Molding process and the porogen method | To induce the EOF for efficient TDD and extraction of ISF | 80 |
| PDMS and silica nanoparticle | Casting and etching method | Potential as platforms for biomedical applications such as drug delivery | 17 |
| Starch, PVA solution, sulfuric acid, formaldehyde solution (37 wt%), and pentane | Micro-molding and gas blowing (foaming) | Promising application prospects in antibacterial MNs-based sampling and medical devices for biomarker monitoring | 81 |
| PGMA | Molding and leaching methods | A wearable patch device for minimally invasive monitoring of trans-epidermal potential | 82 |
| PGA | Nonsolvent-induced phase separation (NIPS) method | Great potential as a diagnostic device for interstitial fluid (ISF) sampling and diagnosis | 83 |
| PP and CBA. | 3D PPP technology based on chemical blowing process | Application to various fields | 52 |
3-Intended applications
A plethora of research in recent decades has provided substantial evidence for the versatile potential of the MN system, noted for its key attributes in various biomedical applications, such as immunobiological administration, disease diagnosis, cosmetics, and primarily, drug delivery through the skin.1 Furthermore, PPMNs have been widely used in DD and medical diagnosis owing to their abundant interconnected pores.18Challenges and limitations
Porous MNs are viewed as a more agile alternative to hollow MNs because they are produced from a porous material that seamlessly integrates MNs with a reservoir function.1 However, numerous factors, such as the drug binding affinity, geometric design, and surface characteristics of the porous medium, considerably influence the drug adsorption and release processes.1I-PPMN design and insertion ability
The MN structure is another important factor for MN designs because it determines the drug delivery and sampling mechanisms.15 MN design determines drug release by adjusting several factors, such as the polymeric material composition, fabrication methods, and MN array geometry, including the base diameter, tip radius, height, aspect ratio, inter-needle distance, needle surface density, and base thickness.85 The PPMN dimensions should be designed according to the biological characteristics of human skin. PPMNs must penetrate the SC to access the epidermis, while avoiding the dermal layer. Therefore, the MN length must exceed the thickness of the SC layer, while remaining less than the combined thickness of the SC and epidermis.86 In addition, changing the PPMN geometry can alter the mechanical strength and insertion depth, which determines the force required for inserting into the skin.3 Because of the inherent elasticity of the skin, penetration of PPMNs through the skin is a major challenge influencing the reproducibility of drug release.85 PPMN skin insertion force is also dictated by the polymer composition and MN geometry, such as the MN wall thickness, wall angle, tip radius, length, and inter-needle space.85II-Porosity and mechanical strength
As a channel-based MN system,1 pore size and porosity are important parameters of PPMNs that influence the efficacy of drug delivery. The pore size influences the type of drug that can be incorporated because MNs with smaller pore sizes are unsuitable for loading high molecular weight drugs. Consequently, during MN preparation, it is essential to select an appropriate pore size according to specific requirements to ensure that PPMNs possess adequate pore size for drug adsorption. Porosity affects MN loading capacity, with manufacturers favoring larger porosity, particularly for drugs requiring high doses for efficacy. Porosity influences the mechanical strength of PPMNs, with hardness and Young's modulus decreasing as porosity increases. Increased porosity facilitates crack formation, thereby lowering hardness and Young's modulus, which hinders the penetration of PPMNs through the skin.11Because mechanical strength is generally negatively correlated with surface porosity,18 numerous studies have shown that the mechanical strength of PPMNs can be increased by selecting materials with superior mechanical properties, minimizing overall MN porosity, strategically introducing pores in specific regions of the needle body, or applying coatings to ensure sufficient mechanical strength for effective skin penetration.11 Another approach to increase the mechanical strength is to fabricate a denser network structure; however, this usually results in a loss of porosity and permeability of the drug load,11,28 which is not ideal for drugs that require high doses to function.11 To overcome this issue, hybrid PPMN arrays fabricated using a hard scaffolding material and soft permeable material could provide both greater mechanical stability and efficient drug release abilities.28 The coating enhances the mechanical strength of MNs while maintaining porosity and functions as a drug carrier to improve the drug loading capacity of MNs. Notably, selecting the appropriate coating formula and coating process is essential to prevent the coating from affecting the sharpness and piercing effect of the MNs.11 Another suggested approach involves filling the pores of a PPMN array with a hydrogel to facilitate both higher mechanical stability and continuous drug release.28 The physical properties of PPMNs, such as heat resistance, stiffness, and mechanical strength, are critical for efficient drug delivery.85 Therefore, improving the mechanical properties of the PPMN material without compromising sample quality or volume is a goal for researchers in this realm.18,85
III-Hydrophilicity of the polymeric matrix
