Synthesis of MoS2/SrZrO3 heterostructures and their photocatalytic H2 evolution under UV irradiation
Qingwen Tian†
,
Li Zhang†,
Jiahui Liu,
Naixu Li,
Quanhong Ma,
Jiancheng Zhou* and
Yueming Sun
School of Chemistry and Chemical Engineering, Southeast University, Nanjing, 211189, P. R. China. E-mail: jczhou@seu.edu.cn; Fax: +86 025 52090620; Tel: +86 025 52090621
First published on 19th November 2014
Abstract
A novel heterojunction of a MoS2/SrZrO3 photocatalyst was successfully synthesized via a simple hydrothermal process and applied to photocatalytic H2 evolution under UV light irradiation. The samples were characterized by X-ray diffraction, UV-vis absorption spectroscopy, scanning and transmission electron microscopy, X-ray photoemission spectroscopy, energy dispersive X-ray spectroscopy and EDX mapping. The heterostructure with an optimal content of 0.05 wt% MoS2 exhibits the highest H2 evolution rate of 5.31 mmol h−1. This is due to the junction between SrZrO3 and MoS2, which suppresses the recombination of photogenerated electrons and holes. Our work indicated that the prepared MoS2/SrZrO3 heterostructured photocatalyst can be used as an effective material for water splitting.
1. Introduction
Photocatalytic water splitting has attracted considerable attention due to the depletion of fossils and energy crisis challenging science,1–3 and hydrogen has been considered as a clean, economical and environmentally friendly energy in modern society.4–6 Since the first report by Fujishima and Honda on electrochemical photolysis of water at a TiO2 electrode,7 great efforts have been made to explore hydrogen evolution to solve energy and environmental issues.4,8 Selecting novel photocatalysts possessing a wide range of light response and higher quantum efficiency, such as, heterogeneous photocatalysts, metal coordination photocatalysts,9 hierarchically structured nanocrystals10,11 etc., have been intensively and extensively investigated because of their special band structure and carrier transportation.12,13Photocatalysts containing d0 and d10 metal irons, such as, Ti4+, Zr4+, Nb5+, Ga3+ and Sb5+ have been demonstrated as higher photocatalytic activities for H2 evolution under UV irradiation.4,14 Among them, perovskite type oxide ABO3, where A is an alkaline metal or an alkaline earth metal and B is a transition metal,15 such as, SrZrO3, BaZrO3, SrTiO3, BiVO4 and etc., have been considered as one of the most promising photocatalysts due to its unique properties as high stability and nontoxicity.16 Furthermore, perovskite-type SrZrO3, with a wide band gap of 5.6 eV, has attracted much attention owing to its applications in high-temperature materials,17 luminescence properties,18 hydrogen gas sensors19 and catalysts.20 And excellent stabilities and more photocatalytic active sites make SrZrO3 a suitable photocatalyst applied to the water splitting and dye degradation reactions under UV light irradiation.
Heterogeneous photocatalysis is considered as one of the most promising methods solving energy and environmental crisis, and perovskite type oxide compounds have been selected as one of the suitable materials.4,14,21 On the other hands, layer-structured transition metal sulfide especially for MoS2, which is consist of metal Mo attached with two S and stacking together to furnish sandwich-like structure, is also exploited for photocatalytic applications owing to its narrow band gap and high thermal stability,22,23 and a great number of heterogeneous photocatalysts containing MoS2 have been synthesized during the past decades.22,24–28 Li et al. synthesized MoS2/CdS catalyst and investigated the photocatalytic activities before and after loading MoS2 under visible light irradiation, founding a greatly enhancement of H2 evolution after loading MoS2.29 Liu et al. prepared a novel photocatalyst with 3D hierarchical heterostructure, MoS2/TiO2, exhibiting an excellent photocatalytic H2 evolution and dye degradation, impling the matched energy band of MoS2/TiO2 heterostructure favoring the charge transfer and suppression the recombination of photogenerated electron and hole between MoS2 and TiO2.27
Herein, a novel heterostructured MoS2/SrZrO3 photocatalyst was synthesized by facile hydrothermal method. Then the as-prepared sample was employed to investigated photocatalytic H2 evolution from aqueous solutions containing Na2S and Na2SO3 under UV irradiation, and 0.05 wt% MoS2 loading on the SrZrO3 crystal was found to show best performance with H2 evolution rate of 5.31 mmol h−1. MoS2 was proved to be an effective separation of photogenerated carriers in the MoS2/SrZrO3 heterojunction and the possible mechanism of H2 evolution was discussed. To the best of our knowledge, this is the first report on the preparation of MoS2/SrZrO3 heterojunction and their application in the photocatalytic activity for water splitting under UV light irradiation.
