Open Access Article
Open Access ArticleCyclic (amino)(barrelene)carbene Ru-complexes: synthesis and reactivity in olefin metathesis†
Jakub
Talcik
a,
Melinda R.
Serrato
 b,
Antonio
Del Vecchio‡
b,
Antonio
Del Vecchio‡
 a,
Sophie
Colombel-Rouen
a,
Jennifer
Morvan
a,
Thierry
Roisnel
a,
Sophie
Colombel-Rouen
a,
Jennifer
Morvan
a,
Thierry
Roisnel
 a,
Rodolphe
Jazzar
a,
Rodolphe
Jazzar
 *b,
Mohand
Melaimi
b,
Guy
Bertrand
*b,
Mohand
Melaimi
b,
Guy
Bertrand
 *b and
Marc
Mauduit
*b and
Marc
Mauduit
 *a
*a
aUniv. Rennes, Ecole Nationale Supérieure de Chimie de Rennes, CNRS, ISCR UMR 6226, F-35000 Rennes, France. E-mail: marc.mauduit@ensc-rennes.fr
bUCSD-CNRS Joint Research Chemistry Laboratory (IRL 3555), Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, California 92093-0358, USA. E-mail: rjazzar@ucsd.edu; gbertrand@ucsd.edu
First published on 28th February 2024
Abstract
The synthesis of ruthenium-complexes with cyclic (amino)(barrelene)carbenes (namely CABCs) as ligands is reported. Isolated in moderate to good yields, these new complexes showed impressive thermal stability at 110 °C over several days. Good catalytic performances were demonstrated in various ring-closing metathesis (RCM), macrocyclic–RCM, ring-closing enyne metathesis (RCEYM), cross-metathesis (CM), and ring-opening cross metathesis (ROCM) reactions.
Considered as an eco-friendly process to build carbon–carbon bonds, olefin metathesis1 represents one of the most efficient synthetic tools in modern organic chemistry.2 The recent development of well-defined ruthenium complexes containing a cyclic (alkyl)(amino)carbene, namely the CAAC ligand,3 led to significant breakthroughs in this field by reaching the highest TONs reported so far (up to 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in ethenolysis4 and 68
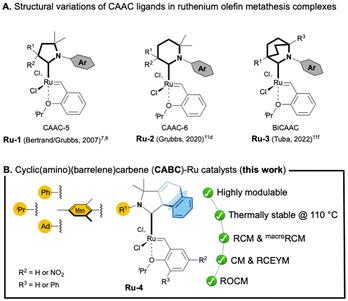
000 in ethenolysis4 and 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in RCM5). It should be noted that this impressive productivity relies on the improved stability of the corresponding Ru–methylidene species toward bimolecular decomposition.6 While numerous catalysts bearing five-membered CAACs (called CAAC-5) have been intensively developed through the modification of the N-aryl fragment and/or the substituents of the quaternary centre (Ru-1, Fig. 1A),7 the structural variation of the heterocyclic backbone has scarcely been investigated. In 2020, Grubbs and co-workers reported a ruthenium complex containing a six-membered CAAC8 (CAAC-6; Ru-2, Fig. 1A) while Tuba and co-workers described a bicyclic CAAC Ru-3 complex9 (BiCAAC, Fig. 1A). Unfortunately, these complexes demonstrated lower catalytic performances than the original Ru-1 type catalysts. Based on these reports, we decided to examine a new type of CAAC-5 ligand, namely cyclic (amino)(barrelene)carbene or CABC, recently reported in 2022.10
000 in RCM5). It should be noted that this impressive productivity relies on the improved stability of the corresponding Ru–methylidene species toward bimolecular decomposition.6 While numerous catalysts bearing five-membered CAACs (called CAAC-5) have been intensively developed through the modification of the N-aryl fragment and/or the substituents of the quaternary centre (Ru-1, Fig. 1A),7 the structural variation of the heterocyclic backbone has scarcely been investigated. In 2020, Grubbs and co-workers reported a ruthenium complex containing a six-membered CAAC8 (CAAC-6; Ru-2, Fig. 1A) while Tuba and co-workers described a bicyclic CAAC Ru-3 complex9 (BiCAAC, Fig. 1A). Unfortunately, these complexes demonstrated lower catalytic performances than the original Ru-1 type catalysts. Based on these reports, we decided to examine a new type of CAAC-5 ligand, namely cyclic (amino)(barrelene)carbene or CABC, recently reported in 2022.10
 | ||
| Fig. 1 Structural modifications of CAACs (A) and the development of a new CAAC featuring a barrelene scaffold (B, this work). | ||
Readily accessible through an intramolecular [4 + 2] cycloaddition between an alkyne and an anthracene derivative, this ligand features a barrelene skeleton, which provides a unique steric environment.10 Herein, we report the synthesis of related CABC–ruthenium complexes (Ru-4) and their catalytic performances in various olefin metathesis transformations (Fig. 1B).
