An expedient, mild and aqueous method for Suzuki–Miyaura diversification of (hetero)aryl halides or (poly)chlorinated pharmaceuticals†‡
Abstract
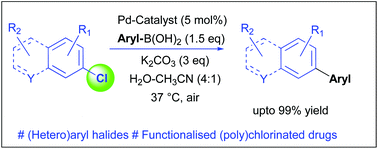
The development of mild, aqueous conditions for the cross-coupling of highly functionalized (hetero)aryl chlorides or bromides is attractive, enabling their functionalization and diversification. Herein, we report a general method for Suzuki–Miyaura cross-coupling at 37 °C in aqueous media in the presence of air. We demonstrate application of this general methodology for derivatisation of (poly)chlorinated, medicinally active compounds and halogenated amino acids. The approach holds the potential to be a useful tool for late-stage functionalization or analogue generation.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...