A porous polymeric structure is generally composed of a polymer matrix and interconnected pores.87 PPMNs, characterized by their interlinked architectures, hold significant promise for dermal interstitial fluid (ISF) extraction.71 Hydrophilicity, defined as the affinity of a material for water molecules, plays a crucial role in this context.65 Specifically, the hydrophilicity of the PPMN array matrix is vital for effective drug adsorption.11 Conversely, poor hydrophilicity and inadequate adhesion of polymeric microneedles (PMNs) hinder the extraction rate and recovery of ISF.71 It is noteworthy that water adsorption in such systems can induce structural alterations, often manifested macroscopically as hygroscopic expansion and a reduction in mechanical stiffness.88 Importantly, mechanical strength remains a key factor, as it directly impacts product performance, durability, and safety.87 Furthermore, polymers with hydrophobic surfaces struggle to adsorb drugs and ISF solely through capillary action, thereby limiting their efficiency, particularly with hydrophilic drugs,19 and greatly restricting their use in microneedles for transdermal delivery and ISF extraction.71 Therefore, tailoring the surface functionality of the porous matrix is essential, especially for applications requiring host–guest interactions or stimuli-responsive behavior.89 To enhance fluid extraction rates and ensure the stability of PPMNs, hydrophilic modification of hydrophobic polymers is a critical and necessary process.71IV-Fabrication technique and industrialization
The main advantage of PPMNs is that their porous structure does not require the micromachining processes used for hollow structures, allowing the use of biodegradable polymers without the risk of broken MNs. However, the formation of the porous structure of PPMN using certain techniques such as phase separation or emulsion methods necessitates strict process conditions, including temperature and time control. Compared to other techniques used to create porous structures, the particle leaching method, which involves blending a matrix material with insoluble particles that are removed after the MNs are formed, has been developed as a simpler method.90 The variation in pore sizes and porosities of PPMNs can be attained through the use of diverse materials and preparation methods, thereby enhancing the adaptability of PPMNs for various applications.84Organic reagents are an essential component of the pharmaceutical industry and play an important role in manufacturing. The concentration of organic reagent must be carefully controlled in the final pharmaceutical product, as excessive levels can pose health risks to humans. These reagents are frequently used as solvents or porogen leaching processes for the fabrication of PPMNs; however, these reagents may leave residual traces within the microchannels of the PPMNs, potentially introducing hidden risks in drug delivery applications, particularly when loading proteins or other sensitive bioactive compounds. Currently, organic solvents are avoided primarily by altering the preparation method. For example, polymer particles are welded together via ultrasonic welding, resulting in a porous structure that does not require the use of organic solvents. However, this approach only welds the particle surfaces together, resulting in MNs with low mechanical strength. To achieve piercing requirements, additional adjustments of preparation conditions are necessary. Similarly, freeze-drying is another nonorganic solvent-based PPMN preparation method. In addition to modifying the preparation process, developing a test technique for determination of organic reagent residues in PPMNs is crucial for ensuring product safety, stability, and efficacy.11 Indeed, research on PPMNs is still in its early stages, and their industrial production and practical clinical application still show limitations that hinder their development.18
Despite certain technical breakthroughs, the industrialization and commercialization of PPMN devices have been impeded for the following reasons. The current PPMN patches are designed to be small, primarily for utilization in in vivo experimental tests. Thus, large MN patches should be considered for practical applications involving humans. Moreover, the PPMN patch substrate should be thin and flexible enough to be adhered to the skin. In addition, certain fabrication methods that are suitable for laboratory-scale production may not be feasible for industrial-scale manufacturing. For instance, the relatively high cost of matrix materials and production processes may present significant challenges to large-scale commercialization. Furthermore, sophisticated and time-consuming manufacturing processes with several phases may raise prices while diminishing production efficiency. When integrated into biosensors for early diagnosis, a low price and appropriate reliability are also important in gaining a competitive edge in the current POC device market. In addition, regulations and rules mandate long-term clinical experiments to assess accuracy and reliability that take several years of design time for MN-based sensing systems before commercialization. Furthermore, sterilization, usage, and disposal should be standardized so that customers or patients can handle medical devices appropriately and safely at home.26
Conclusion
PPMN systems represent a promising advancement in the field of TDD and biosampling. Polymeric materials have emerged as a promising alternative to conventional MN fabrication materials due to their biocompatibility, biodegradability, and versatility.31 Porous MNs have lately been explored due to their distinctive and unique qualities. Porous structures inside MNs with continuous nano- or micro-sized holes can transport medications or biofluids by capillary action.26 This review focused on the fabrication techniques of PPMNs and the associated challenges. Key methods such as phase separation, hot embossing, leaching, etching, and chemical and gas blowing were discussed and compared based on their underlying mechanisms and structural outcomes. Emphasis was placed on the principles guiding pore-formation, alongside an overview of published studies that highlighted the applications of PPMNs in drug delivery, diagnostic biosampling, and transdermal sensing.In spite of their promise, the PPMN technology form laboratory to commercial scale production faces many challenges. Chief among these is fabricating and developing PPMN arrays with sufficient mechanical strength to pierce the skin without fracturing, and ensuring structural integrity suitable for their intended clinical applications. Nevertheless, PPMNs offer a minimally invasive, patient-compliant, and highly adaptable versatile platform for administering a wide range of therapeutic agents, positioning them as a transformative tool in the future of transdermal therapies.
