Open Access Article
Open Access ArticleStudies on nitrate acid based imidazolium ionic liquids: synthesis and application in electrochemical desulfurization of oil†
Xingrui Chena,
Yingying Zhang *b and
Yanhong Kang*a
*b and
Yanhong Kang*a
aCollege of Chemistry and Chemical Engineering, Shenyang Normal University, Shenyang 110034, China. E-mail: ydkang@synu.edu.cn
bCollege of Chemistry, Liaoning University, Shenyang 110036, China. E-mail: 4022310167@smail-lnu.edu.cn
First published on 2nd June 2025
Abstract
The electro-polymerization desulfurization process is a promising technology for oil desulfurization. However, using ionic liquids as electrolytes for exploring deep desulfurization through the electro-polymerization method has not yet been well studied. In this study, four imidazole nitrate ionic liquids were successfully synthesized using a typical two-step method and characterized by 1H-NMR and TGA element analysis. Then, the electro-polymerization desulfurization experiment is conducted using thiophene as model oil and the ionic liquid [C5mim][NO3] as electrolyte. The effects of the oil–agent ratio, electro-polymerization potential, temperature, and reaction time on the desulfurization rate were invested. The results showed that the maximum desulfurization rate of the ionic liquid [C5mim][NO3] reached 68.7% under the optimal conditions: oil–agent ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mL), electro-polymerization potential of 2.5 V, temperature of 80 °C, and reaction time of 150 min. Moreover, the effects of temperature and reaction time on the desulfurization performance of the imidazole nitrate ionic liquid homologs [Cnmim][NO3] (n = 2, 3, 6) were also explored. The results showed that extending the time or increasing the temperature can enhance desulfurization. Finally, the electrochemical desulfurization mechanism and the desulfurization performance of the real oil were further investigated, with a maximum desulfurization rate of 51.2%. The desulfurization rate of ionic liquid can reach 55.1% after five cycles. This study is of great significance for further study of electrochemical desulfurization and promoting clean oil production.
1 (mL), electro-polymerization potential of 2.5 V, temperature of 80 °C, and reaction time of 150 min. Moreover, the effects of temperature and reaction time on the desulfurization performance of the imidazole nitrate ionic liquid homologs [Cnmim][NO3] (n = 2, 3, 6) were also explored. The results showed that extending the time or increasing the temperature can enhance desulfurization. Finally, the electrochemical desulfurization mechanism and the desulfurization performance of the real oil were further investigated, with a maximum desulfurization rate of 51.2%. The desulfurization rate of ionic liquid can reach 55.1% after five cycles. This study is of great significance for further study of electrochemical desulfurization and promoting clean oil production.
1 Introduction
With the rapid development of technology and the economy, petroleum products have been widely employed in various industries, including new batteries, vehicles, ships, and aviation aircraft.1 In gasoline, sulfides consist of mercaptan, thioether, thiophene, and their derivatives.2 However, sulfur oxide is produced when sulfur-containing fossil fuels are combusted at high temperatures.3 The presence of these substances can lead to corrosion, which can cause scaling damage to the equipment.4 Besides, these oxides are involved in creating acid rain and acid smog, causing great harm to the environment and human health.5–7 As such, regulatory agencies have established more rigorous standards on the amount of sulfur in fuel oil. The U.S., Japan, and the European Union request the use of ultra-low sulfur diesel and gasoline fuels.8,9 In China, the National VI standard for light-duty vehicles has been implemented since 2020, and according to the GB 19147-2016 standard, the sulfur content of automotive diesel fuel is 10 ppm.10 Consequently, lowering or eliminating sulfur content in fuel oil is an unstoppable trend, which is crucial for enhancing the global environment and promoting sustainable development.11At present, oil desulphurization technology can be divided into two broad categories: conventional hydrodesulfurization (HDS) and non-HDS.12 HDS is a conventional technique for desulfurization and is the most widely used method for removing sulfur from oil products.13 In HDS, the sulfur compounds are converted to hydrogen sulfide and corresponding by-products of hydrocarbons. It is extensively utilized in refineries due to its high efficiency,14 but it is conducted at elevated temperatures and pressures, using expensive hydrogen gas and catalysts. Therefore, it is highly desirable to develop alternative desulfurization approaches to reduce the sulfur content in fuel oils. Here are various methods such as electro-polymerization,15,16 oxide desulfurization (ODS), extractive desulfurization (EDS),17 adsorptive desulfurization (ADS),18 and bio-desulfurization (BDS).19 Compared with other technologies, the electro-polymerization technique possesses several advantages, such as a simple process, no pollution, less investment, rapid response, and high sensitivity.20,21 It is a polymerization technique that utilizes an applied potential to initiate the polymerization of sulfur compounds directly at the surface,22 and the purpose of desulfurization is achieved. With electro-polymerization, polymers can be rapidly deposited on electrodes in one step.23,24 Thus, the electro-polymerization desulfurization process is a promising technology for desulfurization of oil products. Besides, using volatile organic solvents as electrolytes could cause further environmental and safety problems because of their strongly volatile and toxic properties in the desulfurization process.25–27 Hence, it is crucial to discover sustainable and efficient solvents to replace traditional organic solvents.