2. Experimental section
2.1 Chemicals and materials
Zirconium(IV) oxychloride octahydrate (ZrOCl2·8H2O), strontium nitrate (Sr(NO3)2), potassium hydroxide (KOH), sodium molybdate dehydrate (Na2MoO4·2H2O), thioacetamide (C2H5NS), acetic acid and ethanol. All of the chemicals were purchased from Shanghai Chemical Reagent Company in analytical grade, and were used without further purification.2.2 Synthesis of SrZrO3 photocatalyst
SrZrO3 photocatalyst were synthesized by hydrothermal method according to the previous literature.30 Typically, 1.164 g Sr(NO3)2 and 1.608 g ZrOCl2·8H2O were dissolved in 60 mL 12 mol L−1 KOH solution and stirred 1 h at ambient temperature by using a magnetic stirrer. Then the mixture was transferred to a 100 mL Teflon-lined stainless steel autoclave, heated up to 200 °C and kept for 24 h. After cooling naturally, the product was centrifuged and washed with distilled water, diluted acetic acid to remove some SrCO3 by-products, and ethanol several times. After that, the sample was vacuum dried at 60 °C for 6 h. It is worth noting that the KOH solution was pre-prepared or the temperature would rise up to over 100 °C resulting in the inhomogeneous formation of SrZrO3 crystal.2.3 Synthesis of MoS2 crystal
The MoS2 crystal was synthesized by hydrothermal method. Typically, 0.807 g Na2MoO4·2H2O and 1.2522 g C2H5NS was dissolved in a 100 mL beaker containing 20 mL distilled water and 20 mL ethanol, and the mixture was stirred 30 min under a magnetic stirrer. Then the solution was transferred in a 100 mL Teflon-lined stainless-steel autoclave, sealed and heated to 200 °C for 48 h. After cooling naturally, the product was centrifuged, washed with distilled water and ethanol several times. The sample was vaccum dried at 60 °C for 6 h.2.4 Synthesis of MoS2/SrZrO3 heterocatalyst
The formation process of MoS2/SrZrO3 heterocatalyst was described as follows. 0.5 g as-prepared SrZrO3 was dissolved in the mixture containing 20 mL distilled water and 20 mL ethanol, a certain quality Na2MoO4·2H2O and C2H5NS was then added into the solution and the molar ration of Na2MoO4·2H2O and a certain quality Na2MoO4·2H2O and C2H5NS was then added into the solution and the molar ration of Na2MoO4·2H2O and C2H5NS was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The obtained suspension was sonicated for 10 min and stirred 30 min in order to disperse uniformly. The solution was sealed in a 100 mL Teflon-lined stainless-steel autoclave and heated in an electric oven at 200 °C for 48 h. After cooling to the room temperature, the product was separated by centrifugation, washed with distilled water and ethanol several times. Finally, the catalysts were dried in vacuum at 60 °C for 6 h. The prepared samples with 0.01, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 7, 10 wt% MoS2, defined as the weight ration of MoS2 to SrZrO3, were named as SM1, SM2, SM3, SM4, SM5, SM6, SM7, SM8, SM9, SM10.
