Arginyl-glutamine dipeptide attenuates experimental colitis by enhancing antioxidant function and inhibiting nuclear factor-kappaB†
Hua Yu,
Mingjun Dong,
Yidong Xu,
Ning He,
Xiaoyu Dai* and
Keqiang Li*
Department of Analogy, Ningbo No. 2 Hospital, Ningbo 315010, China
First published on 15th October 2015
Abstract
This study aimed to investigate the effect and underlying mechanism of Arginyl-glutamine (AG) dipeptide on dextran sulfate sodium (DSS)-induced colitis by in vivo and in vitro models. Acute colitis was induced in ICR mice by administering 5% DSS. 1% AG and 0.5% glutamine and 0.5% arginine were fed via mixing in the basic diet after colitis induction. The results showed that AG could reverse the diverse effects on average daily weight gain, colon weight, rectal bleeding score, diarrhea score, and histological score caused by DSS. Meanwhile, dietary AG supplementation attenuated DSS-induced oxidative stress and improved the antioxidant system in mice. Although AG failed to improve intestinal dysfunction, inflammatory response was markedly reduced in DSS-induced colitis after AG treatment. Ultimately, AG inhibited IKK phosphorylation and subsequent nuclear factor-kappaB (NF-κB) activity and its translocation into the nucleus. From the in vitro study, AG was found to improve cell viability, antioxidant function, and monolayer permeability and reduce inflammatory response in DSS-challenged Caco-2 cells. In conclusion, AG protected mice against DSS-induced colitis via increasing the antioxidant system and down-regulating proinflammatory cytokines. The inhibition of NF-κB activation might be part of the mechanism underlying the effects of AG on the colitis model.
Introduction
Inflammatory bowel disease (IBD) is a chronic and inflammatory disorder primarily involving the mucosa and submucosa of the gastrointestinal tract and has become increasingly prevalent all over the world.1 Patients with IBD exhibit neutrophil accumulation, gastrointestinal inflammation with villus atrophy, loss of crypts, and these are accompanied by diarrhea, blood in stools and weight loss.2,3 Recent reports have demonstrated that oxidative stress and inflammation correlates with occurrence of IBD.4,5 Oxidative stress correlates with protein, lipid and DNA oxidative injury and therefore is thought to be involved in the development of many diseases or may exacerbate their symptoms, including inflammation.5Arginyl-glutamine (AG) dipeptide is an aqueous stable source of glutamine with greater solubility compared with each individual amino acid.6 Li et al. reported that AG serves as a protective role in the neonatal intestine against oxygen-induced injury by reducing inflammation and apoptosis.7 Meanwhile, glutamine or arginine containing dipeptides also have been demonstrated to exhibit an antioxidant function.8,9 However, the effects of supplementation of the AG dipeptide on IBD are unknown. We hypothesized that AG dipeptide would protect mice and Caco-2 cells against DSS exposure by increasing antioxidant function and inhibiting nuclear factor-kappaB (NF-κB) signaling pathway.
Material and methods
Animal model and groups
Thirty two male ICR mice weighting 22–24 g were used in the experiment. Mice were divided into four groups: a control group (n = 8), a DSS-treated group (DSS, n = 8), an arginine + glutamine + DSS-treated group (arg + gln, n = 8), and an AG + DSS-treated group (n = 8). In the control group, each mouse was allowed free access to tap water for 7 d. In other three groups, each mouse was allowed free access to 5% DSS solution (KAYON Bio. Technology Co. Ltd), supplied as drinking water, for 7 d. In the arg + gln group, each mouse was fed with 0.1% arginine and 0.1% glutamine mixed in the basic diet. Mice in AG group were fed 0.2% AG mixed in basic diet, AG (98%) was obtained from DgPettides Co., Ltd., Hangzhou, China. The dosage of dipeptide used in this study was according to previous report.10 All mice were housed in polycarbonate cages in a room with controlled temperature (25 ± 3 °C), humidity (50 ± 5%) and a 12 hour cycle of light and dark. They were allowed free access to laboratory strip chows throughout the experimental period. This study was conducted according to the guidelines of the declaration of Helsinki and all procedures involving animal subjects were approved by the Animal Welfare Committee of the Ningbo No. 2 Hospital.After the experimental period, each animal was weighted to calculate average weight gain and then each mouse was sacrificed and colon length and weight were measured. In addition, colon tissues from each mice were harvested and immediately frozen in liquid nitrogen and stored at −70 °C for subsequent gene expression and western blotting analyses.