Ionic liquids are primarily characterized as electrolytes that are liquid, composed of ions, and have a melting point below 100 °C.28,29 They possess distinct and beneficial characteristics, including high ionic conductivity, thermal stability, a broad electrochemical window, and extremely low vapor pressure.30,31 Recent studies have shown that the use of ionic liquids as electrolytes, instead of traditional liquid electrolytes, is very beneficial to improve the efficiency of polymerization.32–34 There is growing interest in the potential benefits of employing ILs as reaction media for conducting polymers.35 Imidazolium-based ionic liquids are known for their low viscosity, high ionic conductivity, and customizable design.36,37 Shi et al.38 prepared polythiophene (PTh) via the direct electrochemical synthesis in an ionic liquid ([Bmim]PF6) containing 0.1 mol L−1 thiophene, and in Sekiguchi's research,39 the pyrrole, thiophene, and aniline were successfully polymerized in 1-ethyl-3-methylimidazolium trifluoromethanesulfonate (EMICF3SO3). In addition to imidazolium-based ionic liquids (ILs), in Pringle's research,40 thiophene, bithiophene, and terthiophene have been successfully electropolymerized in pyrrolidinium-based bis(trifluoromethanesulfonyl)amide (TFSA) ionic liquids. Above all, ionic liquid as an electrolyte for sulfur compounds polymerization has a certain research. Besides, ILs and oil can be easily separated after electro-polymerization desulfurization, and the ILs can be recycled and reused. However, using ILs as electrolytes for exploring deep desulfurization through the electro-polymerization method has not yet been well studied.
In this paper, imidazole nitrate ionic liquid was synthesized, and the desulfurization of thiophene model oil with imidazole nitrate ionic liquids as an electro-polymerization electrolyte was explored. Four factors affecting desulfurization efficiency were considered. This experiment is a new electrochemical desulfurization technology based on nitric acid ionic liquid as an electrolyte, which is of great significance for further study of electrochemical desulfurization and promoting clean oil production.
2 Experimental
2.1 Materials and reagents
Acetonitrile (C2H3N, AR), ethyl acetate (C4H8O2, AR), bromoethane (C2H5Br, CP), bromopropane (C3H7Br, CP), bromopentane (C5H11Br, CP), bromohexane (C6H13Br, CP), dichloromethane (CH2Cl2, AR), sodium nitrate (NaNO3, CP), thiophene (C4H4S, AR), acetic acid glacial (C2H4O2, AR), n-pentane (C5H12, AR), n-hexane (C6H14, AR), cyclohexane (C6H12, AR), toluene (C7H8, AR), cyclooctene (C8H14, AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. N-Methylimidazole (C4H6N2, CP) was obtained from Zhejiang Shuangyang Chemical Factory. Acetone (C3H6O, AR) was obtained from Beijing Yili Fine Chemicals Ltd. Sulfur standard samples, potassium iodide (KI, GR), and sodium azide (N3Na, GR) were purchased from Jiangsu Electric Analytical Instrument Co., Ltd. All reagents and solvents were purified according to standard methods before use, then, the sample was kept in a desiccator. Nitrate ionic liquids were homemade in the laboratory.2.2 Synthesis of [Cnmim] NO3
This work successfully synthesized four imidazole nitrate ionic liquids using a typical two-step method.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were added to the reflux reactor and heated in an oil bath for reflux. Heating temperature at 35–40 °C, after 1 hour, the solution turned turbid, and the refluxing continued at 70–80 °C for 8 hours. After the reaction, the reactor was sealed to isolate the air and cooled in a desiccant dryer for 15 hours to obtain white crystals of bromine salt. After 15 hours, a mixture of 2
1 were added to the reflux reactor and heated in an oil bath for reflux. Heating temperature at 35–40 °C, after 1 hour, the solution turned turbid, and the refluxing continued at 70–80 °C for 8 hours. After the reaction, the reactor was sealed to isolate the air and cooled in a desiccant dryer for 15 hours to obtain white crystals of bromine salt. After 15 hours, a mixture of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate and acetonitrile was added to the reactor at 70 °C and again refluxed for 1 hour. At this time, the white crystals are quickly transferred to a dry beaker after being completely dissolved, sealed, and left to cool and crystallize. When the bromide salt was completely crystallized, the filter was carried out, and the filtered crystals were transferred to the reflux reactor, where a mixture of 2
1 ethyl acetate and acetonitrile was added to the reactor at 70 °C and again refluxed for 1 hour. At this time, the white crystals are quickly transferred to a dry beaker after being completely dissolved, sealed, and left to cool and crystallize. When the bromide salt was completely crystallized, the filter was carried out, and the filtered crystals were transferred to the reflux reactor, where a mixture of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate and acetonitrile was added to dissolve the crystals completely, again refluxed at 70 °C for 1 hour. After the above operations were repeated 3 times, the [Cnmim]Br (n = 2, 3) white crystals were obtained. The final products were dried under vacuum for 2 days and placed in a desiccator for backup.
1 ethyl acetate and acetonitrile was added to dissolve the crystals completely, again refluxed at 70 °C for 1 hour. After the above operations were repeated 3 times, the [Cnmim]Br (n = 2, 3) white crystals were obtained. The final products were dried under vacuum for 2 days and placed in a desiccator for backup.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were mixed and heated at 80 °C, after 30 minutes, the solution turned turbid, again refluxed for 8 hours. After the reaction, the solvent was removed with a rotary evaporator, and the crude product was recrystallized from an ethyl acetate and acetonitrile solution. Finally, a pale yellow liquid was obtained as an intermediate [Cnmim]Br (n = 5, 6), they were dried under a vacuum and placed in a desiccator for backup. The synthesis route is presented in Scheme 1.
