Engineered cyclodextrin-based supramolecular hydrogels for biomedical applications
Yuqi
Zhao†
,
Zhi
Zheng†
*,
Cui-Yun
Yu
 * and
Hua
Wei
* and
Hua
Wei
 *
*
Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study & School of Pharmaceutical Science, Hengyang Medical School, University of South China, 28 W Changsheng Road, Hengyang 421001, Hunan, China. E-mail: zhengzhi@usc.edu.cn; yucuiyunusc@hotmail.com; weih@usc.edu.cn
First published on 27th November 2023
Abstract
Cyclodextrin (CD)-based supramolecular hydrogels are polymer network systems with the ability to rapidly form reversible three-dimensional porous structures through multiple cross-linking methods, offering potential applications in drug delivery. Although CD-based supramolecular hydrogels have been increasingly used in a wide range of applications in recent years, a comprehensive description of their structure, mechanical property modulation, drug loading, delivery, and applications in biomedical fields from a cross-linking perspective is lacking. To provide a comprehensive overview of CD-based supramolecular hydrogels, this review systematically describes their design, regulation of mechanical properties, modes of drug loading and release, and their roles in various biomedical fields, particularly oncology, wound dressing, bone repair, and myocardial tissue engineering. Additionally, this review provides a rational discussion on the current challenges and prospects of CD-based supramolecular hydrogels, which can provide ideas for the rapid development of CD-based hydrogels and foster their translation from the laboratory to clinical medicine.
1. Introduction
Cyclodextrins (CDs), first discovered by Villers in 1891, are a class of naturally occurring water-soluble macrocyclic compounds consisting of multiple D-glucopyranose units linked by α-1,4 glycosidic bonds.1 α-CDs, β-CDs, and γ-CDs are the three most common types of CDs, composed of six, seven, and eight D-glucopyranose units, respectively, with different cavity sizes.2 The hydrophilicity of CDs is conferred by the secondary hydroxyl group located at the C2/3 position of the larger open end of the outer surface and the primary hydroxyl group located at the C6 position of the smaller open end, while the hydrophobicity is conferred by the shielding effect of the C–H bonds that form the hydrophobic cavities.3,4 Due to the unique properties of CDs’ hydrophilic outer edges and hydrophobic inner cavities, they can effectively serve as hydrophobic binding sites for various guest molecules.5 The interactions between CDs and guest molecules involve van der Waals (homogeneous, dispersive, and induced forces), coulombic, hydrophobic, and hydrogen bonding forces.6 Furthermore, the abundance of reactive hydroxyl groups in CDs can be chemically modified to result in a wide variety of functional cyclodextrin derivatives.7 These unique properties of CDs have been of widespread interest in the construction of supramolecular injectable hydrogels.8There are two main methods for the preparation of CD-based supramolecular hydrogels. One method involves forming a poly(pseudo)rotaxane (PPR) structure, where linear polymer chains penetrate into the cavities of the CD.9 The other method involves forming an inclusion complex between the CD and the guest molecule. CD-based supramolecular hydrogels are distinguished by dynamically reversible non-covalent bonding, such as host–guest interactions and hydrogen bonding, making them shear-thinning and self-healing.10 For example, the hydrogel transitions to a flowable state when sufficient shear stress is applied during the injection process, and then recovers to a gel state when the high shear stress is removed.11 This makes supramolecular hydrogels suitable for forming drug reservoirs in target tissues in a mini-invasive manner and allows for continuous and controllable drug release.12 However, the weak mechanical strength and large pore structure of CD-based supramolecular hydrogels severely limit their effectiveness in controlled drug release, stability, and therapeutic ability. To overcome this limitation, a frequent strategy is to regulate the mechanical behavior of supramolecular hydrogels by adjusting the number of host and guest molecules.13,14 However, due to the special structure of macromolecule CDs, the efficiency of CD modification of the macromolecule chain is limited, and fails to significantly improve the mechanical properties of hydrogels. Therefore, in-depth elaboration of the cross-linking of CD-based supramolecular hydrogels is essential to optimize their mechanical properties and drug loading modes, which are major priorities for their effective use in the treatment of various diseases.
Several recently published reviews on CD-based supramolecular materials primarily focus on the preparation, stimuli-responsive drug delivery, and other biological applications of these materials.8,15–18 However, there is, to our knowledge, a lack of detailed discussion regarding the optimization of the mechanical properties and the different drug loading and release modalities of CD-based supramolecular hydrogels.19–21 CD-based supramolecular hydrogels have garnered significant attention due to their self-healing and shear-thinning properties. Nonetheless, the tradeoff between the self-healing properties and mechanical strength of these hydrogels severely limits their practical applications in various biomedical fields.22 Therefore, there is considerable scope to clarify the underlying relationship between the self-healing properties and mechanical strength for the development of CD-based supramolecular hydrogels with balanced properties. For example, supramolecular hydrogel dressings with excellent self-healing and mechanical properties can prevent bacterial infection or premature drug leakage caused by structural damage.23 High-strength supramolecular hydrogels can provide mechanical support in bone/cartilage repair.24 Hydrogels with favorable mechanical strength enable more controllable, stable, and effective drug delivery. Furthermore, the appropriate drug loading and release modalities of supramolecular hydrogels are crucial for the effective treatment of various diseases.12
As detailed above, this review aims to fill the existing gap in the current systematized knowledge by conducting comprehensive investigations on the crosslinking mechanism, optimization of mechanical properties, drug loading and release modalities of CD-based supramolecular hydrogels. In addition, the advantages of CD-based supramolecular hydrogels for targeted drug delivery in specific diseases, such as tumor, bone/cartilage defect, and myocardial infarction, are systematically summarized. Finally, the challenges and opportunities for promoted clinical translations of CD-based supramolecular hydrogels are proposed (Scheme 1). This review is expected to serve as valuable guidance for the next generation of advanced CD-based supramolecular hydrogels in the field of biomedicine from bench to bedside.
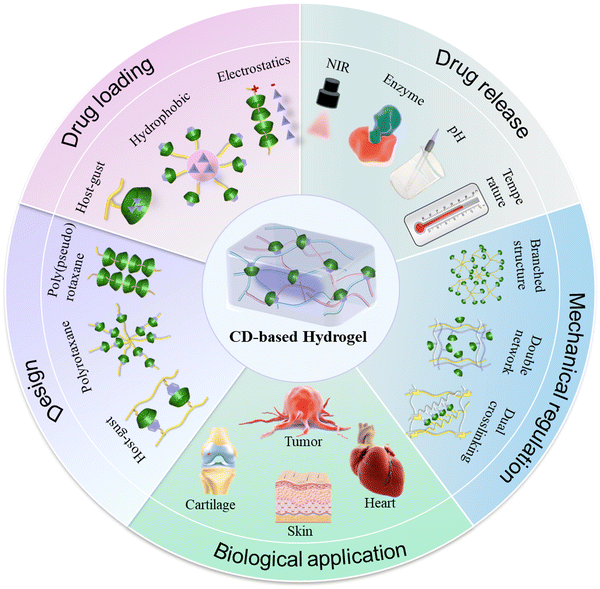 | ||
| Scheme 1 Schematic illustration of the design and biological applications of CD-based supramolecular hydrogels. | ||
2. Design strategy of CD-based supramolecular hydrogels
2.1 Hydrogen bonding
Polyrotaxane (PR) and poly(pseudo)rotaxane (PPR) are unique supramolecular structures that consist of a “necklace” of molecules. These structures are formed by threading multiple cyclodextrin (CD) molecules onto polymer chains, which may or may not have bulky end groups. The presence of cyclodextrins on neighboring polymer chains allows for strong hydrogen bonding interactions, leading to cross-linking of the PR or PPR structures. This cross-linking is responsible for the formation of CD-based PR or PPR supramolecular hydrogels.25–27The size match between the polymer chain and the CD cavity is crucial for the formation of PPR. α-, β-, and γ-CDs have different cavity sizes and can form PPR structures with various polymer chains.28–31 Among them, the combination of α-CD and PEG is currently the most frequently used. Harada et al. initially reported that α-CD can form α-CD/PEG supramolecular hydrogels using PEG with a molecular weight greater than 2 kDa in aqueous solution. The mechanical properties of hydrogels gradually enhanced with the increasing molecular weight of PEG.32,33
These α-CD/PEG PPR hydrogels often face challenges such as rapid drug release and inadequate mechanical properties due to weak non-covalent cross-linking. Although the mechanical properties of hydrogels can be improved with an increased molecular weight of PEG, the poor biodegradability associated with the high molecular weight of PEG in turn limits their biological applications. To achieve a balanced performance between the biodegradability and mechanical properties, various biodegradable linear block copolymers, star polymers, PEG-grafted polymers, or nanoparticles have been frequently incorporated into PPR hydrogels to overcome these limitations.34–37 The incorporation of diverse polymers or nanoparticles enhances the multifunctionality of PPR hydrogels, thereby expanding their applications in biomedicine.37 For example, Tween 80 (T80) is a surfactant that can self-assemble and form micellar structures containing a hydrophilic PEG shell at a specific concentration.38 Tang et al. demonstrated the development of a PPR hydrogel with tunable stiffness via the complexation between T80 and α-CDs, as well as the hydrogen bonding between α-CDs. The hydrogen bonding between CDs and low molecular weight heparin (LMWH) enabled the effective loading and sustained release of LMWH (Fig. 1A).39 Additionally, PEG can be modified on the polymer chain to further enhance the cross-linking density of the hydrogel due to its branching structure. Hwang et al. synthesized hyaluronic acid modified with PEG and dopamine, and then constructed a physical–chemical double cross-linked injectable self-healing hydrogel using the PPR structure formed by PEG and α-CD, as well as the autopolymerization of dopamine in an alkaline environment (Fig. 1B).40
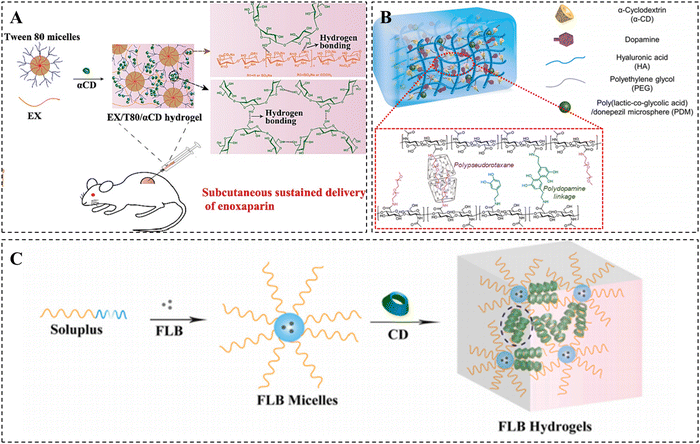 | ||
| Fig. 1 Construction of CD-based supramolecular hydrogels based on PPR. (A) Enoxaparin-loaded supramolecular hydrogel prepared via inclusion complexation between Tween 80 and α-CDs, as well as hydrogen bonding between α-CDs. Reproduced with permission from ref. 39. Copyright 2022, Elsevier. (B) PDM-loaded hydrogel based on PPR and polydopamine. Reproduced with permission from ref. 40. Copyright 2021, Elsevier. (C) FLB hydrogel cross-linked by host–guest recognition between the Soluplus micelles and γ-CDs, as well as the strong hydrogen bonding between γ-CDs. Reproduced with permission from ref. 43. Copyright 2022, Elsevier. | ||
In contrast to α-CD, γ-CD allows for the simultaneous crossing of two PEG chains due to its larger cavity size.41,42 For instance, Fang et al. prepared a shear-thinning supramolecular hydrogel platform based on γ-CD for efficient and safe delivery of ocular drugs (Fig. 1C).43 The crosslinking of this hydrogel primarily relies on the host–guest recognition between the PEG of Soluplus micelles and γ-CDs, as well as the hydrogen bonding interactions between γ-CDs. The drug flurbiprofen (FLB) was loaded into the hydrophobic core of Soluplus micelles. The results demonstrated that FLB hydrogels significantly decreased the dosing frequency and effectively suppressed intraocular inflammation compared to drug solutions and micelles.
2.2 Host–guest interactions
Besides PPR hydrogels formed by hydrogen bonding, a significant component of CD-based supramolecular hydrogel is host–guest supramolecular hydrogels. This type of hydrogel is commonly cross-linked through host–guest recognition between a range of size-matched guest molecules and the unique hydrophobic cavities of CDs.6,12 β-CD is the most commonly utilized in the preparation of host–guest inclusion due to its moderate cavity size, good modifiability, and low cost. The major guest molecules compatible with β-CD include adamantane, azobenzene, ferrocene, and cholesterol.44,45 CD-based host–guest supramolecular hydrogels are increasingly employed in biomedical and other fields due to the strong attraction provided by the dynamic reversibility of host–guest interactions.18,46 In the next sections, an introduction to CD-based supramolecular hydrogels based on host–guest interactions will be provided.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with β-CD.47 β-CD/Ad supramolecular hydrogels are commonly cross-linked through reversible host–guest interactions between β-CD and Ad-modified polymers. These supramolecular hydrogels possess injectable and self-healing properties, making them promising materials for the delivery of bioactive molecules and human tissues.48–50
1 complex with β-CD.47 β-CD/Ad supramolecular hydrogels are commonly cross-linked through reversible host–guest interactions between β-CD and Ad-modified polymers. These supramolecular hydrogels possess injectable and self-healing properties, making them promising materials for the delivery of bioactive molecules and human tissues.48–50
However, the mechanical properties of hydrogels based on CD host–guest interactions are significantly hampered by the weak noncovalent interactions and the low cross-linking density owing to the generally low modification efficiency of β-CD on the macromolecular chains. Unlike previous investigations where β-CD/Ad host–guest hydrogels were formed by modifying CDs and Ads on polymer chains, Chen et al. successfully constructed a robust and self-healing β-CD/Ad host–guest PAM hydrogel using novel cyclodextrin topology nanoparticles (TNPs) as physical cross-linking agents.51 The unique topology of TNPs and the host–guest cross-linking mechanism contributed significantly to the improvement of the hydrogel's mechanical strength and self-healing capabilities. This innovative approach presents a novel method for enhancing the mechanical properties of CD-based hydrogels.
More interestingly, Li et al. utilized the different strengths of the β-CD/Ad host–guest interactions to convert nanoparticles into hydrogels. They developed a multifunctional hydrogel platform that can respond to both physiological and pathological acidic microenvironments (Fig. 2A).52 To achieve this, they synthesized a pH-responsive multivalent hydrophobic host compound, AHCD, by conjugating β-CD to cyclic hexachlorocyclotriphosphazene (HCTP) and acetalization. Subsequently, they prepared pH-responsive ACPA NPs by nanoprecipitation of AHCD with Ad-modified 8-armed PEG, relying on weak host–guest interactions. Under an acidic environment, AHCD underwent hydrolysis, resulting in the production of the hydrophilic host compound HCD. The enhanced host–guest interactions resulted in the conversion of the nanoparticles into a hydrogel. These pH-responsive nanoparticles were successful in protecting mice from ethanol- or drug-induced gastric injury by forming a protective hydrogel barrier on the gastric mucosa after oral administration. This finding has significant clinical implications.