5. The obtained suspension was sonicated for 10 min and stirred 30 min in order to disperse uniformly. The solution was sealed in a 100 mL Teflon-lined stainless-steel autoclave and heated in an electric oven at 200 °C for 48 h. After cooling to the room temperature, the product was separated by centrifugation, washed with distilled water and ethanol several times. Finally, the catalysts were dried in vacuum at 60 °C for 6 h. The prepared samples with 0.01, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 7, 10 wt% MoS2, defined as the weight ration of MoS2 to SrZrO3, were named as SM1, SM2, SM3, SM4, SM5, SM6, SM7, SM8, SM9, SM10.
2.5 Characterization of photocatalyst
X-ray diffraction (XRD) patterns were recorded on Bruker D8-Discover with Cu Kα radiation (λ = 1.54178 Å). The transmission electron microscopy (TEM, Hitachi H-600) and the scanning electron microscopy (SEM, FEI Inspect F50) were used to characterize the morphologies and size of the synthesized product. UV-vis absorption spectra were measured on a Shimadzu UV-3100 spectrometer. The chemical composition analysis of the sample surfaces was characterized by X-ray photoelectron spectra (XPS) (VG Multiab-2000) using a PHI Quantum 2000 XPS system with a monochromatic Al Kα source and charge neutralizer. All of the spectra were calibrated to C 1s peak at 284.6 eV.2.6 Photocatalytic activity test
Photocatalytic reactions of hydrogen evolution were carried out in a 250 mL Pyrex flask at room temperature and atmospheric pressure, and 100 W mercury lamp (365 nm, Shanghai Special Lighting Factory) was used as the light source. Typically, photocatalyst (0.2 g) was dissolved in 250 mL aqueous solution containing Na2S (0.35 M) and Na2SO3 (0.25 M) as electron donors. Prior to irradiation, the suspension was sonicated for 20 min and bubbled with N2 for 30 min to remove the dissolved oxygen. Then the photocatalyst was irradiated with ultraviolet light from the Hg lamp. The amount of H2 was determined by gas chromatograph (GC-14C, Shimadzu, Japan, TCD, nitrogen as a carrier gas and 5 Å molecular sieve column).3. Results and discussion
In order to investigate the changes of phase structures and crystal form of the as-prepared samples, X-ray diffraction measurement was performed. Fig. 1 shows the XRD patterns of SrZrO3, MoS2 and MoS2/SrZrO3 synthesized by hydrothermal method. All the peaks were assigned as orthorhombic perovskite SrZrO3 structure (JCPDS card 44-0161) with cell parameter a = 5.819 Å, b = 8.204 Å and c = 5.797 Å in Pnma (62) space group, and several typical peaks at 2θ = 21.6°, 24.2°, 30.7°, 36.2°, 37.9°, 44.1°, 45.5°, 54.7°, 64.0°, 72.7° were observed and corresponding to (020), (111), (200), (211), (220), (202), (212), (240), (400) and (402) planes, respectively. For pure MoS2 crystal, peaks are ascribed to (002), (100) and (110) planes with hexagonal phase MoS2 (JCPDS card 37-1492).27,31 However, no obvious peaks of heterojunctions attributed to MoS2 phase were observed in the MoS2/SrZrO3 samples mainly due to its low loading content, high distribution of the MoS2 component and weak crystallization in these catalysts.32–34Fig. 2 demonstrated the UV-vis absorption spectra of pure SrZrO3 and MoS2/SrZrO3 heterostructures. For pure SrZrO3 sample, the absorption at 230 nm was supposed to the instinct band gap absorption, and the band gap of SrZrO3 is 5.4 eV which is close to 5.6 eV that literature reported.18,35,36 And the absorption of MoS2 was blue-shifted relative to the bulk MoS2 having a band edge at 1040 nm.27,37 Noticeably, a similar UV-vis absorption curves were observed, and the absorption edges of heterojunction is red-shifted gradually when the increasing amount of MoS2 crystal was loaded on the surface of SrZrO3. Further, the absorption band of MoS2/SrZrO3 was located at the UV region, indicating an excellent UV absorption at this area.