Abbreviations
| 3D: | Three-dimensional |
| API: | Active pharmaceutical ingredient |
| CA: | Cellulose acetate |
| CMC: | Carboxymethylcellulose |
| CSA: | Chondroitin sulfate A sodium salt |
| DSMO: | Dimethyl sulfoxide |
| GelMA: | Gelatin methacryloyl |
| HA: | Hyaluronic acid |
| MNs: | Microneedles |
| PAA: | Polyacrylic acid |
| PCL: | Polycaprolactone |
| PDA: | Polydopamine |
| PDMS: | Polydimethylsiloxane |
| PEG: | Polyethylene glycol |
| PES: | Polyethersulfone |
| PEGDA: | Poly(ethylene glycol)diacrylate |
| PGA: | Polyglycolic acid |
| PGMA: | Poly(glycidyl methacrylate) |
| PLA: | Polylactic acid |
| PLGA: | Poly(lactic-co-glycolic acid) |
| PP: | Polypropylene |
| PPMNs: | Polymeric porous microneedles |
| PMMA: | Poly(methyl methacrylate) |
| PSF: | Polysulfone |
| PVA: | Polyvinyl alcohol |
| PVP: | Polyvinylpyrrolidone |
| PVDF: | Polyvinylidene fluoride |
| SC: | Stratum corneum |
| TDD: | Transdermal drug delivery |
| TDDS: | Transdermal drug delivery system |
| TRIM: | Trimethylolpropane trimethacrylate |
| TEGDMA: | Triethylene glycol dimethacrylate |
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was funded by the Deanship of Graduate Studies and Scientific Research at Jouf University under grant No. (DGSSR-2024-01-01065).References
- B. Z. Chen, Y. T. He, Z. Q. Zhao, Y. H. Feng, L. Liang, J. Peng, C. Y. Yang, H. Uyama, M. Shahbazi and X. D. Guo, Strategies to develop polymeric microneedles for controlled drug release, Adv. Drug Delivery Rev., 2023, 203, 115109 CrossRef CAS.
- Z. Zhao, Y. Chen and Y. Shi, Microneedles: a potential strategy in transdermal delivery and application in the management of psoriasis, RSC Adv., 2020, 1, 144–1449 Search PubMed.
- Y. Zhang, Y. Xu, H. Kong, J. Zhang, H. F. Chan, J. Wang, D. Shao, Y. Tao and M. Li, Microneedle system for tissue engineering and regenerative medicine, Exploration, 2023, 3, 20210170 CrossRef CAS.
- F. K. Aldawood, A. Andar and S. Desai, A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications, Polymers, 2021, 13, 2815 CrossRef CAS.
- M. Abbasi, D. A. Boka and H. DeLoit, Nanomaterial-Enhanced Microneedles: Emerging Therapies for Diabetes and Obesity, Pharmaceutics, 2024, 16, 1344 CrossRef CAS.
- E. Caffarel-Salvador, A. J. Brady, E. Eltayib, T. Meng, A. Alonso-Vicente, P. Gonzalez-Vazquez, B. M. Torrisi, E. M. Vicente-Perez, K. Mooney, D. S. Jones, S. E. J. Bell, C. P. McCoy, H. O. McCarthy, J. C. McElnay and R. F. Donnelly, Hydrogel-Forming Microneedle Arrays Allow Detection of Drugs and Glucose In Vivo: Potential for Use in Diagnosis and Therapeutic Drug Monitoring, PLoS One, 2015, 10, e0–e145644 CrossRef.
- J. H. Jung and S. G. Jin, Microneedle for transdermal drug delivery: current trends and fabrication, J. Pharm. Invest., 2021, 51, 503–517 CrossRef PubMed.
- E. M. Eltayib, A. Himawan, U. Detamornrat, W. K. Muhtadi, H. Li, L. Li, L. Vora and R. F. Donnelly, Porous microneedle arrays as promising tools for the quantification of drugs in the skin: a proof of concept study, Pharm. Dev. Technol., 2024, 29, 164–175 CrossRef PubMed.