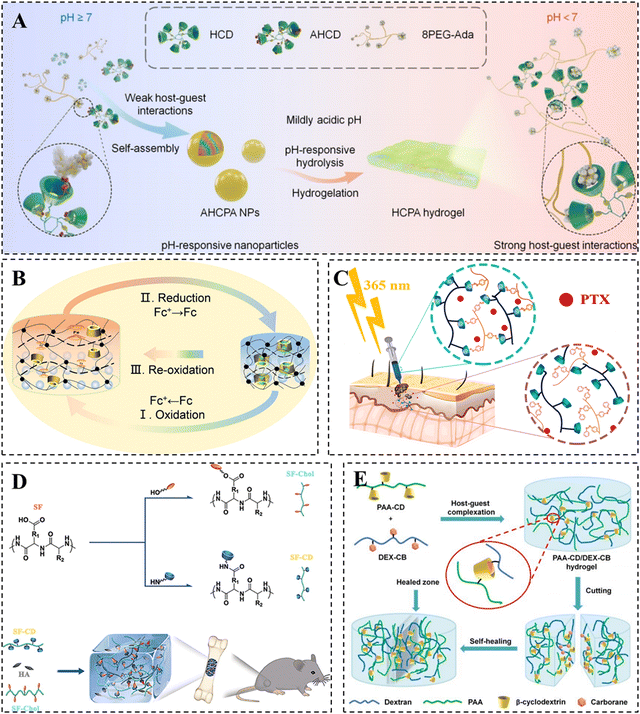 | ||
| Fig. 2 Construction of CD-based supramolecular hydrogels based on host–guest interactions. (A) β-CD/Ad host–guest supramolecular hydrogel converted from nanoparticles under acidic conditions. Reproduced with permission from ref. 52. Copyright 2022, Wiley. (B) Supramolecular photonic hydrogel based on β-CD/Fc host–guest interactions for use in a biosensor. Reproduced with permission from ref. 60. Copyright 2023, Elsevier. (C) PTX-loaded photo/thermal dual-responsive supramolecular hydrogel based on β-CD/Azo host–guest interactions for use in tumor therapy. Reproduced with permission from ref. 69. Copyright 2023, Elsevier. (D) Hydroxyapatite-loaded hydrogel based on β-CD/chol host–guest interactions for use in bone repair. Reproduced with permission from ref. 82. Copyright 2020, Elsevier. (E) Supramolecular hydrogel based on β-CD/CB host–guest interactions. Reproduced with permission from ref. 88. Copyright 2020, Royal Society of Chemistry. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with β-CD or γ-CD, while tending to form 1
1 complexes with β-CD or γ-CD, while tending to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with α-CD.53 This difference can be attributed to the varying sizes of the hydrophobic cavities of CDs. Furthermore, Fc has garnered significant attention due to its excellent oxidation–reduction reversibility. It can undergo a reversible transition between the hydrophobic reduced state and the hydrophilic oxidation state under external stimuli, allowing for the assembly and dissociation of CD/Fc inclusion complexes.54–56
2 complexes with α-CD.53 This difference can be attributed to the varying sizes of the hydrophobic cavities of CDs. Furthermore, Fc has garnered significant attention due to its excellent oxidation–reduction reversibility. It can undergo a reversible transition between the hydrophobic reduced state and the hydrophilic oxidation state under external stimuli, allowing for the assembly and dissociation of CD/Fc inclusion complexes.54–56
The introduction of Fc and its derivatives in CD-based supramolecular hydrogels has the potential to facilitate the development of redox-responsive smart hydrogel systems with promising applications in biomedicine.57–59 For example, Qin et al. designed a supramolecular photonic hydrogel with self-regulation based on the reversible β-CD/Fc host–guest complexation (Fig. 2B).60 In the presence of horseradish peroxidase/H2O2 and glucose oxidase/D-glucose, the contraction or swelling of the hydrogel can be observed as a result of the complexation or dissociation of the β-CD/Fc host–guest complex. The volume change of the hydrogel was simultaneously accompanied by its color variation due to the presence of photonic structures, which confirmed the hydrogel to be a potential candidate as a biosensor for detecting the concentration of H2O2 and glucose as well as enzyme activity.
Currently, most CD/Fc supramolecular hydrogels achieve drug release or other functions by inducing a sol–gel transition with externally provided redox stimuli. Chiang et al. took an interesting approach by utilizing the release of reducing agents loaded in the hydrogel to accelerate both the self-healing procedure and the restoration of mechanical properties. It was considered to be an effective approach to the problem of substantial reduction of the host–guest hydrogel's mechanical properties with the swelling process or external stimuli. In their study, they developed an injectable HA-pAA hydrogel with CD/Fc supramolecular interactions to deliver GSH-loaded LbL-PPMM magnetic microcapsules and chondrocytes.61 By slowly releasing the reducing agent GSH from LbL-PPMM, the self-healing rate of the hydrogel was significantly accelerated, and the mechanical strength of the hydrogel was restored to its initial level due to the gradual reduction of the oxidized Fc.
Azo-based UV-responsive hydrogels show promise as platforms for smart drug delivery in superficial tissues, allowing for remote and precise spatiotemporal modulation of drug release.67,68 For example, Pourbadiei et al. synthesized the photo/thermal responsive copolymer NIPAZO by using Azo and NIPAM. They then prepared the DAS@SCD/NIPAZO hydrogel through host–guest recognition between NIPAZO and CD-modified starch (Fig. 2C).69 At physiological temperature, the paclitaxel-loaded hydrogel exhibited a slow and sustainable release of paclitaxel within 96 hours. However, the CD/Azo host–guest interaction in the hydrogel gradually broke when exposed to 365 nm UV illumination, resulting in an increased release rate of paclitaxel and a significant inhibitory effect on tumor growth.
Unfortunately, the limited tissue penetration of UV light has hindered extensive research on CD/Azo hydrogels in various biomedical applications, including deep tissue repair. Red or near-infrared light (600–900 nm) offers deeper penetration and causes less photodamage compared to UV light.70 Previous studies have demonstrated that the activated light of Azo is able to be red-shifted via chemical modification of the electron-donating or electron-withdrawing groups on the benzene ring.71,72 Wu et al. designed a red light-sensitive hyaluronic acid (HA) hydrogel by utilizing tetra-ortho-methoxy-substituted azobenzene (mAzo) and β-CD through host–guest recognition.73 The combination of red-shifted-photoisomerized Azo and HA confers enhanced hydrogen bonding and reduced photoisomerization of the polymeric guest. Compared to conventional CD/Azo hydrogels, this hydrogel avoids the problem of rapid drug release brought about by a complete red light-responsive gel–sol transition, making it a promising candidate for sustained drug release.
However, polymers such as PEG and polyacrylamide, while exhibiting good biocompatibility, suffer from disadvantages such as poor biodegradability and lack of bioactivity. Poly-L-glutamic acid (PLGA), similar to proteins found in the extracellular matrix, is an ideal peptide material due to its excellent biocompatibility, biodegradability, and ability to promote tissue repair and cell growth through its degradation products.77 Li et al. developed a degradable and self-healing β-CD/chol host–guest peptide hydrogel using PLGA. The hydrogel demonstrated the highest energy storage modulus when the β-CD/chol molar ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the mechanical properties of the hydrogel were progressively enhanced with increasing molecular weight of PLGA.78
1, and the mechanical properties of the hydrogel were progressively enhanced with increasing molecular weight of PLGA.78
Although the β-CD/chol host–guest hydrogels offer the ability to adjust their mechanical properties, hydrogels formed from a single network of host–guest crosslinking remain mechanically weak. Compared to other natural polymers, silk fibroin (SF) demonstrates exceptional mechanical strength as SF molecules can transition from randomly curled to a stable β-folded structure, forming β-sheet nanocrystalline structures primarily driven by hydrogen bonding.79,80 To address the mechanical limitations of β-CD/chol hydrogels, Bai et al. developed HG-SF hydrogels by utilizing the β-CD/chol dynamic host–guest interactions with the layered structure of SF. The hydrogel demonstrated outstanding mechanical strength, self-healing, with a maximum compressive stress of up to 3.16 MPa, and achieved a healing efficiency of 93.78% after 2 hours.81 In addition, the research team incorporated hydroxyapatite nanoparticles with good osteoconductivity into HG-SF hydrogels to form organic–inorganic hybrid hydrogels, which were utilized for bone repair (Fig. 2D).82 In summary, cholesterol, commonly used as a guest molecule for cyclodextrins, offers advantages in biological applications because of its outstanding biocompatibility and biodegradability.
In addition to CBs, many other naturally occurring hydrophobic small molecules are capable of host–guest complexation with CDs.89 Coumarin (COU) is a light-sensitive small molecule that exhibits interesting changes when irradiated with light at wavelengths of 365 nm and 254 nm.90 Furthermore, COU has been extensively studied as a guest molecule in host–guest complexation.91 Liu et al. constructed a bimodal supramolecular hydrogel by forming a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex between COU and γ-CD.92 Notably, the mechanical strength of the hydrogel is able to be controlled by switching the weak physical cross-linking sites to strong chemical cross-linking sites using UV irradiation. Specifically, the encapsulated COU in γ-CD undergoes dimerization under 365 nm UV irradiation, resulting in a change of the cross-linking interaction from host–guest recognition to COU–COU covalent bonding. As a result, the hydrogel's storage modulus increases to 2.3 MPa. Nevertheless, the COU–COU covalent bonds are broken, and the hydrogel returns to its soft physically cross-linked state. This unique property makes the hydrogel a promising material for self-healing applications due to its reversible stiffness tunability and self-healing properties.
1 host–guest complex between COU and γ-CD.92 Notably, the mechanical strength of the hydrogel is able to be controlled by switching the weak physical cross-linking sites to strong chemical cross-linking sites using UV irradiation. Specifically, the encapsulated COU in γ-CD undergoes dimerization under 365 nm UV irradiation, resulting in a change of the cross-linking interaction from host–guest recognition to COU–COU covalent bonding. As a result, the hydrogel's storage modulus increases to 2.3 MPa. Nevertheless, the COU–COU covalent bonds are broken, and the hydrogel returns to its soft physically cross-linked state. This unique property makes the hydrogel a promising material for self-healing applications due to its reversible stiffness tunability and self-healing properties.
2.3 Ionic interactions
Compared to other noncovalent interactions, such as hydrogen bonding and host–guest interactions, ionic interactions are more robust and play an essential role in the formation of CD-based supramolecular hydrogels.93 Host–guest interactions have been extensively recognized to make a substantial contribution to the favorable self-healing properties of hydrogels while limiting their mechanical properties. To address the trade-off between these two properties in CD-based supramolecular hydrogels, ionic interactions have often been combined with the most extensively used host–guest interactions. There are two primary strategies for constructing hydrogels of this nature.One approach involves modifying CD molecules or guest molecules with charged groups, such as guanidino groups, to create charged polymers or nanoparticles through host–guest interactions. Subsequently, supramolecular hydrogels are prepared by utilizing electrostatic interactions between charged nanoparticles and charged inorganic materials, such as LAPONITE®. The incorporation of inorganic nanomaterials can further enhance the mechanical properties of the hydrogels.94 Zhang et al. developed a supramolecular hydrogel that is highly loaded with drugs and responsive to near-infrared (NIR) light by using upconverting nanoparticles (UCNP), αCD/Azo, and LAPONITE® (Fig. 3A).95 Specifically, UCNP@αCD-E-azo nanoparticles were obtained by host–guest recognition of α-CD covalently coated UCNP and azobenzene quaternary ammonium salt. This was immediately followed by the preparation of a supramolecular hydrogel via electrostatic interactions between the quaternary ammonium salt and LAPONITE®. UCNP has the ability to convert absorbed NIR light into UV light and heat, causing a gel–sol phase transition and facilitating the effective release of drugs. The combination of photothermal therapy and chemotherapy in this hydrogel system effectively inhibits tumor growth, making it a promising controlled drug delivery platform for cancer treatment.
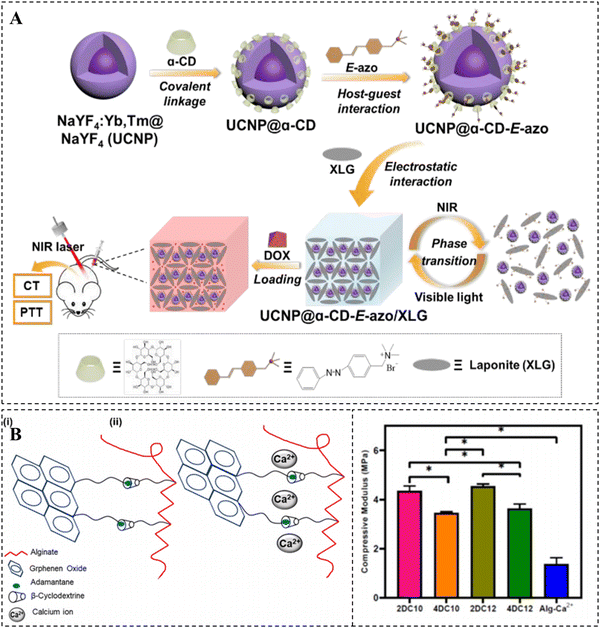 | ||
| Fig. 3 Construction of CD-based supramolecular hydrogels based on ionic interactions. (A) NIR-responsive hydrogel via electrostatic interaction between UCNP@α-CD-E-azo and LAPONITE® and its pH/NIR-responsive DOX release. Reproduced with permission from ref. 95. Copyright 2020, Royal Society of Chemistry. (B) Crosslinking mechanism of the nanohybrid Alg-GO hydrogel with adjustable mechanical properties. Reproduced with permission from ref. 96. Copyright 2021, Elsevier. | ||
Another approach is to utilize host–guest interactions as the initial cross-linking mechanism in hydrogels. In this method, electrostatic interactions occur between polymer chains that are enriched with charged groups, such as carboxyl groups, and ions serve as the second cross-linking agent. It should be noted that ionic interactions do not take place on CDs or their guest molecules. For example, Kharaziha et al. successfully prepared a nanohybrid double crosslinked hydrogel by employing host–guest interactions between β-CD grafted sodium alginate (Alg-CD) and Ad-modified graphene oxide (Ad-GO), as well as the ionic interactions between Ca2+ and Alg (Fig. 3B).96 The incorporation of Ca2+ significantly strengthened the shear-thinning properties of the hydrogel. Moreover, the hydrogel demonstrated adjustable mechanical and biological properties due to the ability to control the crosslink density and network structure. This characteristic makes it highly promising as a minimally invasive injectable material.
Overall, the introduction of ionic interactions in host–guest supramolecular hydrogels is a well-established and effective method for enhancing their mechanical properties. Moreover, these ionic interactions facilitate the efficient encapsulation of charged drugs in CD-based supramolecular hydrogels.
3. Strategies to improve the mechanical properties of CD-based supramolecular hydrogels
Currently, increasing research interests have been concentrated on CD-based supramolecular hydrogels due to their shear-thinning and self-healing properties. However, their generally weak mechanical properties limit the potential applications in certain fields.22 For wound healing applications, supramolecular hydrogel dressings are expected to possess not only favorable self-healing properties but also appropriate mechanical properties, thus avoiding bacterial infections or premature drug leakage caused by structural damage from external pressure during the application process.23 In the field of bone and cartilage tissue engineering, it is necessary for hydrogels to have mechanical strength comparable to those of natural bone and cartilage to improve the quality of repair.24 The elastic modulus of articular cartilage can reach up to 950 kPa, while that of bone is even higher than 1 MPa.97 Unfortunately, CD-based supramolecular hydrogels typically fail to achieve such high mechanical strength values due mainly to the following three factors, (i) the crosslinking of supramolecular hydrogels is primarily based on the reversible non-covalent interactions, (ii) the low grafting efficiency of the host and guest molecules onto the polymer chain results in a low cross-linking density, and (iii) the steric hindrance of the polymer backbone also accounts somewhat for the compromised mechanical properties of the hydrogels.One of the simplest and most direct methods to improve the mechanical properties of hydrogels is to increase the polymer concentration or the amount of host–guest crosslinking sites. For instance, Ren et al. designed a supramolecular hydrogel with high adhesion properties by the host–guest interactions between β-CD and dopamine co-grafted alginate and adamantane-grafted polyacrylamide (Fig. 4A).98 The results demonstrated the effectiveness of this approach in enhancing the mechanical properties of the hydrogel. In another study, Lee et al. developed F127-Ad/CDP hydrogels for protein drug delivery by crosslinking Pluronic F127 modified with single or multiple Ads and β-CD polymers via host–guest interactions (Fig. 4B).99 The formation of F127 micelles not only conferred thermally responsive sol–gel transition properties, but also enhanced the mechanical properties of the hydrogel. As the number of Ads attached to F127 increased, the polymer concentration required for hydrogel formation gradually decreased, resulting in improved mechanical properties of the hydrogel.
