Synthesis and formation mechanism of 1D hollow SiO2 nanomaterials using in situ formed 1D NaCl crystal templates†
Jingting Zhuac,
Bingbing Wang*a and
Ping Jin*ab
aResearch Center for Industrial Ceramics, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Dingxi 1295, Changning, Shanghai 200050, China. E-mail: wangbingbing@mail.sic.ac.cn; p-jin@mail.sic.ac.cn
bNational Institute of Advanced Industrial Science and Technology (AIST), Moriyama, Nagoya 463-8560, Japan
cState Key Laboratory of Silicate Materials for Architectures (Wuhan University of Technology), Wuhan, 430070, P. R. China
First published on 21st October 2015
Abstract
One dimensional (1D) hollow silica nanomaterials are, for the first time, successfully prepared using in situ formed water-soluble NaCl 1D crystals as templates. The formation mechanism of the 1D NaCl crystals and the 1D hollow SiO2 nanomaterials is systematically studied. This new approach may open up new opportunities in the synthesis of novel anisotropic nanomaterials, the construction of nanodevices, and have potential applications in drug delivery.
Recently, one dimensional (1D) nanomaterials, including nanotubes, nanobelts, and nanowires, have attracted significant research interest due to their unique mechanical, electrical, and optical properties.1,2 These intriguing properties of 1D nanomaterials enabled by the one dimensional structure allow them to be used in a variety of applications, such as high-strength nanocomposites, field-emitting surfaces, nanotransistors, biomaterial delivery tools, sensors, optical devices, energy storage devices, and catalysis materials.3–12 Among the widely reported 1D nanomaterials, 1D hollow silica nanomaterials have gained special interest arising from their distinct optical, electrical, and mechanical properties.13 Moreover, 1D hollow silica nanomaterials, possessing a large surface area, are easily combined with other nanomaterials. Also, the 1D hollow silica nanomaterials are highly biocompatible, making them promising in applications in catalysis, drug and gene delivery, and as nanosensors and templates.14
Numerous methods, such as surfactant stabilization,15–18 anodized aluminum oxide (AAO), and vapor phase deposition templating, have been developed to fabricate silica nanotubes. Surfactant stabilization methods usually require an optimized ratio between the surfactant and tetraethylorthosilicate (TEOS) to obtain size-controllable products, which is a time-consuming trying process, and requires extra washing steps to remove the surfactant due to the toxicity/contamination for further applications. The AAO template method utilizes an AAO membrane with hexagonally arranged pores as a template for the preparation of silica nanotubes.19 However, this method has a poor control over the diameter and the length of the silica nanotubes. Silica nanotubes can also be synthesized using various metal powders as initiating materials under high temperature and low pressure to grow as 1D nanostructured materials.20,21 These methods also have technical challenges in controlling the diameter of the silica nanotubes besides the high temperature and low pressure, and expensive equipment that hinders the possibility of large-scale production.
Because on the above, the template-assisted method has become an attractive and popular alternative to overcome these problems. Several 1D inorganic nanomaterials, including Ag nanowires, Au nanorods, CNT, and V3O7·H2O nanowires, have been employed as templates to synthesize silica nanotubes due to the easy removal of the templates under alkaline or acidic conditions.17,22–25 These templates can provide binding sites during the silica formation. The thickness of the silica can be controlled by the reaction time, temperature, and/or the concentration of TEOS usually used as a silica precursor. However, the template-based method still has not addressed some of the major challenges, including the handling of toxic organic chemicals or surfactants and the difficulties in controlling their shape and size. More importantly, most of these 1D templates themselves are difficult to synthesize and valuable. The one-time-use nature of these templates makes the cost issue even worse. As an alternative, ionic crystals such as NaCl, usually uniform in geometry and size, have attracted great interest due to their water-soluble and environmentally benign properties. Recently, NaCl nanocubes were used as a template for the facet-selective epitaxial growing of NaYF4 nanocages.26 In addition, hollow SiO2 cubes have been synthesized by a one-step in situ coating on NaCl nanocubes.27 However, in most cases, it remains a great challenge to obtain 1D silica nanomaterials because of the enormous difficulty in generating 1D NaCl templates.28–32
Sodium chloride is one of the most representative examples of water-soluble salts that usually appears in the form of highly regular cubes in both natural and artificial environments.28,31 Production of NaCl in other shapes and structures has been proven difficult and has seldom been achieved. To the best of our knowledge, there are no reports on the preparation of silica layer-coated NaCl nanowires (NaCl@SiO2).29,30,33
In this work, we have demonstrated a novel method for the synthesis of 1D hollow silica nanomaterials with structures by using the in situ formed 1D NaCl crystals behaving as effective sacrificial templates. Typically, a certain amount (0.1 mL, can produce 10 mg of NaCl crystals) of NaCl/glycerinum (1.7 mol NaCl dissolved in 1 L glycerinum) solution was added into 20 mL of isopropanol during agitation for 5 min. Consequently, TEOS (0.21 mL), NH3·H2O (0.14 mL) and H2O (0.14 mL) were added into the above solution in sequence. The mixture was then stored at 25 °C for 2 h to allow the reactions to take place. After that, TEOS (0.24 mL), NH3·H2O (0.1 mL), and H2O (0.45 mL) were added one by one and reacted at room temperature for another 4 h (this is the second growth process of SiO2 coating). The products were collected by centrifugation, washed with deionized water several times, and then dried at 200 °C under air atmosphere overnight. At last, the hollow silica nanobelts were obtained by using the in situ formed water-soluble NaCl nanobelts as templates, for the first time.
