Aggregates of a hydrazono-sulfonamide adduct as picric acid sensors†
Vaithiyanathan Mahendran and
Sivakumar Shanmugam*
Department of Organic Chemistry, School of Chemistry, Madurai Kamaraj University, Madurai 625021, India. E-mail: shivazzen@mkuniversity.org
First published on 14th October 2015
Abstract
A novel hydrazono-sulfonamide adduct (AVM) was designed and synthesized. Due to the inhibition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization with a higher water content, the adduct shows remarkable aggregation induced emission enhancement (AIEE) properties in a THF
N isomerization with a higher water content, the adduct shows remarkable aggregation induced emission enhancement (AIEE) properties in a THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solvent system. The formation of nanoaggregates was confirmed by transmission electron microscopy (TEM) analysis. Theoretical DFT calculations supported the observed photophysical changes. These aggregates of AVM act as selective and sensitive sensors for picric acid via a fluorescence quenching mechanism. The efficiency of the quenching process was calculated using the Stern–Volmer equation. The detection limit was found to be 80 nM. In addition, contact mode detection using fluorescent test strips was developed to demonstrate the solid phase sensing of picric acid.
water solvent system. The formation of nanoaggregates was confirmed by transmission electron microscopy (TEM) analysis. Theoretical DFT calculations supported the observed photophysical changes. These aggregates of AVM act as selective and sensitive sensors for picric acid via a fluorescence quenching mechanism. The efficiency of the quenching process was calculated using the Stern–Volmer equation. The detection limit was found to be 80 nM. In addition, contact mode detection using fluorescent test strips was developed to demonstrate the solid phase sensing of picric acid.
Introduction
Owing to its strong acidity and high water solubility,1 picric acid (PA) is considered as a threat to human beings and a great hazard to the environment.2 Consequently, for the past few years, the detection of picric acid has been a field of enormous interest to researchers.3 Due to the explosive nature of PA and the increasing terrorist activities all over the world, it is necessary to develop sensitive and selective fluorescent probes for the detection of PA. In spite of the more explosive nature of PA compared to other nitro aromatic compounds (NACs), the development of sensors for the detection of PA has received less attention than the development of sensors for other NACs. Several methods and materials such as metal complexes,4 ionic liquids,5 organic gels,6 polymers,7 metal–organic frameworks,8 and quantum dots9 have been reported and are useful for the detection of NACs. As fluorescent signalling involves simple instrumentation, high sensitivity, selectivity, fast response and importantly low cost, the merits of fluorescent based detection of NACs among various other methods have been discussed in a good number of publications.10 Owing to the impact of PA, Kumar et al.,11 Mukherjee et al.,12 Tang et al.,13 and others14 have made significant contributions towards the preparation of various ensembles for the detection of PA.Aggregation induced emission, which is exactly opposite to concentration quenching or aggregation caused quenching, was first reported by Tang et al. in 2001.15 In the solution state, certain molecules are weakly emissive but emit strongly in the condensed phase or the aggregated state.16 In the aggregation phenomenon, molecules tend to form highly emissive nanoparticles, which are effectively utilized as sensors for various analytes.17 The restriction of intramolecular motion has been proven to be a primary reason for the AIE of many luminogens. In addition, many of the reported probes for PA sensors were big molecules with high molecular weights, needed costly raw materials for preparation, and involved multistep syntheses. Therefore, cost effective, highly-selective and sensitive small molecule based sensors for PA are still in demand. In our recent publication,18 we had disclosed a novel method for the preparation of a hydrazono-sulfonamide adduct using a heterogeneous catalyst, which shows remarkable aggregation induced emission enhancement (AIEE) properties. We said that the inhibition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization with a higher water content accounted for the observed AIEE character of the adduct. In continuation of our previous work, we herein report a novel hydrazono-sulfonamide adduct AVM as a fluorescent chemosensor for PA, which employs easy synthetic operation and shows superior selectivity towards PA over other NACs with a high sensitivity and quenching constant and a low detection limit. To the best of our knowledge, a PA sensor using the C
N isomerization with a higher water content accounted for the observed AIEE character of the adduct. In continuation of our previous work, we herein report a novel hydrazono-sulfonamide adduct AVM as a fluorescent chemosensor for PA, which employs easy synthetic operation and shows superior selectivity towards PA over other NACs with a high sensitivity and quenching constant and a low detection limit. To the best of our knowledge, a PA sensor using the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization concept is unprecedented.
N isomerization concept is unprecedented.
