Homolysis/mesolysis of alkoxyamines activated by chemical oxidation and photochemical-triggered radical reactions at room temperature†
Abstract
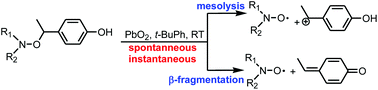
Alkoxyamines, which are connected with a phenol moiety by a (substituted) methylene bridge undergo homolytic cleavage upon chemical oxidation or a photo-induced hydrogen transfer. This selectively triggered reaction yields a nitroxide radical. In the presence of an excess of lead dioxide as the oxidant in tert-butylbenzene as solvent, spontaneous, instantaneous and almost quantitative generations of nitroxides from various alkoxyamines are observed at room temperature, which support activation energies for the cleavage lower than 100 kJ mol−1. The rate and the amount of released nitroxide depend on the amount of “catalyst” and the structure of alkoxyamines.
- This article is part of the themed collections: Recent Open Access Articles in Frontiers Journals and FOCUS: Radical-involved chemical transformations


 Please wait while we load your content...
Please wait while we load your content...