- S. Lyu, Z. Dong, X. Xu, H. Bei, H. Yuen, C. James Cheung, M. Wong, Y. He and X. Zhao, Going below and beyond the surface: Microneedle structure, materials, drugs, fabrication, and applications for wound healing and tissue regeneration, Bioact. Mater., 2023, 27, 303–326 Search PubMed.
- X. Luo, L. Yang and Y. Cui, Microneedles: materials, fabrication, and biomedical applications, Biomed. Microdevices, 2023, 25, 20 CrossRef PubMed.
- G. Gao, L. Zhang, Z. Li, S. Ma and F. Ma, Porous Microneedles for Therapy and Diagnosis: Fabrication and Challenges, ACS Biomater. Sci. Eng., 2023, 9, 85–105 CrossRef PubMed.
- D. Kulkarni, D. Gadade, N. Chapaitkar, S. Shelke, S. Pekamwar, R. Aher, A. Ahire, M. Avhale, R. Badgule, R. Bansode and B. Bobade, Polymeric Microneedles: An Emerging Paradigm for Advanced Biomedical Applications, Sci. Pharm., 2023, 91, 27 CrossRef.
- Y. T. He, L. Liang, Z. Q. Zhao, L. F. Hu, W. M. Fei, B. Z. Chen, Y. Cui and X. D. Guo, Advances in porous microneedle systems for drug delivery and biomarker detection: A mini review, J. Drug Delivery Sci. Technol., 2022, 74, 103518 CrossRef.
- C. Oliveira, J. A. Teixeira, N. Oliveira, S. Ferreira and C. M. Botelho, Microneedles' device: design, fabrication, and applications, Macromol, 2024, 4, 320–355 Search PubMed.
- Z. Le, J. Yu, Y. J. Quek, B. Bai, X. Li, Y. Shou, B. Myint, C. Xu and A. Tay, Design principles of microneedles for drug delivery and sampling applications, Mater. Today, 2023, 63, 137–169 CrossRef.
- J. Li, Y. Zhou, J. Yang, R. Ye, J. Gao, L. Ren, B. Liu, L. Liang and L. Jiang, Fabrication of gradient porous microneedle array by modified hot embossing for transdermal drug delivery, Mater. Sci. Eng., C, 2019, 96, 576–582 CrossRef PubMed.
- R. Maia; P. Sousa; V. Pinto; R. Lima; G. Minas and R. O. Rodrigues In In Development and Characterization of Porous PDMS Microneedles; IEEE: Piscataway, Jun 22, 2023; , pp. 44–47 Search PubMed.
- Q. Yan, S. Shen, L. Liu, J. Weng, G. Zheng, X. Dong, J. Yang, Q. Yang and J. Xie, Fabrication of controlled porous and ultrafast dissolution porous microneedles by organic-solvent-free ice templating method, Int. J. Pharm., 2024, 660, 124220 CrossRef PubMed.
- S. Yun, Y. Choi, S. Choi, T. An and W. Choi, Porous Polymer Microneedles with Superhydrophilic Surface for Rapid Fluid Transport, Int. J. Precis. Eng. Manuf., 2024, 25, 1279–1287 CrossRef.
- E. Fakeih, A. A. Aguirre-Pablo, S. T. Thoroddsen and K. N. Salama, Fabrication and Characterization of Porous Microneedles for Enhanced Fluid Injection and Suction: A Two-Photon Polymerization Approach, Adv. Eng. Mater., 2023, 25(16), 2300161 CrossRef CAS.
- K. Takeuchi, N. Takama, R. Kinoshita, T. Okitsu and B. Kim, Flexible and porous microneedles of PDMS for continuous glucose monitoring, Biomed. Microdevices, 2020, 22, 79 CrossRef CAS.
- N. T. Chevala, S. R. Jitta, S. M. Marques, V. M. Vaz and L. Kumar, Polymeric microneedles for transdermal delivery of nanoparticles: Frontiers of formulation, sterility and stability aspects, J. Drug Delivery Sci. Technol., 2021, 65, 102711 CrossRef.
- R. Jijie, A. Barras, R. Boukherroub and S. Szunerits, Nanomaterials for transdermal drug delivery: beyond the state of the art of liposomal structures, J. Mater. Chem. B, 2017, 5, 8653–8675 RSC.
- R. Jamaledin, P. Makvandi, C. K. Y. Yiu, T. Agarwal, R. Vecchione, W. Sun, T. K. Maiti, F. R. Tay and P. A. Netti, Engineered Microneedle Patches for Controlled Release of Active Compounds: Recent Advances in Release Profile Tuning, Adv. Ther., 2020, 3(12), 2000171 CrossRef CAS.
