Facile synthesis of penta-substituted pyrroles and pyrrole-fused piperidin-4-ones via four component reactions of 2,3-diketoesters, anilines and enaminones†
Abstract
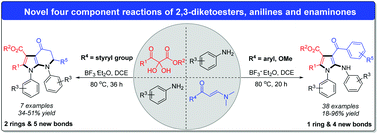
Given the importance of pyrroles in pharmaceuticals, agrochemicals and functional materials, the development of efficient strategies for their construction continues to be a hot area. Herein, we present a novel BF3·Et2O mediated four component reaction of 2,3-diketoesters, anilines and enaminones, providing highly functionalized pyrroles and pyrrole-fused piperidin-4-ones in moderate to good yields. The key to success lies in the utilization of enaminone as the substrate and precise capture of the carbocation intermediate by anilines, allowing the amination to occur at the 5-position smoothly. This strategy is potentially applicable to diverse nucleophilic reagents. Moreover, an intramolecular version of this strategy can be utilized to form fused heterocycles.


 Please wait while we load your content...
Please wait while we load your content...