1) were mixed and heated at 80 °C, after 30 minutes, the solution turned turbid, again refluxed for 8 hours. After the reaction, the solvent was removed with a rotary evaporator, and the crude product was recrystallized from an ethyl acetate and acetonitrile solution. Finally, a pale yellow liquid was obtained as an intermediate [Cnmim]Br (n = 5, 6), they were dried under a vacuum and placed in a desiccator for backup. The synthesis route is presented in Scheme 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 and NaNO3 were mixed and added to an appropriate amount of acetone, stirring vigorously. After 50 hours, the NaBr was removed by pumping filtration. The acetone was removed by rotary evaporator, and washed with dichloromethane, filtered, and evaporated. The above operations were repeated 3 times to obtain the purified products [Cnmim][NO3] (n = 2, 3, 5, 6), which were dried in a vacuum for 24 hours and placed in a desiccator for backup. The synthesis route is presented in Scheme 2.
1.2 and NaNO3 were mixed and added to an appropriate amount of acetone, stirring vigorously. After 50 hours, the NaBr was removed by pumping filtration. The acetone was removed by rotary evaporator, and washed with dichloromethane, filtered, and evaporated. The above operations were repeated 3 times to obtain the purified products [Cnmim][NO3] (n = 2, 3, 5, 6), which were dried in a vacuum for 24 hours and placed in a desiccator for backup. The synthesis route is presented in Scheme 2.
2.3 Desulfurization experiment
2.4 Characterization and analysis
3 Results and discussion
3.1 Electrochemical desulfurization potential of model oil
The cyclic voltammetry of the electro-polymerization of thiophene model oil (200 ppm) was studied in a three-electrode system with platinum sheet electrode and platinum wire electrode as working electrode and counter electrode, Ag/AgCl as reference electrode, and ionic liquid [C5mim][NO3] as electrolyte. The potential was scanned with the scan speed of 50 mV s−1 from −500 to positive. As can be seen from Fig. 1, thiophene begins to oxidize at around 2.0 V, which is consistent with the initial oxidation potential of thiophene reported in the literature. With the progress of the reaction, the peak current increases, and the peak current reaches its maximum at 2.5 V, which is the optimum electro-polymerization potential for thiophene. Therefore, for the electrochemical desulfurization experiment of thiophene model oil, the reaction potential can be selected at about 2.5 V for the experiment.3.2 Effect of oil ratio on desulfurization efficiency
The constant potential was provided by a high-precision DC voltage-regulated power supply, the reaction temperature was set at 80 °C, a certain proportion of [C5mim][NO3] ionic liquid and thiophene model oil (200 ppm) was added to the electrolytic cell, and the potential for the polymerization was set at 2.5 V, and the reaction was maintained in stirring for 150 min. The results are shown in Fig. 2.From the result, the desulfurization rate increased from 40.5% to 68.7%, which indicated that the larger the proportion of ionic liquid, the better the desulfurization effect. As the content of the ionic liquid increases, the concentration of thiophene reactants near the electrode decreases, resulting in a smaller amount of electricity required for oxidation and lower mass transfer resistance. This makes the electrochemical oxidation reaction easier to proceed, leading to an increase in the desulfurization rate. However, when the ratio of oil to agent is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the desulfurization rate does not increase further. This may be due to excessive electrode reaction capacity and a decrease in reactant concentration, leading to significant concentration polarization, meaning the reactants cannot be replenished quickly enough, preventing further improvement in desulfurization efficiency. This phenomenon was related to the design of the electrolytic cell and the effective reaction area of the electrode reaction, so the optimum ratio of model oil to ionic liquid [C5mim][NO3] was 3
1, the desulfurization rate does not increase further. This may be due to excessive electrode reaction capacity and a decrease in reactant concentration, leading to significant concentration polarization, meaning the reactants cannot be replenished quickly enough, preventing further improvement in desulfurization efficiency. This phenomenon was related to the design of the electrolytic cell and the effective reaction area of the electrode reaction, so the optimum ratio of model oil to ionic liquid [C5mim][NO3] was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
3.3 Effect of electrochemical polymerization potential on desulfurization
Under the condition of reaction temperature of 80 °C, the thiophene model oil and ionic liquid [C5mim][NO3] with a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were added to the electrolytic cell, and the potential for the polymerization was set at 2.5 V, and the reaction was maintained in stirring for 150 min. The experimental results are shown in Fig. 3.
1 were added to the electrolytic cell, and the potential for the polymerization was set at 2.5 V, and the reaction was maintained in stirring for 150 min. The experimental results are shown in Fig. 3.
As can be seen from Fig. 3, the residual sulfur content of the model oil was measured with little change, and the desulfurization effect was better at 2.5 V with the electrode potential increased from 1.8 V to 3.0 V. The results showed that the change of electrode potential in the appropriate potential range had a certain effect on the electro-polymerization reaction, but were of minor impact. It was related to the optimum polymerization potential of thiophene, and the change of electrode potential near the optimum polymerization potential had a certain effect on the electro-polymerization effect of thiophene.
3.4 Effect of reaction temperature on desulfurization efficiency
The reaction temperature was set under certain conditions, and the thiophene model oil and ionic liquid [C5mim][NO3] with a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were added to the electrolytic cell. The polymerization potential was set at 2.5 V, and the reaction was stirred continuously for 150 min. The experimental results are shown in Fig. 4.
1 were added to the electrolytic cell. The polymerization potential was set at 2.5 V, and the reaction was stirred continuously for 150 min. The experimental results are shown in Fig. 4.