- L. Naves, C. Dhand, L. Almeida, L. Rajamani, S. Ramakrishna and G. Soares, Poly(lactic-co-glycolic) acid drug delivery systems through transdermal pathway: an overview, Prog. Biomater., 2017, 6, 1–11 CrossRef CAS.
- L. Bao, J. Park, G. Bonfante and B. Kim, Recent advances in porous microneedles: materials, fabrication, and transdermal applications, Drug Delivery Transl. Res., 2022, 12, 395–414 CrossRef PubMed.
- X. Qu, X. Guo, T. Zhu, Z. Zhang, W. Wang and Y. Hao, Microneedle patches containing mesoporous polydopamine nanoparticles loaded with triamcinolone acetonide for the treatment of oral mucositis, Front. Bioeng. Biotechnol., 2023, 11, 1203709 CrossRef.
- K. Barthelmes, K. Sathitaphiwan, N. Janwimaluang, K. Ikehara and A. Matsumoto, Increased mechanical stability and permeability by filling the interconnected pores of porous microneedles, Jpn. J. Appl. Phys., 2024, 63, 2 CrossRef.
- N. Sultana, A. Waheed, A. Ali, S. Jahan, M. Aqil, Y. Sultana and M. Mujeeb, Exploring new frontiers in drug delivery with minimally invasive microneedles: fabrication techniques, biomedical applications, and regulatory aspects, Expert Opin. Drug Delivery, 2023, 20, 739–755 CrossRef CAS PubMed.
- S. Bhatnagar, P. R. Gadeela, P. Thathireddy and V. V. K. Venuganti, Microneedle-based drug delivery: materials of construction, J. Chem. Sci., 2019, 131, 1–28 CrossRef.
- E. Larrañeta, R. E. M. Lutton, A. D. Woolfson and R. F. Donnelly, Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development, Mater. Sci. Eng., R, 2016, 104, 1–32 CrossRef.
- S. Yun, Y. Choi, S. Choi, T. An and W. Choi, Porous Polymer Microneedles with Superhydrophilic Surface for Rapid Fluid Transport, Int. J. Precis. Eng. Manuf., 2024, 25, 1279–1287 CrossRef.
- Y. T. He, L. Liang, Z. Q. Zhao, L. F. Hu, W. M. Fei, B. Z. Chen, Y. Cui and X. D. Guo, Advances in porous microneedle systems for drug delivery and biomarker detection: A mini review, J. Drug Delivery Sci. Technol., 2022, 74, 103518 CrossRef.
- A. Rajput, M. Kulkarni, P. Deshmukh, P. Pingale, A. Garkal, S. Gandhi and S. Butani, A key role by polymers in microneedle technology: a new era, Drug Dev. Ind. Pharm., 2021, 47, 1713–1732 CrossRef.
- M. Arruebo, Drug delivery from structured porous inorganic materials, WIREs Nanomedicine and Nanobiotechnology, 2012, 4, 16–30 CrossRef PubMed.
- Z. Faraji Rad, R. E. Nordon, C. J. Anthony, L. Bilston, P. D. Prewett, J. Arns, C. H. Arns, L. Zhang and G. J. Davies, High-fidelity replication of thermoplastic microneedles with open microfluidic channels, Microsystems & nanoengineering, 2017, 3, 17034 Search PubMed.
- A. Tucak, M. Sirbubalo, L. Hindija, O. Rahić, J. Hadžiabdić, K. Muhamedagić, A. Čekić and E. Vranić, Microneedles: Characteristics, Materials, Production Methods and Commercial Development, Micromachines, 2020, 11, 961 CrossRef PubMed.
- J. Park, S. Choi, R. Kamath, Y. Yoon, M. G. Allen and M. R. Prausnitz, Polymer particle-based micromolding to fabricate novel microstructures, Biomed. Microdevices, 2007, 9, 223–234 CrossRef.
- M. Zhang, B. Yang, X. Luan, L. Jiang, C. Lu, C. Wu, X. Pan and T. Peng, State of the Art in Constructing Gas-Propelled Dissolving Microneedles for Significantly Enhanced Drug-Loading and Delivery Efficiency, Pharmaceutics, 2023, 15, 1059 CrossRef.
- B. Stoeber and D. Liepmann, In Fluid Injection through Out-Of-Plane Microneedles, IEEE, 2000, pp. 224–228 Search PubMed.
- S. H. Lee, Y.-J. Cha, S. S. Choi, S.-H. Ha and H. Ho Lee, In Fabrication of Nanoparticle-Based Microneedle for Potential Drug Delivery, IEEE, Dec 2010, pp. 164–167 Search PubMed.