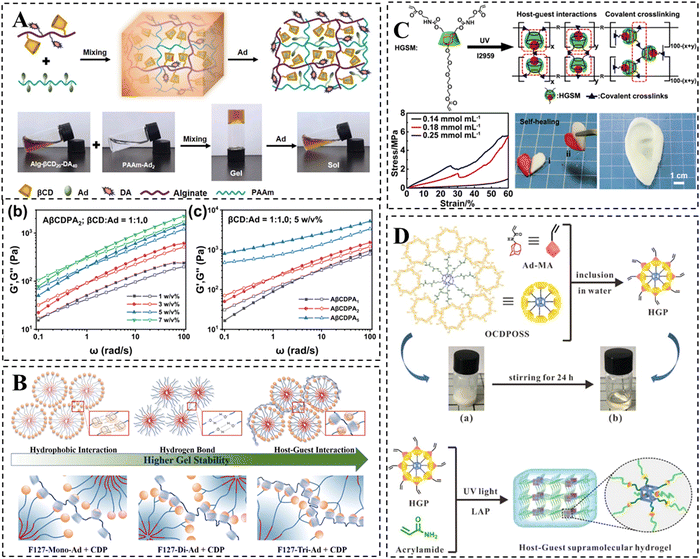 | ||
| Fig. 4 Strategies to improve the mechanical properties of CD-based supramolecular hydrogels. (A) Schematic representation of alginate/polyacrylamide host–guest supramolecular hydrogels. The mechanical properties are improved by increasing the concentration or adamantane substitution of PAAm-Ad polymer. Reproduced with permission from ref. 98. Copyright 2023, Elsevier. (B) Schematic representation of ADA-F127 supramolecular hydrogel. The mechanical properties may be improved by increasing the substitution of adamantane and using the F127 micellar structure as secondary cross-linking. Reproduced with permission from ref. 99. Copyright 2021, Elsevier. (C) Schematic representation of the self-healing HGSM supramolecular hydrogel. The mechanical properties are improved via a combination of multiple cross-linking mechanisms. Reproduced with permission from ref. 100. Copyright 2018, Wiley. (D) Schematic representation of the preparation for the HGP hydrogel. The mechanical properties are improved via the incorporation of POSS and a combination of multiple cross-linking mechanisms. Reproduced with permission from ref. 102. Copyright 2020, Royal Society of Chemistry. | ||
In addition, incorporating multiple cross-linking mechanisms is a common method for enhancing the mechanical properties of CD-based supramolecular hydrogels. One strategy involves introducing chemical covalent crosslinking to create a highly crosslinked rigid network in addition to a relatively relaxed host–guest crosslinked network. Wang et al. initially synthesized three-armed host–guest supramolecules (HGSMs) through appropriate host–guest interactions between β-CD-AOI2 (the host molecule) and A-TEG-Ad (the guest molecule), thus avoiding the spatial site-barriers of the polymer chains that lead to inadequate cross-linking. Subsequently, they covalently polymerized HGSM with double bonds under UV light initiation to obtain HGSM hydrogels (Fig. 4C).100 This physical/chemical dual crosslinked network significantly enhanced the mechanical strength of CD-based hydrogels, making them promising candidates for regenerative medicine applications such as cartilage repair. However, the irreversible nature of covalent interactions leads to a partial loss of the self-healing properties of hydrogels. To strengthen the mechanical properties while realizing the intact retention of the inherent dynamic properties of supramolecular hydrogels, the combination of multiple non-covalent interactions is more popular. Chen et al. employed a kinetic interlocking multiple unit (KIMU) strategy to introduce two types of host–guest interactions, CB[8]-Phe and βCD-Ad, into a hyaluronic acid hydrogel system.48 This approach successfully incorporated the hydrogel into the system without sacrificing self-healing and shear dilution properties, while increasing the hydrogel's storage modulus by 78%. This KIMU effect inhibits the cascade disintegration of the two host–guest complexes, leading to higher dynamic stability.
Finally, the incorporation of rigid inorganic nanomaterials into CD-based hydrogels has been found to strengthen their mechanical properties. Among the various organic–inorganic hybrid silica nanoparticles, POSS, as the smallest, has been shown to play a crucial role in improving the mechanical properties of hydrogels.101 Zhou et al. developed star-type HGP crosslinkers using POSS, β-CD and adamantane. The star-type HGP crosslinkers were created through host–guest interaction, and subsequently, HGP supramolecular hydrogels were formed via covalent polymerization of double bonds (Fig. 4D).102 Comparatively, the HGP hydrogels exhibited a 2.7-fold increase in tensile modulus and demonstrated excellent ductility when compared to the HGM hydrogels lacking POSS. Moreover, the storage modulus of hydrogels improved with a higher HGP cross-linker concentration.
4. Drug loading and release of CD-based supramolecular hydrogels
4.1 Drug loading
Drug-loading methods for CD-based supramolecular hydrogels can be categorized into two types: (1) loading the drug into the hydrogel via non-covalent interactions, like electrostatic interactions, hydrophobic interactions, and hydrogen-bonding interactions; and (2) covalent modification of the drug onto the molecular chains of the hydrogel.103–105 Hydrophilic drugs can be directly physically encapsulated into hydrogels, and their release rate is related to the dimension of the drugs and the affinity between the drugs and the hydrogel matrix. Compared to hydrophilic drugs, hydrophobic drugs are less efficiently loaded into hydrogels due to difficulties in dissolution leading to their poor loading.12,106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, and formed an EA-loaded hydrogel through the photoinitiated click reaction between the inclusion complex as cross-linking agents and tetra-armed poly(ethylene glycol)-norbornene (Fig. 5A).109 The release rate of the EA depended on the stability of the hydrogen bond between the EA and cyclodextrin, the pore size of the hydrogel, and the degradation of the hydrogel.
2 molar ratio, and formed an EA-loaded hydrogel through the photoinitiated click reaction between the inclusion complex as cross-linking agents and tetra-armed poly(ethylene glycol)-norbornene (Fig. 5A).109 The release rate of the EA depended on the stability of the hydrogen bond between the EA and cyclodextrin, the pore size of the hydrogel, and the degradation of the hydrogel.
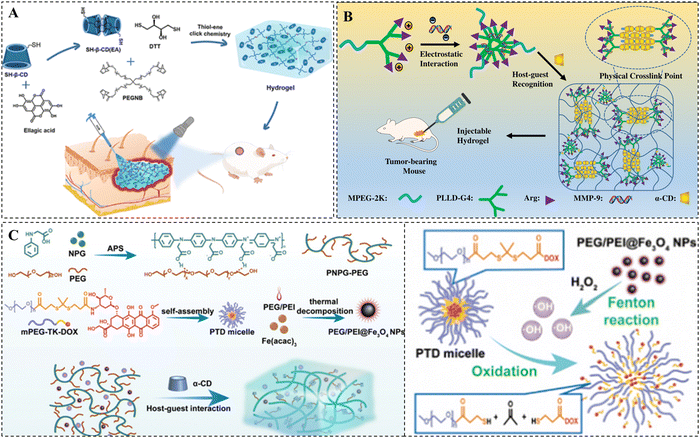 | ||
| Fig. 5 Drug loading methods of CD-based supramolecular hydrogels. (A) Schematic representation of EA-loaded thiol–ene hydrogels for wound healing. EA is loaded into hydrogels via host–guest complexation with β-CD. Reproduced with permission from ref. 109. Copyright 2023, American Chemical Society. (B) Schematic representation of pMMP-9-loaded PPR supramolecular hydrogels for tumor therapy. pMMP-9 is loaded into hydrogels based on electrostatic interaction with MPEG-PLLD-Arg. Reproduced with permission from ref. 114. Copyright 2017, Elsevier. (C) Schematic representation of NIR-responsive supramolecular hydrogels containing mPEG-TK-DOX micelles and PEG/PEI@Fe3O4 nanoparticles for tumor therapy. DOX is loaded into hydrogels by linking to mPEG via TK bonds. Reproduced with permission from ref. 130. Copyright 2023, Royal Society of Chemistry. | ||
In addition, hydrophobic drugs can also be efficiently loaded into nanocarriers, such as micelles, liposomes, and metal nanoparticles, through covalent or noncovalent interactions, and then these nanocarriers can be further loaded into supramolecular hydrogels for efficient loading of hydrophobic drugs.110–112 Domiński et al. developed pH-responsive drug-loaded nanomicelles by encapsulating DOX into their hydrophobic core. Next, they mixed these nanomicelles with a solution of α-CD and 8-hydroxyquinoline glycoconjugate affixes to form supramolecular hydrogels loaded with both hydrophobic DOX and hydrophilic 8-hydroxyquinoline glycoconjugate affixes.113 The structures showed that this drug-loaded hydrogel displayed accelerated drug release under acidic conditions.
For negatively charged gene drugs, they are often loaded into hydrogels by forming complexes with cationic polymers in the hydrogel matrix. Xue et al. synthesized poly(L-lysine) dendrimer molecules functionalized with PEG and arginine (MPEG–PLLD-Arg), which were bound to pMMP-9 via electrostatic interactions to form nanocomplexes. After mixing these complexes with α-CD, a PPR hydrogel for local delivery of pMMP-9 was prepared (Fig. 5B).114 This hydrogel not only maintains the high bioactivity and stability of pMMP-9 for a long time, but also has a well-established slow-release effect on pMMP-9.
In addition to easily hydrolysable ester bonds, there are numerous other covalent bonds that can be cleaved in response to external environmental changes. For example, pH-responsive imine bonds, acylhydrazone bonds, and acetal bonds.120–122 Li et al. modified DOX onto β-CD via imine bonding, and prepared DOX-loaded PPR supramolecular hydrogels by utilizing the host–guest interactions between Pluronic F-127 and β-CD and α-CD.123 As a result of the presence of acylhydrazone bonds, these hydrogels displayed pH-sensitive DOX release. Furthermore, the acylhydrazone bonds demonstrated better stability at lower pH levels. Li et al. prepared DOX-loaded supramolecular hydrogels by utilizing interactions between acid-sensitive PEGylated polyphosphoester-doxorubicin precursors (PBYP-g-PEG-g-DOX) and α-CD.124 The acid sensitivity of the precursor was mainly attributed to the introduced acylhydrazone and acetal bonds. The results showed that under acidic conditions, the acetal and acylhydrazone bonds facilitated cleavage, leading to degradation of the hydrogel and the release of DOX.
Redox-responsive covalent bonds, such as disulfide, diselenide, and thioketal bonds, are frequently employed for drug modification.125–127 Thioketal (TK) bonds are considered to be one of the most efficient reactive oxygen species (ROS)-responsive bonds and can rapidly oxidize to thiols under high levels of ROS.128,129 Huang et al. developed a NIR-responsive supramolecular hydrogel using conjugated poly(N-phenylglycine)–poly(ethylene glycol) (PNPG–PEG) and α-CD. They encapsulated PEG/PEI@Fe3O4 nanoparticles with DOX-loaded nanomicelles within the hydrogel (Fig. 5C).130 The drug-loaded nanomicelles were self-assembled by the amphiphilic precursors mPEG-TK-DOX linked via TK bonds. The PEG/PEI@Fe3O4 nanoparticles in this hydrogel could utilize the Fenton reaction to generate ˙OH, which led to an increase of ROS and induced the breakage of the ROS-responsive TK bond, thereby promoting sustained DOX release.
Overall, the covalent modification of drugs on CD-based supramolecular hydrogels substantially extends the duration of drug release and enhances intelligent responsiveness.131 This modification also prevents the elimination of drug molecules (particularly peptide drugs) by the immune system.132,133
4.2 Drug release
The release rate of drugs physically encapsulated in hydrogels relies mainly on the pore size of hydrogels and the dimension of drugs.134–136 If the hydrogel possesses a larger pore size than the dimension of drugs, the release rate of drugs has nothing to do with the pore size of the hydrogel, but is associated with the affinity between the hydrogel matrix.137,138 If the hydrogel possesses a pore size close to the dimension of the drugs, the release rate of drugs depends mainly on the degradation rate of the hydrogel and the interaction between drugs and the hydrogel.139,140 If the hydrogel possesses a smaller pore size than the dimension of the drugs, the hydrogel pores may cause a strong spatial site resistance, preventing the diffusion of drugs, and the release rate of drugs mainly depends on the swelling or shrinkage and degradation of the hydrogel.136 For example, after a change in the external environment, the electrostatic or hydrogen bonding interactions between hydrophilic groups and water molecules in the hydrogel are enhanced, resulting in water-absorbing swelling of the hydrogel, which accelerates the release of the drug.141,142 Conversely, if there are more hydrophobic groups in the hydrogel, dominated by hydrophobic interactions, the formation of hydrogen bonds between the hydrophilic groups and external water molecules is hindered. This leads to shrinkage of the hydrogel, generating a squeezing effect, which promotes drug release.143 In addition, when there are easily hydrolyzable bonds in the hydrogel structure, such as ester and peptide bonds, the pore size of the hydrogel gradually increases with the degradation of the hydrogel network, which in turn achieves a slow release of the drug.144–146Drugs are loaded into hydrogels as chemical couplings, and their release rate depends greatly on the breakage rate of covalent bonds. Stable covalent bonds, such as amide bonds, achieve slow drug release mainly through degradation of the hydrogel network, whereas covalent bonds that can respond to cleavage, such as imine and disulfide bonds, can achieve controlled drug release in response to external environmental changes.10 Currently pH-responsive bonds such as imine bonds and acylhydrazone bonds, redox-responsive bonds including disulfide bonds and thioketal bonds, as well as enzyme-responsive bonds, have been extensively utilized for covalently modifying drugs on hydrogels.123,130,147–149 These modified drug-loaded hydrogels exhibit more precise on-demand drug release and higher therapeutic efficiency compared to physically embedded drug-loaded hydrogels. For instance, Ha et al. developed amphiphilic prodrugs by conjugating podophyllotoxin with PEG and self-assembled them to form nanomicelles. Subsequently, the nanomicelles were further self-assembled into supramolecular hydrogels using the strong hydrogen bonding force between PEG and α-CD. The water-soluble anticancer drug 5-Fu was loaded in a physically embedded manner within these supramolecular hydrogels (Fig. 6A).150 It is demonstrated that α-CD was slowly degraded by amylase, resulting in the disruption of the hydrogel structure and, consequently, an accelerated release of 5-Fu. Additionally, the presence of Na2S2O4, a biomimetic azo reductase, caused the cleavage of the Azo bond in the amphiphilic prodrug, leading to the formation of 4′-O-demethyl-4β-(4′′-aminoanilino)-4-desoxy-podophyllotoxin (AdP). This also disrupted the nanocellular structure and further facilitated the release of both 5-Fu and active AdP. Due to the specific secretion of amylase and azo reductase by colonic flora, this hydrogel enables responsive drug release at the colonic site, thereby demonstrating more precise on-demand effects in the therapy of diseases related to the colon.