As shown in Fig. 1, rhombic dodecahedron (RD)-NaCl crystals and RD-NaCl@SiO2 (Fig. 1b) of about 2 μm in length (Fig. 1a) were firstly synthesized according to our previous report.30 After the second SiO2 coating process, NaCl@SiO2 nanobelts and hollow RD-SiO2 particle hybrid materials (see Fig. 1c) were obtained. The inset in Fig. 1c proves the hollow structure of the RD-SiO2 particles. Based on the apparent density difference between the NaCl@SiO2 nanobelts and the hollow RD-SiO2 particles, the NaCl@SiO2 nanobelts can be easily separated from the hollow RD-SiO2 particles through centrifugation. Fig. 1d shows typical low-magnification scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (the upper inset in Fig. 1d) images of the pure NaCl@SiO2 nanobelts (with a productivity of >95%) and NaCl@SiO2 nanowires. The images reveal that the NaCl@SiO2 nanobelts are 500–800 nm in width 8–15 μm in length, and 20–80 nm in thickness (see Fig. S3†). The EDS image (inset in Fig. 1d) and XRD pattern (Fig. 1f) verify the structure of the NaCl@SiO2 nanobelts. After the NaCl templates were completely removed by water, the hollow SiO2 nanobelts retained excellent morphology integrity (Fig. 1e). Fig. S1a† shows the nitrogen adsorption–desorption isotherms. The as-synthesized samples gave only one sharp adsorption–desorption loop near P/P0 = 1. The Barrett–Joyner–Halenda (BJH) pore diameter was estimated to be 3.08 nm in the samples. The Brunauer–Emmett–Teller (BET) surface area was determined to be 19.2 m2 g−1 and the volume of the mesopores in the samples was about 34.1 mm3 g−1.
To study the growing mechanism of the hollow SiO2 nanobelts, we investigated the influence of water and TEOS amount on the formation of NaCl@SiO2 nanobelts. First, the role of deionized water was investigated. The RD-NaCl@SiO2 particles of about 2 μm in size were prepared (see Fig. 1b and S2a†). Subsequently, a fixed amount of TEOS (0.15 mL), ammonia (0.1 mL) and various amounts of deionized water (0.05, 0.25, 0.45 to 0.65 mL) were introduced to trigger the growth of the silica cell and the dissolving of the NaCl template (see Table S1†). Only a small amount of 1D NaCl@SiO2 nanomaterials containing both nanobelts (major product) and nanowires (minor product) was obtained when the amount of water added was 0.05 mL (see Fig. S2b and S3†). The final products contained hollow RD-SiO2 particles, RD-NaCl@SiO2 particles, and yolk–shell structured RD-NaCl@SiO2 particles (see Fig. S2f†). This indicates that the RD-NaCl crystals encapsulated in the RD-SiO2 shells were only partially resolved due to the insufficient supply of water. In addition, the dissolved NaCl solution recrystallized into NaCl nanobelt templates, which acted as templates again and coated by SiO2 shells. Interestingly, the productivity of the hollow SiO2 nanobelts increased from 2 to 6 to 10 mg when the amount of water increased from 0.05, to 0.25, to 0.45 mL, respectively (see Fig. S2c and d†). The yield of hollow SiO2 nanobelts will reach the highest value at a certain water amount. When the amount of deionized water was increased to 0.65 mL, pure hollow RD-SiO2 particles were prepared with no appearance of NaCl@SiO2 nanobelts (see Fig. S2e†). At this condition, RD-NaCl crystals encapsulated in RD-SiO2 shells were completely dissolved into solution, and the released NaCl did not recrystallize again into NaCl nanobelts. This may be due to the abundance of water in the reaction system, which allows all of the NaCl to be dissolved in water and in turn, the formation requirement (e.g., oversaturation) of NaCl crystals can not be satisfied. The above phenomenon demonstrates that NaCl nanobelt templates are formed through recrystallization of the released NaCl from the NaCl@SiO2 structures in an isopropanol-rich solution. Moreover, we also found that the maximum productivity of hollow SiO2 nanobelts is obtained when an optimized amount of deionized water present in the reaction solution.