The morphologies of as-prepared catalysts were characterized by scanning electron microscope (SEM) and transmission electron microscope (TEM), which was shown in Fig. 3. The morphology of SrZrO3 (Fig. 3a) shows a cubic structure with an average side length of 2 μm, and the sizes and shapes of pure SrZrO3 and SM8 remained almost the same after loading MoS2, which is demonstrated by the comparison of Fig. 3c with Fig. 3a. For pure MoS2 (Fig. 3b), the successfully synthesized MoS2 was layer-structured, which is flower-like nanostructure with average diameter of 200 nm. Furthermore, the MoS2 small nanoflakes loading on the surface of SrZrO3 were observed from Fig. 3d. The elemental composition of the photocatalyst was examined by energy dispersive X-ray spectroscopy (EDX) analysis, Fig. 3f. The presence of Sr, Zr, O, Mo and S elements were confirmed by EDX. The HRTEM image of MoS2/SrZrO3 in Fig. 3e indicates the lattice spacing with an interplanar distance of 0.291 nm and 0.61 nm, which are corresponding to the (200) and (002) d-spacing of the orthorhombic phase of SrZrO3 and hexagonal MoS2,5,27,38 respectively. Herein, the HRTEM image is just an indirect proof demonstrating the existence of MoS2 in MoS2/SrZrO3 heterojunctions.
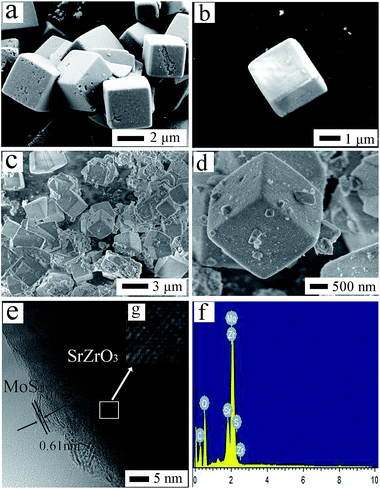 | ||
| Fig. 3 (a) SEM images of SrZrO3, (b) SEM images of MoS2, (c and d) SEM images of SM8, (e and g) HRTEM images of SM8, (f) EDX pattern of SM8 photocatalyst in (c). | ||
To confirm the formation of MoS2/SrZrO3 heterojunction, the SEM and TEM analysis of SrZrO3 have been characterized before and after loading MoS2. In addition to this, SEM-EDX mapping has also been detected to show the elemental distribution of Sr, Zr, O, Mo and S, which was presented in Fig. 4 and the selected area of EDX-mapping analysis was shown in Fig. 4a. Obviously, homogeneous distributions of Sr, Zr and O were observed in the particle (Fig. 4b–d) which is consistent to the SEM image. The distribution of elements of Mo and S were also displayed uniformly with low density (Fig. 4e and f).
 | ||
| Fig. 4 (a) SEM image of SM8, and (b–f) the corresponding EDX mapping of MoS2/SrZrO3 at the region shown in (a), indicating spatial distribution of Sr, Zr, O, Mo, S, respectively. | ||
To further investigated the chemical composition and elemental status of SrZrO3 and MoS2/SrZrO3, XPS analysis was carried out and presented in Fig. 5. The content of MoS2 lower than 0.1% cannot be detected by XPS, and the different of all heterojunctions is the smoothness with different MoS2 content, thus, we chose SM8 to investigate the XPS of the elements. The XPS peak for C 1s at 284.6 eV is due to adventitious carbon from the XPS instrument. Fig. 5a gives the comparison of XPS spectra of SrZrO3 and SM8 samples. The compositions of high resolution XPS of Sr, Zr and O elements for SrZrO3 and SM8 were shown in Fig. 5b–d. No apparent differences except O were observed when MoS2 loading on the surface of SrZrO3, indicating the introduced MoS2 has little influence on the chemical valence.33 Two peaks of Sr 3d in Fig. 4b at 135.3 and 133.7 eV are assigned to Sr 3d3/2 and Sr 3d5/2, and the binding energies corresponding to Zr 3d3/2 and Zr 3d5/2 are 182.2 and 179.9 eV, respectively.32 The binding energy difference of O 1s was 533.7 eV due to the absorbed oxygen in SrZrO3 sample,34 and the binding energy located at 531.8 eV is assigned to O 1s. The peaks of Mo 3d (Fig. 5e) at 232.6 and 229.4 eV are attributed to Mo(+4) 3d3/2 and Mo(+4) 3d5/2, respectively.22 It can be clearly seen from Fig. 5f that the measured binding energies of S 2p1/2 and S 2p3/2 are corresponding to 163 and 161.9 eV, respectively.22,27,39 The atomic percentage of Mo and S analyzed by XPS was 3.876![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.481, which is close to 1
7.481, which is close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Taken all, the existence of MoS2 and SrZrO3 in SM8 sample was further confirmed by high resolution of XPS spectra, which is consistent of XRD and TEM analysis.
