Ag–Cu2O composite microstructures with tunable Ag contents: synthesis and surface-enhanced (resonance) Raman scattering (SE(R)RS) properties†
Lihua Yanga,
Jian Lva,
Yongming Suia,
Wuyou Fua,
Xiaoming Zhoub,
Jinwen Maa,
Qian Lia,
Meiling Suna,
Yannan Muac,
Yanli Chena,
Jun Wanga and
Haibin Yang*a
aState Key Laboratory of Superhard Materials, Jilin University, Changchun, 130012, China. E-mail: yanghb@jlu.edu.cn; Fax: +86 431 85168763; Tel: +86 431 85168763
bCollege of Physics, Beihua University, Jilin 132013, PR China
cDepartment of Physics and Chemistry, Heihe University, Heihe 164300, PR China
First published on 28th March 2014
Abstract
Metal–semiconductor composite microstructures have recently been demonstrated to possess potential applications due to their unique structures. Ag–Cu2O composite microstructures (Ag–Cu2O CMSs) with tunable silver content have been synthesized with a facile in situ method. Ag contents on the surface of Cu2O can be tuned through the variation of the concentration of AgNO3, which further greatly affected the surface-enhanced (resonance) Raman scattering (SE(R)RS) performance. The Ag–Cu2O CMSs prepared with 0.4 mM AgNO3 show the optimum SE(R)RS properties, better than pure Ag NPs. Furthermore, the enhancement mechanism and uniformity of Ag–Cu2O CMSs are investigated in detail.
1. Introduction
Cu2O is a p-type semiconductor oxide with a band gap of 2.17 eV, which makes it an excellent candidate for catalysis, gas sensors, chemical templates, CO oxidation, solar driven water splitting, fuel cells and solar cells.1–8 Over the past decades, an impressive effort has been devoted to the synthesis of Cu2O with designed shapes and desired functions, including polyhedral nanoparticles,9–12 nanosheets/rods/wires/tubes,13–16 hollows, nanocages and nanoframes.17–21 However, just a few studies have reported the use of Cu2O as Raman active substrate and Cu2O can usually generate weak Raman activity.22–25Surface-enhanced (resonance) Raman scattering (SE(R)RS) is a powerful analytical tool for determining chemical information of molecules on substrates for surface science, analytical chemistry, and biology.26–30 With SE(R)RS, extremely small amounts of substances can be detected; even single molecule detection has been reported.31–33 During the past few decades, SE(R)RS active substrate has been developed significantly. Noble metals and transition metals, such as Au,34 Ag,35 Cu,36 Pt,37 Pd,38 Co39 and Ni40 have been extensively employed owing to their large enhancement. Semiconductor materials have been used as SE(R)RS active substrate and caused increasing attention due to the widespread application in both SE(R)RS spectroscopy and material fields. Zhao's group got the SERS signal on the surface of TiO2 and ZnO nanoparticles.41–43 Other semiconductors such as Fe3O4, Ag2O, NiO, MoO3 and a-Fe2O3 have also been investigated.44–48 However, it is found that semiconductors can only directly generate weak SERS activity, much lower than metals such as Ag and Au. It is widely accepted that composite nanostructures can incorporate multiple functions into one system for specific applications and can induce fascinating new properties by the heterointerfaces.49 Therefore, semiconductor–metal composites are studied for their improved SERS effect. To further improve the SE(R)RS activity on Cu2O semiconductor, for practical applications, Ag is chosen as the other domain to prepare Ag–Cu2O composite microstructures (Ag–Cu2O CMSs). The Ag–Cu2O CMSs may exhibit the following advantages: (i) Ag is a more effective plasmonic material which can exhibit excellent Raman enhancement ability; (ii) Cu2O provide advantages in terms of chemical stability (inhibiting the aggregation of Ag nanostructures) and tune the localized surface plasmonic resonance (LSPR) of metallic nanostructures; (iii) the Ag–Cu2O CMSs with heterostructures will show fascinating SE(R)RS properties better than both Cu2O and pure Ag nanoparticles (NPs).