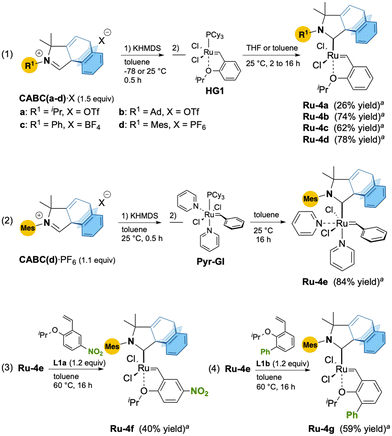
We initiated our study by preparing ruthenium CABC complexes Ru-4a–d from the corresponding iminium salts of CABC(a–d)·X bearing isopropyl, adamantyl, phenyl and mesityl N-substituents, respectively (Scheme 1, eqn (1)). To our delight, deprotonation of CABC(a–d)·X with potassium hexamethyldisilazide (KHMDS) in toluene followed by reaction with the Hoveyda–Grubbs catalyst 1st generation (HG1) over 2 to 16 hours afforded the corresponding CABC Ru-4a–d in low to good isolated yields (26–78%). Following the same protocol, bis-pyridine Ru-4e was isolated in 85% yield from MesCABC(d)·PF6 and Pyr-GI precursors (Scheme 1, eqn (2)).11 Finally, the reaction between Ru-4e and styrenylether ligands L1a,b led to the corresponding nitro-Grela-12 and Blechert-type13,14Ru-4f and Ru-4g in 40% and 59% yield, respectively (Scheme 1, eqn (3) and (4)).15
Suitable crystals of Ru-4a–d,f allowed us to perform X-ray diffraction analyses (Fig. 2). Unfortunately, all attempts to obtain suitable crystals for Ru-4e,-4g were unsuccessful. Unexpectedly, the solid-state structures showed that the barrelene fragment is above the styrenylether moiety, in contrast to most of the previously reported CAAC–Ru complexes, for which the N-aryl unit is above the styrenylether.7,16 It is noteworthy that relatively less intramolecular hydrogen bonding interactions were observed between barrelene CPh–H and ClRu atoms (d = 2.49–2.72 Å) across all complexes. It should be noted that for Ru-4b, these interactions are also observed in solution as shown by a significant downfield shift of the corresponding proton by 1H NMR spectroscopy with respect to the corresponding iminium salt (see the ESI† for details).
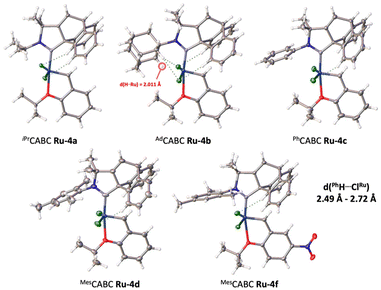 | ||
| Fig. 2 Solid-state structures of CABC-complexes Ru-4a–d,-f from single crystal X-ray diffraction. Displacement ellipsoids are drawn at 30% probability. | ||
For the adamantyl CABC Ru-4b, additional CAd–H bond interactions lock the chlorine atoms into a constrained configuration, which explains the unusually large Cl–Ru–Cl bond angle (∡ = 154.44°) and the very short intramolecular hydrogen–ruthenium (2.01 Å) distance within the range of classical agostic interactions (≈1.8–2.3 Å). With respect to thermal stability, all complexes are stable to air and moisture as solids. We also studied the stability of MesCABC Ru-4d at 110 °C in aerated toluene-d8 solution and observed less than 4% degradation after ten days (see ESI† section 2.2. for further details).