The effect of electrochemical desulfurization at different temperatures can be seen in Fig. 4. With the increase of temperature from 25 °C to 80 °C, the desulfurization rate of thiophene model oil increased from 47% to 68.5%, indicating that the desulfurization effect increased with the increase of temperature. The reason for this trend was related to the change in the viscosity of the ionic liquid. As the temperature increased, the viscosity of ionic liquid showed a decreasing trend. According to literature reports,41–45 the viscosity of ionic liquid was closely related to its electrochemical activity. The smaller the viscosity of ionic liquid, the higher the electrochemical activity of ionic liquid, and the faster the diffusion of thiophene monomer in it. Therefore, when the temperature increased, the electrical conductivity of the ionic liquid as the electrolyte increased, which promoted the occurrence of the electro-polymerization reaction, and correspondingly, the desulfurization rate was also improved at the same time.
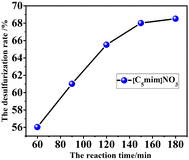
3.5 Effect of reaction time on desulfurization effect
Under the condition that the reaction temperature was set at 80 °C, the thiophene model oil and ionic liquid [C5mim][NO3] with the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were added to the electrolytic cell, the polymerization potential was set at 2.5 V, and the stirring was maintained for a certain time. The experimental results are shown in Fig. 5.
1 were added to the electrolytic cell, the polymerization potential was set at 2.5 V, and the stirring was maintained for a certain time. The experimental results are shown in Fig. 5.
The experimental results showed that the longer the reaction time of electro-polymerization, the better the desulfurization effect of the model oil, the reason was that electrochemical polymerization was a chain initiation process, and extending the reaction time caused the polymerization of thiophene to continue further, resulting in the reduction of thiophene in the model oil. When the reaction time was 180 min, the desulfurization rate of model oil was the best, which was 68.6%. However, the desulfurization effect changed little after 150 minutes, which can be considered that the reaction was basically completed. To save cost, the optimum reaction time was determined to be 150 min.
Furthermore, the ESI-MS spectra of the ionic liquid [C5mim][NO3] before and after desulfurization are shown in the ESI (Fig. S13 and S14†). The results show that the purity of the synthesized ionic liquid [C5mim][NO3] is relatively good, and the data demonstrate that NO3− remains chemically stable throughout the electrochemical process and does not participate in the oxidative desulfurization of model oil.
3.6 Reusability of ionic liquid
The advantage of ionic liquid used as electrolyte for electrochemical desulfurization of oil products is its reproducibility and recycling characteristics. The reuse performance of ionic liquid as electrolyte was investigated. Removal of polythiophenes or formation of polymer precipitates by centrifugation and filtration, and then the lower ionic liquid phase was taken after the layering. Re-join the model oil at the volume ratio of model oil to ionic liquid is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ionic liquid recycling experiment was carried out at electric potential is 2.5 V, the temperature is 80 °C, and the time is 150 min. The influence of ionic liquid [C5mim][NO3] cycle times on desulfurization rate is shown in Fig. 6.
1. The ionic liquid recycling experiment was carried out at electric potential is 2.5 V, the temperature is 80 °C, and the time is 150 min. The influence of ionic liquid [C5mim][NO3] cycle times on desulfurization rate is shown in Fig. 6.
As can be seen from Fig. 6, the desulphurization rate gradually decreased with the increase of ionic liquid recycling times. The desulfurization rate was 55.1% after the fifth cycle. The reason may be that the recovery of ionic liquid will be artificially incomplete in the process of ionic liquid recovery, and decomposed to a certain extent, resulting in a slight decrease in catalytic yield. The results show that ionic liquid as electrolyte has good repeatability and recyclability for electrochemical desulfurization of oil products.
3.7 Desulfurization performance of nitric acid ionic liquid homologs [Cnmim][NO3] (n = 2, 3, 6)
Nitric acid ionic liquid [C5mim][NO3] showed certain effects in the electrochemical polymerization desulfurization experiment, and it was speculated that its homologs may also have similar properties, and this property will show certain rules according to the change of the structure of the homologs. According to this idea, the desulfurization experiment of nitric acid ionic liquid homologs [Cnmim][NO3] (n = 2, 3, 6) was designed, and the influence of reaction time and reaction temperature on the desulfurization effect was mainly investigated. The experimental method was consistent with the above. The temperature was set under certain conditions, and the ratio of [Cnmim][NO3] nitric acid ionic liquid homologs and thiophene model oil with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were added to the electrolytic cell. The potential for the polymerization was set at 2.5 V, and the reaction was maintained in stirring for 150 min. The experimental results are shown in Fig. 7.
3 were added to the electrolytic cell. The potential for the polymerization was set at 2.5 V, and the reaction was maintained in stirring for 150 min. The experimental results are shown in Fig. 7.
As can be seen from Fig. 7, the reaction temperatures of nitric acid ionic liquid homologs [Cnmim][NO3](n = 2, 3, 6) all increased from 25 °C to 80 °C, respectively, and the desulphurization rates of thiophene model oils showed an increasing trend. These results showed that the higher the reaction temperature, the lower the sulfur content of thiophene model oil. The reason was that as the temperature increased, the viscosity of ionic liquids decreased, making its conductivity increase, the diffusion rate of thiophene in the ionic liquid was accelerated, thiophene was more prone to polymerization, and the desulfurization effect was improved.