Experimental section
Materials and methods
Tosyl azide19 was prepared by adopting the method reported in the literature. Preparation and characterization data for Cu(BTC) MOF are presented in our reported protocol.18 Unless otherwise stated, all solvents and chemicals were obtained from commercial sources and used without further purification. Analytical thin layer chromatography (TLC) was performed on pre-coated silica gel-G plates (Merck) using a mixture of petroleum ether (60–80 °C) and ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent. The 1H and 13C NMR spectra were recorded on a Bruker (Advance) 300 MHz instrument using TMS as an internal standard and CDCl3 as a solvent. Chemical shifts are expressed in parts per million (ppm) and the coupling constants (J values) are expressed in hertz (Hz). The following abbreviations are used to indicate spin multiplicities: s (singlet), m (multiplet). Elemental analyses were carried out using a Perkin-Elmer 2400 series II analyzer. Melting points were determined using open capillaries and were uncorrected. Absorption measurements were carried out in an Agilent single beam UV-Diode Array spectrophotometer. Fluorescence spectra were recorded using an Agilent Cary Eclipse Fluorescence spectrophotometer. Electrospray ionization mass spectrometry (ESI-MS) was recorded using a LCQ Fleet, Thermo Fisher Instruments Limited, US. The morphological characterization of the nanoaggregates was determined using a JEOL JEM-2010 Transmission Electron Microscope. Single crystal XRD analysis was performed using a 3-circle Bruker Apex X-ray diffractometer. The slit width was 5 nm for both excitation and emission. HPLC grade solvents were used for photophysical measurements.
3) as the eluent. The 1H and 13C NMR spectra were recorded on a Bruker (Advance) 300 MHz instrument using TMS as an internal standard and CDCl3 as a solvent. Chemical shifts are expressed in parts per million (ppm) and the coupling constants (J values) are expressed in hertz (Hz). The following abbreviations are used to indicate spin multiplicities: s (singlet), m (multiplet). Elemental analyses were carried out using a Perkin-Elmer 2400 series II analyzer. Melting points were determined using open capillaries and were uncorrected. Absorption measurements were carried out in an Agilent single beam UV-Diode Array spectrophotometer. Fluorescence spectra were recorded using an Agilent Cary Eclipse Fluorescence spectrophotometer. Electrospray ionization mass spectrometry (ESI-MS) was recorded using a LCQ Fleet, Thermo Fisher Instruments Limited, US. The morphological characterization of the nanoaggregates was determined using a JEOL JEM-2010 Transmission Electron Microscope. Single crystal XRD analysis was performed using a 3-circle Bruker Apex X-ray diffractometer. The slit width was 5 nm for both excitation and emission. HPLC grade solvents were used for photophysical measurements.
Preparation of aggregates
A stock solution of AVM was prepared in THF (10−4 M). An aliquot (1 mL) of this stock solution was transferred to a volumetric flask. An appropriate amount of THF and water was added to the flask under vigorous stirring to get the 10−5 M THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures with water fractions of 0–90%. The spectral analyses of the resultant mixtures were measured immediately.
water mixtures with water fractions of 0–90%. The spectral analyses of the resultant mixtures were measured immediately.
Caution! The nitroaromatic compounds used in this study, specially TNT and picric acid, are very powerful explosives. They must be handled with care and also in very small quantities.
Synthesis of probe AVM
Adduct AVM has been synthesized by following our recently reported protocol.18 To the stirring mixture of N,N-dimethylamino benzaldehyde 1 (1 mmol) and phenylhydrazine 3 (1 mmol) in DCM (3 mL) phenyl acetylene 2 (1 mmol), tosyl azide 4 (1 mmol) and activated Cu(BTC) MOF (1 mol%) were added. To the above mixture, triethylamine (1.1 mmol) was added slowly. The whole reaction mixture was agitated for 10 min at room temperature and then filtered to separate the catalyst from the reaction mixture. To the filtrate petroleum ether and an ethyl acetate mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mL) were added and the resulting mixture was stirred for 10 min. About 75% of the solvent mixture was distilled off from the filtrate under vacuum at 70 °C. The above crude product was cooled to 0 to 5 °C and triturated immediately for 5–8 min to afford a light yellow solid which was collected by filtration. The recovered catalyst was thoroughly washed with DCM and air dried for 10 min before using it for the next reaction. Isolated yield 80% (1.4 g); light yellow solid; mp 191–193 °C; 1H NMR (300 MHz, CDCl3): δ 7.54–7.44 (m, 7H), 7.35–7.25 (m, 4H), 7.20–7.16 (m, 2H), 7.07 (t, J = 7.5 Hz, 4H), 6.59 (d, J = 9.0 Hz, 2H), 4.95 (s, 2H), 2.98 (s, 6H), 2.33 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 167.5, 151.8, 146.3, 141.6, 140.8, 136.6, 136.0, 129.9, 129.1, 129.1, 128.9, 128.7, 128.7, 128.4, 126.3, 125.9, 121.1, 111.6, 40.0, 36.9, 21.2; MS (ESI) m/z [M + H]+: 511.2; anal. calcd for: C30H30N4O2S: C, 70.56; H, 5.92; N, 10.97%. Found C, 70.60; H, 5.87; N, 10.92%.
1, 20 mL) were added and the resulting mixture was stirred for 10 min. About 75% of the solvent mixture was distilled off from the filtrate under vacuum at 70 °C. The above crude product was cooled to 0 to 5 °C and triturated immediately for 5–8 min to afford a light yellow solid which was collected by filtration. The recovered catalyst was thoroughly washed with DCM and air dried for 10 min before using it for the next reaction. Isolated yield 80% (1.4 g); light yellow solid; mp 191–193 °C; 1H NMR (300 MHz, CDCl3): δ 7.54–7.44 (m, 7H), 7.35–7.25 (m, 4H), 7.20–7.16 (m, 2H), 7.07 (t, J = 7.5 Hz, 4H), 6.59 (d, J = 9.0 Hz, 2H), 4.95 (s, 2H), 2.98 (s, 6H), 2.33 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 167.5, 151.8, 146.3, 141.6, 140.8, 136.6, 136.0, 129.9, 129.1, 129.1, 128.9, 128.7, 128.7, 128.4, 126.3, 125.9, 121.1, 111.6, 40.0, 36.9, 21.2; MS (ESI) m/z [M + H]+: 511.2; anal. calcd for: C30H30N4O2S: C, 70.56; H, 5.92; N, 10.97%. Found C, 70.60; H, 5.87; N, 10.92%.