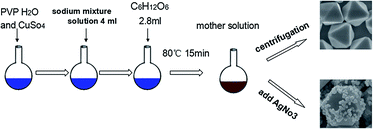
In this paper, we report a facile in situ method for the homogeneous growth of Ag NPs on the surfaces of Cu2O truncated octahedra by directly adding AgNO3 into Cu2O-containing mother solution. Ag+ is reduced to Ag, and then Ag NPs are in situ deposited onto primary Cu2O nanomaterials (shown in Scheme 1). The SE(R)RS properties of Ag–Cu2O CMSs using Rhodamine B (RB) as probing molecules is investigated. Through the variation of the concentration of AgNO3, Ag contents on the Cu2O truncated octahedra can be controllably tuned, which further greatly affect the SE(R)RS performance. When 0.4 mM AgNO3 is used, the prepared Ag–Cu2O CMSs exhibit good sensitivity SE(R)RS enhancements and excellent uniform response. Furthermore, the SE(R)RS enhancement mechanisms is discussed.
2. Experimental
2.1. Preparation of Ag–Cu2O composite microstructures
The preparation of Ag–Cu2O CMSs was based on our previous report with little modification.50 Briefly, 0.68 g copper sulfate was dispersed in 76 ml of deionized water, followed by addition of 4 ml of sodium mixture solution (0.74 M sodium citrate and 1.2 M sodium carbonate mixed solution) slowly. After the mixture was stirred for 10 min, 6 g PVP (K-30; Mw = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was added with vigorous stirring. After the complete dissolution of the PVP powder, 4 ml of 1.4 M glucose solution was slowly dropped into them. The solution was kept in a water bath at a temperature of 80 °C for 15 min (mother solution). In order to coat walls of the Cu2O with different density of Ag, AgNO3 was directly added into the mother solution with vigorous stirring for 20 min. Immediate color changed from deep red to deep gray, suggesting uniform Ag–Cu2O CMSs were formed.
000) was added with vigorous stirring. After the complete dissolution of the PVP powder, 4 ml of 1.4 M glucose solution was slowly dropped into them. The solution was kept in a water bath at a temperature of 80 °C for 15 min (mother solution). In order to coat walls of the Cu2O with different density of Ag, AgNO3 was directly added into the mother solution with vigorous stirring for 20 min. Immediate color changed from deep red to deep gray, suggesting uniform Ag–Cu2O CMSs were formed.
2.2. Characterization
X-ray powder diffraction (XRD) analysis was conducted on a Rigaku D/max-2500 X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). Field-emission scanning electron microscopic (FESEM) images were performed on a JEOL JEM-6700F microscope operating at 5 kV. Transmission electron microscopic (TEM) images and high-resolution transmission electron microscopic (HRTEM) images were obtained on a JEOL JEM-2000EX microscope with accelerating voltage of 200 kV and a JEOL JEM-3010 microscopy operated at 200 kV, respectively. UV-vis absorption spectra were recorded using a spectrophotometer (Shimadzu, 3100 UV-vis-NIR).2.3. SE(R)RS measurements
For SE(R)RS experiments, Rhodamine B (RB) dye was used as a Raman probe. The Ag–Cu2O CMSs substrate was incubated in the dark for 10 h in an aqueous solution containing 1 × 10−5 M RB. After the precipitate was centrifuged and dried, the Raman spectrum of the samples drop-casted onto glass slides was measured with a Renishaw Raman system model 1000 spectrometer. The 514.5 nm radiation from a 20 mW air-cooled argon ion laser was used as the exciting source. The laser power at the samples position was typically 400 μW. Data acquisition involved 30 s accumulations. For comparison purposes, the Ag NPs with the similar size as that coated on Cu2O were prepared in similar conditions by sodium citrate reducing AgNO3 aqueous solution (ESI†).3. Results and discussion
Fig. 1a and b show representative FESEM and TEM images of the product. It is quite intriguing to observe that Cu2O truncated octahedra composed of eight {111} planes and six {100} planes is formed, and Ag NPs with size of 80–100 nm are deposited on the surface of Cu2O truncated octahedra. To further study the structures of the samples, a typical HRTEM image of Ag and Cu2O taken from the interface of Ag–Cu2O CMSs is shown in Fig. 1c. The fringes with value of 0.24 nm is in good agreement with the (111) lattice spacing of Ag NPs, whereas the fringes with value of 0.30 nm is in good agreement with the (110) lattice spacing of Cu2O. Fig. 1d displays the XRD pattern of as-prepared Ag–Cu2O CMSs, the diffraction peaks can be assigned perfectly to cubic phase Cu2O (standard card JCPDS no. 05-0667; space group: Pn![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 0.4269 nm) and cubic phase Ag (JCPDS no. 04-0783; space group: Fm3m, a = 0.4086 nm). The results indicate that Ag NPs have been coated on the surface of Cu2O, consistent with the FESEM and HRTEM observations.
m, a = 0.4269 nm) and cubic phase Ag (JCPDS no. 04-0783; space group: Fm3m, a = 0.4086 nm). The results indicate that Ag NPs have been coated on the surface of Cu2O, consistent with the FESEM and HRTEM observations.
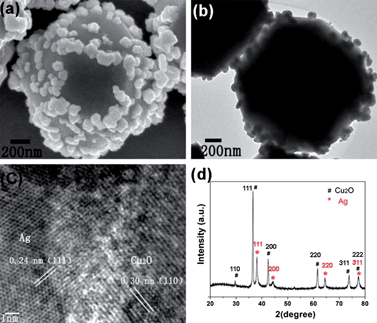 | ||
| Fig. 1 FESEM image (a), TEM image (b), HRTEM image (c) and XRD pattern (d) of as-prepared Ag–Cu2O CMSs by 0.4 mM AgNO3. | ||
3.1. Effect of AgNO3
It is found that AgNO3 plays a crucial role in the formation of the Ag–Cu2O CMSs. As shown in Fig. 2a, only Cu2O truncated octahedra is formed when no AgNO3 is used. When 0.2 mM AgNO3 is used, less Ag NPs are formed on surfaces of Cu2O truncated octahedral (Fig. 2b). Upon increasing the concentration of AgNO3 to 0.4 mM, the density of Ag and the effective coverage area of Ag on Cu2O truncated octahedra are enhanced significantly (Fig. 2c). When the concentration of AgNO3 is further increased to 0.6 mM, FESEM allowed us to identify the existence of Ag NPs aggregation, and the size of Ag NPs further increased to 300–500 nm (Fig. 2d). These results imply that Ag NPs coverage on the surfaces of Cu2O can be conveniently controlled by tuning the concentration of the Ag precursor. | ||
| Fig. 2 FESEM images of the Ag–Cu2O CMSs prepared by (a) 0 mM (b) 0.2 mM, (c) 0.4 mM and (d) 0.6 mM AgNO3. | ||
3.2. Growth mechanism
On the basis of our previously development, at the beginning of the reaction, the truncated octahedral Cu2O precursor particles were formed.50 After AgNO3 was directly added into the mother solution, glucose and sodium citrate in the mother solution are capable of reducing Ag+ to Ag.51–53 At an initial stage, large amounts of small Ag nucleate, and then they quickly deposited onto the Cu2O to form Ag–Cu2O CMSs in order to reduce the surface energy in the system. With the increase of AgNO3 concentrations, the evolution of Ag contents on the Cu2O particle from sparse dispersion to appropriate density and favourable interparticle spacing of Ag nanoparticles and finally entire coverage.3.3. SE(R)RS of Ag–Cu2O CMSs and Ag NPs
The SE(R)RS spectra of RB adsorbed on these Ag–Cu2O CMSs with different density of Ag NPs are compared in Fig. 3A. It is found that no SE(R)RS signal of RB is observed for Cu2O truncated octahedra (curve a), indicating no SE(R)RS effect is observed on Cu2O. But, distinct SE(R)RS signals are observed from curve b, c and d. All of above bands are associated with the RB at around 618, 1196, 1280, 1357, 1504, 1526, 1563, 1599, and 1649 cm−1.54 Obviously, (curve c) the Ag–Cu2O CMSs prepared by 0.4 mM AgNO3 exhibits the highest SE(R)RS performance. This may be due to the fact that the plasmonic interactions of Ag NPs due to the favorable interparticle spacing produced by the reaction conditions yield a higher density of SE(R)RS hot spots.55,56 Furthermore, the SE(R)RS signals of (curve c) Ag–Cu2O CMSs prepared by 0.4 mM AgNO3 are compared with that of bare Ag NPs as show in Fig. 3B. It is striking that the Ag–Cu2O CMSs prepared by 0.4 mM AgNO3 exhibit higher SE(R)RS signals even than pure Ag. This enhanced performance may derive from the synergistic effect between Ag and Cu2O, and more Raman hot spots are introduced not only due to the Ag hot spots but also the hot spots formed at the interface between Ag NPs and the Cu2O.573.4. Enhancement mechanisms
There are two main SE(R)RS enhancement mechanisms: electromagnetic (EM) and chemical. The EM enhancement is due to a large increase of the electric field caused by localized surface plasmon resonance (LSPR) induced by the laser light in nanosized metal clusters on the surface. LSPR makes a major contribution to the electromagnetic field enhancement and therefore SE(R)RS.58 In order to investigate the LSPR effect in Ag–Cu2O CMSs substrate, the Ag–Cu2O CMSs prepared by 0.4 mM AgNO3 was recorded by UV-vis spectroscopy, as shown in Fig. 4. It is noticed that the plasmon peak of Ag–Cu2O CMSs, compared to the Cu2O, shows an observed red shift. Shan et al. show that the surface plasmon absorption may change due to the interaction between Ag and semiconductor ZnO quantum dots.59 After the deposition of Ag NP onto Cu2O, a metal–semiconductor heterostructure is formed. As the Fermi energy level of Ag (4.26 eV) is lower than that of Cu2O (5.1 eV), electrons will transfer from Ag to Cu2O, leading to a consequent red shift of the plasmon absorption. The charge redistribution results in a positively charged Ag NPs and negatively charged Cu2O (as shown in Scheme 2 ESI†), which induces a larger electromagnetic field, as validated by the XPS spectra and related density functional theory (DFT) calculations of Li's group.60 Under the larger electromagnetic field, Ag NPs would excite a more intense LSPR under the irradiation of a suitable laser, and therefore makes a major contribution to the SE(R)RS enhancement. The RB molecules adsorbed on the Ag surface are located in this enhanced electromagnetic field and consequently exhibit a stronger Raman signal.3.5. Uniformity, sensitivity and enhancement factor
To test the sensitivity of Ag–Cu2O CMSs substrate, SE(R)RS spectra of RB with different concentrations (10−3 to 10−8 M) adsorbed on Ag–Cu2O CMSs substrate prepared by 0.4 mM AgNO3 were obtained. As shown in Fig. 5, the intensity of the peaks decreases with the decreasing of the RB concentration. The peak at 1649 cm−1 can be observed as low as 1 × 10−8 M. The EF of Ag–Cu2O CMSs substrate is ∼7.8 × 104, which is calculated using (ISERS/INR)(CNR/CSERS). The ISERS could be obtained from 10−8 M RB on the Ag–Cu2O CMSs sample. The INR was obtained from the normal Raman intensity of RB (10−2 M) solution. CNR and CSERS represent the concentrations of RB solution. These results indicate that the prepared substrate has good sensitivity. | ||
| Fig. 5 Raman spectra of RB with different concentrations adsorbed on Ag–Cu2O CMSs prepared by 0.4 mM AgNO3. | ||
The uniform response is another essential parameter for quantitative SE(R)RS substrate. Fig. 6 displays the Raman spectra recorded from seven randomly selected positions on Ag–Cu2O CMSs substrate prepared by 0.4 mM AgNO3. The peak at 1649 cm−1 is chosen to calculate the relative standard deviation (RSD) of Raman intensity of spot-to-spot. The RSD is lower than 9.3% from seven points, which reveals that the uniform response of the measurements is excellent. These results suggest that Ag–Cu2O CMSs substrate prepared by 0.4 mM AgNO3 has a good SE(R)RS enhancements and a uniform response, which are important for practical assays.
 | ||
| Fig. 6 The uniform response of Ag–Cu2O CMSs substrate prepared by 0.4 mM AgNO3, the inset shows the intensity change of peak at 1649 cm−1. | ||
4. Conclusion
In summary, Ag–Cu2O CMSs have been synthesized with a facile in situ method. The density of Ag NPs coated on Cu2O can be controlled by tuning the concentration of AgNO3. The SE(R)RS property of Ag–Cu2O CMSs was investigated. There was an optimum amount of Ag NPs. When 0.4 mM AgNO3 was used, the prepared Ag–Cu2O CMSs showed the highest SE(R)RS properties, even better than pure Ag. Besides the good sensitivity SE(R)RS enhancements, our substrates display good reproducibility suggest that this substrate has a potential application in SE(R)RS detection. It is expected that this work can provide valuable information for design of new composites.Acknowledgements
This work was financially supported by the Science and Technology Development Program of Jilin Province (20100417), the Postdoctoral Science Foundation of China (no. 801130120411) and the National Natural Science Foundation of China (51272086, 3A512Q191460).Notes and references
- (a) Z. Zhang, C. Zhong, Y. Deng, L. Liu, Y. Wu and W. Hu, RSC Adv., 2013, 3, 6763 RSC; (b) X. Meng, G. Tian, Y. Chen, Y. Qu, J. Zhou, K. Pan, W. Zhou, G. Zhang and H. Fu, RSC Adv., 2012, 2, 2875 RSC; (c) C. Chen, H. Xu, L. Xu, F. Zhang, J. Dong and H. Wang, RSC Adv., 2013, 3, 25010–25018 RSC; (d) T. Kou, C. Jin, C. Zhang, J. Sun and Z. Zhang, RSC Adv., 2012, 2, 12636 RSC.
- (a) X. W. Liu, F. Y. Wang, F. Zhen and J.-R. Huang, RSC Adv., 2012, 2, 7647 RSC; (b) X. W. Liu, RSC Adv., 2011, 1, 1119 RSC; (c) Y. M. Sui, Y. Zeng, W. T. Zheng, B. B. Liu, B. Zou and H. B. Yang, Sens. Actuators, B, 2012, 171, 135 CrossRef PubMed; (d) C. P. Zhao, X. W. Zhang, Y. P. Zhang, Y. L. Xing, X. J. Zhang, X. H. Zhang and J. H. Jie, CrystEngComm, 2012, 14, 819 RSC.
- (a) W. C. Huang, L. M. Lyu, Y. C. Yang and M. H. Huang, J. Am. Chem. Soc., 2012, 134, 1261 CrossRef CAS PubMed; (b) S. Sun, X. Zhang, X. Song, S. Liang, L. Wang and Z. Yang, CrystEngComm, 2012, 14, 3545 RSC.
- Z. Y. Wang, D. Y. Luan, C. M. Li, F. B. Su, S. Madhavi, F. Y. C. Boey and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 16271 CrossRef CAS PubMed.
- A. Paracchino, V. Laporte, K. Sivula, M. Grätzel and E. Thimsen, Nat. Mater., 2011, 10, 456 CrossRef CAS PubMed.
- M. Leng, M. Z. Liu, Y. B. Zhang, Z. Q. Wang, C. Yu, X. G. Yang, H. J. Zhang and C. Wang, J. Am. Chem. Soc., 2010, 132, 17084 CrossRef CAS PubMed.
- X. Wang, C. Liu, B. J. Zheng, Y. Q. Jiang, L. Zhang, Z. X. Xie and L. S. Zheng, J. Mater. Chem. A, 2013, 1, 282 CAS.
- (a) K. Chen, S. Song and D. Xue, CrystEngComm, 2013, 15, 10028–10033 RSC; (b) Y.-K. Hsu, C.-H. Yu, Y.-C. Chen and Y.-G. Lin, RSC Adv., 2012, 2, 12455 RSC; (c) M. Hara, T. Kondo, M. Komoda, S. Ikeda, K. Shinohara, A. Tanaka, J. N. Kondo and K. Domen, Chem. Commun., 1998, 357 RSC.
- (a) M. J. Siegfried and K.-S. Choi, Angew. Chem., 2005, 117, 3282 CrossRef PubMed; (b) M. J. Siegfried and K.-S. Choi, Adv. Mater., 2004, 16, 19 CrossRef PubMed.
- (a) S. L. Shinde and K. K. Nanda, RSC Adv., 2012, 2, 3647 RSC; (b) B. J. Li, Y. Y. Li, Y. B. Zhao and L. Sun, J. Phys. Chem. Solids, 2013, 74, 1842 CrossRef CAS PubMed.
- (a) Y. B. Cao, J. M. Fan, L. Y. Bai, F. L. Yuan and Y. F. Chen, Cryst. Growth Des., 2010, 10, 232 CrossRef CAS; (b) L. H. Yang, Y. M. Sui, W. Y. Zhao, W. Y. Fu and H. B. Yang, et al., CrystEngComm, 2011, 13, 6265 RSC.
- (a) S. D. Sun, X. P. Song, C. C. Kong and Z. M. Yang, CrystEngComm, 2011, 13, 6616 RSC; (b) J. Watt, S. Cheong and R. D. Tilley, Nano Today, 2013, 8, 198 CrossRef CAS PubMed.
- J. H. Zhong, G. R. Li, Z. L. Wang, Y. N. Ou and Y. X. Tong, Inorg. Chem., 2011, 50, 757 CrossRef CAS PubMed.
- (a) L. Gou and C. J. Murphy, Nano Lett., 2003, 3, 231 CrossRef CAS; (b) M. J. Siegfried and K.-S. Choi, J. Am. Chem. Soc., 2006, 128, 10356 CrossRef CAS PubMed.
- (a) X. Liang, L. Gao, S. Yang and J. Sun, Adv. Mater., 2009, 21, 1 Search PubMed; (b) R. Liu, F. Oba, E. W. Bohannan, F. Ernst and J. A. Switzer, Chem. Mater., 2003, 15, 4882 CrossRef CAS.
- (a) S. Sahoo, S. Husale, B. Colwill, T.-M. Lu, S. Nayak and P. M. Ajayan, ACS Nano, 2009, 3, 3935 CrossRef CAS PubMed; (b) W. Wang, G. Wang, X. Wang, Y. Zhan, Y. Liu and C. Zheng, Adv. Mater., 2002, 14, 67 CrossRef CAS.
- Y. M. Sui, W. Y. Fu, Y. Zeng, H. B. Yang, Y. Y. Zhang, H. Chen, Y. X. Li, M. H. Li and G. T. Zou, Angew. Chem., Int. Ed., 2010, 49, 4282 CrossRef CAS PubMed.
- (a) H. Liu, Y. Zhou, S. A. Kulinich, J. J. Li, L. L. Han, S. Z. Qiao and X. W. Du, J. Mater. Chem. A, 2013, 1, 302 RSC; (b) H. Li, Y. H. Ni, Y. F. Cai, L. Zhang, J. Z. Zhou, J. M. Hong and X. W. Wei, J. Mater. Chem., 2009, 19, 594 RSC.
- C. P. Zhao, X. W. Zhang, Y. P. Zhang, Y. L. Xing, X. J. Zhang, X. H. Zhang and J. S. Jie, CrystEngComm, 2012, 14, 819 RSC.
- (a) H. Xu and W. Wang, Angew. Chem., Int. Ed., 2007, 46, 1489 CrossRef CAS PubMed; (b) W. Wang, Y. Tu, P. Zhang and G. Zhang, CrystEngComm, 2011, 13, 1838 RSC.
- (a) Y. Chang, J. Teo and H. Zeng, Langmuir, 2005, 21, 1074 CrossRef CAS PubMed; (b) W. Z. Wang, P. C. Zhang, L. Peng, W. J. Xie, G. L. Zhang, Y. Tu and W. J. Mai, CrystEngComm, 2010, 12, 700 RSC.
- A. Kudelski, W. Grochala, M. Janik-Czachor, J. Bukowska, A. Szummer and M. Dolata, J. Raman Spectrosc., 1998, 29, 431 CrossRef CAS.
- C. Qiu, Y. Bao, N. L. Netzer and C. Y. Jiang, J. Mater. Chem. A, 2013, 1, 8790 CAS.
- R. C. Wang and H. Y. Lin, Mater. Chem. Phys., 2012, 136, 661 CrossRef CAS PubMed.
- L. Jiang, T. You, P. Yin, Y. Shang, D. Zhang, L. Guo and S. Yang, Nanoscale, 2013, 5, 2784 RSC.
- (a) G. Mcnay, D. Eustace, W. E. Smith, K. Faulds and D. Graham, Appl. Spectrosc., 2011, 65, 825–837 CrossRef CAS PubMed; (b) M. Fleischmann, P. J. Hendra and A. J. Mcquillan, Chem. Phys. Lett., 1974, 26, 163 CrossRef CAS; (c) J. Reed, P. Eisenberger, B. K. Teo and B. M. Kincaid, J. Am. Chem. Soc., 1977, 99, 5217 CrossRef CAS.
- (a) Y. C. Cao, R. Jin, J.-M. Nam, C. S. Thaxton and C. A. Mirkin, J. Am. Chem. Soc., 2003, 125, 14676 CrossRef CAS PubMed; (b) D. Zhang, Y. Xie, M. F. Mrozek, C. Ortiz, V. J. Davisson and D. Ben-Amotz, Anal. Chem., 2003, 75, 5703 CrossRef CAS PubMed.
- (a) T. Takahashi, S. Kuroiwa, T. Ogura and S. Yoshikawa, J. Am. Chem. Soc., 2005, 127, 9970 CrossRef CAS PubMed; (b) S. D. Hudson and G. Chumanov, Anal. Bioanal. Chem., 2009, 394, 679 CrossRef CAS PubMed.
- A. M. Mohs, M. C. Mancini, S. Singhal, J. M. Provenzale, B. Leyland Jones, M. D. Wang and S. M. Nie, Anal. Chem., 2010, 82, 9058 CrossRef CAS PubMed.
- S. Feng, D. Lin, J. Lin, B. Li and Z. Huang, et al., Analyst, 2013, 138, 3967 RSC.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. Dasari and M. S. Feld, Phys. Rev. Lett., 1998, 78, 1667–1670 CrossRef.
- (a) S. M. Nie and S. R. Emory, Science, 1977, 275, 1102–1106 CrossRef PubMed; (b) A. Otto, J. Raman Spectrosc., 2002, 33, 593–398 CrossRef CAS PubMed.
- I. Delfino, A. R. Bizzarri and S. Cannistraro, Biophys. Chem., 2005, 113, 41–51 CrossRef CAS PubMed.
- (a) J. Fang, S. Lebedkin, S. Yang and H. Hahn, Chem. Commun., 2011, 47, 5157 RSC; (b) M. Z. Si, Y. P. Kang and Z. G. Zhang, J. Raman Spectrosc., 2009, 40, 1319 CrossRef CAS PubMed; (c) A. C. Manikas, A. S. Beobide and G. A. Voyiatzis, Analyst, 2009, 134, 587–592 RSC.
- (a) Y. Wang, T. Gao, K. Wang, X. Wu, X. Shi, Y. Liu, S. Lou and S. Zhou, Nanoscale, 2012, 4, 7121 RSC; (b) C. Wang, J. Fang, Y. Jin and M. Cheng, Appl. Surf. Sci., 2011, 258, 1144 CrossRef CAS PubMed; (c) M. Zhang, Z. Cao and L. Yobas, Sens. Actuators, B, 2013, 184, 235 CrossRef CAS PubMed.
- (a) C. Kong, S. Sun, X. Zhang, X. Song and Z. Yang, CrystEngComm, 2013, 15, 6136 RSC; (b) E. B. Santos, F. A. Sigoli and I. O. Mazali, Mater. Lett., 2013, 108, 172 CrossRef PubMed.
- B. Ren, G. K. Liu, X. B. Lian, Z. L. Yang and Z. Q. Tian, Anal. Bioanal. Chem., 2007, 388, 29 CrossRef CAS PubMed.
- L. M. Chen and Y. N. Liu, CrystEngComm, 2011, 13, 6481 RSC.
- D. Tsoutsi, L. Guerrini, J. M. Hermida-Ramon and V. Giannini, et al., Nanoscale, 2013, 5, 5841 RSC.
- (a) B. Ren, X. F. Lin, J. W. Yan, B. W. Mao and Z. Q. Tian, J. Phys. Chem. B, 2003, 107, 899 CrossRef CAS; (b) Z. Liu, Z. L. Yang, L. Cui, B. Ren and Z. Q. Tian, J. Phys. Chem. C, 2007, 111, 1170 Search PubMed.
- L. Yang, X. Jiang, W. Ruan, B. Zhao, W. Xu and J. R. Lombardi, J. Phys. Chem. C, 2008, 112, 20095 CAS.
- Z. H. Sun, B. Zhao and J. R. Lombardi, Appl. Phys. Lett., 2007, 91, 221106 CrossRef PubMed.
- Y. Wang, Z. Sun, Y. Wang, H. Hu, S. Jing, B. Zhao, W. Xu, C. Zhao and J. R. Lombardi, J. Raman Spectrosc., 2007, 38, 34 CrossRef CAS PubMed.
- Q. Gao, A. W. Zhao, Z. B. Gan, W. Y. Tao, D. Li, M. F. Zhang, H. Guo and D. Wang, et al., CrystEngComm, 2012, 14, 4834 RSC.
- X. Q. Wang, H. Wen, T. J. He, J. Zuo, C. Y. Xu and F. C. Liu, Spectrochim. Acta, Part A, 1997, 53, 2495 CrossRef.
- B. H. Loo, J. Electroanal. Chem., 1982, 136, 209 CrossRef CAS.
- X. Q. Fu, F. L. Bei, X. Wang, X. J. Yang and L. Lu, J. Raman Spectrosc., 2009, 40, 1290 CrossRef CAS PubMed.
- X. Fu, F. Bei, X. Wang, X. Yang and L. Lu, Mater. Lett., 2009, 63, 185 CrossRef CAS PubMed.
- R. Costi, A. E. Saunders and U. Banin, Angew. Chem., Int. Ed., 2010, 49, 4878 CrossRef CAS PubMed.
- Y. Sui, W. Fu, H. Yang, Y. Zeng and Y. Zhang, et al., Cryst. Growth Des., 2010, 10, 99 CAS.
- (a) P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391 CrossRef CAS; (b) M. Z. Si, Y. P. Kangc and Z. G. Zhanga, J. Raman Spectrosc., 2009, 40, 1319 CrossRef CAS PubMed.
- (a) C. Xue, G. S. Métraux, J. E. Millstone and C. A. Mirkin, J. Am. Chem. Soc., 2008, 130, 8337 CrossRef CAS PubMed; (b) J. Zhang, M. R. Langille and C. A. Mirkin, Nano Lett., 2011, 11, 2495 CrossRef CAS PubMed.
- X. Sun and Y. Li, Adv. Mater., 2005, 17, 2626–2630 CrossRef CAS PubMed.
- (a) C. Fang, A. V. Ellis and N. H. Voelcker, Electrochim. Acta, 2012, 59, 346 CrossRef CAS PubMed; (b) Z. Zheng, S. Tang, S. Vongehr and X. Meng, Mater. Chem. Phys., 2011, 129, 594–598 CrossRef CAS PubMed.
- J. Li, Q. Kan, C. Wang and H. Chen, Chin. Opt. Lett., 2011, 9, 090501–090504 CrossRef.
- (a) U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Springer, Berlin, 1995 Search PubMed; (b) C. F. Bohren and D. R. Huffman, Absorption and Scattering of Light by Small Particles, John Wiley and Sons, New York, 1983 Search PubMed.
- S. L. Kleinman, R. R. Frontiera, A.-I. Henry, J. A. Dieringer and R. P. Van Duyne, Phys. Chem. Chem. Phys., 2013, 15, 21–36 RSC.
- (a) Y. K. Mishra, S. Mohapatra, R. Singhal, D. K. Avasthi, D. C. Agarwal and S. B. Ogale, Appl. Phys. Lett., 2008, 92, 043107 CrossRef PubMed; (b) Y. X. Wang, W. Song, W. D. Ruan, J. X. Yang, B. Zhao and J. R. Lombardi, J. Phys. Chem. C, 2009, 113, 8065 CrossRef CAS.
- G. Y. Shan, L. H. Xu, G. R. Wang and Y. C. Liu, J. Phys. Chem. C, 2007, 111, 3290–3293 CAS.
- L. L. Li, X. B. Chen, Y. Wu, D. S. Wang, Q. Peng, G. Zhou and Y. D. Li, Angew. Chem., Int. Ed., 2013, 52, 11049 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00675e |
| This journal is © The Royal Society of Chemistry 2014 |