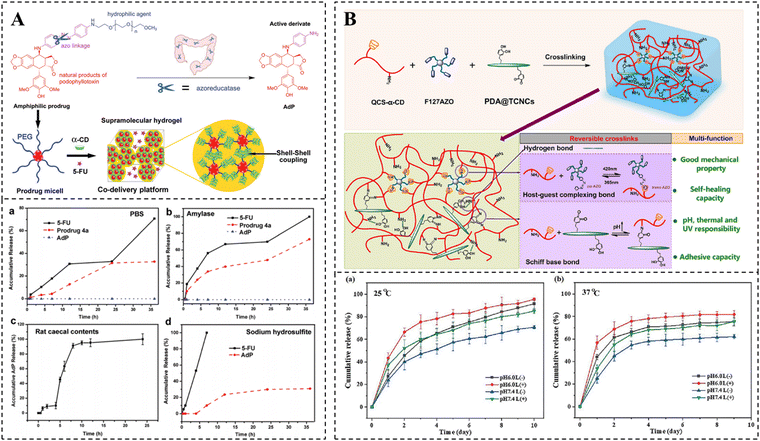 | ||
| Fig. 6 Drug release of CD-based supramolecular hydrogels. (A) Schematic diagram of the preparation of PPR hydrogel coloaded with AdP and 5-FU and its amylase or azo reductase responsive release kinetics. Reproduced with permission from ref. 150. Copyright 2021, Royal Society of Chemistry. (B) Schematic illustration of the preparation of curcumin-loaded hydrogel and its multi-responsive release kinetics (light, pH, and temperature). Reproduced with permission from ref. 155. Copyright 2023, Elsevier. | ||
Achieving controlled release based on effective loading and release of drugs is essential for disease treatment. It is fascinating that stimuli-responsive hydrogels allow for the responsive release of drugs in specific environments, thus improving the efficacy of drugs. CD-based supramolecular hydrogels are an ideal smart drug delivery system that can form drug reservoirs in target tissues in a mini-invasive manner and realize controlled release of therapeutic drugs in time and space by responding to stimuli such as pH, redox, and light. Studies have shown that CD-based supramolecular self-assembly systems can effectively react to external stimuli, leading to the assembly and dissociation of the system. For example, the pH sensitivity of the β-CD/benzimidazole system,151–153 the redox responsiveness of the β-CD/Fc,56,154 and the UV light sensitivity of the β-CD/Azo have been investigated.67,68 These host–guest interactions offer promising conditions for the exploitation of stimuli-responsive CD-based supramolecular hydrogels.
In contrast to hydrogels that respond to single stimuli, multi-responsive hydrogels based on CD are capable of responding to multiple stimuli simultaneously. Such hydrogels can also demonstrate greater sensitivity to small environmental changes at the lesion site, resulting in more accurate drug release and more effective disease treatment. For example, Liu et al. prepared a supramolecular hydrogel that is responsive to both light and heat by using α-CD-grafted quaternized chitosan and Azo-modified Pluronic F127. Furthermore, polydopamine-coated tunicate cellulose nanocrystals were dispersed homogeneously in the hydrogel through Schiff base bonding and hydrogen bonding. This not only introduced pH responsiveness to the hydrogel, but also improved its mechanical properties and adhesion (Fig. 6B).155 As a multi-responsive delivery platform for curcumin, this supramolecular hydrogel can release curcumin on demand in response to low pH and high-temperature environments at the wound site, as well as external UV irradiation, thereby effectively promoting wound healing. However, current research on CD-based multi-stimulus-responsive hydrogels is still in its early stages and requires further development.
5. Biological applications of CD-based hydrogels
Hydrogels have been broadly utilized in regenerative medicine, drug delivery, and biosensing due to their excellent biocompatibility and adjustable pore size and stiffness.156,157 Among hydrogels, supramolecular hydrogels crosslinked by noncovalent interactions exhibit unique properties, such as injectability, self-healing, and stimulus responsiveness, which greatly expand the biological applications of hydrogels.20,158,159 CDs are of vital importance in the development of drug carriers, which can not only increase the solubility and bioavailability of insoluble drugs, but also enable sustained drug release, thereby optimizing therapeutic effects.15–17,160,161 In summary, the combination of multifunctional CD units and supramolecular hydrogels represents an effective strategy for maximizing the safety and efficacy of therapeutic molecules, providing a promising approach for the exploitation of functional hydrogels and broadening the applications of hydrogels in disease treatment.12 Recent advancements in CD-based supramolecular hydrogels for tumor therapy, bone and cartilage repair, myocardial repair, and wound healing are briefly described below.5.1 Tumor treatment
One of the most pressing health issues in contemporary society is cancer, with the number of cancer-related deaths steadily increasing each year. The current approach to treating cancer is shifting towards a synergistic model that combines multiple therapies, with chemotherapy remaining the most crucial strategy.162,163 However, the clinical applications of most anticancer drugs are limited due to their low solubility, poor stability, and inadequate tumor targeting. A solution to these challenges is provided by CD-based supramolecular hydrogels. These hydrogels enable the effective loading of anticancer drugs and facilitate local sustained release through intratumoral or peritumoral injection.164 By delivering anticancer drugs locally, the concentration of the drugs can be maximized, enhancing their effectiveness against tumor cells while minimizing unnecessary side effects on healthy tissues.165Hydrophobic chemotherapeutic drugs such as paclitaxel (PTX) and doxorubicin (DOX) are often loaded into hydrogels for the treatment of various tumors.166–169 Song et al. developed thermo-responsive supramolecular hydrogels using two host–guest interactions to achieve sustained delivery of anticancer drugs, including DOX (Fig. 7A).170 Specifically, the researchers assembled thermo-responsive pseudo-block copolymers (βCD-(PNIPAAm)4/Ad-PEG) through host–guest recognition between βCD-core PNIPAAm 4-armed star polymers and Ad-modified PEG. These copolymers formed supramolecular micelles when the temperature exceeded the LCST. The bis-supramolecular hydrogels were then created by aggregating the PPR structure formed between PEG and α-CD. The resulting bis-supramolecular hydrogels exhibited significantly improved mechanical properties and enhanced slow-release properties of DOX compared to the single PPR supramolecular hydrogel at temperatures up to 37 °C. Moreover, the hydrogel demonstrated superior antitumor effects in multi-drug resistant cancer cells.
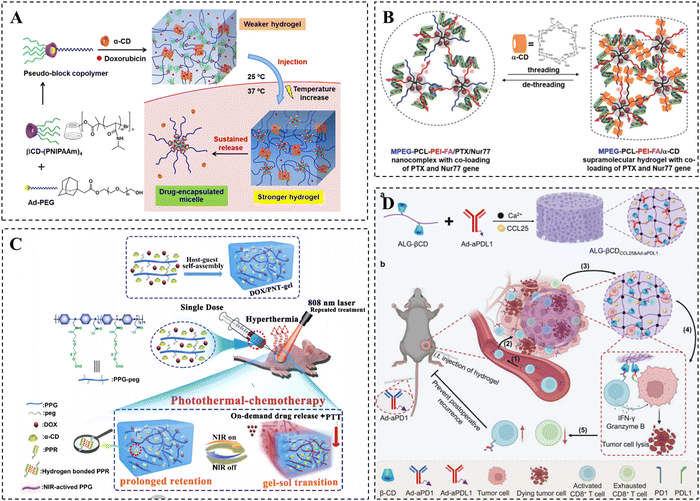 | ||
| Fig. 7 CD-based supramolecular hydrogels for tumor therapy. (A) Schematic diagram of DOX-loaded PPR supramolecular hydrogels for tumor therapy and its thermo-responsive drug release. Reproduced with permission from ref. 170. Copyright 2020, American Chemical Society. (B) Schematic representation of PTX and Nur77 co-loaded PPR hydrogels for tumor therapy. Reproduced with permission from ref. 175. Copyright 2019, Wiley. (C) Schematic representation of the DOX/PNT-gel for photothermal-chemotherapy treatment. Reproduced with permission from ref. 178. Copyright 2019, Elsevier. (D) Schematic representation of the ALG-βCD hydrogels for tumor therapy. Reproduced with permission from ref. 180. Copyright 2023, Wiley. | ||
Compared to the treatment of a single chemotherapy drug, combination therapy with multiple chemotherapeutic drugs is considered a more effective anti-tumor strategy. The combination of hydroxycamptothecin (HCPT) and betulinic acid (BA) has been speculated to enhance tumor inhibition. Therefore, Dai et al. utilized the amphiphilic precursor 8 arm-PEG-BA to self-assemble with hydrophobic HCPT, forming drug-loaded micelles and achieving simultaneous and efficient loading of the two drugs.171 The temperature-sensitive hydrogel was then formed through the package interaction between α-CD and PEG in the micelles. The hydrogel could be injected intratumorally for local delivery and temperature-sensitive controlled release of BA and HCPT, exhibiting more favorable anti-tumor effects compared to monotherapy.
In addition to delivering chemotherapeutic drugs, CD-based supramolecular hydrogels can also serve as an attractive biomaterial for the delivery of antitumor gene drugs.172 Gene drugs can form complexes with PEG-modified cationic polymers or polycations, as they have a negative charge. Consequently, CD-based PPR hydrogels can be prepared via the host–guest interaction between these complexes and α-CD.173 These CD-based PPR hydrogels can act as local gene reservoirs, ensuring the sustained availability of DNA vectors in specific locations over a long period, thus demonstrating their potential as a platform for gene drug delivery. Liu et al. utilized MPEG–PCL–PEI triblock copolymers to bind the Bcl-2 converted Nur77 gene, resulting in the formation of MPEG–PCL–PEI/Nur77 complex micelles through hydrophobic interaction.174 Subsequently, a PPR hydrogel was obtained through mixing complex micelles with α-CD. This hydrogel could be formed in situ at the tumor site and exhibited a desirable slow-release effect on pDNA (Nur77) for up to 7 days. Notably, it demonstrated significant inhibitory effects on drug-resistant tumors with high expression of Bcl-2.
To achieve the synergistic delivery of chemotherapeutic drugs and gene drugs, as well as more precise drug therapy, the research group made further modifications to the MPEG–PCL–PEI triblock copolymers. They incorporated a folic acid targeting moiety and mixed it with α-CD to create a supramolecular hydrogel. This hydrogel allowed for the co-loading of the chemotherapeutic drug PTX and Nur77 gene (Fig. 7B).175 After injecting the hydrogel near the tumor, the MPEG–PCL–PEI–FA/PTX/Nur77 complex was released and targeted the tumor cells with high FR expression. The hydrogel degraded and gradually released PTX and Nur77 genes. Compared to the untargeted hydrogel, the targeted hydrogel showed significantly enhanced inhibition of drug-resistant tumors.
In addition, the synergistic effects of photothermal therapy and chemotherapy have been shown to enhance anti-tumor effects.176,177 Shen et al. prepared NIR-responsive injectable hydrogels via supramolecular assembly between α-CD and PEG using PEG-modified poly-N-phenylglycine (PPG) as a photothermal backbone (Fig. 7C). The PPG backbone endowed the hydrogel with photothermal conversion capabilities, which can induce gel–sol conversion by absorbing external NIR light (808 nm) and converting it into heat, thus realizing the precise release of DOX on demand.178 It was indicated that this hydrogel achieved almost complete eradication of 4T1 breast cancer by synergizing photothermal therapy and chemotherapy, making it an attractive and multifunctional tumor therapeutic platform.
Immunotherapy has emerged as a novel and effective anti-tumor therapy in recent years, which can enhance the recognition and killing of tumor cells by modulating the immune system.179 Zhu et al. presented an alginate hydrogel for the multifaceted promotion of the recruitment, engagement, and rejuvenation of T cells (Fig. 7D).180 It was demonstrated that following the recruitment of CCR9+CD8+ T cells by the chemokine CCL25 released from the hydrogel, the engagement of CD8+ T cells with tumor cells was further facilitated by the anti-PDL1 antibody and anti-PD1 antibody immobilized in the hydrogel via CD/Ad host–guest interactions. Meanwhile, CD8+ T cells were rejuvenated to avoid depletion, ultimately achieving enhanced T cell-mediated immunotherapy efficacy. Overall, CD-based supramolecular hydrogels show broad application prospects in tumor therapy, and more of their applications in tumor therapy are summarized in Table 1.
| Hydrogel components | Drugs delivered | Therapeutic strategies | Tumor types | Ref |
|---|---|---|---|---|
| βCD-PNIPAAm/Ad-PEG/α-CD | DOX | Sustained controlled release of DOX to overcome multidrug resistance (MDR) in tumor cells | AT3B-1 cells | 170 |
| DAS@SCD/NIPAZO | PTX | UV-responsive PTX delivery | Melanoma therapy | 69 |
| HPG–PCL–MPEG/α-CD | CPT/DOX | Combination therapy with multiple chemotherapy drugs | HNE-1 tumor | 181 |
| 8 arm-PEG-BA/α-CD | BA/HCPT | Combination therapy with multiple chemotherapy drugs | LLC tumor | 171 |
| MPEG-PLLD-Arg/α-CD | pMMP-9 | Gene therapy | HNE-1 tumor | 114 |
| MPEG–PCL–PEI/α-CD | Bcl-2 conversion Nur77 gene | Gene therapy | Hepatocarcinoma | 174 |
| MPEG–PCL–PEI–FA/α-CD | PTX/Nur77 | Combination therapy with chemotherapy drugs and therapeutic genes | Hepatocarcinoma | 175 |
| 4-PEG/α-CD | G4/Adv | Viral immunotherapy | Murine melanoma | 182 |
| PNPG-PEG/α-CD | CDDP | NIR-triggered cisplatin delivery and combined chemo-photothermal therapy | Triple-negative breast cancer | 183 |
| PPG-peg/α-CD | DOX | NIR-triggered DOX delivery and combined chemo-photothermal therapy | 4T1 breast cancer | 178 |
| PNPG-PEG/PTD micelles/PEG/PEI@Fe3O4 NPs/α-CD | DOX | ROS-responsive DOX release and synergistic chemo-photothermal therapy | 4T1 breast cancer | 130 |
| ALG-βCD/Ad-aPDL1/Ca2+ | aPDL1/CCL25 | T cell-mediated immunotherapy | B16-F10 tumor | 180 |
| OSA-βCD/Ca2+ | WA-cRGD/(aPD-L1) | Ferroptosis-immunotherapy | B16-F10 tumors | 179 |
5.2 Bone/cartilage repair
Throughout the regeneration of bone and cartilage, the extracellular matrix (ECM) is known to be critical in facilitating signal and material exchange. CD-based supramolecular hydrogels exhibit similarities to the natural ECM and can create a suitable microenvironment for cell and tissue repair.184,185 Moreover, these hydrogels possess shear-thinning properties, enabling them to be administered through minimally invasive injections, thereby replacing traditional implantation procedures.105 Furthermore, their malleability allows them to be molded into various shapes to accommodate irregular bone defect sites.186To enhance the regeneration of bone and cartilage defects, various approaches have been explored, involving the integration of growth factors, metal ions, and nanomaterials into hydrogels. These additives serve to improve the osteogenic activity of the hydrogels. For example, metal ions such as Ca2+, Mg2+, Zn2+, Cu2+, and Sr2+ have been found to possess favorable pro-angiogenic and osteogenic properties.187,188 Yu et al. developed supramolecular hydrogels for bone repair with the use of host–guest interactions between gelatin aromatic residues and CDs (Fig. 8A).189 Meanwhile, the hydrogel was reinforced with the strong coordination between alendronate (ALN) and Ca2+/Mg2+ to enhance its osteogenic activity and mechanical properties. The study demonstrated that the presence of ALN and Ca2+/Mg2+ significantly facilitated osteogenic differentiation of stem cells and bone regeneration within the hydrogel, thus establishing it as an ideal platform for bone repair.