2. Taken all, the existence of MoS2 and SrZrO3 in SM8 sample was further confirmed by high resolution of XPS spectra, which is consistent of XRD and TEM analysis.
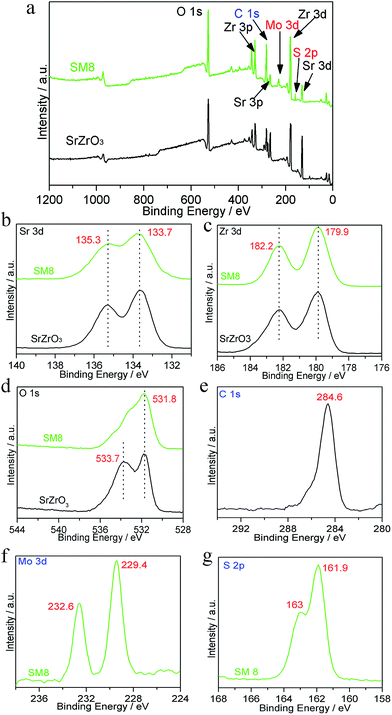 | ||
| Fig. 5 (a) XPS survey spectra of sample SrZrO3 and SM8, (b–f) high resolution XPS spectra of Sr, Zr, O, Mo and S. | ||
In order to investigated the photocatalytic performance of SrZrO3 photocatalyst before and after loading MoS2, a serious of experiments have been carried out and the results have been shown in Fig. 6a. It is clear that the rate of H2 evolution was obviously enhanced when loading a relative small amount of MoS2 with 0.05 wt%. The rate of H2 evolution for pure SrZrO3 photocatalyst was 1.95 mmol h−1, while the rate of H2 evolution of MoS2/SrZrO3 was 5.31 mmol h−1, nearly 2.7 times of pure samples, which is due to the effect of heterojunction between MoS2 and SrZrO3. As can be seen, H2 production was gradually decreased or even lower than pure SrZrO3 as the increasing amount of MoS2. It is mainly due to several reasons: the decrease of SrZrO3 surface active sites resulting from the excessive loading MoS2 cluster; deterioration of catalytic properties of SrZrO3 cluster; decrease of irradiation passing through the sacrifice solution.40 The stability and recycling performance of photocatalysts are important factors in the commercial applications, thus, time course of H2 production over MoS2/SrZrO3 was performed (Fig. 6b). Clearly, the average rate of H2 evolution was nearly unchanged after 30 h reaction, indicating an excellent stability of photocatalyst.
 | ||
| Fig. 6 (a) Photocatalytic activity of MoS2/SrZrO3 catalysts with loading different amount of MoS2, and (b) time course of H2 evolution over 0.05 MoS2 (0.05 wt%)/SrZrO3 photocatalyst. | ||
A possible mechanism of photocatalytic H2 evolution over MoS2/SrZrO3 heterojunction was proposed and depicted in Fig. 7. The SrZrO3 photocatalyst absorbed enough energy greater than band gap under UV light irradiation, and generated electron was easily transferred to conduction band, leaving photo-generated holes in the valence band. The catalytic activities without MoS2 loading were lower because of the recombination between photo-generated electrons and holes. While, the H2 evolution was significantly enhanced after loading MoS2 because of the rapid transfer of the photo-generated electrons from the conduction band of SrZrO3 samples to MoS2 which is due to the higher conduction band of SrZrO3 than that of MoS2. At the same time, the H+ was reduced to H2 with the MoS2 trapping of photo-generated electrons, and whereas holes of SrZrO3 crystals in the valence band oxidize S2− and SO32−on the surface of SrZrO3. The heterojunctions between SrZrO3 and MoS2 was considered as an important factor for the remarkably improved H2 evolution.