- S. Mazinani, S. Darvishmanesh, A. Ehsanzadeh and B. Van der Bruggen, Phase separation analysis of Extem/solvent/non-solvent systems and relation with membrane morphology, J. Membr. Sci., 2017, 526, 301–314 CrossRef.
- P. Liu, H. Du, Y. Chen, H. Wang, J. Mao, L. Zhang, J. Tao and J. Zhu, Polymer microneedles with interconnected porous structures via a phase inversion route for transdermal medical applications, J. Mater. Chem. B, 2020, 8, 2032–2039 RSC.
- H. Matsuyama, T. Maki, M. Teramoto and K. Asano, Effect of polypropylene molecular weight on porous membrane formation by thermally induced phase separation, J. Membr. Sci., 2002, 204, 323–328 CrossRef.
- H. Matsuyama, Y. Takida, T. Maki and M. Teramoto, Preparation of porous membrane by combined use of thermally induced phase separation and immersion precipitation, Polymer, 2002, 43, 5243–5248 CrossRef.
- Y. C. Kim, J. W. Lee, E. S. Esser, H. Kalluri, J. C. Joyce, R. W. Compans, I. Skountzou and M. R. Prausnitz, Fabrication of microneedle patches with lyophilized influenza vaccine suspended in organic solvent, Drug Delivery Transl. Res., 2021, 11, 692–701 CrossRef.
- A. Rezvankhah, Z. Emam-Djomeh and G. Askari, Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review, Drying Technol., 2020, 38, 235–258 CrossRef.
- C. R. S. Siow, P. Wan Sia Heng and L. W. Chan, Application of freeze-drying in the development of oral drug delivery systems, Expert Opin. Drug Delivery, 2016, 13, 1595–1608 CrossRef PubMed.
- T. Su, Z. Tang, J. Hu, Y. Zhu and T. Shen, Innovative freeze-drying technique in the fabrication of dissolving microneedle patch: Enhancing transdermal drug delivery efficiency, Drug Delivery Transl. Res., 2024, 14(11), 3112–3127 CrossRef PubMed.
- L. Bao, G. Bonfante, H. Lee, N. Takama, J. park and B. Kim, Biodegradable Porous Microneedles via PLA Microspheres for Rapid ISF Extraction, in Proceedings of JSPE Semestrial Meeting 2021 JSPE Spring Conference, The Japan Society for Precision Engineering, 2021, pp. 731–732 Search PubMed.
- E. Babaie and S. B. Bhaduri, Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review, ACS Biomater. Sci. Eng., 2018, 4, 1–39 CrossRef.
- C. J. Yoo, B. S. Shin, B. S. Kang, D. H. Yun, D. B. You and S. M. Hong, Manufacturing a Porous Structure According to the Process Parameters of Functional 3D Porous Polymer Printing Technology Based on a Chemical Blowing Agent, IOP Conf. Ser.:Mater. Sci. Eng., 2017, 229, 12027 Search PubMed.
- J. A. Reglero Ruiz, M. Vincent, J. Agassant, T. Sadik, C. Pillon and C. Carrot, Polymer foaming with chemical blowing agents: Experiment and modeling, Polym. Eng. Sci., 2015, 55, 2018–2029 CrossRef.
- E. Aram and S. Mehdipour-Ataei, A review on the micro- and nanoporous polymeric foams: Preparation and properties, Int. J. Polym. Mater. Polym. Biomater., 2016, 65, 358–375 CrossRef.
- D. J. Mooney, D. F. Baldwin, N. P. Suh, J. P. Vacanti and R. Langer, Novel approach to fabricate porous sponges of poly(d,l-lactic-co-glycolic acid) without the use of organic solvents, Biomaterials, 1996, 17, 1417–1422 CrossRef PubMed.
- A. Almirall, G. Larrecq, J. A. Delgado, S. Martı́nez, J. A. Planell and M. P. Ginebra, Fabrication of low temperature macroporous hydroxyapatite scaffolds by foaming and hydrolysis of an α-TCP paste, Biomaterials, 2004, 25, 3671–3680 CrossRef PubMed.
- D. Chen, B. Yang, C. Yang, J. Wu and Q. Zhao, Macroporous Hydrogels Prepared By Ice Templating: Developments And Challenges, Chin. J. Chem., 2023, 41, 3082–3096 CrossRef.
- T. Bedir, S. Kadian, S. Shukla, O. Gunduz and R. Narayan, Additive manufacturing of microneedles for sensing and drug delivery, Expert Opin. Drug Delivery, 2024, 21, 1053–1068 CrossRef PubMed.