The effect of TEOS amount on the synthesis of NaCl@SiO2 nanobelts could be deduced from Fig. S4.† Various amounts of TEOS (0.21, 0.24, and 0.27 mL) were added into the system (see in Table S1†) when the amounts of deionized water and ammonia added were constant at 0.45 mL and 0.1 mL, respectively. Under these conditions, the amount of the secondary recrystallized NaCl nanobelt templates is fixed. As the amount of TEOS increased, the yield the hollow SiO2 nanobelts was increased from 14 to 19 and to 21 mg (see Fig. S4a and b). This suggests that not all of the recrystallized NaCl nanobelt templates are coated by SiO2 shells when the adding amount of TEOS is insufficient. Those recrystallized NaCl nanobelt templates without the protection of the silica shell were resolved into water phase again. The SiO2 shells coated on the NaCl nanobelt templates can efficiently protect the NaCl nanobelt templates from being re-dissolved into solution. When the amount of TEOS was increased to 0.27 mL, the yield of hollow SiO2 nanobelts did not increase apparently. The morphology of the obtained hollow SiO2 nanobelts under this condition was very poor (see Fig. S4c†). There are many SiO2 particles attached on the hollow SiO2 nanobelts. Based on the above results, the maximum yield of hollow SiO2 nanobelts can be obtained only when the amount of deionized water and TEOS added are exactly appropriate.
Interestingly, among the NaCl@SiO2 nanobelts and hollow RD-SiO2 particle hybrid materials, numerous ultrafine NaCl@SiO2 nanowires with a diameter of about 20–30 nm was observed (Fig. S5†). In order to obtain SiO2 nanotubes with a diameter of less than 100 nm, the size of each RD-silica shell with around 2 μm should be decreased to the nanometer range.
For further confirmation, NaCl@SiO2 small cubic composites of about 150 nm in size were prepared (Fig. 2a and b). And then, TEOS (0.4 mL), NH3·H2O (0.1 mL), and H2O (0.45 mL) were added step by step and reacted at room temperature for another 4 h. Then the products were collected by centrifugation, washed with deionized water several times. After dissolving NaCl templates, SiO2 nanotubes with a diameter of about 60–100 nm were obtained (Fig. 3, S6e and f†).
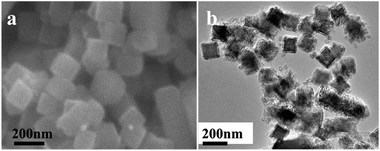 | ||
| Fig. 2 (a) Typical SEM image of the rhombic dodecahedron NaCl cubic crystals with size of about 150 nm and (b) TEM image of the NaCl@SiO2 cubic composites. | ||
Fig. 3a shows a typical SEM image of the as-synthesized NaCl@SiO2 nanowires, which demonstrates that the nanowires are very uniform with diameters predominantly in the range of 60–100 nm and lengths of about 10 μm. TEM images (Fig. 3b and c) confirm the morphology and the size of the as-prepared NaCl@SiO2 nanowires. The SAED pattern (the upper inset in Fig. 3c) suggests the single crystal nature of face centered cubic structured NaCl. The EDS image (the lower inset in Fig. 3c) confirms the elementary composition of the NaCl@SiO2 nanowires. The XRD pattern (Fig. S7†) further confirms the elementary composition of the NaCl@SiO2 nanowires and SiO2 nanotubes after washing the NaCl template. After removal of the NaCl templates, the hollow SiO2 nanotubes main the original morphology (Fig. 3d). The TEM images (Fig. 3e and f) confirm that the SiO2 nanotubes have a uniform shell thickness. Also, the diameter of the NaCl nanowire templates is about 40–60 nm. The as-synthesized samples have only one sharp adsorption–desorption loop near P/P0 = 1. The BJH pore diameter is determined to be 3.28 nm in the samples (Fig. S1b†). The BET surface area is about 23.2 m2 g−1 and the volume of the mesopores in the samples is around 29.1 mm3 g−1.
Based on the microstructure evolution process, the formation process of the 1D hollow SiO2 nanomaterials is schematically illustrated in Scheme 1. The process can be divided into four steps. (1) The NaCl (cubic or RD) crystals were encapsulated into SiO2 shells. (2) Subsequently, when an appropriate amount of water was added into the system, the NaCl (cubic or RD) crystals were dissolved into solution from the SiO2 (cubic or RD) shells. Then, the bulk NaCl solution was separated into many 1D NaCl solution by the SiO2 (cubic or RD) shells and outflowed. At the same time, the 1D NaCl solution was recrystallized into 1D NaCl crystals in the isopropanol-rich solution due to the oversaturation. (3) The hydrolyzed TEOS adsorbed onto the surface of the in situ formed NaCl 1D crystals and formed SiO2 layers after a period of reaction. (4) 1D hollow SiO2 nanomaterials were obtained through centrifugation and washing with deionized water several times. These methods were used to separate the 1D hollow SiO2 nanomaterials from the hollow SiO2 particles and remove the NaCl crystal templates.
 | ||
| Scheme 1 Schematic illustration of the formation process of 1D hollow silica nanomaterials using the in situ formed 1D NaCl crystals. | ||
1D hollow silica nanomaterials with different morphologies, such as nanowires and nanobelts, were successfully fabricated in solution by using the in situ formed 1D NaCl crystals as templates. The 1D hollow silica nanomaterials possess a good biocompatibility, as well as can be easily combined with other nanomaterials. These characteristics endow these structures to be used as building blocks for nanofluidic devices, high-quality nanoreactors for catalysis and confined synthesis, and well-defined containers for drug delivery.3–12 Furthermore, this work provided insights into the crystal formation of 1D NaCl crystals and the growing mechanism of 1D hollow SiO2 nanomaterials. Because of the ubiquitous and environmentally friendly nature of the NaCl template here used, this method is expected to become a green alternative to the conventional hard or soft template-based methods in the fabrication of diverse 1D hollow multi-functional materials, e.g., TiO2, ZrO2, and SnO2.
Acknowledgements
This study was financially supported by the high-tech project of MOST (2012BAA10B03, 2014AA032802), the national sci-tech support plan the National Natural Science Foundation of China (NSFC, No. 51272273, 51172265, 51372264), and the Science and Technology Commission of Shanghai Municipality (STCSM, No. 13PJ1409000, 13NM1402200).Notes and references
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Science, 2002, 297, 787–792 CrossRef CAS PubMed.
- S. Gupta, Q. Zhang, T. Emrick and T. P. Russell, Nano Lett., 2006, 6, 2066 CrossRef CAS PubMed.
- S. Guo, J. Li, W. Ren, D. Wen, S. Dong and E. Wang, Chem. Mater., 2009, 21, 2247 CrossRef CAS.
- Q. Ji, R. Iwaura, M. Kogiso, J. H. Jung and T. Shimizu, Chem. Mater., 2004, 16, 250 CrossRef CAS.
- Q. Ji, R. Iwaura, M. Kogiso, J. H. Jung, K. Yoshida and T. Shimizu, Chem. Mater., 2004, 16, 250 CrossRef CAS.
- B. C. Satishkumar, S. K. Doorn, G. A. Baker and A. M. Dattelbaum, ACS Nano, 2008, 2, 2283 CrossRef CAS PubMed.
- R. S. Norman, J. W. Stone, A. Gole, C. J. Murphy and T. L. Sabo-Attwood, Nano Lett., 2008, 8, 302 CrossRef CAS PubMed.
- B. Sun and H. Sirringhaus, J. Am. Chem. Soc., 2006, 128, 16231 CrossRef CAS PubMed.
- S. Kim, S. K. Kim and S. Park, J. Am. Chem. Soc., 2009, 131, 8380 CrossRef CAS PubMed.
- M. S. Fuhrer, B. M. Kim, T. Dürkop and T. Brintlinger, Nano Lett., 2002, 2, 755 CrossRef CAS.
- T. Fukui, H. Atomi, T. Kanai, R. Matsumi, S. Fujiwara and T. Imanaka, Genome Res., 2005, 15, 352–363 CrossRef CAS PubMed.
- P. X. Huang, F. Wu, B. L. Zhu, X. P. Gao, H. Y. Zhu, T. Y. Yan and W. P. Huang, J. Phys. Chem. B, 2005, 109, 19169 CrossRef CAS PubMed.
- L. P. Dávila, V. J. Leppert and E. M. Bringa, Scr. Mater., 2009, 60, 843–846 CrossRef PubMed.
- J. A. Dykens and Y. Will, Drug discovery today, 2007, 12, 777–785 CrossRef CAS PubMed.
- H. Makoto and A. Motonari, Adv. Mater., 2000, 12, 839 CrossRef.
- E. A. Whitsitt and A. R. Barron, Nano Lett., 2003, 3, 775 CrossRef CAS.
- K. G. Lee, R. Wi, M. Imran, T. J. Park, J. Lee, S. Y. Lee and D. H. Kim, ACS Nano, 2010, 3933 CrossRef CAS PubMed.
- Y. Yu, H. Qiu, X. Wu, H. Li, Y. Li, Y. Sakamoto, Y. Inoue, K. Sakamoto, O. Terasaki and S. Che, Adv. Funct. Mater., 2008, 18, 541–550 CrossRef CAS PubMed.
- C. Chen and Y. Wu, Adv. Mater., 2005, 17, 404 CrossRef CAS PubMed.
- J. Hu and S. Lee, Adv. Mater., 2003, 15, 70 CrossRef CAS PubMed.
- Y. Li and Y. Bando, Adv. Mater., 2004, 16, 37 CrossRef CAS PubMed.
- S. O. Obare, N. R. Jana and C. J. Murphy, Nano Lett., 2001, 1, 601 CrossRef CAS.
- Y. Yin, Y. Lu, Y. Sun and Y. Xia, Nano Lett., 2002, 2, 427 CrossRef CAS.
- J. Zygmunt and R. Nesper, Adv. Mater., 2003, 15, 1538 CrossRef CAS PubMed.
- C. Gao, Z. Lu and Y. Yin, Langmuir, 2011, 27, 12201–12208 CrossRef CAS PubMed.
- F. Wang, L. D. Sun, J. Gu, Y. F. Wang, W. Feng, Y. Yang, J. Wang and C. H. Yan, Angew. Chem., Int. Ed., 2012, 51, 8796–8799 CrossRef CAS PubMed.
- X. M. Jiang and C. J. Brinker, J. Am. Chem. Soc., 2006, 128, 4512–4513 CrossRef CAS PubMed.
- J. Zhang, S. Zhang, Z. Wang, Z. Zhang and S. Wang, Angew. Chem., Int. Ed., 2011, 50, 6044–6047 CrossRef CAS PubMed.
- K. Y. Suh, A. Khademhosseini, G. Eng and R. Langer, Langmuir, 2004, 20, 6080 CrossRef CAS PubMed.
- B. Wang, P. Jin and Y. Yue, RSC Adv, 2015, 5, 5072–5076 RSC.
- Y.-C. Pu, J. R. Hwu, W.-C. Su, D.-B. Shieh, Y. Tzeng and C.-S. Yeh, J. Am. Chem. Soc., 2006, 128, 11606 CrossRef CAS PubMed.
- H. X. Lin, Z. C. Lei, Z. Y. Jiang, C. P. Hou, D. Y. Liu, M. M. Xu, Z. Q. Tian and Z. X. Xie, J. Am. Chem. Soc., 2013, 135, 9311–9314 CrossRef CAS PubMed.
- X. M. Jiang and C. J. Brinker, J. Am. Chem. Soc., 2006, 128, 4512 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18744c |
| This journal is © The Royal Society of Chemistry 2015 |