Results and discussion
The synthetic route for the preparation of adduct AVM is outlined in Scheme 1. The adduct was fully characterized using NMR, ESI-MS and elemental analysis. Single crystals of AVM were obtained by the slow evaporation of a solution of AVM in ethyl acetate and pet ether solvent mixtures (Fig. 1 and CCDC 1404294†). X-ray diffraction analysis revealed that the adduct is crystallised in the monoclinic crystal system with the P21/c space group. The crystallographic parameters of the adduct are provided in Table 1. From the crystal structure, it was found that both the imines are in E configurations. The adduct is highly soluble in common organic solvents such as THF, chloroform, dichloromethane, etc. and insoluble in water. We envisaged that AVM could be weakly emissive because of the active C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, which can be arrested in the aggregated state.18,20
N isomerization, which can be arrested in the aggregated state.18,20
| Parameters | AVM |
|---|---|
| a R1 = ∑|Fo − Fc|/∑|Fo|.b wR2 = {∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]}1/2. | |
| Formula | C30H30N4O2S |
| Formula weight | 510.2 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 13.4218(17) |
| b/Å | 10.5641(11) |
| c/Å | 19.169(3) |
| α/° | 90 |
| β/° | 106.172(7) |
| γ/° | 90 |
| V/Å3 | 2610.4(6) |
| Z | 4 |
| Temperature (K) | 100 |
| D, g cm−3 | 1.308 |
| μ (Mo-Kα) mm−1 | 0.160 |
| F(000) | 1086 |
| Size (mm) | 0.08 × 0.15 × 0.25 |
| θ range (°) | 1.6–25.0 |
| Reflection collected | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 348 348 |
| R1a [I > 2σ(I)] | 0.0671 |
| wR2b [I > 2σ(I)] | 0.1817 |
| Goodness-of-fit | 1.10 |
We screened the photophysical properties of adduct AVM using UV-Vis and fluorescence spectroscopy techniques in THF and THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures by maintaining the concentration at 1 × 10−5 M. The UV-Vis spectrum (ESI†) of adduct AVM in THF shows an absorption maximum at 354 nm. When water (90% volume fraction) was added to the THF solution of AVM, the absorption band was slightly red shifted to 371 nm with the appearance of a leveling-off long wavelength tail due to the Mei scattering effect, which confirms the formation of nanoparticles.21 The photoluminescence (PL) spectra of AVM in THF and THF
water mixtures by maintaining the concentration at 1 × 10−5 M. The UV-Vis spectrum (ESI†) of adduct AVM in THF shows an absorption maximum at 354 nm. When water (90% volume fraction) was added to the THF solution of AVM, the absorption band was slightly red shifted to 371 nm with the appearance of a leveling-off long wavelength tail due to the Mei scattering effect, which confirms the formation of nanoparticles.21 The photoluminescence (PL) spectra of AVM in THF and THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures are shown in Fig. 2. As depicted in Fig. 2, AVM is weakly emissive when it is dissolved in THF and exhibits an emission band at 435 nm. When 10 to 50% of water was added, the PL intensity gradually increased with a shift in the wavelength from 434 to 460 nm. At the same time, the PL intensity of the samples with water fractions of 60, 70 and 80% decreased. In contrast, the PL intensity of the sample with a 90% water fraction reached a maximum and the emission maximum was located at 438 nm. A similar kind of spectral change was frequently observed for compounds with AIEE properties, but the reason for it remains unclear.22 We assumed that the inhibition of C
water mixtures are shown in Fig. 2. As depicted in Fig. 2, AVM is weakly emissive when it is dissolved in THF and exhibits an emission band at 435 nm. When 10 to 50% of water was added, the PL intensity gradually increased with a shift in the wavelength from 434 to 460 nm. At the same time, the PL intensity of the samples with water fractions of 60, 70 and 80% decreased. In contrast, the PL intensity of the sample with a 90% water fraction reached a maximum and the emission maximum was located at 438 nm. A similar kind of spectral change was frequently observed for compounds with AIEE properties, but the reason for it remains unclear.22 We assumed that the inhibition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization could be the primary reason for the observed spectral changes. Because of the C
N isomerization could be the primary reason for the observed spectral changes. Because of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization of AVM was weakly emissive in THF solution, when water was added the C
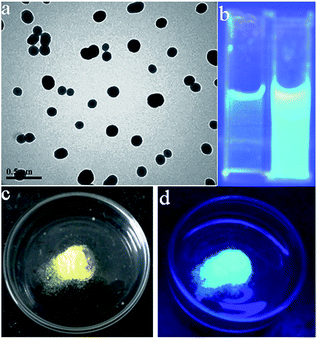
N isomerization of AVM was weakly emissive in THF solution, when water was added the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization gets blocked causing the suppression of non-radiative decay from the excited state.18 After the addition of water, the solute molecules can aggregate into various kinds of nanoparticle suspensions: crystal particles and amorphous particles.22f The former leads to an enhancement in the PL intensity, while the latter leads to a reduction in intensity. Thus, the measured overall PL intensity data depends on the combined actions of the two kinds of nanoparticles. However, it is difficult to control the formation of nanoparticles when the water content is high. Thus, the measured PL intensity often shows no regularity in samples with a high water content. Transmission electron microscopy (TEM) further confirmed the formation of spherical nanoparticles (Fig. 3a).