Under the condition that the reaction temperature was set at 80 °C, the ratio of thiophene model oil and nitric acid ionic liquid homologs [Cnmim][NO3] (n = 2, 3, 6) were added to the electrolytic cell at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the polymerization potential was set at 2.5 V, and the stirring time was certain. The experimental results are shown in Fig. 8.
1, the polymerization potential was set at 2.5 V, and the stirring time was certain. The experimental results are shown in Fig. 8.
As can be seen from Fig. 8, the longer the reaction time of electro-polymerization desulfurization, the better the removal effect of thiophene in thiophene model oil. When the reaction time was extended from 60 min to 150 min, the desulfurization effects of the model oils of [Cnmim][NO3] (n = 2, 3, 6) were significantly improved. The reason was that the longer the reaction time the more fully the contact between the thiophene model oil and ionic liquid. Finally, the content of thiophene in the model oil was reduced and the desulfurization rate of the model oil was increased.
3.8 Comparative analysis
The comprehensive comparative analysis between the electrochemical desulfurization method and other desulfurization techniques (oxidative desulfurization, extraction desulfurization, and biocatalytic desulfurization) are listed in Table 1.| Method | Oil type | Sulfurization rate/% | References |
|---|---|---|---|
| Electrochemical desulfurization | Thiophene | 68.7% | This work |
| Oxidative desulfurization | Sn 650 base oil | 32% (one extraction) | 46 |
| 60% (three extraction) | |||
| Extraction desulfurization | Dibenzothiophene (DBT) | 57.41% (acetonitrile extraction) | 47 |
| 63.59% (acetonitrile extraction and H2O2 oxidant) | |||
| Biocatalytic desulfurization | Diesel oils | 48.5% | 48 |
| Extractive desulfurization | Benzothiophene (BT) | 61.2% | 49 |
Compared with other desulfurization techniques, nitrate acid-based imidazolium ionic liquids electrochemical desulfurization achieves a superior desulfurization efficiency of 68.7%. This improvement may be attributed to the dual-functional nature of the ionic liquid, which acts as both an efficient extractant and electrochemical medium, selectively enriching aromatic sulfides via π–π interactions; furthermore, a heterogeneous reaction interface at the electrode surface, which optimizes the microenvironment to overcome mass transfer limitations inherent in traditional homogeneous oxidation systems. Besides, this electrochemical desulfurization technology achieves green superiority by replacing volatile organic solvents (ACN, DMSO, DMF) with a recyclable ionic liquid system, eliminating toxic emissions while sustaining 55.1% efficiency after five cycles. Above all, using ionic liquids for desulfurization is a successful approach to achieve environmentally friendly and recyclable desulfurization.
3.9 Electrochemical desulfurization mechanism
The mechanism of the thiophene electrochemical polymerization reaction is as follows: the first step is the thiophene monomer loses an electron and oxidizes into a thiophene radical cation. The second step is two radical cations underwent coupling to form a dihydrodimer double cations and then lost 2 protons, again aromatization to generate dimer. The mechanism is illustrated in Scheme 3.The dimer oxidation potential is lower than the thiophene monomer. Therefore, the dimer is more easily oxidized than the thiophene monomer to form a dimer radical, which again leads to loss of electrons, and the electrochemical step was again carried out. Successively, the dimer radical is further coupled with the thiophene monomer radical, and the above two-step process is repeated. The mechanism is illustrated in Scheme 4.
3.10 Coexistence interference experiment of [C5mim][NO3] for desulfurization of model oil
The components of real oil are very complex, the main components are alkanes, alkenes, cycloalkanes, and aromatic hydrocarbons. Sulfur compounds are mainly thiophene, followed by mercaptan, and a small amount of thioether and disulfide. In the model oil composed of thiophene, other components existing in the real oil were added, and 6 groups of experiments were carried out in parallel. The first group was added with n-pentane, the second group was added with n-hexane, the third group was added with toluene, the fourth group was added with cyclooctene, the fifth group was a mixture of n-pentane and n-hexane, and the sixth group was a blank experiment. The addition of these coexisting substances made it closer to the composition of real oil, and the electro-polymerization desulfurization experiment was conducted to investigate the influence of other substances on the desulfurization effect of model oil. The volume ratio of model oil to ionic liquid is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the electric potential is 2.5 V, the temperature is 60 °C, and the time is 80 min. The coexistence interference experiment was carried out and the results are shown in Fig. 9.
1, the electric potential is 2.5 V, the temperature is 60 °C, and the time is 80 min. The coexistence interference experiment was carried out and the results are shown in Fig. 9.
As can be seen from Fig. 9, the four interfering substances added have a slight impact on the desulfurization effect, these effects can be practically ignored. The reason for this phenomenon was that the concentration of thiophene model oil was slightly changed by the amount of interfering substances added; when the desulfurization effect was tested by coulometry, the volume of the model oil added unchanged, which makes the sulfur content of the model oil of the same volume slightly change, and the experiment has a certain error. Based on the above analysis, it can be concluded that the addition of the above four interfering substances has no substantial impact on the desulfurization effect of model oil.
3.11 Desulfurization performance of real oil
The basic experimental conditions of imidazole nitrate ionic liquid for the desulfurization of model oil were applied to the desulfurization of real oil. The reaction conditions were as follows: ratio of oil to agent 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, polymerization potential 2.5 V, reaction temperature at room temperature, reaction time 180 min. According to the above reaction conditions, the desulfurization experiment was carried out. Before the experiment, the sulfur content in the real oil was determined by using the microcoulomb comprehensive analyzer, and the sulfur content was 52.012 ppm. After the experiment, the sulfur content was determined. The sulfur content was 25.377 ppm, and the desulfurization rate reached 51.21%.