4. Conclusions
In summary, the MoS2/SrZrO3 heterojunction was synthesized for the first time through two-step hydrothermal reaction. The highest of H2 evolution rate of 5.31 mmol h−1 was obtained with 0.05 wt% MoS2 loading on the surface of SrZrO3 under UV irradiation, and the mechanism of process of H2 evolution was also proposed. Furthermore, the amount of MoS2 plays an important in photocatalytic activities, which were might due to the changes of active sites, band gaps and irradiation opacity. Obviously, the influences of MoS2 in MoS2/SrZrO3 heterogeneous photocatalyst were matching the band energy and suppressing the recombination between photogenerated electron and holes, resulting in the enhancement of H2 evolution.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (no. 31070517) and the National Basic Research Program of China (973 Program, no. 2013CB932902).Notes and references
- Y. Q. Zhong, K. Ueno, Y. Mori, X. Shi, T. Oshikiri, K. Murakoshi, H. Inoue and H. Misawa, Angew. Chem., Int. Ed., 2014, 53, 1 CrossRef.
- Z. J. Han, F. Qiu, R. Eisenberg, P. L. Holland and T. D. Krauss, Science, 2012, 338, 1321 CrossRef CAS PubMed.
- H. Kisch, Angew. Chem., Int. Ed., 2013, 52, 812 CrossRef CAS PubMed.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC.
- X. Zong, H. J. Yan, G. P. Wu, G. J. Ma, F. Y. Wen, L. Wang and C. Li, J. Am. Chem. Soc., 2008, 130, 7176 CrossRef CAS PubMed.
- T. F. Jaramillo, K. P. Jorgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- H. I. Karunadasa, C. J. Chang and J. R. Long, Nature, 2010, 464, 1329 CrossRef CAS PubMed.
- F. Bai, Z. C. Sun, H. M. Wu, R. E. Haddad, X. Y. Xiao and H. Y. Fan, Nano Lett., 2011, 11, 3759 CrossRef CAS PubMed.
- Y. Zhong, Z. X. Wang, R. F. Zhang, F. Bai, H. M. Wu, R. Haddad and H. Y. Fan, ACS Nano, 2014, 8, 827 CrossRef CAS PubMed.
- F. Bai, Z. C. Sun, H. M. Wu, R. E. Haddad, E. N. Coker, J. Y. Huang, M. A. Rodriguez and H. Y. Fan, Nano Lett., 2011, 11, 5196 CrossRef CAS PubMed.
- J. C. Colmenares and R. Luque, Chem. Soc. Rev., 2014, 43, 765 RSC.
- Y. Q. Qu and X. F. Duan, Chem. Soc. Rev., 2013, 42, 2568 RSC.
- Y. Miseki, H. Kato and A. Kudo, Energy Environ. Sci., 2009, 2, 306 CAS.
- Y. J. Su, K. L. Pan and M. B. Chang, Int. J. Hydrogen Energy, 2014, 39, 4917 CrossRef CAS PubMed.
- L. Q. Jing, Y. C. Qu, H. J. Su, C. H. Yao and H. G. Fu, J. Phys. Chem. C, 2011, 115, 12375 CAS.
- E. A. Slonimskaya and A. V. Belyakov, Glass Ceram., 2001, 58, 1 CrossRef.
- A. Y. Zhang, M. K. Lü, S. F. Wang, G. J. Zhou, S. M. Wang and Y. Y. Zhou, J. Alloys Compd., 2007, 433, 7 CrossRef PubMed.