N isomerization gets blocked causing the suppression of non-radiative decay from the excited state.18 After the addition of water, the solute molecules can aggregate into various kinds of nanoparticle suspensions: crystal particles and amorphous particles.22f The former leads to an enhancement in the PL intensity, while the latter leads to a reduction in intensity. Thus, the measured overall PL intensity data depends on the combined actions of the two kinds of nanoparticles. However, it is difficult to control the formation of nanoparticles when the water content is high. Thus, the measured PL intensity often shows no regularity in samples with a high water content. Transmission electron microscopy (TEM) further confirmed the formation of spherical nanoparticles (Fig. 3a).
The ground state geometry of AVM was optimized using a density functional theory (DFT) method using B3LYP/6-31G basis sets. The DFT calculations were carried out using the Gaussian 09 program.23 The ground state optimized geometries and absorption behaviours of the corresponding transitions of the compounds were obtained from time dependent (TD) DFT using the above mentioned functional and basis set. The DFT optimized structure indicates a similar E configuration for both the imine groups in the gas phase which is the same as the crystal structure of the adduct (Fig. 4b). The frontier molecular orbital diagram (Fig. 4a) shows that the highest occupied molecular orbital (HOMO) is mainly delocalized on the N,N-dimethyphenyl moiety indicating the n–π* transition. The lowest unoccupied molecular orbital (LUMO) is delocalized on the N,N-dimethyphenyl and sulfonyl groups. The calculated energy gap between the HOMO and LUMO is 3.9 eV.
 | ||
| Fig. 4 HOMO and LUMO energy levels of AVM calculated using TD-DFT/B3LYP/6-31G basic sets (a) and the optimized geometry (b). | ||
Picric acid detection
The highly emissive nature of AVM in the condensed phase prompted us to explore the possibility of its application as a sensor. In general, electron deficient NACs tend to interact with electron rich molecules and electron donating groups such as free amines and N,N-dialkyl or aryl amino groups. Based on this concept several fluorescent sensors have been developed for NACs.24 We hypothesized that AVM could act as a probe for NACs as it possesses a N,N-dimethyl amino group. To confirm our hypothesis, we commenced our work using PA as a model NAC. We used nanoaggregates from 50% and 90% water mixtures as picric acid probes along with the pure THF solution of AVM for comparison.Emission bands of the pure THF solution of AVM, and the nanoaggregates of the samples with 50% and 90% water fractions were located at 434, 460, and 438 nm when excited at the corresponding absorption maxima. Fluorescence spectroscopic titrations of the nanoaggregates and the pure THF solution of AVM with picric acid revealed that the PL intensity of the corresponding solution decreased with the incremental addition of picric acid (Fig. 5). The highly emissive nature of the nanoaggregates of the samples with 50% and 90% water fractions was completely quenched with 30 equivalents of picric acid, without any appreciable shift in the wavelength. However, the pure THF solution of AVM needed 50 equivalents of picric acid for complete quenching which indicates the utility of the nanoaggregates towards the sensing of PA compared to the pure THF solution. During the addition of PA to the probe, the colorless solution rapidly turned yellow (ESI†).