1, polymerization potential 2.5 V, reaction temperature at room temperature, reaction time 180 min. According to the above reaction conditions, the desulfurization experiment was carried out. Before the experiment, the sulfur content in the real oil was determined by using the microcoulomb comprehensive analyzer, and the sulfur content was 52.012 ppm. After the experiment, the sulfur content was determined. The sulfur content was 25.377 ppm, and the desulfurization rate reached 51.21%.
The electrochemical desulfurization experiment of ionic liquid for real oil is not sufficiently mature, because the gasoline component is very complex, has no fixed boiling point, and the real oil is volatile, and can also volatilize at low temperatures. Therefore, if the experimental temperature is increased, the volatilization of substances with low boiling points or even substances with high boiling points will be caused, and thiophene sulfur compounds mainly exist in high boiling point fractions. This will cause a change in sulfur content, and there are some hidden dangers in the experiment. Therefore, in this experiment, the reaction temperature was set to room temperature to avoid the above situation. However, due to the high viscosity of nitric acid ionic liquid at room temperature, the conductivity of the ionic liquid is affected, so the desulfurization effect may be affected to a certain extent. In addition, the volatility of gasoline has an impact on the determination of sulfur content, because in the process of using micro coulometry to determine sulfur content, high-temperature combustion accelerates the volatilization of gasoline, so the sulfur content of the sample increases gradually during the measurement process.
4 Conclusions
In this paper, the desulfurization performance of imidazole nitrate ionic liquid in model oil electro-polymerization is studied, and the desulfurization process is optimized. The imidazole nitrate ionic liquid [Cnmim][NO3] (n = 2, 3, 5, 6) prepared by the experiment was used for electro-polymerization desulfurization experiment for the electrolyte to remove thiophene in the model oil. The experimental results show that all four ionic liquids can remove thiophene from the model oil, but the removal effect is different. In the electrochemical polymerization desulfurization experiment using imidazole nitrate acid ionic liquids [C5mim][NO3] as the electrolyte, the experimental conditions such as oil–agent ratio, polymerization potential, temperature, and time were optimized, and the optimal process conditions were determined as oil–agent ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mL), polymerization potential 2.5 V, temperature 80 °C, time 150 min, and the desulfurization rate is 68.7%. The desulfurization performance of the imidazole nitrate ionic liquid homologs [Cnmim][NO3] (n = 2, 3, 6) was further explored. It was found that the desulfurization rate of ionic liquids [C5mim][NO3] is the best. The desulfurization rate was affected by the steric hindrance effect with the extension or shortening of carbon chain length. The desulfurization performance of [C2mim][NO3], [C3mim][NO3], [C6mim][NO3] was relatively poor. Simulated other components in real oil except thiophene, co-existing interference experiments were carried out, and the results showed that the four substances such as cyclooctene had no substantial effect on the desulfurization effect. The electrochemical desulphurization of real oil was studied, and the primary desulphurization rate reached 51.21%. The desulfurization rate was 55.1% after the fifth cycle. The results show that ionic liquid as electrolyte has good repeatability and recyclability for electrochemical desulfurization of oil products.
1 (mL), polymerization potential 2.5 V, temperature 80 °C, time 150 min, and the desulfurization rate is 68.7%. The desulfurization performance of the imidazole nitrate ionic liquid homologs [Cnmim][NO3] (n = 2, 3, 6) was further explored. It was found that the desulfurization rate of ionic liquids [C5mim][NO3] is the best. The desulfurization rate was affected by the steric hindrance effect with the extension or shortening of carbon chain length. The desulfurization performance of [C2mim][NO3], [C3mim][NO3], [C6mim][NO3] was relatively poor. Simulated other components in real oil except thiophene, co-existing interference experiments were carried out, and the results showed that the four substances such as cyclooctene had no substantial effect on the desulfurization effect. The electrochemical desulphurization of real oil was studied, and the primary desulphurization rate reached 51.21%. The desulfurization rate was 55.1% after the fifth cycle. The results show that ionic liquid as electrolyte has good repeatability and recyclability for electrochemical desulfurization of oil products.
Data availability
All data were communicated in the paper.Author contributions
Xingrui Chen: investigation; writing – original draft; and writing – review and editing. Yingying Zhang: investigation; analysis, writing – original draft; and writing – review and editing. Yanhong Kang: conceptualization, methodology, investigation and supervision.Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Wei, Y. Jin and W. Zhang, Treatment of high-concentration wastewater from an oil and gas field via a paired sequencing batch and ceramic membrane reactor, Int. J. Environ. Res. Public Health, 2020, 17, 1953 Search PubMed.
- M. L. Gorbaty and S. R. Kelemen, Characterization and reactivity of organically bound sulfur and nitrogen fossil fuels, Fuel Process. Technol., 2001, 71, 71–78 CrossRef CAS.
- H. M. Ali, C. Sitinjak and M. H. Md Said, Model predicting social acceptance behavior to implement ELV policy: Exploring the role of knowledge toward ELV policy on social acceptance in Malaysia, Front. Public Health, 2023, 10, 1093732 Search PubMed.
- E. Paris, P. Avino and E. Guerriero, Activated porous carbon fiber: new adsorbent for sampling and analysis by thermal desorption of siloxanes in biogas and biomethane, Int. J. Environ. Res. Public Health, 2022, 19, 10890 CrossRef CAS PubMed.
- W. Zhang, H. Zhang and J. Xiao, Carbon nanotube catalysts for oxidative desulfurization of a model diesel fuel using molecular oxygen, Green Chem., 2013, 16, 211220 Search PubMed.
- C. P. Li, D. Li and S. S. Zou, Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents, Green Chem., 2013, 15, 27932799 Search PubMed.
- S. Wei, H. He, Y. Cheng, C. Yang, G. Zeng and L. Qiu, Performances, kinetics and mechanisms of catalytic oxidative desulfurization from oils, RSC Adv., 2016, 6, 103253–103269 RSC.
- I. V. Babich and J. A. Moulijn, Science and technology of novel processes for deep desulfurization of oil refinery streams: a review, Fuel, 2003, 82, 607–631 CrossRef CAS.
- L. L. Mguni, Y. Yao and X. Liu, Ultra-deep desulphurization of both model and commercial diesel fuels by adsorption method, J. Environ. Chem. Eng., 2019, 7, 102957 CrossRef CAS.
- O. Zhou, China Implements Euro 6-equivalent Fuel Standards, Platt's Oilgram News, 2019, vol. 97 Search PubMed.
- X. Li, J. Zhang, F. Zhou, Y. Wang, X. Yuan and H. Wang, Oxidative desulfurization of dibenzothiophene and diesel by hydrogen peroxide: Catalysis of H3PMo12O40 immobilized on the ionic liquid modified SiO2, Mol. Catal., 2018, 452, 93–99 Search PubMed.
- F. A. Duarte, P. de and A. Mello, Sulfur removal from hydrotreated petro leum fractions using ultrasound-assisted oxidative desulfurization process, Fuel, 2011, 90, 2158–2164 Search PubMed.
- Q. Wu, Q. Zhang and X. Chen, Integrated assessment of waste tire pyrolysis and upgrading pathways for production of high-value products, ACS Omega, 2022, 7, 30954–30966 Search PubMed.
- S. O. Ribeiro, B. Duarte and B. De Castro, Improving the catalytic performance of Keggin [PW12O40] 3- for oxidative desulfurization: ionic liquids versus SBA-15 composite, Mater, 2018, 11, 1196 CrossRef PubMed.
- C. Shu, T. Sun, Q. Guo, J. Jia and Z. Lou, Desulfurization of diesel fuel with nickel boride in situ generated in an ionic liquid, Green Chem., 2014, 16, 3881–3889 Search PubMed.
- D. P. Harrison, L. S. Carpenter and J. T. Hyde, Reductive electro-polymerization of a vinyl-containing poly-pyridyl complex on glassy carbon and fluorine-doped tin oxide electrodes, J. Visualized Exp., 2015, 95, 52035 Search PubMed.
- T. J. Ren, J. Zhang, Y. H. Hu, J. L. Pan, M. S. Liu and D. S. Zhao, Extractive desulfurization of fuel oil with metal-based ionic liquids, Chin. Chem. Lett., 2015, 26, 1169–1173 CrossRef CAS.
- M. Zheng, H. Hu, Z. Ye, Q. Huang and X. Chen, Adsorption desulfurization performance and adsorption-diffusion study of B2O3 modified Ag-CeOx/TiO2-SiO2, J. Hazard. Mater., 2019, 362, 424–435 CrossRef CAS PubMed.
- L. Gonsalvesh, S. P. Marinov, M. Stefanova, R. Carleer and J. Yperman, Biodesulphurized low rank coal: Maritza east lignite and it's “humus-like” byproduct, Fuel, 2013, 103, 1039–1050 CrossRef CAS.
- C. Lu, Z. Li and L. Ren, In situ oxidation of Cu2O crystal for electrochemical detection of glucose, Sensors, 2019, 19, 2926 CrossRef CAS PubMed.
- J. Shao, C. Wang and Y. Shen, Electrochemical sensors and biosensors for the analysis of tea components: A bibliometric review, Front. Chem., 2022, 9, 818461 CrossRef PubMed.
- B. B. Cui, Z. Mao and Y. Chen, Tuning of resistive memory switching in electropolymerized metallopolymeric films, Chem. Sci., 2015, 6, 1308–1315 RSC.
- T. Kilic, Y. K. Cho and N. Jeong, Multielectrode spectroscopy enables rapid and sensitive molecular profiling of extracellular vesicles, ACS Cent. Sci., 2022, 8, 110–117 CrossRef CAS PubMed.
- V. G. Sree, J. I. Sohn and H. Im, Pre-anodized graphite pencil electrode coated with a poly (thionine) film for simultaneous sensing of 3-nitrophenol and 4-nitrophenol in environmental water samples, Sensors, 2022, 22, 1151 CrossRef CAS PubMed.
- H. Pan, M. Zhang and Z. Cheng, Carbon-free and binder-free Li-Al alloy anode enabling an all-solid-state Li-S battery with high energy and stability, Sci. Adv., 2022, 8, 4372 CrossRef PubMed.
- F. Bella, S. Galliano and M. Falco, Unveiling iodine-based electrolytes chemistry in aqueous dye-sensitized solar cells, Chem. Sci., 2016, 7, 4880–4890 RSC.
- S. Qi, D. Wu, Y. Dong and J. Liao, Cobalt-based electrode materials for sodium-ion batteries, Chem. Eng. J., 2019, 370, 185–207 CrossRef CAS.
- K. Mishra, N. Devi and S. S. Siwal, Ionic liquid-based polymer nanocomposites for sensors, energy, biomedicine, and environmental applications: roadmap to the future, Adv. Sci., 2022, 9, 2202187 CrossRef CAS PubMed.
- M. Yi, J. Ma and Y. Ren, Ionic liquid meets MOF: a facile method to optimize the structure of CoSe2-NiSe2 heterojunctions with N, P, and F triple-doped carbon using ionic liquid for efficient hydrogen evolution and flexible supercapacitors, Adv. Sci., 2023, 10, 2206029 CrossRef CAS PubMed.
- O. S. Morozov, A. V. Babkin and A. V. Ivanchenko, Ionomers based on addition and ring opening metathesis polymerized 5-phenyl-2-norbornene as a membrane material for ionic actuators, Membranes, 2022, 12, 316 CrossRef CAS PubMed.
- P. Nellepalli, L. C. Tomé and K. Vijayakrishna, Imidazolium-based copoly (ionic liquid) membranes for CO2/N2 separation, Ind. Eng. Chem. Res., 2019, 58, 2017–2026 CrossRef CAS.
- W. Lu, A. G. Fadeev and B. Qi, Use of ionic liquids for π-conjugated polymer electrochemical devices, Science, 2002, 297, 983–987 CrossRef CAS PubMed.
- J. Ding, D. Zhou and G. Spinks, Use of ionic liquids as electrolytes in electromechanical actuator systems based on inherently conducting polymers, Chem. Mater., 2003, 15, 2392–2398 CrossRef CAS.
- V. I. Volkov, O. V. Yarmolenko and A. V. Chernyak, Polymer electrolytes for lithium-ion batteries studied by NMR techniques, Membranes, 2022, 12, 416 CrossRef CAS PubMed.
- J. M. Pringle, O. Winther-Jensen and C. Lynam, One-step synthesis of conducting polymer–noble metal nanoparticle composites using an ionic liquid, Adv. Funct. Mater., 2008, 18, 2031–2040 CrossRef CAS.
- K. Liu, Z. Wang and L. Shi, Ionic liquids for high performance lithium metal batteries, J. Energy Chem., 2021, 59, 320–333 CrossRef CAS.
- S. Kazemiabnavi, Z. Zhang and K. Thornton, Electrochemical stability window of imidazolium-based ionic liquids as electrolytes for lithium batteries, J. Phys. Chem. B, 2016, 120, 5691–5702 CrossRef CAS PubMed.
- J. H. Shi, X. Sun and C. H. Yang, Electrochemical synthesis of polythiophene in an ionic liquid, Chin. Chem. Lett., 2002, 13, 306–307 CAS.
- K. Sekiguchi, M. Atobe and T. Fuchigami, Electrooxidative polymerization of aromatic compounds in 1-ethyl-3-methylimidazolium trifluoromethanesulfonate room-temperature ionic liquid, J. Electroanal. Chem., 2003, 557, 1–7 CrossRef CAS.
- J. M. Pringle, M. Forsyth and D. R. MacFarlane, The influence of the monomer and the ionic liquid on the electrochemical preparation of polythiophene, Polymer, 2005, 46, 2047–2058 CrossRef CAS.
- C. D. Stachurski, J. J. H. Davis and T. Cosby, Physical and electrochemical analysis of N-Alkylpyrrolidinium-Substituted boronium ionic liquids, Inorg. Chem., 2023, 62, 18280–18289 CrossRef CAS PubMed.
- W. L. Yuan, X. Yang and L. He, Viscosity, conductivity, and electrochemical property of dicyanamide ionic liquids, Front. Chem., 2018, 6, 59 CrossRef PubMed.
- S. Zhang, H. Baba and T. Sakka, Interfacial viscosity and ionic reorientation probed using electrochemical surface plasmon resonance at the gold electrode interface of ionic liquids, J. Electroanal. Chem., 2022, 913, 116299 Search PubMed.
- Q. Zhang, Q. Li and D. Liu, Density, dynamic viscosity, electrical conductivity, electrochemical potential window, and excess properties of ionic liquid N-butyl-pyridinium dicyanamide and binary system with propylene carbonate, J. Mol. Liq., 2018, 249, 1097–1106 CrossRef CAS.
- M. A. Ploss, M. W. Rutland and S. Glavatskih, Influence of electric potential on the apparent viscosity of an ionic liquid: facts and artifacts, Phys. Chem. Chem. Phys., 2016, 38, 26609–26615 Search PubMed.
- A. Mortezaee, M. A. Sobati and S. Movahedirad, An experimental investigation on the oxidative desulfurization of a mineral lubricant base oil, J. Environ. Health Sci. Eng., 2021, 19, 1951–1968 CrossRef CAS PubMed.
- Q. Wu, Q. Shi and J. Shang, Synthesis of surface-active heteropolyacid-based ionic liquids and their catalytic performance for desulfurization of fuel oils, ACS Omega, 2020, 5, 31171–31179 CrossRef CAS PubMed.
- S. Maghsoudi, M. Vossoughi and A. Kheirolomoom, Biodesulfurization of hydrocarbons and diesel fuels by Rhodococcus sp. strain P32C1, Biochem. Eng. J., 2001, 8, 151–156 CrossRef CAS.
- Z. Ren, Z. Zhou and M. Li, Deep desulfurization of fuels using imidazole anion-based ionic liquids, ACS Sustain. Chem. Eng., 2018, 7, 1890–1900 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01724f |
| This journal is © The Royal Society of Chemistry 2025 |