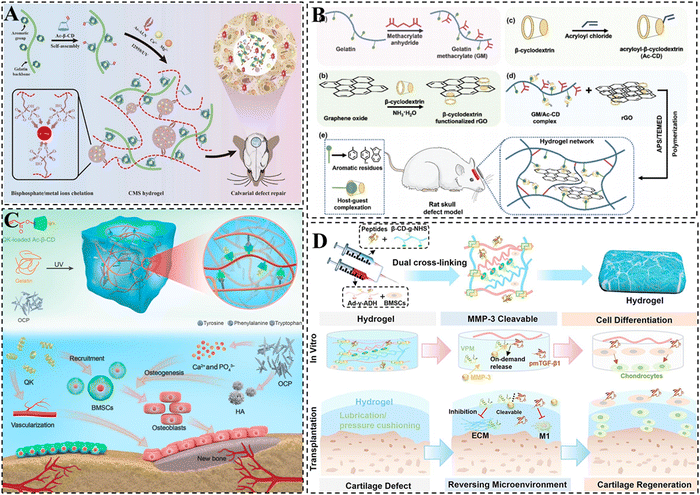 | ||
| Fig. 8 CD-based supramolecular hydrogels for bone/cartilage repair. (A) Schematic representation of CMS supramolecular hydrogel with dual physical cross-linking for cranial bone restoration. Reproduced with permission from ref. 189. Copyright 2023, Elsevier. (B) Schematic illustration of the GM/Ac-CD/rGO hydrogel for cranial bone repair. Reproduced with permission from ref. 193. Copyright 2022, Royal Society of Chemistry. (C) Schematic representation of dual network supramolecular hydrogel loaded with OCP and QK and its mechanism of building angiogenic and osteogenic microenvironments for bone repair. Reproduced with permission from ref. 197. Copyright 2023, American Association for the Advancement of Science. (D) Schematic representation of peptide-based supramolecular hydrogel encapsulated with BMSCs and MMP-3 cleavable VPM-pmTGF-β1 integrative peptide. Reproduced with permission from ref. 206. Copyright 2023, Elsevier. | ||
In addition, previous studies have demonstrated that the incorporation of conductive materials can effectively enhance the bone repair capabilities of hydrogels.190 Among these materials, reduced graphene oxide (rGO) stands out as an ideal choice due to its ability to not only promote the osteogenic differentiation of MSCs but also contribute to a significant improvement in the mechanical properties of hydrogels.191,192 Taking this into account, Li et al. developed a multifunctional hydrogel scaffold that combines good mechanical, photothermal, conductive, and low swelling properties by incorporating CDs, rGO, and gelatin. The formation of the hydrogel primarily relied on double-bond polymerization and host–guest cross-linking between CDs and gelatin (Fig. 8B).193 It is noteworthy that the non-covalent supramolecular interactions within the hydrogel were found to enhance its toughness, while the presence of rigid rGO conferred improved mechanical, electrical conductivity, and photothermal antimicrobial properties to the hydrogel. The efficacy of the multifunctional hydrogel in promoting the proliferation and differentiation of MC3T3-E1 cells was further confirmed, thus facilitating the repair of skull defects.
Octacalcium phosphate (OCP) exhibits enhanced osteoinduction as a precursor of hydroxyapatite (HA). It is capable of converting to HA and releasing Ca2+ at physiological pH, which promotes the osteogenic differentiation of stem cells.194–196 Therefore, Li et al. prepared an injectable hydrogel for bone repair through the double-bond polymerization of Ac-β-CD and the host–guest interaction between CD and aromatic groups (Fig. 8C).197 OCP (an HA precursor that releases Ca2+) and QK (a VEGF-mimetic peptide) were simultaneously added into the hydrogel to further enhance its osteoconductive properties, angiogenic capacity and mechanical properties. It was demonstrated that the integration of QK and OCP into the dual-network hydrogel could synergistically contribute to the regeneration of skull bone defects via the construction of angiogenic and osteogenic microenvironments, indicating promising application prospects in the field of bone repair.
Due to the fact that there are no blood vessels, lymphatic or nervous systems, stem cells face difficulties migrating to the cartilage injury site, resulting in limited self-repair of damaged cartilage tissue.198–200 It is generally accepted that the delivery of stem cells is of crucial importance in the regeneration of cartilage tissue.201 The use of CD-based supramolecular hydrogels offers multiple properties that can potentially enable highly active delivery of stem cells.202–204 Furthermore, CDs can form complexes with the aromatic residues of natural collagen or hydrophobic drug molecules via host–guest interaction, which enhances the tissue adhesion of the hydrogel, as well as the loading efficiency of hydrophobic drugs. Xu et al. formed a supramolecular gelatin hydrogel using β-CDs. The excess β-CD cavities in the hydrogel facilitated the effective loading and slow release of the hydrophobic small molecule drug kartogenin (KGN).205 Additionally, the hydrogel was able to directly encapsulate hydrophilic protein growth factor (TGF-β1) and BMSCs. In a knee cartilage defect model, it was demonstrated that the hydrogel exhibited a significant contribution to cartilage regeneration. In addition, our group prepared a peptide-based hydrogel with both favorable self-healing and mechanical properties by integrating β-CD/Ad host–guest interactions, hydrogen bonding as well as amide bonding (Fig. 8D). The hydrogel effectively inhibited cartilage inflammation and promoted BMSC differentiation with the delivery of BMSC and MMP-3 cleavable VPM-pmTGF-β1 integrative peptide, achieving efficient restoration of damaged cartilage tissue.206
To achieve the simultaneous repair of cartilage and subchondral bone, an injectable, in situ-forming biphasic hydrogel was developed by Liu et al. This was accomplished by covalently photo-crosslinking double-bond-modified CDs with hyaluronic acid methacrylate (HAMA) and gelatin methacryloyl (GelMA), respectively.207 To promote chondrogenic and osteogenic differentiation, the drugs KGN and melatonin (MLT) were separately encapsulated into the CD cavities within the hydrogel through host–guest interactions. The slow release of KGN and MLT from the cartilage and subchondral bone layers induced chondrogenic and osteogenic differentiation, ultimately realizing the simultaneous regeneration of both cartilage and subchondral bone. Table 2 provides a thorough summarization of the recent applications of CD-based supramolecular hydrogels in bone and cartilage repair.
| Hydrogel components | Osteogenic/chondrogenic active ingredients | Therapeutic strategies | Animal models | Ref. |
|---|---|---|---|---|
| PAMAM-Ad/β-CD-g-PNIPAM/ChS-F/PEG-AMI | ChS | Mechanical properties of hydrogels under dynamic conditions can mimic dynamic bone tissue and ChS can confer hydrogel bone repair capabilities | Rat right limb bone defect model | 208 |
| Gelatin/Ac-β-CD | Icaritin/MSCs | Icariin promotes endogenous cell recruitment and infiltration, as well as osteogenic differentiation of MSCs, thereby accelerating the bone regeneration. | Steroid-associated osteonecrosis | 209 |
| GelMA/Ac-β-CD | Kartogenin/TGF-β1/hBMSCs | Continuous release of KGN and TGF-β1 promotes chondrogenic differentiation of hBMSC for cartilage regeneration. | Osteochondral defects in rat knee joints | 205 |
| HA-CD/HA-Ad | MSCs | Spatio-temporal controlled delivery of MSC for cartilage regeneration. | Rat osteochondral defect model | 204 |
| HAMA/GelMA/β-CD-AOI2 | Melatonin/Kartogenin/MSCs | The release of KGN and MLT induces chondrogenic and osteogenic differentiation for simultaneous repair of cartilage and subchondral bone. | Rabbit osteochondral interface defect model | 207 |
| SF-CD/SF-Chol/HA | Hydroxyapatite | The introduction of HA promotes the differentiation of stem cells to osteoblasts. | Rat femoral defect model | 82 |
| GM/Ac-CD/rGO | rGO | The rigid rGO endowed the hydrogel with enhanced mechanical, electrical conductivity, and photothermal antimicrobial properties, which effectively promoted the repair of skull defects. | Rat skull defect model | 193 |
| Gelatin/Ac-β-CD/Ac-ALN/Ca2+/Mg2+ | ALN/Ca2+/Mg2+ | The addition of ALN with metal ions such as Ca2+/Mg2+ significantly improves bone regeneration because of the excellent osteogenic activity. | Rat skull defect model | 189 |
| Gelatin/Ac-β-CD | Octacalcium phosphate (HA precursor)/QK (VEGF-mimicking peptide) | The introduction of OCP and QK improves the mechanical properties, osteoconductive properties, and angiogenic capacity of hydrogels, which can synergistically promote the repair and regeneration of cranial bone defects by constructing angiogenic and osteogenic microenvironments | Rat skull defect model | 197 |
5.3 Myocardial repair
Myocardial infarction (MI) is a prevalent cardiovascular disease characterized by impaired vascularization and the loss of cardiomyocytes, ultimately leading to ventricular remodeling or sudden death.210 The use of biomaterials for sustainable delivery of bioactive agents or stem cells to damaged tissues is an effective approach to treating MI, as it reduces side effects and improves therapeutic efficacy.211–213 CD-based supramolecular hydrogels show promise as prospective platforms for drug or stem cell delivery because of their injectability and favorable biocompatibility.214 While erythropoietin (EPO) has been found to have anti-apoptotic and angiogenic effects in the treatment of MI, it can also lead to adverse effects such as erythrocytosis.215 To minimize these adverse effects, Wang et al. incorporated linear MPEG–PCL–MPEG polymers into α-CD cavities and formed a supramolecular hydrogel through hydrogen bonding between the α-CDs for the sustained delivery of EPO to the myocardial site.216 The study demonstrated that this supramolecular hydrogel enhanced the therapeutic efficacy of EPO and improved cardiac function without causing erythrocytosis. Similarly, Wang et al. encapsulated BMSCs into α-CD/MPEG–PCL–MPEG hydrogels for the treatment of MI.217 It was shown that the supramolecular hydrogel loaded with BMSCs increased the left ventricular ejection function and attenuated left ventricular dilatation.In addition, previous studies have demonstrated that extracellular vesicles (EVs) derived from endothelial progenitor cells (EPC) play a beneficial role in cardiac repair.218 Chen et al. conducted a study in which EPC-derived EVs were loaded into a hyaluronic acid hydrogel formed by β-CD/Ad supramolecular interactions.219 This study showed that intramyocardial delivery of EPC-derived EVs through shear-thinning hydrogels not only improved angiogenesis and hemodynamic function at the site of MI, but also maintained the ventricular geometry intact, thereby facilitating myocardial repair.
Gene drugs have emerged as a highly promising therapeutic approach for MI in recent years.220 To enhance the efficacy of gene drugs, supramolecular hydrogels have already been employed for the local and continuous release of gene drugs. For instance, Burdick et al. designed a self-healing hyaluronic acid hydrogel using CD/Ad host–guest interactions (Fig. 9A).221 Chol-modified miR-302 was embedded into the hydrogel via host–guest recognition. The results demonstrated that the sustained delivery of miR-302 in hydrogels effectively promoted the proliferation and regeneration of cardiomyocytes after MI. Another study by the same research group designed a dynamic acylhydrazone-bonded cross-linked hydrogel for local delivery of siMMP2 (Fig. 9B).222 In this case, CDs were modified on HA to form complexes with chol-modified siRNAs, thereby restricting the passive diffusion of siRNAs within the hydrogels. Moreover, MMP-responsive peptide was also integrated into the crosslinks, enabling the hydrogels to erode and release siMMP2 in response to MMP2. The findings demonstrated that siMMP2 could slow down hydrogel erosion by silencing MMP2 expression, thereby reducing infarct enlargement and remodeling, and improving myocardial function.
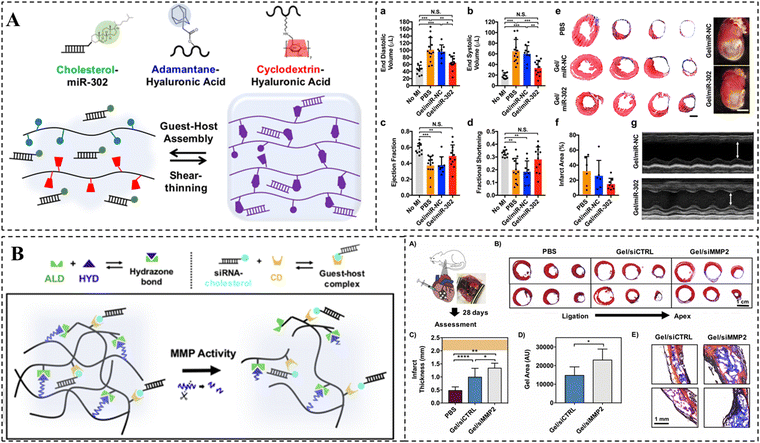 | ||
| Fig. 9 CD-based supramolecular hydrogels for myocardial repair. (A) Schematic representation of the CD/Ad supramolecular hydrogel and its in vivo therapeutic effect. miR-302-chol was loaded into the hydrogel via CD/Chol host–guest interactions. Reproduced with permission from ref. 221. Copyright 2017, Springer Nature. (B) Schematic illustration of a protease-degradable, injectable hydrogel for delivery of siRNA to the heart site and its in vivo therapeutic effect. Reproduced with permission from ref. 222. Copyright 2018, Elsevier. | ||
5.4 Wound repair
CD-based supramolecular hydrogels have garnered significant attention in the field of wound dressings and have demonstrated considerable application value in recent years.223 In comparison to traditional hydrogels, CD-based supramolecular hydrogels offer distinct advantages in the field of wound repair due to their injectability, shape adaptability, and favorable self-healing ability.224 The utilization of anti-inflammatory drugs or bioactive molecules, such as epidermal growth factors (EGF) at the wound site has emerged as an effective therapeutic approach to accelerate wound healing.225–227 CD-based supramolecular hydrogels can serve as drug reservoirs for the continuous delivery of drugs or bioactive molecules, resulting in the acceleration of the wound healing process.228 Zhao et al. constructed a light-responsive hyaluronic acid hydrogel by harnessing the photoisomerization property of Azo (Fig. 10A).229 The study demonstrated that the CD/Azo host–guest cross-links in the hydrogel were disrupted under the irradiation of UV light, resulting in the rapid release of EGF at the wound site and consequently facilitating wound healing. Similarly, a curcumin-loaded chitin supramolecular hydrogel was prepared by Shi et al. via β-CD/Ad host–guest interactions as well as dynamic Schiff base bonding (Fig. 10B).230 Curcumin, a hydrophobic drug with antioxidant and anti-inflammatory activities, was loaded into the excess CD cavity in the hydrogel. The study indicated that the curcumin-loaded hydrogel effectively promoted wound healing with favorable drug release kinetics.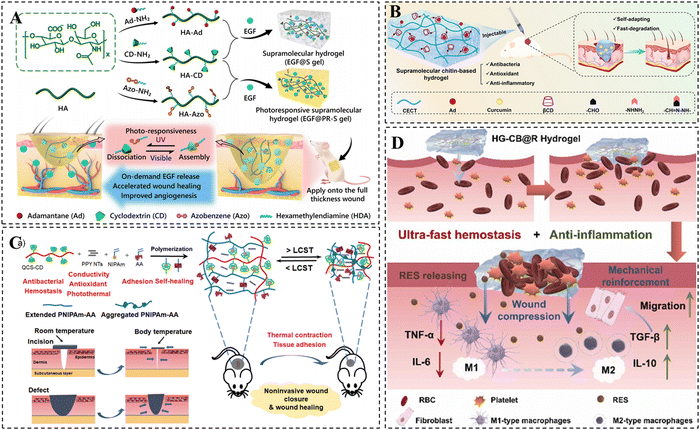 | ||
| Fig. 10 CD-based supramolecular hydrogels for wound repair. (A) Schematic diagram of UV-responsive hyaluronic acid supramolecular hydrogels loaded with EGF for skin repair. Reproduced with permission from ref. 229. Copyright 2020, Elsevier. (B) Schematic representation of curcumin-loaded chitin-based hydrogels for wound healing. Reproduced with permission from ref. 230. Copyright 2023, Elsevier. (C) Schematic illustration of PNIPAm-AA/QCS-CD/PPY hydrogel for non-invasive mouth closure and wound healing. Reproduced with permission from ref. 234. Copyright 2022, Wiley. (D) Schematic representation of HG-CB@R hydrogels for therapeutic rapid hemostasis and wound healing. Reproduced with permission from ref. 235. Copyright 2023, Elsevier. | ||
In addition to delivering anti-inflammatory drugs for their anti-inflammatory effects, ideal hydrogel dressings are expected to possess antimicrobial, angiogenic, and conductive properties to prevent wound infections and accelerate wound healing.231,232 Currently, hydrogel platforms that integrate multiple functions are becoming popular in the field of wound healing.233 Yu et al. developed an injectable thermosensitive hydrogel by utilizing the host–guest complexation of N-isopropylacrylamide (NIPAM) with CD and hydrogen bonding between adenine (Fig. 10C).234 This hydrogel was endowed with various bioactivities such as photo-thermal antimicrobial, electrical conductivity, antioxidant, and hemostatic properties through the incorporation of CD-grafted quaternized chitosan and polypyrrole nanotubes. These properties effectively reduced inflammation and promoted skin repair. Moreover, the presence of adenine facilitated excellent tissue adhesion, while the thermal contraction behavior provided by PNIPAM allowed for rapid contraction of the wound defect site, enabling non-invasive wound closure. The hydrogel shows great promise for the non-invasive closure of large open wounds and enhanced wound healing. A resveratrol-loaded HG-CB@R hydrogel for therapeutic rapid hemostasis was prepared by Tan et al. using host–guest interactions and double-bond radical polymerization (Fig. 10D). It was shown that blood coagulation could be accelerated by rapid absorption of water from the wound site. In addition, the cross-linking density of the hydrogel can be further increased via the coordination of bisphosphonate groups with Fe3+ in the blood, improving its mechanical properties and avoiding secondary bleeding from hydrogel rupture.235
6. Summary and outlook
In this review, we present a comprehensive summary of the preparation of CD-based supramolecular hydrogels and the optimization strategy for enhancing their mechanical properties. Additionally, we provide a detailed description of the different methods for loading drugs into CD-based supramolecular hydrogels and their responsive drug release modes, highlighting their applications as stimuli-responsive drug carriers in various biomedical fields including oncology therapeutics, bone/cartilage repair, myocardial repair, and skin repair. CD-based supramolecular hydrogels exhibit intriguing features like self-healing, injectability, shear-thinning, and stimulus responsiveness, which contribute to their practicality and intelligence. It is evident that with the continuous optimization of the mechanical properties, loading methods, and the introduction of stimuli-responsive units, CD-based supramolecular hydrogels have vast potential and will find broader applications in the future. Although numerous functions of CD-based supramolecular hydrogels have been validated in laboratory settings, and positive results have been obtained in animal models for various biological applications, theoretical studies of these hydrogels are still in their early stages, and there remain significant clinical requirements that have not been addressed. Consequently, the translation of CD-based supramolecular hydrogels from laboratory research to clinical medicine poses a substantial challenge.To further advance the biomedical application of CD-based supramolecular hydrogels and expedite their clinical transformation, it is imperative to enhance research in the following areas: (i) Strengthening research on the encapsulation performance of different CD hosts and guests is necessary. This will help elucidate the various factors that affect encapsulation efficiency and gradually develop theories to provide theoretical guidance for the fabrication of CD-based hydrogels. Additionally, the advantages of CDs in the long-term controlled release of drug molecules should be fully utilized, combined with stimuli-responsive building units, and extended to the application of these materials in biomedicine or tissue engineering. (ii) Further research should be conducted on CD-based supramolecular hydrogels with superior mechanical properties to enhance their applicability in tissue engineering, particularly in bone repair, where mechanical support is crucial. Moreover, efforts should be made to improve the functionalization and intelligence of CD-based supramolecular hydrogels, enabling them with a wider variety of characteristics like electrical conductivity, multi-stimulus responsiveness, and antimicrobial properties, thereby moving them towards true functional diversification. (iii) In the early stages of designing CD-based supramolecular hydrogels, it is crucial to fully consider the biodegradability and biocompatibility of the hydrogels. Additionally, efforts should be made to minimize the foreign body reaction (FBR) of the hydrogels in vivo in order to ensure their in vivo safety and enhance their potential for clinical translation. (iv) To facilitate clinical translation, research systems should steer clear of overly complex designs and prioritize the safety and feasibility of the materials used. It is also of importance to take the reproducibility and stability of experimental results into consideration, as well as the suitability of the hydrogels for scaled-up industrial production in the early stages of development. Although numerous investigations focus on the design of CD-based supramolecular hydrogels and their initial application to specific diseases, clinical translation remains a distant goal. However, it is worth trusting that CD-based supramolecular hydrogels hold promise for future multifunctionality, intelligence, and disease-specific adaptability. These hydrogels have the potential to integrate diagnostics, treatment, and detection, thereby improving patient compliance, meeting growing clinical needs, and ultimately achieving clinical translation.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by Hunan Science and Technology Innovation Leading Talent Project (2022RC3080), the National Natural Science Foundation of China (82304429, 82373826, 82304433), the Key R & D Program of Hunan Province (2021SK2036), Post-doctoral Funding from the University of South China (220XQD106), and the Foundation of Hunan Provincial Natural Science Foundation of China (2023JJ40570).References
- G. Crini, Chem. Rev., 2014, 114, 10940–10975 CrossRef CAS PubMed.
- S. V. Kurkov and T. Loftsson, Int. J. Pharm., 2013, 453, 167–180 CrossRef CAS PubMed.
- A. Harada, Acc. Chem. Res., 2001, 34, 456–464 CrossRef CAS PubMed.
- N. G. Hădărugă, G. N. Bandur, I. David and D. I. Hădărugă, Environ. Chem. Lett., 2019, 17, 349–373 CrossRef.
- M. A. Przybyla, G. Yilmaz and C. R. Becer, Polym. Chem., 2020, 11, 7582–7602 RSC.
- G. Liu, Q. Yuan, G. Hollett, W. Zhao, Y. Kang and J. Wu, Polym. Chem., 2018, 9, 3436–3449 RSC.
- A. Roy, K. Manna, S. Dey and S. Pal, Carbohydr. Polym., 2023, 306, 120576 CrossRef CAS PubMed.
- M. Mohamadhoseini and Z. Mohamadnia, Coord. Chem. Rev., 2021, 432, 213711 CrossRef CAS.
- J. Li, NPG Asia Mater., 2010, 2, 112–118 CrossRef.
- S. Bernhard and M. W. Tibbitt, Adv. Drug Delivery Rev., 2021, 171, 240–256 CrossRef CAS.
- H. J. Lee, P. T. Le, H. J. Kwon and K. D. Park, J. Mater. Chem. B, 2019, 7, 3374–3382 RSC.
- G. Fang, X. Yang, S. Chen, Q. Wang, A. Zhang and B. Tang, Coord. Chem. Rev., 2022, 454, 214352 CrossRef CAS.
- M. Osaki, S. Yonei, C. Ueda, R. Ikura, J. Park, H. Yamaguchi, A. Harada, M. Tanaka and Y. Takashima, Macromolecules, 2021, 54, 8067–8076 CrossRef CAS.
- D. J. Whitaker, J. Park, C. Ueda, G. Wu, A. Harada, G. Matsuba, Y. Takashima and O. A. Scherman, Polym. Chem., 2022, 13, 5127–5134 RSC.
- R. Mejia-Ariza, L. Graña-Suárez, W. Verboom and J. Huskens, J. Mater. Chem. B, 2017, 5, 36–52 RSC.
- Y. Yuan, T. Nie, Y. Fang, X. You, H. Huang and J. Wu, J. Mater. Chem. B, 2022, 10, 2077–2096 RSC.
- W. Xu, X. Li, L. Wang, S. Li, S. Chu, J. Wang, Y. Li, J. Hou, Q. Luo and J. Liu, Front. Chem., 2021, 9, 635507 CrossRef CAS PubMed.
- J. Wankar, N. G. Kotla, S. Gera, S. Rasala, A. Pandit and Y. A. Rochev, Adv. Funct. Mater., 2020, 30, 1909049 CrossRef CAS.
- Y. Wang, L. He, L. Ding, X. Zhao, H. Ma, Y. Luo, S. Ma and Y. Xiong, Chem. Mater., 2023, 35, 5723–5743 CrossRef CAS.
- S. Wang, P. J. Ong, S. Liu, W. Thitsartarn, M. Tan, A. Suwardi, Q. Zhu and X. J. Loh, Chem. – Asian J., 2022, 17, e202200608 CrossRef CAS.
- M. Jain, B. P. Nowak and B. J. Ravoo, ChemNanoMat, 2022, 8, e202200077 CrossRef CAS.
- J. Xu, X. Zhu, J. Zhao, G. Ling and P. Zhang, Adv. Colloid Interface Sci., 2023, 321, 103000 CrossRef CAS.
- X. Han, Y. Su, G. Che, Q. Wei, H. Zheng, J. Zhou and Y. Li, ACS Appl. Mater. Interfaces, 2022, 14, 50199–50214 CrossRef CAS.
- W. Wang, Y. Shi, G. Lin, B. Tang, X. Li, J. Zhang, X. Ding and G. Zhou, Macromol. Biosci., 2023, 23, e2200539 CrossRef PubMed.
- S. Loethen, J. M. Kim and D. H. Thompson, Polym. Rev., 2007, 47, 383–418 CrossRef CAS.
- J. J. Li, F. Zhao and J. Li, Appl. Microbiol. Biotechnol., 2011, 90, 427–443 CrossRef CAS.
- M. Arunachalam and H. W. Gibson, Prog. Polym. Sci., 2014, 39, 1043–1073 CrossRef CAS.
- A. Harada, Coord. Chem. Rev., 1996, 148, 115–133 CrossRef CAS.
- M. Okada, M. Kamachi and A. Harada, J. Phys. Chem. B, 1999, 103, 2607–2613 CrossRef CAS.
- C. Yang, X. Wang, H. Li, S. H. Goh and J. Li, Biomacromolecules, 2007, 8, 3365–3374 CrossRef CAS.
- X. Li and J. Li, J. Biomed. Mater. Res., Part A, 2008, 86A, 1055–1061 CrossRef CAS PubMed.
- J. Li, A. Harada and M. Kamachi, Polym. J., 1994, 26, 1019–1026 CrossRef CAS.
- A. Harada, J. Li and M. Kamachi, Macromolecules, 1993, 26, 5698–5703 CrossRef CAS.
- M. Serres-Gómez, G. González-Gaitano, D. B. Kaldybekov, E. D. H. Mansfield, V. V. Khutoryanskiy, J. R. Isasi and C. A. Dreiss, Langmuir, 2018, 34, 10591–10602 CrossRef PubMed.
- J. Yu, W. Ha, J. N. Sun and Y. P. Shi, ACS Appl. Mater. Interfaces, 2014, 6, 19544–19551 CrossRef CAS PubMed.
- A. J. Poudel, F. He, L. Huang, L. Xiao and G. Yang, Carbohydr. Polym., 2018, 194, 69–79 CrossRef CAS PubMed.
- G. Bovone, E. A. Guzzi, S. Bernhard, T. Weber, D. Dranseikiene and M. W. Tibbitt, Adv. Mater., 2022, 34, e2106941 CrossRef.
- T. Mehmood and A. Ahmed, Langmuir, 2020, 36, 2886–2892 CrossRef CAS PubMed.
- B. Tang, X. Yang, A. Zhang, Q. Wang, L. Fan and G. Fang, Carbohydr. Polym., 2022, 297, 120002 CrossRef CAS.
- C. Hwang, S. Y. Lee, H. J. Kim, K. Lee, J. Lee, D. D. Kim and H. J. Cho, Carbohydr. Polym., 2021, 266, 118104 CrossRef CAS.
- P. Gao, J. Wang, L. Ye, A.-Y. Zhang and Z.-G. Feng, Macromol. Chem. Phys., 2011, 212, 2319–2327 CrossRef CAS.
- A. Harada, J. Li and M. Kamachi, Nature, 1994, 370, 126–128 CrossRef CAS.
- G. Fang, Q. Wang, X. Yang, Y. Qian, G. Zhang and B. Tang, Carbohydr. Polym., 2022, 277, 118889 CrossRef CAS.
- X. Wang, Z. Luo and Z. Xiao, Carbohydr. Polym., 2014, 101, 1027–1032 CrossRef CAS.
- K. M. Sahu, S. Patra and S. K. Swain, Int. J. Biol. Macromol., 2023, 240, 124338 CrossRef CAS PubMed.
- S. Tan, K. Ladewig, Q. Fu, A. Blencowe and G. G. Qiao, Macromol. Rapid Commun., 2014, 35, 1166–1184 CrossRef CAS.
- A. Harada, R. Kobayashi, Y. Takashima, A. Hashidzume and H. Yamaguchi, Nat. Chem., 2011, 3, 34–37 CrossRef CAS PubMed.
- R. Chen, Y. Li, Y. Jin, Y. Sun, Z. Zhao, Y. Xu, J. F. Xu, Y. Dong and D. Liu, Carbohydr. Polym., 2023, 310, 120703 CrossRef CAS PubMed.
- C. Loebel, C. B. Rodell, M. H. Chen and J. A. Burdick, Nat. Protoc., 2017, 12, 1521–1541 CrossRef CAS.
- Z. Wang, Y. Ren, Y. Zhu, L. Hao, Y. Chen, G. An, H. Wu, X. Shi and C. Mao, Angew. Chem., Int. Ed., 2018, 57, 9008–9012 CrossRef CAS.
- J. Chen, X. Xu, M. Liu, Y. Li, D. Yu, Y. Lu, M. Xiong, I. Wyman, X. Xu and X. Wu, Carbohydr. Polym., 2021, 264, 117978 CrossRef CAS PubMed.
- Z. Li, G. Li, J. Xu, C. Li, S. Han, C. Zhang, P. Wu, Y. Lin, C. Wang, J. Zhang and X. Li, Adv. Mater., 2022, 34, e2109178 CrossRef.
- A. Harada and S. Takahashi, J. Chem. Soc., Chem. Commun., 1984, 10, 645–646 RSC.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef PubMed.
- Q. Ling, F. Zhen, D. Astruc and H. Gu, Macromol. Rapid Commun., 2021, 42, e2100049 CrossRef.
- X. Liu, L. Zhao, F. Liu, D. Astruc and H. Gu, Coord. Chem. Rev., 2020, 419, 213406 CrossRef CAS.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef PubMed.
- M. Jain and B. J. Ravoo, Angew. Chem., Int. Ed., 2021, 60, 21062–21068 CrossRef CAS PubMed.
- W. Zhao, X. Zhang, R. Zhang, K. Zhang, Y. Li and F. J. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 56898–56907 CrossRef CAS.
- J. Qin, B. Dong, W. Wang and L. Cao, J. Colloid Interface Sci., 2023, 649, 344–354 CrossRef CAS PubMed.
- M. Y. Chiang, I. Y. Cheng, S. H. Chou, J. H. Tsai, Y. J. Chen, H. E. Lu, S. W. Yang, S. J. Chang and S. Y. Chen, J. Mater. Chem. B, 2021, 9, 9370–9382 RSC.
- H. M. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- I. Tomatsu, A. Hashidzume and A. Harada, Macromolecules, 2005, 38, 5223–5227 CrossRef CAS.
- I. Tomatsu, A. Hashidzume and A. Harada, J. Am. Chem. Soc., 2006, 128, 2226–2227 CrossRef CAS PubMed.
- M. M. Song, Y. M. Wang, B. Wang, X. Y. Liang, Z. Y. Chang, B. J. Li and S. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 15021–15029 CrossRef CAS PubMed.
- F. He, L. Wang, S. Yang, W. Qin, Y. Feng, Y. Liu, Y. Zhou, G. Yu and J. Li, Carbohydr. Polym., 2021, 256, 117595 CrossRef CAS PubMed.
- M. Salzano de Luna, V. Marturano, M. Manganelli, C. Santillo, V. Ambrogi, G. Filippone and P. Cerruti, J. Colloid Interface Sci., 2020, 568, 16–24 CrossRef CAS PubMed.
- Y. Liu, C. Yu, H. Jin, B. Jiang, X. Zhu, Y. Zhou, Z. Lu and D. Yan, J. Am. Chem. Soc., 2013, 135, 4765–4770 CrossRef CAS PubMed.
- B. Pourbadiei, S. Y. Adlsadabad, N. Rahbariasr and A. Pourjavadi, Carbohydr. Polym., 2023, 313, 120667 CrossRef CAS PubMed.
- S. Jia, W.-K. Fong, B. Graham and B. J. Boyd, Chem. Mater., 2018, 30, 2873–2887 CrossRef CAS.
- Z. Mahimwalla, K. G. Yager, J.-I. Mamiya, A. Shishido, A. Priimagi and C. J. Barrett, Polym. Bull., 2012, 69, 967–1006 CrossRef CAS.
- D. Wang, F. Schellenberger, J. T. Pham, H.-J. Butt and S. Wu, Chem. Commun., 2018, 54, 3403–3406 RSC.
- K. Wu, X. Wu, Y. Zhang, S. Chen, Z. Qiao, D. Wei, J. Sun and H. Fan, Biomacromolecules, 2022, 23, 1030–1040 CrossRef CAS PubMed.
- N. M. Cerqueira, E. F. Oliveira, D. S. Gesto, D. Santos-Martins, C. Moreira, H. N. Moorthy, M. J. Ramos and P. A. Fernandes, Biochemistry, 2016, 55, 5483–5506 CrossRef CAS PubMed.
- J. Sun, S. Wang and F. Gao, Langmuir, 2016, 32, 12725–12731 CrossRef CAS PubMed.
- F. van de Manakker, M. van der Pot, T. Vermonden, C. F. van Nostrum and W. E. Hennink, Macromolecules, 2008, 41, 1766–1773 CrossRef CAS.
- C. Li, Adv. Drug Delivery Rev., 2002, 54, 695–713 CrossRef CAS PubMed.
- G. Li, J. Wu, B. Wang, S. Yan, K. Zhang, J. Ding and J. Yin, Biomacromolecules, 2015, 16, 3508–3518 CrossRef CAS PubMed.
- S. Keten, Z. Xu, B. Ihle and M. J. Buehler, Nat. Mater., 2010, 9, 359–367 CrossRef CAS PubMed.
- D. López Barreiro, Z. Martín-Moldes, J. Yeo, S. Shen, M. J. Hawker, F. J. Martin-Martinez, D. L. Kaplan and M. J. Buehler, Adv. Mater., 2019, 31, e1904720 CrossRef PubMed.
- X. Huang, M. Zhang, J. Ming, X. Ning and S. Bai, ACS Appl. Bio Mater., 2020, 3, 7103–7112 CrossRef CAS PubMed.
- S. Bai, M. Zhang, X. Huang, X. Zhang, C. Lu, J. Song and H. Yang, Chem. Eng. J., 2021, 413, 127512 CrossRef CAS.
- C. Ma, Y. Shi, D. A. Pena, L. Peng and G. Yu, Angew. Chem., Int. Ed., 2015, 54, 7376–7380 CrossRef CAS PubMed.
- Y. Guan, H.-B. Zhao, L.-X. Yu, S.-C. Chen and Y.-Z. Wang, RSC Adv., 2014, 4, 4955–4959 RSC.
- Z. Deng, Y. Guo, X. Zhao, P. X. Ma and B. Guo, Chem. Mater., 2018, 30, 1729–1742 CrossRef CAS.
- P. Stockmann, M. Gozzi, R. Kuhnert, M. B. Sárosi and E. Hey-Hawkins, Chem. Soc. Rev., 2019, 48, 3497–3512 RSC.
- G. Calabrese, A. Daou, E. Barbu and J. Tsibouklis, Drug Discovery Today, 2018, 23, 63–75 CrossRef CAS.
- H. Xiong, Y. Li, H. Ye, G. Huang, D. Zhou and Y. Huang, J. Mater. Chem. B, 2020, 8, 10309–10313 RSC.
- Y. M. Zhang, Y. H. Liu and Y. Liu, Adv. Mater., 2020, 32, e1806158 CrossRef PubMed.
- K. Iliopoulos, O. Krupka, D. Gindre and M. Sallé, J. Am. Chem. Soc., 2010, 132, 14343–14345 CrossRef CAS PubMed.
- J. N. Moorthy, K. Venkatesan and R. G. Weiss, J. Org. Chem., 1992, 57, 3292–3297 CrossRef CAS.
- A. Liu, X. Gao, X. Xie, W. Ma, M. Xie and R. Sun, Dyes Pigm., 2020, 177, 108288 CrossRef CAS.
- C. F. J. Faul and M. Antonietti, Adv. Mater., 2003, 15, 673–683 CrossRef CAS.
- Z. Li, G. Wang, Y. Wang and H. Li, Angew. Chem., Int. Ed., 2018, 57, 2194–2198 CrossRef CAS PubMed.
- T. Zhang, Z. Liu, H. Aslan, C. Zhang and M. Yu, J. Mater. Chem. B, 2020, 8, 6429–6437 RSC.
- S. Soltani, R. Emadi, S. H. Javanmard, M. Kharaziha and A. Rahmati, Int. J. Biol. Macromol., 2021, 180, 311–323 CrossRef CAS.
- K. H. Vining and D. J. Mooney, Nat. Rev. Mol. Cell Biol., 2017, 18, 728–742 CrossRef CAS PubMed.
- P. Ren, L. Yang, D. Wei, M. Liang, L. Xu, T. Zhang, W. Hu, Z. Zhang and Q. Zhang, Int. J. Biol. Macromol., 2023, 242, 124885 CrossRef CAS PubMed.
- S. Y. Lee, S. I. Jeon, S. B. Sim, Y. Byun and C. H. Ahn, Acta Biomater., 2021, 131, 286–301 CrossRef CAS PubMed.
- Z. Wang, Y. Ren, Y. Zhu, L. Hao, Y. Chen, G. An, H. Wu, X. Shi and C. Mao, Angew. Chem., Int. Ed., 2018, 57, 9008–9012 CrossRef CAS PubMed.
- M. Chen, Y. Zhang, Q. Xie, W. Zhang, X. Pan, P. Gu, H. Zhou, Y. Gao, A. Walther and X. Fan, ACS Biomater. Sci. Eng., 2019, 5, 4612–4623 CrossRef CAS PubMed.
- Y. Zhou, Y. Zhang, Z. Dai, F. Jiang, J. Tian and W. Zhang, Biomater. Sci., 2020, 8, 3359–3369 RSC.
- S. H. Jeong, M. Kim, T. Y. Kim, H. Choi and S. K. Hahn, ACS Biomater. Sci. Eng., 2021, 7, 4581–4590 CrossRef CAS PubMed.
- M. C. Amin, N. Ahmad, M. Pandey, M. M. Abeer and N. Mohamad, Expert Opin. Drug Delivery, 2015, 12, 1149–1161 CrossRef CAS PubMed.
- Z. Zheng, C. Yu and H. Wei, Tissue Eng., Part B, 2021, 27, 430–454 CrossRef CAS PubMed.
- B. Yu, A. Zhan, Q. Liu, H. Ye, X. Huang, Y. Shu, Y. Yang and H. Liu, Int. J. Pharm., 2020, 578, 119075 CrossRef CAS PubMed.
- H. Wei and C. Y. Yu, Biomater. Sci., 2015, 3, 1050–1060 RSC.
- Q. Feng, K. Wei, S. Lin, Z. Xu, Y. Sun, P. Shi, G. Li and L. Bian, Biomaterials, 2016, 101, 217–228 CrossRef CAS PubMed.
- T. Zhang, L. Guo, R. Li, J. Shao, L. Lu, P. Yang, A. Zhao and Y. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 4959–4972 CrossRef CAS.
- B. Lu, X. Han, D. Zou, X. Luo, L. Liu, J. Wang, M. F. Maitz, P. Yang, N. Huang and A. Zhao, Mater Today Bio, 2022, 16, 100392 CrossRef CAS.
- L. E. Kass and J. Nguyen, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2022, 14, e1756 CAS.
- M. G. Bezold, A. R. Hanna, B. R. Dollinger, P. Patil, F. Yu, C. L. Duvall and M. K. Gupta, Adv. Funct. Mater., 2023, 33, 2213368 CrossRef CAS.
- A. Domiński, T. Konieczny, M. Godzierz, M. Musioł, H. Janeczek, A. Foryś, M. Domińska, G. Pastuch-Gawołek, T. Piotrowski and P. Kurcok, Pharmaceutics, 2022, 14, 2490 CrossRef PubMed.
- Q. Lin, Y. Yang, Q. Hu, Z. Guo, T. Liu, J. Xu, J. Wu, T. B. Kirk, D. Ma and W. Xue, Acta Biomater., 2017, 49, 456–471 CrossRef CAS PubMed.
- S. A. Fisher, A. E. G. Baker and M. S. Shoichet, J. Am. Chem. Soc., 2017, 139, 7416–7427 CrossRef CAS PubMed.
- S. Ulrich, Acc. Chem. Res., 2019, 52, 510–519 CrossRef CAS PubMed.
- C. M. L. Lau, G. Jahanmir and Y. Chau, Acta Biomater., 2020, 101, 219–226 CrossRef CAS PubMed.
- J. Wang, X. Gao, A. Boarino, F. Célerse, C. Corminboeuf and H.-A. Klok, Macromolecules, 2022, 55, 10145–10152 CrossRef CAS.
- L. Dai, R. Liu, L.-Q. Hu, J.-H. Wang and C.-L. Si, RSC Adv., 2017, 7, 2905–2912 RSC.
- Y. Zhou, Z. Zhai, Y. Yao, J. C. Stant, S. L. Landrum, M. J. Bortner, C. E. Frazier and K. J. Edgar, Carbohydr. Polym., 2023, 300, 120213 CrossRef CAS PubMed.
- X. Jiang, F. Zeng, X. Yang, C. Jian, L. Zhang, A. Yu and A. Lu, Acta Biomater., 2022, 141, 102–113 CrossRef CAS PubMed.
- S. J. Sonawane, R. S. Kalhapure and T. Govender, Eur. J. Pharm. Sci., 2017, 99, 45–65 CrossRef CAS PubMed.
- C. Li, H. Li, J. Guo, L. Li, X. Xi and Y. Yu, RSC Adv., 2020, 10, 689–697 RSC.
- F. Li, J. He, M. Zhang and P. Ni, Polym. Chem., 2015, 6, 5009–5014 RSC.
- Y. Zhao, J. Xu, Y. Zhang, F. Wu, W. Zhao, R. Li, Y. Yang, M. Zhang, Y. Zhang and C. Guo, Chem. Eng. J., 2023, 472, 144911 CrossRef CAS.
- B. Sun, C. Luo, H. Yu, X. Zhang, Q. Chen, W. Yang, M. Wang, Q. Kan, H. Zhang, Y. Wang, Z. He and J. Sun, Nano Lett., 2018, 18, 3643–3650 CrossRef CAS PubMed.
- Z. Shi, J. Liu, L. Tian, J. Li, Y. Gao, Y. Xing, W. Yan, C. Hua, X. Xie, C. Liu and C. Liang, Biomed. Pharmacother., 2022, 155, 113707 CrossRef CAS PubMed.
- D. Tang, Y. Yu, J. Zhang, X. Dong, C. Liu and H. Xiao, Adv. Mater., 2022, 34, e2203820 CrossRef PubMed.
- P. Pei, C. Sun, W. Tao, J. Li, X. Yang and J. Wang, Biomaterials, 2019, 188, 74–82 CrossRef CAS PubMed.
- S. Huang, N. Zhao, Z. Qian and W. Yuan, J. Mater. Chem. B, 2023, 11, 3727–3739 RSC.
- S. Shahi, H. Roghani-Mamaqani, S. Talebi and H. Mardani, Coord. Chem. Rev., 2022, 455, 214368 CrossRef CAS.
- Z. Wei, E. Volkova, M. R. Blatchley and S. Gerecht, Adv. Drug Delivery Rev., 2019, 149–150, 95–106 CrossRef CAS PubMed.
- K. H. Bae and M. Kurisawa, Biomater. Sci., 2016, 4, 1184–1192 RSC.
- G. Jahanmir, C. M. L. Lau, M. J. Abdekhodaie and Y. Chau, ACS Appl. Bio Mater., 2020, 3, 4208–4219 CrossRef CAS PubMed.
- F. Brandl, F. Kastner, R. M. Gschwind, T. Blunk, J. Tessmar and A. Göpferich, J. Controlled Release, 2010, 142, 221–228 CrossRef CAS PubMed.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS PubMed.
- Y. H. Lin, H. F. Liang, C. K. Chung, M. C. Chen and H. W. Sung, Biomaterials, 2005, 26, 2105–2113 CrossRef CAS PubMed.
- C. S. Brazel and N. A. Peppas, Eur. J. Pharm. Biopharm., 2000, 49, 47–58 CrossRef CAS PubMed.
- E. Axpe, D. Chan, G. S. Offeddu, Y. Chang, D. Merida, H. L. Hernandez and E. A. Appel, Macromolecules, 2019, 52, 6889–6897 CrossRef CAS PubMed.
- R. Censi, T. Vermonden, M. J. van Steenbergen, H. Deschout, K. Braeckmans, S. C. De Smedt, C. F. van Nostrum, P. di Martino and W. E. Hennink, J. Controlled Release, 2009, 140, 230–236 CrossRef CAS PubMed.
- M. J. Penn and M. G. Hennessy, Appl. Math. Modelling, 2022, 112, 649–668 CrossRef.
- N. Yavari and S. Azizian, J. Mol. Liq., 2022, 363, 119861 CrossRef CAS.
- N. A. Peppas, P. Bures, W. Leobandung and H. Ichikawa, Eur. J. Pharm. Biopharm., 2000, 50, 27–46 CrossRef CAS PubMed.
- Z. Pan and L. Brassart, J. Mech. Phys. Solids, 2022, 167, 105016 CrossRef CAS.
- P. J. LeValley, R. Neelarapu, B. P. Sutherland, S. Dasgupta, C. J. Kloxin and A. M. Kloxin, J. Am. Chem. Soc., 2020, 142, 4671–4679 CrossRef CAS PubMed.
- G. Jahanmir, M. J. Abdekhodaie and Y. Chau, Macromolecules, 2018, 51, 3941–3952 CrossRef CAS.
- F. Li, J. He, M. Zhang, K. C. Tam and P. Ni, RSC Adv., 2015, 5, 54658–54666 RSC.
- W. Qing, Y. Wang, H. Li, J. Zhu and X. Liu, RSC Adv., 2016, 6, 95812–95817 RSC.
- J. Sheng, Y. Wang, L. Xiong, Q. Luo, X. Li, Z. Shen and W. Zhu, Polym. Chem., 2017, 8, 1680–1688 RSC.
- W. Ha, X. B. Zhao, W. H. Zhao, J. J. Tang and Y. P. Shi, J. Mater. Chem. B, 2021, 9, 3200–3209 RSC.
- W. Xu, Y. Nan, Y. Jin, X. Chen, M. Xie, C. Chen and C. Zhao, Chem. Mater., 2022, 34, 8740–8748 CrossRef CAS.
- J. Wang, D. Li, Y. Fan, M. Shi, Y. Yang, L. Wang, Y. Peng, M. Shen and X. Shi, Nanoscale, 2019, 11, 22343–22350 RSC.
- L. Peng, S. Liu, A. Feng and J. Yuan, Mol. Pharmaceutics, 2017, 14, 2475–2486 CrossRef CAS PubMed.
- J. He, W. Zhang, X. Zhou, F. Xu, J. Zou, Q. Zhang, Y. Zhao, H. He, H. Yang and J. Liu, Bioact. Mater., 2023, 19, 115–126 CAS.
- X. Liu, Y. Zhang, Y. Liu, S. Hua, F. Meng, Q. Ma, L. Kong, S. Pan and Y. Che, Int. J. Biol. Macromol., 2023, 240, 124365 CrossRef CAS PubMed.
- R. Dimatteo, N. J. Darling and T. Segura, Adv. Drug Delivery Rev., 2018, 127, 167–184 CrossRef CAS PubMed.
- Q. Wang, Y. Zhang, Y. Ma, M. Wang and G. Pan, Mater. Today Bio, 2023, 20, 100640 CrossRef CAS.
- J. Omar, D. Ponsford, C. A. Dreiss, T. C. Lee and X. J. Loh, Chem. – Asian J., 2022, 17, e202200081 CrossRef CAS PubMed.
- J. Hoque, N. Sangaj and S. Varghese, Macromol. Biosci., 2019, 19, e1800259 CrossRef.
- J. Szejtli, Med. Res. Rev., 1994, 14, 353–386 CrossRef CAS PubMed.
- Z. Liu, L. Ye, J. Xi, J. Wang and Z.-G. Feng, Prog. Polym. Sci., 2021, 118, 101408 CrossRef CAS.
- T. A. Ahles and J. C. Root, Ann. Rev. Clin. Psychol., 2018, 14, 425–451 CrossRef PubMed.
- M. Sepantafar, R. Maheronnaghsh, H. Mohammadi, F. Radmanesh, M. M. Hasani-sadrabadi, M. Ebrahimi and H. Baharvand, Trends Biotechnol., 2017, 35, 1074–1087 CrossRef CAS.
- B. Tan, L. Huang, Y. Wu and J. Liao, J. Biomed. Mater. Res., Part A, 2021, 109, 404–425 CrossRef CAS PubMed.
- B. Dutta, K. C. Barick and P. A. Hassan, Adv. Colloid Interface Sci., 2021, 296, 102509 CrossRef CAS.
- A. Kasiński, M. Zielińska-Pisklak, E. Oledzka and M. Sobczak, Int. J. Nanomed., 2020, 15, 4541–4572 CrossRef.
- L. Rong, Y. Liu, Y. Fan, J. Xiao, Y. Su, L. Lu, S. Peng, W. Yuan and M. Zhan, Carbohydr. Polym., 2023, 310, 120721 CrossRef CAS PubMed.
- C. Nieto, M. A. Vega, V. Rodríguez, P. Pérez-Esteban and E. M. Martín Del Valle, Carbohydr. Polym., 2022, 294, 119732 CrossRef CAS PubMed.
- L. Yin, K. Zhang, W. Sun, Y. Zhang, Y. Wang and J. Qin, Int. J. Biol. Macromol., 2023, 249, 126012 CrossRef CAS PubMed.
- X. Song, Z. Zhang, J. Zhu, Y. Wen, F. Zhao, L. Lei, N. Phan-Thien, B. C. Khoo and J. Li, Biomacromolecules, 2020, 21, 1516–1527 CrossRef CAS PubMed.
- L. Dai, K. Liu, L. Wang, J. Liu, J. He, X. Liu and J. Lei, Mater. Sci. Eng., C, 2017, 76, 966–974 CrossRef CAS.
- J. Li and X. J. Loh, Adv. Drug Delivery Rev., 2008, 60, 1000–1017 CrossRef CAS PubMed.
- D. Ma, H. B. Zhang, D. H. Chen and L. M. Zhang, J. Colloid Interface Sci., 2011, 364, 566–573 CrossRef CAS PubMed.
- X. Liu, X. Chen, M. X. Chua, Z. Li, X. J. Loh and Y. L. Wu, Adv. Healthcare Mater., 2017, 6, 1700159 CrossRef PubMed.
- X. Liu, Z. Li, X. J. Loh, K. Chen, Z. Li and Y. L. Wu, Macromol. Rapid Commun., 2019, 40, e1800117 CrossRef PubMed.
- H. Huang, X. Wang, W. Wang, X. Qu, X. Song, Y. Zhang, L. Zhong, D. P. Yang, X. Dong and Y. Zhao, Biomaterials, 2022, 280, 121289 CrossRef CAS PubMed.
- Y. Ma, Y. Sun, L. Xu, X. Li, D. Gong, Z. Miao and H. Qian, Adv. Healthcare Mater., 2022, 11, e2201023 CrossRef PubMed.
- C. Liu, X. Guo, C. Ruan, H. Hu, B. P. Jiang, H. Liang and X. C. Shen, Acta Biomater., 2019, 96, 281–294 CrossRef CAS PubMed.
- Z. Cheng, C. Xue, M. Liu, Z. Cheng, G. Tian, M. Li, R. Xue, X. Yao, Y. Zhang and Z. Luo, Acta Biomater., 2023, 169, 289–305 CrossRef CAS PubMed.
- Y. Zhu, L. Jin, J. Chen, M. Su, T. Sun and X. Yang, Adv. Mater., 2023, 2309667 CrossRef CAS.
- W. Zhang, X. Zhou, T. Liu, D. Ma and W. Xue, J. Mater. Chem. B, 2015, 3, 2127–2136 RSC.
- J. Wang, C. Guo, X. Y. Wang and H. Yang, J. Controlled Release, 2021, 329, 328–336 CrossRef CAS PubMed.
- C. Ruan, C. Liu, H. Hu, X. L. Guo, B. P. Jiang, H. Liang and X. C. Shen, Chem. Sci., 2019, 10, 4699–4706 RSC.
- M. Liu, X. Zeng, C. Ma, H. Yi, Z. Ali, X. Mou, S. Li, Y. Deng and N. He, Bone Res., 2017, 5, 17014 CrossRef CAS.
- X. Xue, Y. Hu, S. Wang, X. Chen, Y. Jiang and J. Su, Bioact. Mater., 2022, 12, 327–339 CAS.
- Y. P. Singh, J. C. Moses, N. Bhardwaj and B. B. Mandal, J. Mater. Chem. B, 2018, 6, 5499–5529 RSC.
- L. Wang, H. He, X. Yang, Y. Zhang, S. Xiong, C. Wang, X. Yang, B. Chen and Q. Wang, Mater. Today Adv., 2021, 12, 100162 CrossRef CAS.
- Y. Zhang, C. An, Y. Zhang, H. Zhang, A. F. Mohammad, Q. Li, W. Liu, F. Shao, J. Sui, C. Ren, K. Sun, F. Cheng, J. Liu and H. Wang, Mater. Sci. Eng., C, 2021, 131, 112497 CrossRef CAS PubMed.
- T. Yu, Y. Hu, W. He, Y. Xu, A. Zhan, K. Chen, M. Liu, X. Xiao, X. Xu, Q. Feng and L. Jiang, Mater. Today Bio, 2023, 19, 100558 CrossRef CAS.
- C. Yu, X. Ying, M. A. Shahbazi, L. Yang, Z. Ma, L. Ye, W. Yang, R. Sun, T. Gu, R. Tang, S. Fan and S. Yao, Biomaterials, 2023, 301, 122266 CrossRef CAS PubMed.
- M. H. Norahan, M. Amroon, R. Ghahremanzadeh, N. Rabiee and N. Baheiraei, IET Nanobiotechnol., 2019, 13, 720–725 CrossRef PubMed.
- S. Yu, M. You, K. Zhou and J. Li, Front. Bioeng. Biotechnol., 2023, 11, 1185520 CrossRef PubMed.
- Y. Li, J. He, J. Zhou, Z. Li, L. Liu, S. Hu, B. Guo and W. Wang, Biomater. Sci., 2022, 10, 1326–1341 RSC.
- O. Suzuki, Acta Biomater., 2010, 6, 3379–3387 CrossRef CAS PubMed.
- S. Saito, R. Hamai, Y. Shiwaku, T. Hasegawa, S. Sakai, K. Tsuchiya, Y. Sai, R. Iwama, N. Amizuka, T. Takahashi and O. Suzuki, Acta Biomater., 2021, 129, 309–322 CrossRef CAS PubMed.
- E. Amann, A. Amirall, A. R. Franco, P. S. P. Poh, F. J. Sola Dueñas, G. Fuentes Estévez, I. B. Leonor, R. L. Reis, M. van Griensven and E. R. Balmayor, Adv. Healthcare Mater., 2021, 10, e2001692 CrossRef.
- J. Li, J. Ma, Q. Feng, E. Xie, Q. Meng, W. Shu, J. Wu, L. Bian, F. Han and B. Li, Research, 2023, 6, 0021 CrossRef CAS PubMed.
- S. Khajeh, F. Bozorg-Ghalati, M. Zare, G. Panahi and V. Razban, Curr. Mol. Med., 2021, 21, 56–72 CrossRef CAS PubMed.
- A. R. Armiento, M. Alini and M. J. Stoddart, Adv. Drug Delivery Rev., 2019, 146, 289–305 CrossRef CAS PubMed.
- A. Trengove, C. Di Bella and A. J. O'Connor, Tissue Eng., Part B, 2022, 28, 114–128 CrossRef CAS PubMed.
- K. Johnson, S. Zhu, M. S. Tremblay, J. N. Payette, J. Wang, L. C. Bouchez, S. Meeusen, A. Althage, C. Y. Cho, X. Wu and P. G. Schultz, Science, 2012, 336, 717–721 CrossRef CAS PubMed.
- D. Magne, C. Vinatier, M. Julien, P. Weiss and J. Guicheux, Trends Mol. Med., 2005, 11, 519–526 CrossRef CAS PubMed.
- M. P. Murphy, L. S. Koepke, M. T. Lopez, X. Tong, T. H. Ambrosi, G. S. Gulati, O. Marecic, Y. Wang, R. C. Ransom, M. Y. Hoover, H. Steininger, L. Zhao, M. P. Walkiewicz, N. Quarto, B. Levi, D. C. Wan, I. L. Weissman, S. B. Goodman, F. Yang, M. T. Longaker and C. K. F. Chan, Nat. Med., 2020, 26, 1583–1592 CrossRef CAS PubMed.
- S. H. Jeong, M. Kim, T. Y. Kim, H. Kim, J. H. Ju and S. K. Hahn, ACS Appl. Bio Mater., 2020, 3, 5040–5047 CrossRef CAS PubMed.
- J. Xu, Q. Feng, S. Lin, W. Yuan, R. Li, J. Li, K. Wei, X. Chen, K. Zhang, Y. Yang, T. Wu, B. Wang, M. Zhu, R. Guo, G. Li and L. Bian, Biomaterials, 2019, 210, 51–61 CrossRef CAS PubMed.
- Z. Zheng, J. Sun, J. Wang, S. He, Y. Huang, X. Yang, Y. Zhao, C.-Y. Yu and H. Wei, Chem. Eng. J., 2023, 473, 145228 CrossRef CAS.
- X. Liu, Y. Chen, A. S. Mao, C. Xuan, Z. Wang, H. Gao, G. An, Y. Zhu, X. Shi and C. Mao, Biomaterials, 2020, 232, 119644 CrossRef CAS PubMed.
- X. Bai, S. Lü, Z. Cao, C. Gao, H. Duan, X. Xu, L. Sun, N. Gao, C. Feng and M. Liu, Chem. Eng. J., 2016, 288, 546–556 CrossRef CAS.
- Q. Feng, J. Xu, K. Zhang, H. Yao, N. Zheng, L. Zheng, J. Wang, K. Wei, X. Xiao, L. Qin and L. Bian, ACS Cent. Sci., 2019, 5, 440–450 CrossRef CAS.
- B. Lindahl and N. L. Mills, Nat. Med., 2023, 29, 2200–2205 CrossRef CAS.
- Z. Zheng, Y. Tan, Y. Li, Y. Liu, G. Yi, C.-Y. Yu and H. Wei, J. Controlled Release, 2021, 335, 216–236 CrossRef CAS PubMed.
- Z. Zheng, C. Lei, H. Liu, M. Jiang, Z. Zhou, Y. Zhao, C. Y. Yu and H. Wei, Adv. Healthcare Mater., 2022, 11, e2200990 CrossRef PubMed.
- Z. Zheng, Z. Guo, F. Zhong, B. Wang, L. Liu, W. Ma, C. Y. Yu and H. Wei, J. Controlled Release, 2022, 347, 127–142 CrossRef CAS PubMed.
- Z. Zheng, Y. Tan, Y. Li, Y. Liu, G. Yi, C. Y. Yu and H. Wei, J. Controlled Release, 2021, 335, 216–236 CrossRef CAS PubMed.
- P. Ponikowski and E. A. Jankowska, Eur. Heart J., 2010, 31, 2577–2579 CrossRef PubMed.
- T. Wang, X. J. Jiang, T. Lin, S. Ren, X. Y. Li, X. Z. Zhang and Q. Z. Tang, Biomaterials, 2009, 30, 4161–4167 CrossRef CAS PubMed.
- T. Wang, X. J. Jiang, Q. Z. Tang, X. Y. Li, T. Lin, D. Q. Wu, X. Z. Zhang and E. Okello, Acta Biomater., 2009, 5, 2939–2944 CrossRef CAS PubMed.
- M. Riaud, M. C. Martinez and C. N. J. P. Montero-Menei, Pharmaceutics, 2020, 12, 1195 CrossRef CAS PubMed.
- C. W. Chen, L. L. Wang, S. Zaman, J. Gordon, M. F. Arisi, C. M. Venkataraman, J. J. Chung, G. Hung, A. C. Gaffey, L. A. Spruce, H. Fazelinia, R. C. Gorman, S. H. Seeholzer, J. A. Burdick and P. Atluri, Cardiovasc. Res., 2018, 114, 1029–1040 CrossRef CAS PubMed.
- Q. Zhang, L. Wang, S. Wang, H. Cheng, L. Xu, G. Pei, Y. Wang, C. Fu, Y. Jiang, C. He and Q. Wei, Signal Transduction Targeted Ther., 2022, 7, 78 CrossRef CAS PubMed.
- L. L. Wang, Y. Liu, J. J. Chung, T. Wang, A. C. Gaffey, M. Lu, C. A. Cavanaugh, S. Zhou, R. Kanade, P. Atluri, E. E. Morrisey and J. A. Burdick, Nat. Biomed. Eng., 2017, 1, 983–992 CrossRef CAS PubMed.
- L. L. Wang, J. J. Chung, E. C. Li, S. Uman, P. Atluri and J. A. Burdick, J. Controlled Release, 2018, 285, 152–161 CrossRef CAS PubMed.
- R. Dimatteo, N. J. Darling and T. Segura, Adv. Drug Delivery Rev., 2018, 127, 167–184 CrossRef CAS PubMed.
- C. Alvarez-Lorenzo, C. A. Garcia-Gonzalez and A. Concheiro, J. Controlled Release, 2017, 268, 269–281 CrossRef CAS PubMed.
- H. Kim, W. H. Kong, K. Y. Seong, D. K. Sung, H. Jeong, J. K. Kim, S. Y. Yang and S. K. Hahn, Biomacromolecules, 2016, 17, 3694–3705 CrossRef CAS PubMed.
- C. Wang, Q. Zhang, G. Hou, C. Wang and H. Yan, Eur. Polym. J., 2023, 190, 112003 CrossRef CAS.
- R. Yu, Y. Yang, J. He, M. Li and B. Guo, Chem. Eng. J., 2021, 417, 128278 CrossRef CAS.
- E. Pinho, M. Grootveld, G. Soares and M. Henriques, Crit. Rev. Biotechnol., 2014, 34, 328–337 CrossRef CAS PubMed.
- W. Zhao, Y. Li, X. Zhang, R. Zhang, Y. Hu, C. Boyer and F. J. Xu, J. Controlled Release, 2020, 323, 24–35 CrossRef CAS PubMed.
- W. Shi, D. Zhang, L. Han, W. Shao, Q. Liu, B. Song, G. Yan, R. Tang and X. Yang, Carbohydr. Polym., 2024, 323, 121374 CrossRef CAS PubMed.
- Y. Zhang, X. Gao, X. Tang, L. Peng, H. Zhang, S. Zhang, Q. Hu and J. Li, Int. J. Biol. Macromol., 2023, 253, 126693 CrossRef CAS PubMed.
- K. Zha, W. Zhang, W. Hu, M. Tan, S. Zhang, Y. Yu, S. Gou, P. Bu, B. Zhou, Y. Zou, Y. Xiong, B. Mi, G. Liu, Q. Feng and K. Cai, Adv. Funct. Mater., 2023, 2308145 CrossRef.
- R. Yu, Z. Li, G. Pan and B. Guo, Sci. China: Chem., 2022, 65, 2238–2251 CrossRef CAS.
- R. Yu, M. Li, Z. Li, G. Pan, Y. Liang and B. Guo, Adv. Healthcare Mater., 2022, 11, e2102749 CrossRef PubMed.
- L. Tan, M. Li, H. Chen, Y. Zhang, Y. Liu, M. Chen, Z. Luo, K. Cai and Y. Hu, Nano Today, 2023, 52, 101962 CrossRef.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |