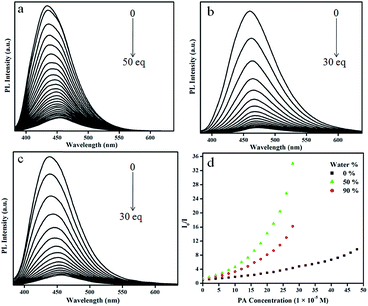 | ||
Fig. 5 PL spectral changes of AVM (1 × 10−5 M) containing different concentrations of PA in neat THF solution (a) and THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures 50 water mixtures 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (b), and 10 50 (b), and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (c). Stern–Volmer plot (d). 90 (c). Stern–Volmer plot (d). | ||
The data from the fluorescence titrations were used to calculate the quenching constant using the Stern–Volmer equation,
| I0/I = 1 + KSV[Q] |
The Stern–Volmer plot (I0/I vs. PA concentration) is presented in Fig. 5d. The Stern–Volmer constant of the quenching process was found to be 1.0 × 105 M−1 which is comparable to the reported values.11h,12a,25 A higher quenching constant indicates an effective interaction between the probe and PA, which is further supported by the spectral overlap between the absorption spectrum of PA and the emission spectrum of the probe in the wavelength region of 381–491 nm (ESI†). Linearity at low concentrations of PA in the Stern–Volmer plot (ESI†) indicates the involvement of a static quenching mechanism. However, at higher concentrations of PA, the plot bent upward due to a super amplified quenching effect.26 Nanoaggregates of the sample with a 50% water fraction show a higher sensing performance than those of the 90% water fraction, which may due the polymer packing in the former being looser than in the latter and the looser packing allows voids to interact with more PA molecules.13c
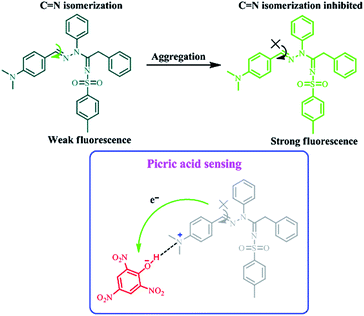
The turn-off mechanism (Fig. 6) may be explained by the electron transfer and/or energy transfer mechanism11a,27,28 between the picrate ion and the AVM adduct. The proton NMR spectral studies show the presence of PA, and shift the sensor peak positions downfield (Fig. 7), which further confirms the electrostatic interaction between the sensor and PA.14c The aromatic protons near to the –NMe2 group were shifted downfield, thus confirming that the –NMe2 group is the receptor site. In addition to PA, we screened other NACs (2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT), 1,4-dinitrobenzene (DNB), 4-nitrobenzene (NB), 1,4-dinitrophenol (DNP), 4-nitrophenol (NP), 4-nitrobenzoic acid (NBA), and benzoquinone (BQ)) with the probe (Fig. 8). Noticeably, the selectivity is very high toward PA compared to other NACs. Nanoaggregates of AVM offer more diffusion channels for the exciton to migrate, allowing them to be more quickly annihilated by picric acid.29
 | ||
Fig. 8 Relative fluorescence quenching of the nanoaggregates of AVM upon addition of various NACs (30 eq.) in a THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (10 water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) mixture. 90) mixture. | ||
The detection limit30 was calculated using the equation 3σ/slope, where σ is the standard deviation. The detection limit was found to be 80 nM, which is almost comparable to the reported values.14i,31 To explain the merits of this work, we demonstrated the solid phase detection of PA using test strips. Test strips were prepared using TLC plates coated by dipping into a solution of the AVM adduct. The coated strips were dried under vacuum and then used for the detection of PA. The emissive nature of the test strips was quenched when they came into contact with PA. The quenching confirms the solid phase interaction between the probe and PA (Fig. 9).
 | ||
| Fig. 9 Test strip based detection of PA using UV light (365 nm): blank TLC plate (a), after coating with AVM (b) and upon interaction with picric acid (c). | ||
Conclusion
We have designed and synthesized a novel hydrazono-sulfonamide (AVM) adduct using a heterogeneous Cu(BTC) MOF catalyst in good yield. An aggregation study in a THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water system reveals that C
water system reveals that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization is the main cause for the observed spectral changes. Theoretical DFT calculations further confirmed this fact. Formation of nanoaggregates was confirmed using TEM analysis. Nanoaggregates of the AVM adduct showed high sensitivity and selectivity towards PA compared to other NACs. Furthermore, a low detection limit and high Stern–Volmer constant shows the efficiency of the quenching process. In addition, it was demonstrated that PA can be detected using test strips made from TLC plates.
N isomerization is the main cause for the observed spectral changes. Theoretical DFT calculations further confirmed this fact. Formation of nanoaggregates was confirmed using TEM analysis. Nanoaggregates of the AVM adduct showed high sensitivity and selectivity towards PA compared to other NACs. Furthermore, a low detection limit and high Stern–Volmer constant shows the efficiency of the quenching process. In addition, it was demonstrated that PA can be detected using test strips made from TLC plates.
Acknowledgements
The authors thank DST and UGC for financial assistance and DST-IRHPA for funding the purchase of a higher resolution NMR spectrometer. VM expresses special gratitude to DST-MRP (Reg. No. SR/FT/CS-63/2010) for research fellowship.References
- H. Muthurajan, R. Sivabalan, M. B. Talawar and S. N. Asthana, J. Hazard. Mater., 2004, 112, 17 CrossRef CAS PubMed.
- (a) V. Pimienta, R. Etchenique and T. Buhse, J. Phys. Chem. A, 2001, 105, 10037 CrossRef CAS; (b) J. Shen, J. Zhang, Y. Zuo, L. Wang, X. Sun, J. Li, W. Han and R. He, J. Hazard. Mater., 2009, 163, 1199 CrossRef CAS PubMed; (c) G. Anderson, J. D. Lamar and P. T. Charles, Environ. Sci. Technol., 2007, 41, 2888–2893 CrossRef CAS PubMed; (d) J. F. Wyman, M. P. Serve, D. W. Hobson, L. H. Lee and D. E. J. Uddin, J. Toxicol. Environ. Health, Part A, 1992, 37, 313–327 CrossRef CAS PubMed.
- (a) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed; (b) M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543–2555 RSC; (c) Y. Salinas, R. Martínez-Máñez, M. D. Marcos, F. Sancenón, A. M. Costero, M. Parra and S. Gil, Chem. Soc. Rev., 2012, 41, 1261–1296 RSC.