- I. R. Shein, V. L. Kozhevnikov and A. L. Ivanovskii, Solid State Sci., 2008, 10, 217 CrossRef CAS PubMed.
- M. Daturi and G. Busca, Chem. Mater., 1995, 7, 2115 CrossRef.
- Y. L. Tian, B. B. Chang, Z. C. Yang, B. C. Zhou, F. N. Xi and X. P. Dong, RSC Adv., 2014, 4, 4187 RSC.
- Y. Liu, Y. X. Yu and W. D. Zhang, J. Phys. Chem. C, 2013, 117, 12949 CAS.
- J. L. Li, X. J. Liu, L. K. Pan, W. Qin, T. Q. Chen and Z. Sun, RSC Adv., 2014, 4, 9647 RSC.
- H. I. Karunadasa, E. Montalvo, Y. Sun, M. Majda, J. R. Long and C. J. Chang, Science, 2012, 335, 698 CrossRef CAS PubMed.
- Y. G. Li, H. L. Wang, L. M. Xie, Y. Y. Liang, G. S. Hong and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 7296 CrossRef CAS PubMed.
- F. Meng, J. T. Li, S. K. Cushing, M. J. Zhi and N. Q. Wu, J. Am. Chem. Soc., 2013, 135, 10286 CrossRef CAS PubMed.
- W. J. Zhou, Z. Y. Yin, Y. P. Du, X. Huang, Z. Y. Zeng, Z. X. Fan, H. Liu, J. Y. Wang and H. Zhang, Small, 2013, 9, 140 CrossRef CAS PubMed.
- G. Zhou, X. Y. Xu, J. Y. Yu, B. Feng, Y. Zhang, J. G. Hu and Y. X. Zhou, CrystEngComm, 2014, 16, 9025 RSC.
- X. Zong, G. P. Wu, H. J. Yan, G. J. Ma, J. Y. Shi, F. Y. Wen, L. Wang and C. Li, J. Phys. Chem. C, 2010, 114, 1963 CAS.
- T. N. Ye, Z. H. Dong, Y. N. Zhao, J. G. Yu, F. Q. Wang, S. K. Guo and Y. C. Zou, CrystEngComm, 2011, 13, 3842 RSC.
- Y. T. Lu, D. D. Wang, P. Yang, Y. K. Du and C. Lu, Catal. Sci. Technol., 2014, 4, 2650 CAS.
- J. R. Ran, J. G. Yu and M. Jaroniec, Green Chem., 2011, 13, 2708 RSC.
- L. F. Qi, J. G. Yu and M. Jaroniec, Phys. Chem. Chem. Phys., 2011, 13, 8915 RSC.
- Y. Lee, J. Lee, T. Noh, D. Byun, K. Yoo, K. Yamaura and E. Takayama-Muromachi, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 113101 CrossRef.
- R. Schafranek, J. D. Baniecki, M. Ishii, Y. Kotaka, K. Yamanka and K. Kurihara, J. Phys. D: Appl. Phys., 2012, 45, 55303 CrossRef.
- N. X. Li, B. Y. Zhou, P. H. Guo, J. C. Zhou and D. W. Jing, Int. J. Hydrogen Energy, 2013, 38, 11268 CrossRef CAS PubMed.
- U. Oemar, P. S. Ang, K. Hidajat and S. Kawi, Int. J. Hydrogen Energy, 2013, 38, 5525 CrossRef CAS PubMed.
- J. H. Liu, L. Zhang, Q. W. Tian, N. X. Li, J. C. Zhou and Y. M. Sun, J. Mater. Chem. A, 2014 10.1039/c4ta04984e.
- V. O. Koroteev, L. G. Bulusheva, I. P. Asanov, E. V. Shlyakhova, D. V. Vyalikh and A. V. Okotrub, J. Phys. Chem. C, 2011, 115, 21199 CAS.
- J. G. Yu, Y. Hai and B. Cheng, J. Phys. Chem. C, 2011, 115, 4953 CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |



