Ruthenium nanoparticle-intercalated montmorillonite clay for solvent-free alkene hydrogenation reaction
Abstract
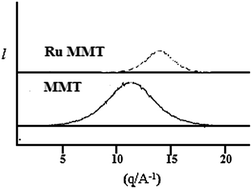
Well-characterized, ruthenium nanoparticle-intercalated montmorillonite clay was used as a catalyst in solvent-free alkene hydrogenation reactions and the corresponding products were obtained in good yields. The catalytic activity of ruthenium nanoparticle-intercalated montmorillonite clay was successfully tested with 16 different functionalized and non-functionalized alkenes. Apart from alkene reduction, the ruthenium nanoparticle-intercalated montmorillonite clay was also tested in Wittig-type reactions for obtaining dehydrobrittonin A, an important intermediate for the synthesis of brittonin A. Ruthenium nanoparticle-intercalated montmorillonite clay was found to be active in the synthesis of dehydrobrittonin A and brittonin A. The ability to recycle the catalyst nine times, together with low catalyst loading, high catalytic activity and catalytic selectivity were noteworthy advantages of the proposed protocol.

 Please wait while we load your content...
Please wait while we load your content...