- (a) G. G. Shan, H. B. Li, H. Z. Sun, D.-X. Zhu, H.-T. Cao and Z.-M. Su, J. Mater. Chem. C, 2013, 1, 1440–1449 RSC; (b) V. Sathish, A. Ramdass, Z.-Z. Lu, M. Velayudham, P. Thanasekaran, K.-L. Lu and S. Rajagopal, J. Phys. Chem. B, 2013, 117, 14358–14366 CrossRef CAS PubMed.
- S. Shaligram, P. P. Wadgaonkar and U. K. Kharul, J. Mater. Chem. A, 2014, 2, 13983–21398 CAS.
- (a) B. G. Bag, G. C. Maity and S. K. Dinda, Org. Lett., 2006, 8, 5457–5460 CrossRef CAS PubMed; (b) K. K. Kartha, A. Sandeep, V. K. Praveen and A. Ajayaghosh, Chem. Rec., 2015, 15, 252–265 CrossRef CAS PubMed.
- (a) H. Sohn, M. J. Sailor, D. Magde and W. C. Trogler, J. Am. Chem. Soc., 2003, 125, 3821–3830 CrossRef CAS PubMed; (b) S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871–2883 RSC; (c) T.-P. Huynh, M. Sosnowska, J. W. Sobczak, B. K. C. Chandra, V. N. Nesterov, F. D’Souza and W. Kutner, Anal. Chem., 2013, 85, 8361–8368 CrossRef CAS PubMed; (d) W. Wu, S. Ye, H. L. Xiao, Y. Fu, Q. Huang, G. Yu, Y. Liu, J. Qin, Q. Lia and Z. Li, J. Mater. Chem., 2012, 22, 6374–6382 RSC; (e) I.-H. Park, R. Medishetty, J.-Y. Kim, S. S. Lee and J. J. Vittal, Angew. Chem., Int. Ed., 2014, 53, 5591–5595 CrossRef CAS PubMed.
- (a) X. Jiang, Y. Liu, P. Wu, L. Wang, Q. Wang, G. Zhu, X.-L. Li and J. Wang, RSC Adv., 2014, 4, 47357–47360 RSC; (b) S. Sanda, S. Parshamoni, S. B. Biswas and S. Konar, Chem. Commun., 2015, 51, 6576–6579 RSC.
- (a) R. Freeman, T. Finder, L. Bahshi, R. Gill and I. Willner, Adv. Mater., 2012, 48, 6416–6421 CrossRef PubMed; (b) B. Liu, C. Tong, L. Feng, C. Wang, H. Yao and C. Lü, Chem.–Eur. J., 2014, 20, 2132–2137 CrossRef CAS PubMed.
- (a) D. S. Moore, Rev. Sci. Instrum., 2004, 75, 2499 CrossRef CAS; (b) E. S. Forzani, D. Lu, M. J. Leright, A. D. Aguilar, F. Tsow, R. A. Iglesias, Q. Zhang, J. Lu, J. Li and N. Tao, J. Am. Chem. Soc., 2009, 131, 1390–1391 CrossRef CAS PubMed; (c) M. Riskin, R. Tel-Vered, T. Bourenko, E. Granot and I. Willner, J. Am. Chem. Soc., 2008, 130, 9726–9733 CrossRef CAS PubMed; (d) R. Hodyss and J. L. Beauchamp, Anal. Chem., 2005, 77, 3607–3610 CrossRef CAS PubMed; (e) C.-Y. Lai, B. G. Trewyn, D. M. Jeftinija, K. Jeftinija, S. Xu, S. Jeftinija and V. S.-Y. Lin, J. Am. Chem. Soc., 2003, 125, 4451–4459 CrossRef CAS PubMed.
- (a) V. Vij, V. Bhalla and M. Kumar, ACS Appl. Mater. Interfaces, 2013, 5, 5373–5380 CrossRef CAS PubMed; (b) V. Bhalla, S. Kaur, V. Vij and M. Kumar, Inorg. Chem., 2013, 52, 4860–4865 CrossRef CAS PubMed; (c) M. Kumar, S. I. Reja and V. Bhalla, Org. Lett., 2012, 14, 6084–6087 CrossRef CAS PubMed; (d) V. Bhalla, A. Gupta and M. Kumar, Org. Lett., 2012, 14, 3112–3115 CrossRef CAS PubMed; (e) V. Bhalla, H. Arora, H. Singh and M. Kumar, Dalton Trans., 2013, 42, 969–974 RSC; (f) S. Kaur, V. Bhalla, V. Vij and M. Kumar, J. Mater. Chem. C, 2014, 2, 3936–3941 RSC; (g) S. Kaur, A. Gupta, V. Bhalla and M. Kumar, J. Mater. Chem. C, 2014, 2, 7356–7363 RSC; (h) S. Pramanik, V. Bhalla and M. Kumar, Anal. Chim. Acta, 2013, 793, 99–106 CrossRef CAS PubMed.
- (a) S. Shanmugaraju, H. Jadhav, Y. P. Patil and P. S. Mukherjee, Inorg. Chem., 2012, 51, 13072–13074 CrossRef CAS PubMed; (b) S. Shanmugaraju, S. A. Joshi and P. S. Mukherjee, Inorg. Chem., 2011, 50, 11736–11745 CrossRef CAS PubMed; (c) A. Chowdhury and P. S. Mukherjee, J. Org. Chem., 2015, 80, 4064–4075 CrossRef CAS PubMed; (d) B. Roy, A. K. Bar, B. Gole and P. S. Mukherjee, J. Org. Chem., 2013, 78, 1306–1310 CrossRef CAS PubMed; (e) K. Acharyya and P. S. Mukherjee, Chem. Commun., 2014, 50, 15788–15791 RSC; (f) V. Vajpayee, H. Kim, A. Mishra, P. S. Mukherjee, P. J. Stang, M. H. Lee, H. K. Kim and K.-W. Chi, Dalton Trans., 2011, 40, 3112–3115 RSC; (g) A. K. Bar, S. Shanmugaraju, K.-W. Chi and P. S. Mukherjee, Dalton Trans., 2011, 40, 2257–2267 RSC; (h) K. Acharyya and P. S. Mukherjee, Chem.–Eur. J., 2015, 21, 6823–6831 CrossRef CAS PubMed; (i) S. Shanmugaraju, Chem.–Eur. J., 2015, 21, 6656–6666 CrossRef CAS PubMed; (j) B. Gole, S. Shanmugaraju, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 10046–10048 RSC; (k) B. Gole, W. Song, M. Lackinger and P. S. Mukherjee, Chem.–Eur. J., 2014, 20, 13662–13680 CrossRef CAS PubMed; (l) B. Gole, A. K. Bar and P. S. Mukherjee, Chem.–Eur. J., 2014, 20, 2276–2291 CrossRef CAS PubMed.
- (a) H. Li, H. Wu, E. Zhao, J. Li, J. Z. Sun, A. Qin and B. Z. Tang, Macromolecules, 2013, 46, 3907–3914 CrossRef CAS; (b) J. Liu, Y. Zhong, J. W. Y. Lam, P. Lu, Y. Hong, Y. Yu, Y. Yu, M. Faisal, H. H. Y. Sung, I. D. Williams, K. S. Wong and B. Z Tang, Macromolecules, 2010, 43, 4921–4936 CrossRef CAS; (c) R. Hu, J. L. Maldonado, M. Rodriguez, C. Deng, C. K. W. Jim, J. W. Y. Lam, M. M. F. Yuen, G. R. Ortiz and B. Z. Tang, J. Mater. Chem., 2012, 22, 232–240 RSC; (d) Y. Zhang, G. Chen, Y. Lin, L. Zhao, W. Z. Yuan, P. Lu, C. K. W. Jim, Y. Zhang and B. Z. Tang, Polym. Chem., 2015, 6, 97–105 RSC; (e) P. Lu, J. W. Y. Lam, J. Liu, C. K. W. Jim, W. Yuan, N. Xie, Y. Zhong, Q. Hu, K. S. Wong, K. K. L. Cheuk and B. Z. Tang, Macromol. Rapid Commun., 2010, 31, 834–839 CrossRef CAS PubMed; (f) C. Y. K. Chan, Z. Zhao, J. W. Y. Lam, J. Liu, S. Chen, P. Lu, F. Mahtab, X. Chen, H. H. Y. Sung, H. S. Kwok, Y. Ma, I. D. Williams, K. S. Wong and B. Z. Tang, Adv. Funct. Mater., 2012, 22, 378–389 CrossRef CAS.
- (a) Y. Peng, A. J. Zhang, M. Dong and Y.-W. Wang, Chem. Commun., 2011, 47, 4505–4507 RSC; (b) Y. Xu, B. Li, W. Li, J. Zhao, S. Sun and Y. Pang, Chem. Commun., 2013, 49, 4764–4766 RSC; (c) P. Vishnoi, S. Sen, G. N. Patwari and R. Murugavel, New J. Chem., 2015, 39, 886–892 RSC; (d) L. Wenfeng, M. H. Chang and L. Ziqiang, RSC Adv., 2014, 4, 39351–39358 RSC; (e) R. Kumar, S. Sandhu, P. Singh, G. Hundal, M. S. Hundal and S. Kumar, Asian J. Org. Chem., 2014, 3, 805–813 CrossRef CAS; (f) H.-T. Feng and Y.-S. Zheng, Chem.–Eur. J., 2014, 20, 195–201 CrossRef CAS PubMed; (g) J. Ye, X. Wang, R. F. Bogale, L. Zhao, H. Cheng, W. Gong, J. Zhao and G. Ning, Sens. Actuators, B, 2015, 210, 566–573 CrossRef CAS; (h) R. Chopra, P. Kaur and K. Singh, Anal. Chim. Acta, 2015, 864, 55–63 CrossRef CAS PubMed; (i) J.-F. Xiong, J.-X. Li, G.-Z. Mo, J.-P. Huo, J.-Y. Liu, X.-Y. Chen and Z.-Y. Wang, J. Org. Chem., 2014, 79, 11619–11630 CrossRef CAS PubMed; (j) X. He, P. Zhang, J.-B. Lin, H. V. Huynh, S. E. Navarro Muñoz, C.-C. Ling and T. Baumgartner, Org. Lett., 2013, 15, 5322–5325 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC; (b) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC; (c) J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed; (d) Z. Zhao, J. W. Y. Lam and B. Z Tang, Curr. Org. Chem., 2010, 14, 2109–2132 CrossRef CAS.
- (a) T. Sanji, K. Shiraishi, M. Nakamura and M. Tanaka, Chem.–Asian. J., 2010, 5, 817–824 CrossRef CAS PubMed; (b) M. Wang, D. Zhang, G. Zhang, Y. Tang, S. Wang and D. Zhu, Anal. Chem., 2008, 80, 6443–6448 CrossRef CAS PubMed; (c) Q. Zhao, K. Li, S. Chen, A. Qin, D. Ding, S. Zhang, Y. Liu, B. Liu, J. Z. Sun and B. Z. Tang, J. Mater. Chem., 2012, 22, 15128–15135 RSC; (d) M. Wang, G. Zhang, D. Zhang, D. Zhu and B. Z. Tang, J. Mater. Chem., 2010, 20, 1858–1867 RSC; (e) E. Wang, E. Zhao, Y. Hong, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem. B, 2014, 2, 2013–2019 RSC.
- V. Mahendran and S. Shanmugam, RSC Adv., 2015, 5, 20003–20010 RSC.
- H. Lu, V. Subbarayan, J. Tao and X. P. Zhang, Organometallics, 2010, 29, 389–393 CrossRef CAS.
- K. Namitharan and K. Pitchumani, Org. Biomol. Chem., 2012, 10, 2937–2941 CAS.
- B. Z. Tang, Y. Geng, J. W. Y. Lam, B. Li, X. Jing, X. Wang, F. Wang, A. B. Pakhomov and X. X. Zhang, Chem. Mater., 1999, 11, 1581–1589 CrossRef CAS.
- (a) X. Zhang, Z. Chi, B. Xu, C. Chen, X. Zhou, Y. Zhang, S. Liu and J. Xu, J. Mater. Chem., 2012, 22, 18505–18513 RSC; (b) G. Zhang, A. Ding, Y. Zhang, L. Yang, L. Kong, X. Zhang, X. Tao, Y. Tian and J. Yang, Sens. Actuators, B, 2014, 202, 209–216 CrossRef CAS; (c) A. Ding, L. Yang, Y. Zhang, G. Zhang, L. Kong, X. Zhang, Y. Tian, X. Tao and J. Yang, Chem.–Eur. J., 2014, 20, 12215–12222 CAS; (d) H. Li, Z. Chi, B. Xu, X. Zhang, Z. Yang, X. Li, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2010, 20, 6103–6110 RSC; (e) S. C. Dong, Z. Li and J. G. Qin, J. Phys. Chem. B, 2009, 113, 434–441 CrossRef CAS PubMed; (f) X. Zhang, Z. Yang, Z. Chi, M. Chen, B. Xu, C. Wang, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2010, 20, 292–298 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- (a) J. M. T. Lin, X. Pan and W. Wang, Chem. Mater., 2014, 26, 4221–4229 CrossRef; (b) B. Xu, X. Wu, H. Li, H. Tong and L. Wang, Macromolecules, 2011, 44, 5089–5092 CrossRef CAS; (c) Y. Xu, B. Li, W. Li, J. Zhao, S. Sun and Y. Pang, Chem. Commun., 2013, 49, 4764–4766 RSC; (d) W. Wei, R. Lu, S. Tang and X. Liu, J. Mater. Chem. A, 2015, 3, 4604–4611 RSC; (e) K. D. Prasad and T. N. G. Row, RSC Adv., 2014, 4, 45306–54531 RSC; (f) G. Sivaraman, B. Vidya and D. Chellappa, RSC Adv., 2014, 4, 30828–30831 RSC; (g) R. Chopra, P. Kaur and K. Singh, Anal. Chim. Acta, 2015, 864, 55–63 CrossRef CAS PubMed.
- (a) J. Wang, J. Mei, W. Yuan, P. Lu, A. Qin, J. Sun, Y. Ma and B. Z. Tang, J. Mater. Chem., 2011, 21, 4056–4059 RSC; (b) D. Li, J. Liu, R. T. K. Kwok, Z. Liang, B. Z. Tang and J. Yu, Chem. Commun., 2012, 48, 7167–7169 RSC.
- N. Venkatramaiah, S. Kumar and S. Patil, Chem.–Eur. J., 2012, 18, 14745–14751 CrossRef CAS PubMed.
- T. Liu, L. Ding, G. He, Y. Yang, W. Wang and Y. Fang, ACS Appl. Mater. Interfaces, 2011, 3, 1245–1253 CAS.
- J. Ma, T. Lin, X. Pan and W. Wang, Chem. Mater., 2014, 26, 4221–4229 CrossRef CAS.
- J. Wang, J. Mei, W. Yuan, P. Lu, A. Qin, J. Sun, Y. Ma and B. Z. Tang, J. Mater. Chem., 2011, 21, 4056–4059 RSC.
- (a) B. Yu, J. Ma, Y. Zhang, G. Zou and Q. Zhang, RSC Adv., 2015, 5, 29262–29265 RSC; (b) N. Venkatesan, V. Singh, P. Rajakumar and A. K. Mishra, RSC Adv., 2014, 4, 53484–53489 RSC.
- Y. Xu, B. Li, W. Li, J. Zhao, S. Sun and Y. Pang, Chem. Commun., 2013, 49, 4764–4766 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1404294. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra17359k |
| This journal is © The Royal Society of Chemistry 2015 |