Intramolecular iron-catalyzed transannulation of furans with O-acetyl oximes: synthesis of functionalized pyrroles†
Abstract
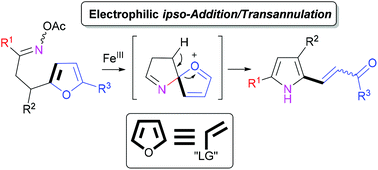
Furyl-tethered O-acetyl oximes undergo a rearrangement to yield densely substituted pyrroles in the presence of FeCl3·6H2O. The reactivity of a furan ring resembles the behaviour of an activated olefin with a formal leaving group that reacts with electrophilic nitrogen in a 5-exo-trig manner to form a spiropyrroline intermediate followed by aromatization of pyrrole via an intermediate ring opening. In contrast to the common activation modes of O-acyl oximes that comprise homolysis, SET-reduction or oxidative addition of low-valent transition metal complexes across the N–O bond, in the present case the catalyst acts as a Lewis acid activating oxime nitrogen for a nucleophilic attack by the reaction partner. The studied reaction was integrated into a telescoping protocol for the synthesis of a broad range of pyrroles, which could find possible applications as flexible matrices for designing push–pull chromophores and as potential building blocks in organic chemistry.


 Please wait while we load your content...
Please wait while we load your content...