- S. D. Gittard, A. Ovsianikov, B. N. Chichkov, A. Doraiswamy and R. J. Narayan, Two-photon polymerization of microneedles for transdermal drug delivery, Expert Opin. Drug Delivery, 2010, 7, 513–533 CrossRef PubMed.
- Q. Yan, S. Shen, L. Liu, J. Weng, G. Zheng, X. Dong, J. Yang, Q. Yang and J. Xie, Fabrication of controlled porous and ultrafast dissolution porous microneedles by organic-solvent-free ice templating method, Int. J. Pharm., 2024, 660, 124220 CrossRef CAS.
- J. Min, J. Park, H. Yoon and Y. Choy, Ultrasonic Welding Method to Fabricate Polymer Microstructure Encapsulating Protein with Minimum Damage, Macromol. Res., 2008, 16, 570–573 CrossRef CAS.
- L. Bao, J. Park, B. Qin and B. Kim, Anti-SARS-CoV-2 IgM/IgG antibodies detection using a patch sensor containing porous microneedles and a paper-based immunoassay, Sci. Rep., 2022, 12, 10693 CrossRef CAS.
- H. Kai and A. Kumatani, A porous microneedle electrochemical glucose sensor fabricated on a scaffold of a polymer monolith, JPhys Energy, 2021, 3, 24006 CrossRef CAS.
- J. Xu, M. Wang, M. Jin, S. Shang, C. Ni, Y. Hu, X. Sun, J. Xu, B. Ji, L. Li, Y. Cheng and G. Wang, Flexible capacitive pressure sensor based on interdigital electrodes with porous microneedle arrays for physiological signal monitoring, Nanotechnol. Precis. Eng., 2024, 7, 013003–013014 CrossRef CAS.
- Y. Lee, S. Park, S. I. Kim, K. Lee and W. Ryu, Rapidly Detachable Microneedles Using Porous Water-Soluble Layer for Ocular Drug Delivery, Adv. Mater. Technol., 2020, 5(5), 1901145 CrossRef CAS.
- G. Wang, K. Kato, S. Ichinose, D. Inoue, A. Kobayashi, H. Terui, S. Tottori, M. Kanzaki and M. Nishizawa, Bilaterally Aligned Electroosmotic Flow Generated by Porous Microneedle Device for Dual-Mode Delivery, Adv. Healthcare Mater., 2024, e2401181 CrossRef PubMed.
- A. Sadeqi, G. Kiaee, W. Zeng, H. Rezaei Nejad and S. Sonkusale, Hard polymeric porous microneedles on stretchable substrate for transdermal drug delivery, Sci. Rep., 2022, 12, 1853 CrossRef CAS PubMed.
- H. Abe, Y. Matsui, N. Kimura and M. Nishizawa, Biodegradable Porous Microneedles for an Electric Skin Patch, Macromol. Mater. Eng., 2021, 306(9), 2100171 CrossRef CAS.
- L. Liu, H. Kai, K. Nagamine, Y. Ogawa and M. Nishizawa, Porous polymer microneedles with interconnecting microchannels for rapid fluid transport, RSC Adv., 2016, 6, 48630–48635 RSC.
- H. Lee, G. Bonfante, Y. Sasaki, N. Takama, T. Minami and B. Kim, Porous microneedles on a paper for screening test of prediabetes, Med. Devices Sens., 2020, 3(4), e10109 CrossRef CAS.
- P. Liu, H. Du, Z. Wu, H. Wang, J. Tao, L. Zhang and J. Zhu, Hydrophilic and anti-adhesive modification of porous polymer microneedles for rapid dermal interstitial fluid extraction, J. Mater. Chem. B, 2021, 9, 5476–5483 RSC.
- Q. Zeng, M. Xu, W. Hu, W. Cao, Y. Zhan, Y. Zhang, Q. Wang and T. Ma, Porous Colorimetric Microneedles for Minimally Invasive Rapid Glucose Sampling and Sensing in Skin Interstitial Fluid, Biosensors, 2023, 13, 537 CrossRef CAS PubMed.
- Y. Pang, Y. Li, K. Chen, M. Wu, J. Zhang, Y. Sun, Y. Xu, X. Wang, Q. Wang, X. Ning and D. Kong, Porous Microneedles Through Direct Ink Drawing with Nanocomposite Inks for Transdermal Collection of Interstitial Fluid, Small, 2024, 20, e2305838 CrossRef PubMed.
- G. Wang, K. Kato, S. Ichinose, D. Inoue, A. Kobayashi, H. Terui, S. Tottori, M. Kanzaki and M. Nishizawa, Bilaterally Aligned Electroosmotic Flow Generated by Porous Microneedle Device for Dual-Mode Delivery, Adv. Healthcare Mater., 2024, 13, e2401181 CrossRef.
- K. Yi, Y. Wang, K. Shi, J. Chi, J. Lyu and Y. Zhao, Aptamer-decorated porous microneedles arrays for extraction and detection of skin interstitial fluid biomarkers, Biosens. Bioelectron., 2021, 190, 113404 CrossRef CAS.
- J. Xu, M. Wang, M. Jin, S. Shang, C. Ni, Y. Hu, X. Sun, J. Xu, B. Ji, L. Li, Y. Cheng and G. Wang, Flexible capacitive pressure sensor based on interdigital electrodes with porous microneedle arrays for physiological signal monitoring, Nanotechnol. Precis. Eng., 2024, 7, 013003–013014 CrossRef CAS.
- L. Fan, X. Zhang, L. Wang, Y. Song, K. Yi, X. Wang, H. Zhang, L. Li and Y. Zhao, Bio-Inspired Porous Microneedles Dwelled Stem Cells for Diabetic Wound Treatment, Adv. Funct. Mater., 2024, 34(28), 2316742 CrossRef CAS.
- A. Fang, Y. Wang, N. Guan, Y. Zuo, L. Lin, B. Guo, A. Mo, Y. Wu, X. Lin, W. Cai, X. Chen, J. Ye, Z. Abdelrahman, X. Li, H. Zheng, Z. Wu, S. Jin, K. Xu, Y. Huang, X. Gu, B. Yu and X. Wang, Author Correction: Porous microneedle patch with sustained delivery of extracellular vesicles mitigates severe spinal cord injury, Nat. Commun., 2023, 14, 4603 CrossRef CAS PubMed.
- K. Nagamine, J. Kubota, H. Kai, Y. Ono and M. Nishizawa, An array of porous microneedles for transdermal monitoring of intercellular swelling, Biomed. Microdevices, 2017, 19, 68 CrossRef PubMed.
- K. Sato, S. Kusama, Y. Matsui, N. Kimura, S. Yoshida and M. Nishizawa, Evaluation of Electroosmotic Flow Promoted By a Porous Microneedle Array, in Electrochemical Society Meeting Abstracts Prime 2020, The Electrochemical Society, Inc., 2020, p. 2796 Search PubMed.
- J. Chen, X. Cai, W. Zhang, D. Zhu, Z. Ruan and N. Jin, Fabrication of Antibacterial Sponge Microneedles for Sampling Skin Interstitial Fluid, Pharmaceutics, 2023, 15, 1730 CrossRef CAS.
- Y. Abe, R. Takizawa, N. Kimura, H. Konno, S. Yoshida and M. Nishizawa, Porous microneedle-based wearable device for monitoring of transepidermal potential, Biomed. Eng. Adv., 2021, 1, 100004 CrossRef.
- H. Jing, J. Park and B. Kim, Fabrication of a Polyglycolic Acid Porous Microneedle Array Patch Using the Nonsolvent Induced Phase Separation Method for Body Fluid Extraction, Nano Sel., 2024,(4), e202400145 Search PubMed.
- G. Gao, L. Zhang, Z. Li, S. Ma and F. Ma, Porous Microneedles for Therapy and Diagnosis: Fabrication and Challenges, ACS Biomater. Sci. Eng., 2023, 9, 85–105 CrossRef CAS.
- P. Singh, A. Carrier, Y. Chen, S. Lin, J. Wang, S. Cui and X. Zhang, Polymeric microneedles for controlled transdermal drug delivery, J. Controlled Release, 2019, 315, 97–113 CrossRef CAS.
- S. S. Abubaker and Y. Zhang, Optimization Design and Fabrication of Polymer Micro Needle by Hot Embossing Method, Int. J. Precis. Eng. Manuf., 2019, 20, 631–640 CrossRef.
- J. Shen, H. Matsumoto, A. Maki, T. Kuriyama, T. Nemoto, S. Koido and H. Takeuchi, A study on the relationship between microstructure and mechanical properties of porous polymer films, Polymer, 2020, 204, 122784 CrossRef CAS.
- K. Kulasinski, Effects of water adsorption in hydrophilic polymers, Polymer Science: Research Advances, Practical Applications and Educational Aspects, ed. M. V. Antonio and S. M. Aurora, Formatex Research center S.L., 2016 Search PubMed.
- D. Wu, F. Xu, B. Sun, R. Fu, H. He and K. Matyjaszewski, Design and Preparation of Porous Polymers, Chem. Rev., 2012, 112, 3959–4015 CrossRef CAS.
- K. Takeuchi, N. Takama, B. Kim, K. Sharma, O. Paul and P. Ruther, Microfluidic chip to interface porous microneedles for ISF collection, Biomed. Microdevices, 2019, 21, 1–10 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |