Palladium–copper catalyzed C(sp3)–C(sp2) bond C–H activation cross-coupling reaction: selective arylation to synthesize 9-aryl-9H-xanthene and 9,9-diaryl-xanthene derivatives†
Guodong Shen*,
Lingyu Zhao,
Yichen Wang,
Wenfang Xia,
Mingsen Yang and
Tongxin Zhang*
School of Chemistry and Chemical Engineering, School of Pharmacy, Liaocheng University, Liaocheng 252000, Shandong, P. R. China. E-mail: shenguodong33@163.com; xintongzhang123@163.com; Fax: +86-635-8239680; Tel: +86-635-8239680
First published on 15th August 2016
Abstract
A novel cooperatively catalyzed C(sp3)–C(sp2) bond C–H activation cross-coupling reaction has been developed. A series of 9-aryl-9H-xanthenes and 9,9-diaryl-xanthenes were selectively synthesized in moderate to good yields by controlling the reaction time and temperature.
Xanthene derivatives are an important class of molecules and heterocyclic scaffolds in biological, medicinal and food research.1 For example (Fig. 1), erythrosine (A), known as Red No. 3, is primarily used for food coloring, commonly used in sweets.2 Mangostin (B) has exhibited excellent biological properties, including antioxidative,3 anti-bacterial,4 anti-inflammatory5 and anticancer activities.6 The uridine derivative (C) is an antiarrhythmic agent7 and can also be used to treat a number of supraventricular tachycardias that do not improve with vagal maneuvers.8 The adenosine derivative (D) can be used for the treatment of liver, cerebrovascular, cardiovascular and other diseases.9 Furthermore, some xanthenes have been used as dyes, and also in fluorescent materials and laser technologies for their useful spectroscopic properties.10
Based on the broad spectrum of applications of xanthene, a lot of methodologies for synthesizing xanthene derivatives have been investigated, some methods particularly for the synthesis of 9-substituted xanthenes. Some good examples are the scandium triflate-catalyzed one-pot domino approach11 and Brønsted acid (TfOH or MsOH) catalyzed oxidative coupling reactions.12 However, these methods are not able to provide effective ways to selectively synthesize 9-aryl-9H-xanthenes and 9,9-diaryl-xanthenes.
Palladium and copper catalyzed C–H activation and cross-coupling (sp2-C and sp3-C) reactions are of paramount importance in the synthesis of organic molecules.12 Numerous C–H activation and cross-coupling systems,13 particularly the cooperative palladium–copper co-catalysed systems,14 have been applied in the preparation of pharmaceuticals, agrochemicals and advanced materials on both laboratory and industrial scales. In the past decades, palladium and copper catalyzed C–X (X = C, N, O, S, etc.) bond formation reactions have drawn considerable attention for their efficiency.15 However, there are few reports about the versatile and selective synthesis of 9-aryl-9H-xanthenes and 9,9-diaryl-xanthenes using the metal catalyzed cross-coupling reaction.16 Herein, we report an efficient method for a highly selective synthesis of 9-aryl-9H- and 9,9-diaryl-xanthene derivatives via the palladium–copper co-catalyzed C(sp3)–C(sp2) bond cross-coupling reaction by controlling the reaction time and temperature.
Initially, xanthene 1a and anisole 1b were selected as the model substrates to identify and optimize the reaction parameters including transition metal catalysts, reaction temperature and reaction time. When the reaction was carried out with Cu(OTf)2 (10 mol%) under a N2 atmosphere at 140 °C, the desired 9-(4-methoxyphenyl)-9H-xanthene 1c could be detected in 5% yield after 15 hours (Table 1, entry 1). Raising the reaction temperature (150 °C) did not improve the yield of the desired compounds (Table 1, entry 2). Only using Pd(OAc)2 to repeat this reaction did not generate the good yield either (Table 1, entry 3). After extensive studies we found that the yield of 1c could be improved significantly by using the co-catalyst system of Pd(OAc)2 and Cu(OTf)2. A new compound 1d was observed at the same time using this co-catalyst system (Table 1, entries 4–5). The structure of 1d was unambiguously elucidated by X-ray crystallography (Fig. 2). When we reduced the reaction time to 2.5 hours, only compound 1c was obtained with a high degree of selectivity (Table 1, entry 6). Further extensive studies were made to improve the reaction yield after we reached a good selectivity. We finally found that the reaction yield was greatly improved if the reaction was carried out in open air (Table 1, entry 7). However, the reaction at lower temperature (120 °C) and using either Pd(OAc)2 or Cu(OTf)2 alone evidently decreased the reaction yield (Table 1, entries 8–10). We also conducted the reaction under an oxygen balloon and found that xanthene can be oxidized to xanthone (Table 1, entry 11). We used TfOH instead of Cu(OTf)2 and the reaction gave a low yield (Table 1, entry 12). To our delight, when the reaction temperature was raised to 145 °C and the reaction time was extended to 24 h, 1d was obtained in 80% yield (Table 1, entry 13). Some other palladium and copper salts such as PdCl2, Pd(dppf)Cl2, Cu(OAc)2·H2O and CuI were also studied (Table 1, entries 14–17). The catalyst system of Pd(OAc)2 and Cu(OTf)2 provided the best result. We also carried out some control experiments for the second arylation for the synthesis of 9,9-diaryl-xanthenes. From the results, we can see that the Pd/Cu co-catalysts are still important for the reaction (Table 1, entries 18–20). Noticeably, the entries 7 and 13 are the best reaction conditions.
| Entry | Catalyst | T (°C) | t (h) | 1cb (%) | 1db (%) | |
|---|---|---|---|---|---|---|
| a Reaction conditions: Pd catalyst (0.025 mmol), Cu catalyst (0.05 mmol), xanthene 1a (0.5 mmol) in anisole (1.5 mL) under N2/air/O2 atmosphere.b Isolated yield after flash chromatography based on 1a.c 35% of xanthone was isolated based on 1a. | ||||||
| 1 | Cu(OTf)2 | N2 | 140 | 15 | 5 | 0 |
| 2 | Cu(OTf)2 | N2 | 150 | 15 | 6 | 0 |
| 3 | Pd(OAc)2 | N2 | 140 | 15 | 4 | 0 |
| 4 | Pd(OAc)2/Cu(OTf)2 | N2 | 130 | 15 | 25 | 5 |
| 5 | Pd(OAc)2/Cu(OTf)2 | N2 | 140 | 15 | 21 | 11 |
| 6 | Pd(OAc)2/Cu(OTf)2 | N2 | 130 | 2.5 | 35 | Trace |
| 7 | Pd(OAc)2/Cu(OTf)2 | Air | 130 | 2.5 | 85 | Trace |
| 8 | Pd(OAc)2/Cu(OTf)2 | Air | 120 | 2.5 | 40 | 0 |
| 9 | Pd(OAc)2 | Air | 130 | 2.5 | 13 | 0 |
| 10 | Cu(OTf)2 | Air | 130 | 2.5 | 41 | 0 |
| 11 | Pd(OAc)2/Cu(OTf)2 | O2 | 130 | 2.5 | 34c | 0 |
| 12 | Pd(OAc)2/TfOH | Air | 130 | 2.5 | 10 | 0 |
| 13 | Pd(OAc)2/Cu(OTf)2 | Air | 145 | 24 | <1 | 80 |
| 14 | PdCl2/Cu(OTf)2 | Air | 130 | 2.5 | 80 | 5 |
| 15 | Pd(dppf)Cl2/Cu(OTf)2 | Air | 130 | 2.5 | 45 | Trace |
| 16 | Pd(OAc)2/Cu(OAc)2 | Air | 130 | 2.5 | 0 | 0 |
| 17 | Pd(OAc)2/CuI | Air | 130 | 2.5 | 0 | 0 |
| 18 | Pd(OAc)2 | Air | 145 | 24 | <5 | 0 |
| 19 | Cu(OTf)2 | Air | 145 | 24 | <5 | 0 |
| 20 | Pd(OAc)2/Cu(OTf)2 | N2 | 145 | 24 | 24 | 15 |
With the optimized reaction conditions in hand, the scope of substrates was further investigated (Table 2). From the experimental results we could conclude that the reaction yields were mainly influenced by electronic effects and the steric hindrance of aromatics b. The aromatics b must have the electron-donating groups such as –OMe, –OH, –SMe, –NH2 etc. The reaction time for mono- and bis-arylation was mainly determined by TLC. When the starting material was consumed completely, the reaction was stopped resulting in the best yield. The reactions of xanthene 1a with anisole 1b, ethoxybenzene 2b, oxydibenzene 3b or 1,2-dimethoxybenzene 4b could generate mono-substituted xanthenes (1c, 2c, 3c and 4c) or di-substituted xanthenes (1d, 2d, 3d and 4d) selectively by controlling the reaction time and temperature. However, when 1-methoxy-2-methylbenzene 5b, 1-bromo-2-methoxybenzene 6b and methyl phenyl sulfide 7b were used, the yields of 5c, 6c and 7c were not high. Therefore, increasing the reaction temperature and extending the reaction time could not improve the yield. Phenol 8b was used to run the reaction at 130 °C for 2.5 h and we did not detect the product 4-(9H-xanthen-9-yl)phenol 8c by TLC, but 4,4′-(9H-xanthene-9,9-diyl)diphenol 8d was afforded at 140 °C for 24 h (for the crystal structure of 8d, see ESI†). Aniline 9b and o-toluidine 10b could proceed by the reaction and the anticipated products 9c and 10c were obtained. Because of the steric hindrance of 1,3,5-trimethoxybenzene 11b, we could not obtain bis-substituted products. The substituted-xanthene derivatives bearing –COPh and –COMe groups were able to react with anisole 1b to afford the corresponding products in moderate yields (12c, 12d, 13c, and 13d). Benzene 12b and toluene 13b could not proceed by the reaction. Benzonitrile 14b and nitrobenzene 15b with electron poor functional groups were not detected in the corresponding products. Furthermore, our method can also be successfully applied to synthesize the 9,9-diaryl-xanthenes with different aromatic functional groups. As shown in Table 3, the reaction of 9-aryl-9H-xanthenes c with aromatics b could afford the anticipated 9,9-diaryl-xanthenes d in moderate yields (Table 3, entries 1–5). The yields for the 3,4-di-(differently)-substituted xanthenes were not high and it may be due to the formation of isomers.
| Entry | c | b | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: palladium acetate (0.025 mmol), copper(II) trifluoromethanesulfonate (0.05 mmol), 9-(4-methoxyphenyl)-9H-xanthene 1c (0.5 mmol) in aromatics b (1.5 mL) as solvent for 17 h under air.b Isolated yield after flash chromatography based on c.c 0.5 mL 1b and 0.5 mL 2b were used. | ||||
| 1 | 1c | 1b |  |
62 |
| 2 | 1c | 2b |  |
48 |
| 3 | 1c | 3b | 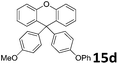 |
55 |
| 4 | 1c | 8b |  |
52 |
| 5 | 2c | 8b |  |
40 |
| 6 | 1a | 1b + 2bc | 1d + 2d + 14d | 53(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
9H-Fluorene 4a can also be reacted with anisole 1b under the reaction conditions, but only the bis-substituted product 9,9-bis(4-methoxyphenyl)-9H-fluorene 17d was isolated in 51% yield (Scheme 1).
In the process of our experiment, xanthone was isolated in low yield under the optimal reaction conditions (Table 1, entry 7). To gain some insight into the reaction mechanism, we used the xanthone to run the reaction and product 1c was not detected.17 Therefore, we speculate that the reaction did not proceed to the intermediate of xanthone. A plausible mechanism which accounts for the cooperative palladium–copper catalyzed C(sp3)–C(sp2) coupling reaction is shown in Fig. 3. Firstly, aromatics bearing electron-donating functional groups and xanthenes can be reacted with the metal catalyst Cu(OTf)2 and Pd(OAc)2, respectively.18 The obtained intermediates A and B proceeded from the cross-coupling reaction to get the intermediate C, which then translated to the final product after the reductive elimination reaction.
To summarize, we have developed a novel and efficient cooperative palladium–copper catalyzed C(sp3)–C(sp2) C–H activation and cross-coupling reaction to synthesize 9-aryl-9H-xanthene and 9,9-diaryl-xanthene derivatives which are very important to the chemical and pharmaceutical industry. The newly-developed co-catalyst system demonstrated moderate yields and good selectivity. We will use this protocol to further expand our research to additional aromatics with other functional groups which might be potentially applicable to the pharmaceutical industry and biochemical research.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21402079) and the Shandong Provincial Natural Science Foundation of China (ZR2015PB004).Notes and references
- (a) K. D. Sajal, S. Ritesh and P. Gautam, Eur. J. Org. Chem., 2009, 4757 Search PubMed; (b) G. Saint-Ruf, H. T. Hieu and J. P. Poupelin, Naturwissenschaften, 1975, 62, 584 CrossRef CAS PubMed; (c) N. P. Buu-Hoi, G. Saint-Ruf, A. De and H. T. Hieu, Chim. Ther., 1972, 7, 83 CAS; (d) R. M. Ion, Prog. Catal., 1997, 2, 55 Search PubMed; (e) R. M. Ion, D. Frackowiak, A. Planner and K. Wiktorowicz, Acta Biochim. Pol., 1998, 45, 833 CAS.
- (a) A. S. Jennings, S. L. Schwartz, N. J. Balter, D. Gardner and R. J. Witorsch, Toxicol. Appl. Pharmacol., 1990, 103, 549 CrossRef CAS PubMed; (b) G. H. Y. Lin and D. J. Brusick, Mutagenesis, 1986, 1, 253 CrossRef CAS PubMed.
- H. Jung, B. Su, W. Keller, R. Mehta and A. Kinghorn, J. Agric. Food Chem., 2006, 54, 2077 CrossRef CAS PubMed.
- T. Hideo, Jpn. Tokkyo Koho, JP 56005480, 1981.
- J. P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf and R. Lacroix, Eur. J. Med. Chem., 1978, 13, 67 CAS.
- D. Shankaranarayan, C. Gopalakrishnan and L. Kameswaran, Arch. Int. Pharmacodyn. Ther., 1979, 239, 257 CAS.
- T. Hiroshi, M. Masayuki, F. Tetsuya, S. Mitsuo and H. Tsujiaki, Nucleic Acids Res., 1989, 17, 8135 CrossRef.
- T. Hiroshi, F. Tetsuya, S. Mitsuo, H. Tsujiaki, E. J. William and Z. Gerald, Tetrahedron Lett., 1988, 29, 577 CrossRef.
- (a) H. Tsujiaki, I. Masahide, M. Mitsuo and M. Katsuhiro, Eur. Pat. Appl., EP 617046 A1 19940928, 1994; (b) O. Tsuneya, T. Masaki, H. Tsujiaki and I. Masahide, Eur. Pat. Appl., EP 624372 A2 19941117, 1994.
- (a) S. M. Menchen, S. C. Benson, J. Y. L. Lam, W. Zhen, D. Sun, B. B. Rosenblum, S. H. Khan and M. Taing, U. S. Pat., US6583168, 2003; (b) A. Banerjee and A. K. Mukherjee, Stain Technol., 1981, 56, 83 CrossRef CAS PubMed.
- (a) S. K. Das, R. Singh and G. Panda, Eur. J. Org. Chem., 2009, 4757 CrossRef CAS; (b) R. Singh and G. Panda, Org. Biomol. Chem., 2010, 8, 1097 RSC.
- (a) X. F. Wu, H. Neumann and M. Beller, Chem. Rev., 2013, 113, 1 CrossRef CAS PubMed; (b) A. PintEr, A. Sud, D. Sureshkumar and M. Klussmann, Angew. Chem., Int. Ed., 2010, 49, 5004 CrossRef CAS PubMed; (c) B. Schweitzer-Chaput, A. Sud, A. PintEr, S. Dehn, P. Schulze and M. Klussmann, Angew. Chem., Int. Ed., 2013, 52, 13228 CrossRef CAS PubMed; (d) A. PintEr and M. Klussmann, Adv. Synth. Catal., 2012, 354, 701 CrossRef CAS; (e) J. M. Curto and M. C. Kozlowski, J. Am. Chem. Soc., 2015, 131, 17 Search PubMed; (f) X. Chen, C. E. Goodhue and J. Q. Yu, J. Am. Chem. Soc., 2006, 128, 12634 CrossRef CAS PubMed.
- C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed.
- (a) K. Semba and Y. Nakao, J. Am. Chem. Soc., 2014, 136, 7567 CrossRef CAS PubMed; (b) C. W. D. Gallop, M. T. Chen and O. Navarro, Org. Lett., 2014, 16, 3724 CrossRef CAS PubMed; (c) K. Semba, K. Ariyama, H. Zheng, R. Kameyama, S. Sakaki and Y. Nakao, Angew. Chem., Int. Ed., 2016, 55, 6275 CrossRef CAS PubMed.
- (a) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed; (b) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2008, 47, 3096 CrossRef CAS PubMed; (c) S. Matsumura, Y. Maeda, T. Nishimura and S. Uemura, J. Am. Chem. Soc., 2003, 125, 8862 CrossRef CAS PubMed; (d) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC.
- (a) S. K. Das, R. Singh and G. Panda, Eur. J. Org. Chem., 2009, 4757 CrossRef CAS; (b) R. Singh and G. Panda, Org. Biomol. Chem., 2010, 8, 1097 RSC.
- S. R. Sheng, T. Li, J. W. Jiang, W. He and C. S. Song, Polym. Int., 2010, 59, 1014 CrossRef CAS.
- S. J. Park, J. R. Price and M. H. Todd, J. Org. Chem., 2012, 77, 949 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1484028. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra17546e |
| This journal is © The Royal Society of Chemistry 2016 |