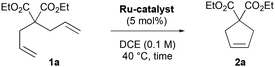
With these results in hand, we next investigated the catalytic performance of all CABC Ru-4a–g complexes in the RCM of DEDAM 1a (Table 1). At 40 °C, poor reactivity was observed with CABC Ru-4a–d (1–7% yield, entries 1–4†) after 18 h of reaction at 5 mol% catalyst loading. In comparison, bis-pyridine Ru-4e led to 41% yield (entry 5), while the nitro-Grela congener MesCABC Ru-4f afforded 13% yield (entry 6). In marked contrast, MesCABC–Blechert Ru-4g afforded higher reactivity, obtaining cyclopentene 2a in 73% yield (entry 7). Gratifyingly at 110 °C, RCM with Ru-4g was completed within 4 hours yielding 2a in 97% (entry 8) in agreement with the excellent thermal stability of this family of carbene complexes.
| Entry | Catalyst | Time (h) | Conv. (yield)b(%) |
|---|---|---|---|
| a Reaction conditions: DEDAM 1a (0.17 mmol), catalyst (0.0085 mmol), DCE (1.7 mL), argon. b Determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as an internal reference (see the ESI†). c Reaction performed in toluene at 110 °C. | |||
| 1 | Ru-4a | 18 | 5 (1) |
| 2 | Ru-4b | 18 | 3 (2) |
| 3 | Ru-4c | 18 | 7 (5) |
| 4 | Ru-4d | 18 | 9 (7) |
| 5 | Ru-4e | 18 | 45 (41) |
| 6 | Ru-4f | 18 | 14 (13) |
| 7 | Ru-4g | 18 | 75 (73) |
| 8 | Ru-4g | 4 | 99 (97) |
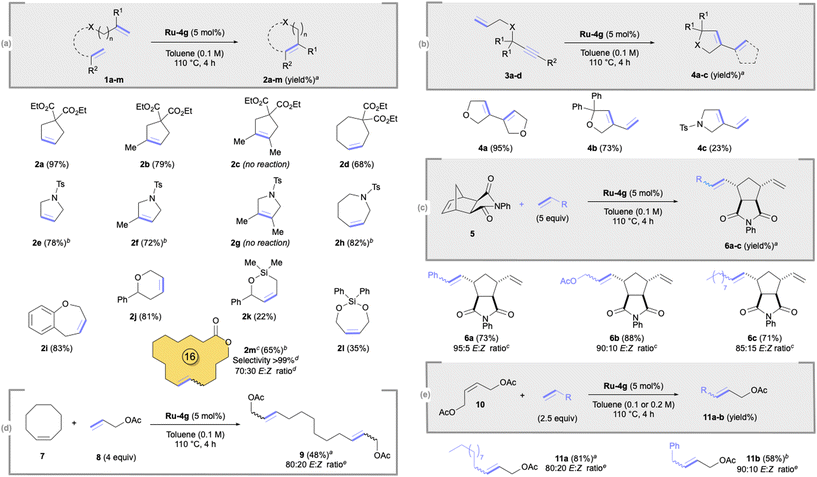
Having identified Blechert MesCABC Ru-4g as the most efficient catalyst, we then investigated its performance in various olefin metathesis transformations under optimised conditions (5 mol%, 110 °C; Scheme 2). Di- or tri-substituted cyclopentenes 2a,b,e,f were formed in good to excellent yields (72–97%). Similar good yields (68–83%) were also observed for seven-membered cycloalkenes 2d,h,i. However, no reaction occurred for tetrasubstituted cyclopentenes 2c,g and low 22–35% yields were obtained for silane derivatives 2k,l. To our delight, Ru-4g was also efficient in macroRCM, leading to a valuable odorant17 16-membered macrocycle 2m in 65% yield without any isomerised side product (>99% selectivity).18 Ring-closing enyne metathesis (RCEYM) was also examined, in which Ru-4g demonstrated good activity (73–95%), except for cyclic diene 4c (23%; Scheme 2b). We next focused on the ring-opening cross-metathesis (ROCM) of exo-norbornene 5 and cyclooctene 7 with different cross-olefin partners.
The corresponding trans-cyclopentanes 6a,b,c were formed in good yields (71–88%; Scheme 2c) while acyclic diene 9 was obtained in a moderate 48% yield (Scheme 2d). Lastly, we investigated the catalytic performance of Ru-4g in cross-metathesis (CM; Scheme 2e). The reaction between 1-dodecene and cis-1,4-diacetoxy-2-butene 8 furnished the corresponding alkene 11a in 81% yield with an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 E
20 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio. Nevertheless, a lower 58% yield and a 90
Z ratio. Nevertheless, a lower 58% yield and a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 E
10 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio were observed for product 11b resulting from the CM between homoallyl benzene and allyl acetate 10.
Z ratio were observed for product 11b resulting from the CM between homoallyl benzene and allyl acetate 10.
According to a broadly accepted mechanism (Fig. 3), productive metathesis involves the formation of a transient unsaturated 14e Ru–methylidene synAS prone to coordinating with incoming alkenes via a π-complex intermediate. Subsequent oxidative [2 + 2] cycloaddition results in the formation of a Ru(IV) ruthenacyclobutane which is able to undergo reductive elimination to reform the reactive unsaturated 14e Ru–methylidene antiAS with the elimination of the corresponding product. As shown by Grubbs and others,1a,19 the configuration of the chlorine atoms around the ruthenium centre is subject to change in order to facilitate this process. Intrigued by the unexpected stability of these complexes and the need for thermal activation (110 °C) with Blechert MesCABC Ru-4g (see Table 1, entry 8), we performed preliminary DFT studies (density functional theory) at the B3LYP-D3 level of theory. Transient unsaturated 14e-Ru–methylidene intermediates resulting from the Hoveyda–Grubbs Ru-4a–d were successfully optimized in the syn- and anti-configurations (synAS-4a–d and antiAS-4a–d respectively) of the chlorine atoms. In all cases, we found anti-configurations to be significantly higher in energy with respect to cisAS-4a–d. This difference is more significant with adamantyl 4b for which the anti-conformer lies significantly above the syn-conformer (19.5 kcal mol−1). To better understand these differences, we also considered the steric map profile of these intermediates, which points to a distorted configuration of the chlorine atoms in the anti-configuration. Together with the relatively less intramolecular hydrogen bonding interactions observed in the solid state (also more pronounced with adamantyl 4b), the unusual stability of these complexes points to a mechanical gridlock situation wherein the steric profile of the barrelene clashing with chlorine atoms impairs the metathesis step.
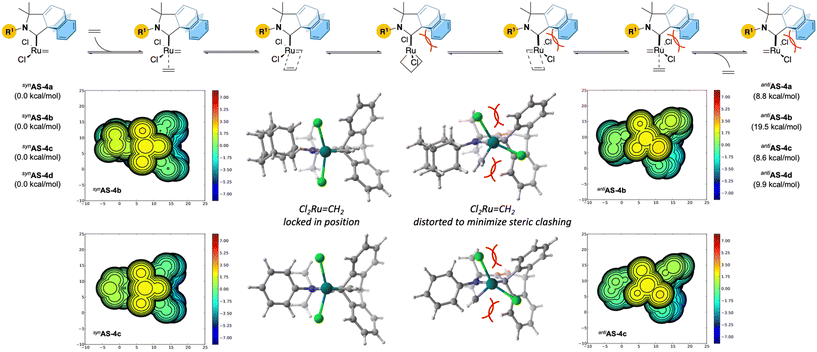 | ||
| Fig. 3 Preliminary DFT studies (performed at the B3LYP-D3 level of theory) and steric maps highlight the importance of steric crowding during the propagation. | ||
Conclusions
A set of seven new Ru-complexes containing cyclic (amino)(barrelene)carbene (CABC) ligands were synthesized in moderate to good yields and fully characterized. This novel class of complexes demonstrated remarkable thermal stability in solution at 110 °C over 10 days. The Blechert-type MesCABC Ru-4g was the most efficient, affording good yields in various metathesis reactions (RCM, macroRCM, RCEYM, CM, and ROCM). In addition, DFT calculations provided key insights into the initiation step and the propagating CABC–Ru–methylidene active species, thus explaining why thermal activation is required for this family of olefin metathesis catalysts.Data availability
All experimental and crystallographic data associated with this work are available in the ESI.†Author contributions
R. J., G. B. and Ma. M. conceptualized and supervised this work. M. R. S. prepared the CABC precursors. J. T., A. D. V., S. C. R. and J. M. developed the catalytic methodologies. Mo. M. performed the DFT studies and T. R. performed the X-ray diffraction analysis. The manuscript was written by R. J. and Ma. M. and reviewed by all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the CNRS, the ENSC de Rennes and the University of California San Diego. This work was supported by the Agence Nationale de la Recherche (ANR-19-CE07-0017 ChiCAAC, grant to J. T.), “France Relance” (for fellowship to A. D. V.) and the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Catalysis Science Program, under Award # DE-SC0009376. The generous gift of ruthenium complexes by Umicore AG & Co is gratefully acknowledged. We thank P. Jéhan for HRMS analysis.References
- (a) Handbook of Metathesis, ed. R. H. Grubbs, A. G. Wenzel, D. J. O'Leary and E. Khosravi, Wiley-VCH, Weinheim, Germany, 2nd edn, 2015 Search PubMed; (b) Olefin Metathesis: Theory and Practice, ed. K. Grela, John Wiley & Sons, Hoboken, N. J., 2014 Search PubMed.
- Metathesis in Natural Product Synthesis: Strategies, Substrates, and Catalysts, ed. J. Cossy, S. Arseniyadis and C. Meyer, Wiley-VCH, Weinheim, Germany, 2010 Search PubMed.
- (a) V. Lavallo, Y. Canac, C. Präsang, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2005, 44, 5705 CrossRef CAS; (b) R. Jazzar, R. D. Dewhurst, J.-B. Bourg, B. Donnadieu, Y. Canac and G. Bertrand, Angew. Chem., Int. Ed., 2007, 46, 2899 CrossRef CAS; (c) F. Vermersch, L. Oliveira, J. Huner, M. Soleilhavoup, R. Jazzar and G. Bertrand, J. Org. Chem., 2022, 87, 3511 CrossRef CAS PubMed. For recent reviews, see: (d) M. Soleilhavoup and G. Bertrand, Acc. Chem. Res., 2015, 48, 256 CrossRef CAS PubMed; (e) M. Melaimi, R. Jazzar, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10046 CrossRef CAS; (f) U. S. D. Paul and U. Radius, Eur. J. Inorg. Chem., 2017, 2017, 3362 CrossRef CAS; (g) R. Jazzar, M. Soleilhavoup and G. Bertrand, Chem. Rev., 2020, 120, 4141 CrossRef CAS PubMed; (h) R. K. Singh, T. K. Khan, S. Misra and A. K. Singh, J. Organomet. Chem., 2021, 956, 122133 CrossRef CAS.
- (a) D. R. Anderson, V. Lavallo, D. J. O'Leary, G. Bertrand and R. H. Grubbs, Angew. Chem., Int. Ed., 2007, 46, 7262 CrossRef CAS; (b) V. M. Marx, A. H. Sullivan, M. Melaimi, S. C. Virgil, B. K. Keitz, D. S. Weinberger, G. Bertrand and R. H. Grubbs, Angew. Chem., Int. Ed., 2015, 54, 1919 CrossRef CAS; (c) R. Gawin, A. Tracz, P. Krajczy, A. Kozakiewicz-Piekarz, J. P. Martínez and B. Trzaskowski, J. Am. Chem. Soc., 2023, 145, 25010 CAS.
- (a) R. Gawin, A. Kozakiewicz, P. A. Guńka, P. Dąbrowski and K. Skowerski, Angew. Chem., Int. Ed., 2017, 56, 981 CrossRef CAS; (b) R. Gawin, A. Tracz, M. Chwalba, A. Kozakiewicz, B. Trzaskowski and K. Skowerski, ACS Catal., 2017, 7, 5443 CrossRef CAS; (c) D. L. Nascimento, A. Gawin, R. Gawin, P. A. Guńka, J. Zachara, K. Skowerski and D. E. Fogg, J. Am. Chem. Soc., 2019, 141, 10626 CrossRef CAS.
- (a) D. L. Nascimento and D. E. Fogg, J. Am. Chem. Soc., 2019, 141, 19236 CrossRef CAS PubMed; (b) D. L. Nascimento, M. Foscato, G. Occhipinti, V. R. Jensen and D. E. Fogg, J. Am. Chem. Soc., 2021, 143, 11072 CrossRef CAS; (c) G. Occhipinti, D. L. Nascimento, M. Foscato, D. E. Fogg and V. R. Jensen, Chem. Sci., 2022, 13, 5107 RSC.
- For a recent review on CAAC-Ru-complexes, see: J. Morvan, M. Mauduit, G. Bertrand and R. Jazzar, ACS Catal., 2021, 11, 1714 CrossRef CAS.
- A. E. Samkian, Y. Xu, S. C. Virgil, K.-Y. Yoon and R. H. Grubbs, Organometallics, 2020, 39, 495 CrossRef CAS.
- M. Nagyházi, Á. Lukács, G. Turczel, J. Hancsók, J. Valyon, A. Bényei, S. Kéki and R. Tuba, Angew. Chem., Int. Ed., 2022, 61, e202204413 CrossRef.
- (a) M. R. Serrato, M. Melaimi and G. Bertrand, Chem. Commun., 2022, 58, 7519 RSC; (b) S. Baguli, S. Sarkar, S. Nath, D. Mallick and D. Mukherjee, Angew. Chem., Int. Ed., 2023, 62, e202312858 CrossRef CAS PubMed.
- For the synthesis of Pyr-GI, see: E. L. Dias, PhD Thesis, California Institute of Technology, Pasadena, CA (USA), 1998.
- A. Michrowska, R. Bujok, S. Harutyunyan, V. Sashuk, G. Dolgonos and K. Grela, J. Am. Chem. Soc., 2004, 126, 9318 CrossRef CAS PubMed.
- (a) H. Wakamatsu and S. Blechert, Angew. Chem., Int. Ed., 2002, 41, 2403 CrossRef CAS; (b) H. Wakamatsu and S. Blechert, Angew. Chem., Int. Ed., 2002, 41, 794 CrossRef CAS.
- For previous synthesis of Blechert-type CAAC Ru-complexes: A. Del Vecchio, J. Talcik, S. Colombel-Rouen, J. Lorkowski, M. R. Serrato, T. Roisnel, N. Vanthuyne, G. Bertrand, R. Jazzar and M. Mauduit, ACS Catal., 2023, 13, 6195 CrossRef CAS.
- It should be noted that Ru-4f could also be formed via the direct addition of MesCABC(d)·BF4 to the phosphine-based nitro-Grela Ru-precursor but with a lower 13% yield. However, any attempts to synthesize Ru-4g through this protocol failed.
- This structural feature was recently reported: (a) J. Morvan, F. Vermersch, Z. Zhang, T. Vives, V. Dorcet, T. Roisnel, C. Crévisy, L. Falivene, L. Cavallo, N. Vanthuyne, G. Bertrand, R. Jazzar and M. Mauduit, Organometallics, 2023, 42, 495 CrossRef CAS; (b) A. Sytniczuk, A. Kajetanowicz and K. Grela, Chem. Catal., 2023, 3, 100713 CrossRef CAS.
- Selected reviews on macrocyclic musks: (a) A. S. Williams, Synthesis, 1999, 1707 CrossRef CAS; (b) P. Kraft, J. A. Bajgrowicz, C. Denis and G. Frater, Angew. Chem., Int. Ed., 2000, 39, 2980 CrossRef CAS.
- For instance, see: (a) T. M. Trnka, J. P. Morgan, M. S. Sanford, T. E. Wilhem, M. Scholl, T.-L. Choi, S. Ding, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 2546 CrossRef CAS PubMed; (b) S. H. Hong, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2004, 126, 7414 CrossRef CAS PubMed; (c) N. J. Beach, J. A. M. Lummins, J. M. Bates and D. E. Fogg, Organometallics, 2012, 31, 2349 CrossRef CAS.
- S. G. Patra and N. K. Das, Polyhedron, 2021, 200, 115096 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra, GC analysis. CCDC 2256504, 2253658–2253660 and 2260084. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00102h |
| ‡ Present address: Department of Chemistry and Industrial Chemistry, University of Pisa, via G. Moruzzi 13, 56124 – Pisa, Italy. |
| This journal is © The Royal Society of Chemistry 2024 |