Clinical evaluation of DSS colitis
Rectal bleeding and diarrhea were monitored daily. The appearance of blood in the stool was measured by haemoccult tests (Beckman Coulter), and was given a score from 0 to 4, defined as follows: 0 for no blood; 2 for positive haemoccult; and 4 for gross bleeding. The severity of diarrhea was given a score from 0 to 4, defined as follows: 0 for well-formed pellets; 2 for pasty and semiformed stools; and 4 for liquid stools.11 All clinical scorings were performed in a blinded fashion.Histomorphometry determination
The morphological evaluation after DSS treatment was used haematoxylin and eosin (HE) staining according to previous report.12 Briefly, one piece of each colon samples (0.5 cm) was kept in 4% neutral buffered 10% formalin, processed using routine histological methods and mounted in paraffin blocks. Six-micrometer-thick sections were cut and stained with haematoxylin and eosin (HE). All specimens were examined under a light microscope (Nikon, Japan).The histological examination was performed in a blinded fashion using a scoring system previously validated and described.13 Three independent parameters were measured: severity of inflammation (0–3: none, slight, moderate, severe), depth of injury (0–3: none, mucosal, mucosal and submucosal, transmural), crypt damage (0–4: none, basal 1/3 damaged, basal 2/3 damaged, only surface epithelium intact, entire crypt and epithelium lost) and percentage of the involved area (0–4: 0%, 1–10%, 10–25%, 25–50%, 50–100%). All scores on the individual parameters together could result in a total score ranging from 0 to 14.
Serum oxidative indexes, amino acid profiles, and NF-κB activity
Serum samples were separated from blood by centrifugation at 3500 × g for 15 min. Serum malondialdehyde (MDA), glutathione (GSH), 8-hydroxyguanosine (8-OHG) concentrations, superoxide dismutase (SOD), total antioxidant capability (T-AOC), and catalase (CAT) activity were measured using an assay kit in accordance with the manufacturer's instructions (Nanjing Jiancheng, China).12 Serum arginine and glutamine were determined by LC-MS/MS (HPLC Ultimate 3000 and 3200 QTRAP LC-MS/MS) using standards from Sigma Chemicals (St. Louis, MO, USA). NF-κB activity in colonic tissue and Caco-2 cells was measured according to by an enzyme-linked immunosorbent assay (ELISA) kits (Cell Biolabs, USA). Serum lipopolysaccharide (LPS) and diamine oxidase (DAO) activity were determined according to previous report.14Cell culture and treatment
Human colorectal adenocarcinoma-derived intestinal epithelial cells (Caco-2) (ATCC, Manassas, VA) were grown in DMEM/F12 supplemented with 1 mM sodium pyruvate, 20% FBS (HyClone, Logan, UT), and 50 U mL−1 penicillin–streptomycin. Cells were with treated with 2% DSS for 4 days to induce inflammation according to previous report.15 Cell viability was evaluated with the MTT assay (Sigma-Aldrich) according to the manufacturer's instructions. Briefly, 8 × 103 cells were seeded in 96-well plates. The following day, cells were incubated with 0.1, 0.2, 0.5, 1, 2, 5 mM AG for 2 days and then assayed.Trans-epithelial electrical resistance (TEER) measurements
A 12-well Transwell system was used for this assay as described previously.16 Briefly, Caco-2 cells were seeded in the apical chamber and the changes of TEER were measured with an epithelial voltohmmeter ERS-2 (Merck Millipore, USA). About 3 weeks after confluence when the filter-grown Caco-2 monolayers reached epithelial resistance of at least 500 Ω cm2, the cells were incubated in different reagents as indicated. Electrical resistance was measured until similar values were recorded on three consecutive measurements. Values were corrected for background resistance due to the membrane insert and calculated as Ω cm2.Paracellular marker FD-4 (FITC-dextran 4 kDa) flux measurements
Paracellular permeability was assessed following a previously mentioned method.16 Caco-2 monolayers were treated as described above. After treatment, cells were rinsed with PBS and incubated in the upper chamber with Hank's balanced salt solution containing 1 mg mL−1 FD-4 solution for 2 h. FD-4 flux was assessed by taking 100 μL from the basolateral chamber. Fluorescent signal was measured with Synergy H2 microplate reader (Biotek Instruments, USA) using 492 nm excitation and 520 nm emission filters. FD-4 concentrations were determined using standard curves generated by serial dilution of FD-4.Measurement of intracellular ROS levels
Generation of intracellular ROS was assessed using MitoSOX (Invitrogen) and CM-H2DCFDA (Invitrogen). Caco-2 cells were incubated with 5 μM MitoSOX and CM-H2DCFDA at 37 °C for 15 min, washed with PBS, and then analyzed with a flow cytometer (BD Biosciences).17cDNA synthesis and quantification mRNA by real-time PCR analysis
Total RNA was isolated from liquid nitrogen pulverized tissues with TRIZOL regent (Invitrogen, USA) and then treated with DNase I (Invitrogen, USA) according to the manufacturer's instructions. Synthesis of the first strand (cDNA) was performed with oligo (dT) 20 and superscript II reverse transcriptase (Invitrogen, USA).Primers were designed with Primer 5.0 according to the gene sequence of mouse (http://www.ncbi.nlm.nih.gov/pubmed/) to produce an amplification product. The primer sets used were shown at ESI Table S1.† Real-time PCR was performed according to previous studies.18,19 Relative expression was normalized and expressed as a ratio to the expression in control group. Therefore, relative expression of target genes in control group was 1.0. Relative gene expressions represented the comparison vs. control group and reported as a fold change from the control value.
Nuclear proteins extraction and western bolt analysis
Colon nuclear proteins were extracted with nuclear and cytoplasmic extraction reagents in accordance with the manufacturer's instructions (Thermo Fisher Scientific Inc., USA).Proteins from each sample (50 μg) were separated by SDS–polyacrylamide gel electrophoresis and electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (BioRad, Hercules, CA, USA). Membranes were blocked in 7% evaporated milk, diluted in tris-buffered saline containing 0.1% Tween 20 (TBS-226 T) at room temperature for at least 2 h, and then incubated overnight at 4 °C with one of the following primary antibodies: occludin, claudin1, ZO1, NF-κB, IKK, p-IKK, CAT, and SOD (Abcam, Inc., USA). Mouse β-actin antibody (Sigma) was used for total and cytoplasmic protein loading control. Rabbit PCNA antibody (Sigma) was used for nuclear protein loading control. After primary antibody incubation, membranes were washed with TBS-T and incubated with alkaline phosphatase-conjugated anti-mouse or anti-rabbit IgG antibodies (Promega, Madison, WI, USA) for 2 h at room temperature. Membranes were washed with TBS-T followed by three washes with TBS; signals were detected by the addition of 5-bromo-4-chloro-3-238 indolylphosphate/nitroblue tetrazolium (BCIP/NBT) solution (Sigma), then quantified and digitally analyzed using the image J program (NIH). The intensity of each band was measured and subtracted from the background. The expression ratio of target proteins was normalized against β-actin.20
Statistical analysis
All statistical analyses were performed using SPSS 17.0 software. Group comparisons were performed using the one-way analysis of variance (ANOVA) to test homogeneity of variances via Levene's test and followed with Tukey's multiple comparison test. Two groups were analyzed via students' T test. Data are expressed as the mean ± standard error of the mean. Values in the same row with different superscripts are significant (P < 0.05), while values with same superscripts are not significant different (P > 0.05).Results
Effects of AG on clinical indexes in DSS-induced colitis
As shown in Fig. 1, DSS treatment significantly decreased final body weight, average daily weight gain, and colon length, and increased colon weight, rectal bleeding score, diarrhea score, and histological score (P < 0.05). Compared with DSS group, dietary supplementation with AG significantly reversed the diverse effects on average daily weight gain, colon weight, rectal bleeding score, diarrhea score, and histological score (P < 0.05), suggesting a protective role of AG in DSS-induced colitis. Meanwhile, co-dietary arginine and glutamine also exhibited a beneficial role in rectal bleeding score, diarrhea score, and histological score (P < 0.05) compared with DSS group (P < 0.05), but failed to affect average daily weight gain and colon weight (P > 0.05). So we next determined serum arginine and glutamine concentrations after challenging DSS, the result showed that dietary AG markedly increased serum arginine and glutamine concentrations compared with DSS group (P < 0.05). Although the difference between AG group and arg + gln group was insignificant, the concentrations of arginine and glutamine in AG group was much higher (P > 0.05).Based on the Caco-2 cell viability treated with variety of concentration of AG (0.01–5 mM) (Fig. 2A), we chose AG concentration at 0.5 mM for the following experiments. We found that DSS treatment significantly reduced Caco-2 cell viability (P < 0.01), while supplementation with AG obviously improved the cell viability compared with the DSS group (P < 0.05) (Fig. 2B).
Effects of AG on oxidative stress in DSS-induced colitis
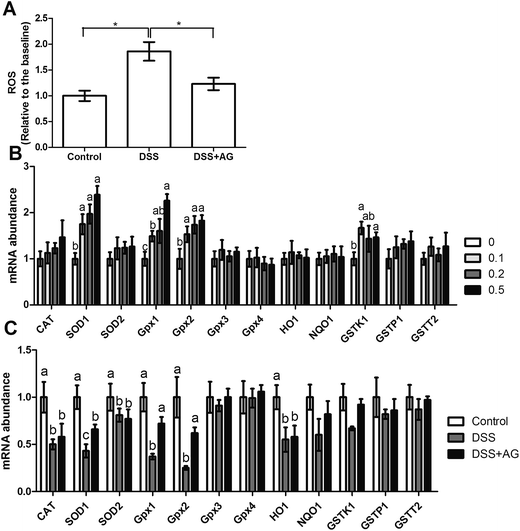
Previous reports have indicated that oxidative stress involves in DSS-induced colitis, so we determined several oxidative indexes in the present study (Fig. 3). The results showed that DSS treatment induced oxidative stress in mice evidenced by the increased MDA and 8-OHG and reduced GSH, T-AOC, and SOD activities (P < 0.05). Dietary supplementation with AG significantly reversed the dysfunction of MDA, 8-OHG, T-AOC, and SOD (P < 0.05). Meanwhile, we further tested antioxidant enzymes SOD and CAT abundances in the colon via western blot (Fig. 3G–I). The results exhibited that AG markedly reversed the inhibitory effect of DSS on SOD expression (P < 0.05), but failed to affect CAT abundance.In vitro model, DSS treatment significantly increased ROS generation in Caco-2 cells and AG exhibited antioxidant function via reducing ROS concentration (P < 0.05) (Fig. 4A). We further preformed RT-PCR to investigate the role of AG and DSS on antioxidant genes (Fig. 4A and B), the results showed that AG treatment enhanced SOD1, Gpx1, Gpx2, and GSTK1 mRNA abundances (P < 0.05), indicating an antioxidant function. DSS exposure markedly down-regulated CAT, SOD1, SOD2, Gpx1, Gpx2, and HO1 expressions (P < 0.05) and AG alleviated DSS-induced SOD1, Gpx1, and Gpx2 down-regulation (P < 0.05).
Effects of AG on intestinal permeability and tight junctions in DSS-induced colitis
Serum LPS level and DAO activity were two maker for intestinal injury. The results exhibited that DSS treatment significantly enhanced serum LPS concentration and reduced DAO activity (P < 0.05) (Fig. 5A and B), indicating that DSS exposure increased intestinal permeability. Tight junctions mainly contribute to intestinal integrety. We also determined several tight junctions (i.e. ZO-1, occludin, claudin1) expression via western blot (Fig. 5C and F). Expressions of ZO-1, occludin, and claudin1 were inhibited after exposure to DSS (P < 0.05). However, dietary supplementation with AG failed to attenuate DSS induced intestinal injury.Both TEER and FD-4 flux were tested to evaluate monolayer permeability in vitro model. After being exposed to DSS for 48 h, the TEER of Caco-2 monolayers dropped to more or less 50% relative to the baseline (P < 0.01) (Fig. 5D). Consistent with the changes of TEER mentioned above, the flux of FD-4 of the monolayers challenged with DSS for 48 h was about 4 times higher relative to the baseline (P < 0.01) (Fig. 5E). These results further demonstrated that DSS induced significant injuries of the intestinal epithelial barrier function. To test the protective effect of AG, AG was added simultaneously with DSS to Caco-2 monolayers for 48 h. As shown in Fig. 5, AG ameliorated both the drop of TEER and the increase of FD-4 flux induced by DSS (P < 0.05).
Effects of AG on inflammatory cytokines expression in DSS-induced colitis
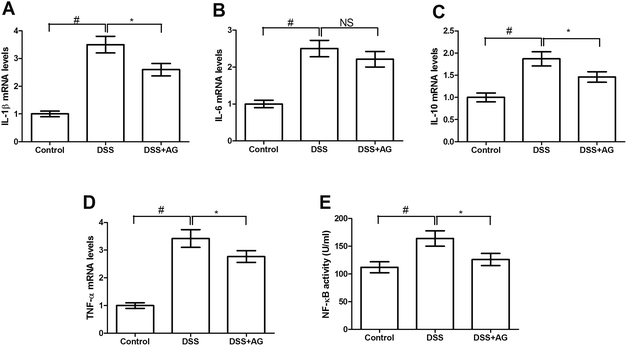
mRNA abundances of cytokines (i.e. IL-1β, IL-6, IL-10, and TNF-α) were determined via RT-PCR in the colon (Fig. 6A–D) and Caco-2 cells (Fig. 7A–D). DSS treatment significantly enhanced colonic IL-1β, IL-6, IL-10, and TNF-α expressions (P < 0.05) in mice, indicating an inflammatory response after challenging DSS. Dietary supplementation with AG markedly down-regulated IL-1β and TNF-α expression compared with DSS group (P < 0.05).Consistent with the changes of cytokines in mice, DSS exposure also markedly up-regulated IL-1β, IL-6, IL-10, and TNF-α expressions (P < 0.05) in Caco-2 cells (Fig. 7A–D). Compared with DSS treated group, AG reduced IL-1β, IL-10, and TNF-α mRNA abundances (P < 0.05), suggesting an anti-inflammatory function in vitro.
Effects of AG on NF-κB signaling pathway in DSS-induced colitis
As shown in Fig. 6, the ELISA result showed that NF-κB activity in DSS group was significant higher (P < 0.05) than other three groups and the western blotting data demonstrated that nuclear NF-κB abundance was increased after exposure to DSS (P < 0.05), while dietary supplementation with AG significantly reduced nuclear NF-κB abundance in the colon (P < 0.05). We further determined colonic IKK, an upstream protein, and p-IKK abundances and found that DSS treatment enhanced colonic IKK phosphorylation (P < 0.05). Dietary AG inhibited IKK phosphorylation (P < 0.05) compared with DSS group, which may mediate NF-κB activation. Meanwhile, we validated the effect of AG on NF-κB signaling pathway in Caco-2 cells and the result showed that AG significantly inhibited NF-κB activity (P < 0.05), which was activated by DSS exposure (Fig. 7E).Discussion
Compelling evidences have demonstrated that DSS treatment can induce diarrhea, blood in stool, weight loss and gastrointestinal inflammation with villus atrophy, loss of crypts and ulceration, which are the main features of IBD.3,21 Thus, DSS-induced colitis in mice was widely applied as a preclinical IBD model. In the present study, DSS treatment significantly affected final body weight, average daily weight gain, colon length, colon weight, rectal bleeding score, diarrhea score, and histological score in mice. Ren et al. demonstrated that dietary arginine (0.4%, 0.8%, and 1.5%) or glutamine (0.5%, 1.0% and 2.0%) supplementation could be a potential therapy for intestinal inflammatory diseases.22 In the present study, we firstly reported dietary supplementation with AG reversed the diverse effects on average daily weight gain, colon weight, rectal bleeding score, diarrhea score, and histological score, suggesting a protective role of AG in DSS-induced colitis.We also compared the beneficial effects of AG with co-dietary arginine and glutamine, and the results showed that both treatments exhibited a positive effect on DSS induced IBD and the beneficial effect of dietary AG was higher compared with co-dietary arginine and glutamine. Previous report suggested that the transport of amino acids in the form of peptides is more effective than amino acids in the free form per unit of time.23 So we speculated that the absorption efficiency contributed to the difference between AG group and arg + gln group, which was confirmed by the higher serum arginine and glutamine concentrations after dietary AG.
As inflammation is intimately associated with reactive oxygen and nitrogen species (ROS/RNS), oxidative stress has been proposed as a mechanism underlying the pathophysiology of IBD.24,25 Some reports suggested that novel anti-oxidative agents may become effective and less-toxic alternatives in IBD and colitis-associated colorectal cancer treatment.26 Compelling evidences have demonstrated that both arginine and glutamine exhibit anti-oxidative function.27–29 Meanwhile, Cruzat et al. reported that oral dipeptide forms of glutamine supplementation attenuated endotoxemia induced oxidative stress in mice via reestablishing GSH content, intracellular redox status, and tissue lipoperoxidation concentration in muscle and liver.9 In the present study, we found that dietary AG significantly reversed DSS induced oxidative stress and improved antioxidant system in vivo and vitro models.
Previous studies have indicated that DSS treatment induces intestinal injury,30,31 we also found that DSS down-regulated tight junctions expression and increased intestinal permeability. Altered expression of tight junctions-related proteins has been considered to be consistent with a loss of epithelial tightness, and provides a molecular mechanism for the enhanced epithelial permeability observed in inflammatory conditions of the gut,32,33 which can act as a trigger for the development of intestinal and systemic diseases. Beutheu et al. reported that arginine and glutamine restored trans-epithelial electrical resistance and prevented methotrexate-induced barrier disruption in Caco-2 cells.34 Although we failed to notice any significant beneficial effect of AG on the DSS induced intestinal tight junction dysfunction in mice, AG markedly ameliorated both the drop of TEER and the increase of FD-4 flux induced by DSS in Caco-2 monolayers.
Compelling investigations in humans and animal models have indicated that production of inflammatory cytokines and inflammation response in the gastrointestinal tract play a vital role in the progress of IBD.4,35,36 In the present study, we found that DSS upregulated IL-1β, IL-6, IL-10, and TNF-α expressions in vivo and in vitro, while dietary AG down-regulated IL-1β, IL-10, and TNF-α expressions, indicating a potential effect in DSS induced inflammation. Similarly, Chu et al. reported that pretreatment with alanyl-glutamine dipeptide also attenuated DSS induced inflammatory response via suppressing cytokine expressions inflammatory mediator production.8 Meanwhile, we also noticed that co-dietary arginine and glutamine reversed IL-1β in DSS induced colitis. Therefore, we speculated that the beneficial function of AG may originate from its effective absorption and metabolism to arginine and glutamine.
Compelling evidences have demonstrated that NF-κB activation involves in the pathological mechanism of IBD and its inhibitors have been widely investigated to ameliorate inflammatory diseases.37,38 Present data suggested that dietary AG markedly reversed colonic NF-κB activity and NF-κB translocation into nucleus caused by DSS treatment. NF-κB activation requires IKK subunits,5 so we determined IKK and p-IKK expression and found that dietary AG significantly reduced IKK phosphorylation. Inhibition of the NF-κB pathway decreases the protein overexpression of the downstream inflammatory mediators TNF-α and IL-6 in IBD mouse model.39 Sunil reported that NF-κB inhibitor, pyrrolidine dithiocarbamate, down-regulates chemokine expressions and may be of use in IBD therapy.40
Conclusion
The present study provides in vivo and in vitro evidence that supplementation with AG improves DSS exposure in mice and Caco-2 cells. Meanwhile, the beneficial role of AG may associate with improving antioxidant system and inhibiting NF-κB signaling pathway. The substantial effect of AG on intestinal inflammatory signaling pathways suggests the potential of dipeptides for broad applicability in other diseases associated with inflammation and oxidative stress.Author contributions
X. D. and K. L. conceived and designed the experiments; H. Y., M. D., Y. X., and N. H. performed the experiments; H. Y. and N. H. analyzed the data; H. Y. wrote the paper; K. L. reviewed the manuscript.Conflict of interest
All authors have no conflict of interest.References
- N. A. Molodecky, I. S. Soon, D. M. Rabi, W. A. Ghali, M. Ferris, G. Chernoff, E. I. Benchimol, R. Panaccione, S. Ghosh, H. W. Barkema and G. G. Kaplan, Gastroenterology, 2012, 142, 46–54 CrossRef PubMed.
- Y. Naito, T. Takagi and T. Yoshikawa, J. Clin. Biochem. Nutr., 2007, 41, 18–26 CrossRef CAS PubMed.
- H. Sann, J. Erichsen, M. Hessmann, A. Pahl and A. Hoffmeyer, Life Sci., 2013, 92, 708–718 CrossRef CAS PubMed.
- S. Sanchez-Fidalgo, A. Cardeno, M. Sanchez-Hidalgo, M. Aparicio-Soto and C. A. de la Lastra, J. Nutr. Biochem., 2013, 24, 1401–1413 CrossRef CAS PubMed.
- J. Yin, W. K. Ren, X. S. Wu, G. Yang, J. Wang, T. J. Li, J. N. Ding, L. C. Cai and D. D. Su, J. Food, Agric. Environ., 2013, 11, 132–139 Search PubMed.
- P. Furst, J. Nutr., 2001, 131, 2562S–2568S CAS.
- N. Li, L. Ma, X. Liu, L. Shaw, S. Li Calzi, M. B. Grant and J. Neu, J. Pediatr. Gastroenterol. Nutr., 2012, 54, 499–504 CrossRef CAS PubMed.
- C. C. Chu, Y. C. Hou, M. H. Pai, C. J. Chao and S. L. Yeh, J. Nutr. Biochem., 2012, 23, 1092–1099 CrossRef CAS PubMed.
- V. F. Cruzat, A. Bittencourt, S. P. Scomazzon, J. S. Leite, P. I. de Bittencourt Jr and J. Tirapegui, Nutrition, 2014, 30, 602–611 CrossRef CAS PubMed.
- Z. Y. Jiang, L. H. Sun, Y. C. Lin, X. Y. Ma, C. T. Zheng, G. L. Zhou, F. Chen and S. T. Zou, J. Anim. Sci., 2009, 87, 4050–4056 CrossRef CAS PubMed.
- K. Vlantis, A. Polykratis, P. S. Welz, G. van Loo, M. Pasparakis and A. Wullaert, Gut, 2015 DOI:10.1136/gutjnl-2014-308323.
- J. Yin, J. Duan, Z. Cui, W. Ren, T. Li and Y. Yin, RSC Adv., 2015, 5, 15479–15486 RSC.
- P. Ranganathan, C. Jayakumar, D. Y. Li and G. Ramesh, J. Cell. Mol. Med., 2014, 18, 1290–1299 CrossRef CAS PubMed.
- J. Yin, W. Ren, J. Duan, L. Wu, S. Chen, T. Li, Y. Yin and G. Wu, Amino Acids, 2014, 46, 883–892 CrossRef CAS PubMed.
- P. Nighot, K. Young, M. Nighot, M. Rawat, E. J. Sung, N. Maharshak, S. E. Plevy, T. Ma and A. Blikslager, Inflammatory Bowel Diseases, 2013, 19, 2867–2877 CrossRef PubMed.
- S. W. Chen, J. Zhu, S. Zuo, J. L. Zhang, Z. Y. Chen, G. W. Chen, X. Wang, Y. S. Pan, Y. C. Liu and P. Y. Wang, Inflammation Res., 2015, 64, 789–797 CrossRef CAS PubMed.
- J. Park, J. S. Min, B. Kim, U. B. Chae, J. W. Yun, M. S. Choi, I. K. Kong, K. T. Chang and D. S. Lee, Neurosci. Lett., 2015, 584, 191–196 CrossRef CAS PubMed.
- J. Yin, W. Ren, G. Liu, J. Duan, G. Yang, L. Wu, T. Li and Y. Yin, Free Radical Res., 2013, 47, 1027–1035 CrossRef CAS PubMed.
- J. Yin, M. M. Wu, H. Xiao, W. K. Ren, J. L. Duan, G. Yang, T. J. Li and Y. L. Yin, J. Anim. Sci., 2014, 92, 612–619 CrossRef CAS PubMed.
- J. Yin, M. Liu, W. Ren, J. Duan, G. Yang, Y. Zhao, R. Fang, L. Chen, T. Li and Y. Yin, PLoS One, 2015, 10, e0122893 Search PubMed.
- J. J. Kim, M. S. Shajib, M. M. Manocha and W. I. Khan, J. Visualized Exp., 2012 DOI:10.3791/3678.
- W. Ren, J. Yin, M. Wu, G. Liu, G. Yang, Y. Xion, D. Su, L. Wu, T. Li, S. Chen, J. Duan, Y. Yin and G. Wu, PLoS One, 2014, 9, e88335 Search PubMed.
- Q. Xu, Y. Wu, H. Liu, Y. Xie, X. Huang and J. Liu, PLoS One, 2014, 9, e88993 Search PubMed.
- H. Zhu and Y. R. Li, Exp. Biol. Med., 2012, 237, 474–480 CrossRef CAS PubMed.
- J. Yin, W. Ren, G. Yang, J. Duan, X. Huang, R. Fang, C. Li, T. Li, Y. Yin, Y. Hou, S. W. Kim and G. Wu, Mol. Nutr. Food Res., 2015 DOI:10.1002/mnfr.201500031.
- A. Piechota-Polanczyk and J. Fichna, Naunyn-Schmiedeberg's Arch. Pharmacol., 2014, 387, 605–620 CrossRef CAS PubMed.
- L. L. Shan, B. Wang, G. Z. Gao, W. G. Cao and Y. K. Zhang, J. Appl. Physiol., 2013, 115, 1146–1155 CrossRef CAS PubMed.
- C. Marques, F. Licks, I. Zattoni, B. Borges, L. E. R. de Souza, C. A. Marroni and N. P. Marroni, World J. Gastroenterol., 2013, 19, 4464–4474 CrossRef PubMed.
- P. H. Tsai, J. J. Liu, C. L. Yeh, W. C. Chiu and S. L. Yeh, Br. J. Nutr., 2012, 107, 1112–1118 CrossRef CAS PubMed.
- D. F. McCole, Inflammatory Bowel Diseases, 2014, 20, 1829–1849 CrossRef PubMed.
- C. Greenhill, Nat. Rev. Gastroenterol. Hepatol., 2014, 11, 75 CrossRef PubMed.
- S. H. Lee, Intestinal Research, 2015, 13, 11–18 CrossRef PubMed.
- J. A. Fernandez-Blanco, J. Estevez, T. Shea-Donohue, V. Martinez and P. Vergara, Journal of Crohn's and Colitis, 2015, 9, 463–476 CrossRef PubMed.
- S. Beutheu, I. Ghouzali, L. Galas, P. Dechelotte and M. Coeffier, Clin. Nutr., 2013, 32, 863–869 CrossRef CAS PubMed.
- M. Scharl, S. R. Vavricka and G. Rogler, Curr. Drug Targets, 2013, 14, 1405–1420 CrossRef CAS.
- A. Beloqui, R. Coco, M. Alhouayek, M. A. Solinis, A. Rodriguez-Gascon, G. G. Muccioli and V. Preat, Int. J. Pharm., 2013, 454, 775–783 CrossRef CAS PubMed.
- T. Funakoshi, K. Yamashita, N. Ichikawa, M. Fukai, T. Suzuki, R. Goto, T. Oura, N. Kobayashi, T. Katsurada, S. Ichihara, M. Ozaki, K. Umezawa and S. Todo, Journal of Crohn's and Colitis, 2012, 6, 215–225 CrossRef PubMed.
- Z. Huang, A. H. Rose, F. W. Hoffmann, A. S. Hashimoto, P. Bertino, T. Denk, J. Takano, N. Iwata, T. C. Saido and P. R. Hoffmann, J. Immunol., 2013, 191, 3778–3788 CrossRef CAS PubMed.
- C. Zeng, J. H. Xiao, M. J. Chang and J. L. Wang, Molecules, 2011, 16, 8552–8568 CrossRef CAS PubMed.
- Y. Sunil, G. Ramadori and D. Raddatzc, International Journal of Colorectal Disease, 2010, 25, 323–333 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1: primers used in this study. See DOI: 10.1039/c5ra16739f |
| This journal is © The Royal Society of Chemistry 2015 |