Structure and optical limiting effects of heterometallic Ag6@Ti12 and Ag8@Ti12 oxo clusters regulated by alkynyl ligands†
Li-Jun
Rong
ab,
Yu-Ting
Ye
ab,
Xin
Lin
b,
Xiaohui
Sun
b,
Shumei
Chen
*a,
Jian
Zhang
 b and
Lei
Zhang
b and
Lei
Zhang
 *b
*b
aCollege of Chemistry, Fuzhou University, Fuzhou, Fujian 350108, P. R. China. E-mail: csm@fzu.edu.cn
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: LZhang@fjirsm.ac.cn
First published on 4th January 2024
Abstract
Heterometallic Ag6@Ti12 and Ag8@Ti12 oxo clusters were prepared through a strategy of protecting polynuclear silver cores by a hollow Ti–O module. The introduction of alkyne ligands has shown significant influence on their structures and optical limiting effects.
In recent years, the prospects of using precious metals in the fields of optoelectronics and catalysis have been widely studied. Silver-based nanomaterials, especially silver clusters, have garnered significant attention due to their lower cost and versatile coordination modes.1–11 However, the instability of silver materials sometimes limits the in-depth study of their properties and physicochemical performance. To enhance the stability of silver metal cores, the use of organic ligand shells has been proved to be an effective approach. For instance, phosphines, thiolates, alkynyls, and some pyridines are protective ligands that can not only prevent infinite aggregation during the cluster synthesis process but also allow for the control of the size, structure, and physicochemical properties of silver nanoclusters.12–17
In our early research work, we found that hollow titanium-oxo moieties could be used to encapsulate silver nanoclusters to form heterometallic core–shell nanostructures.18,19 Within the Ti–O cavity, multiple oxygen atoms are present, which can serve as bridging units for constructing stable silver nanoclusters. Meanwhile, the introduction of silver atoms into titanium-oxo clusters can enhance the optical performance of the obtained cluster materials. Actually, in the field of titanium-oxo clusters, lots of exquisite hollow structures have been synthesized that feature finely crafted internal nanocavities suitable for the embedding of Ag atoms.20–23 Thereinto, the cyclic Ti12-oxo cluster with a double-layered structure might be a suitable protective cavity for the growth of silver clusters.24,25 The alkyne ligands, which serve as anionic protecting agents, have been frequently applied to regulate the structure of silver clusters.26–32 Additionally, the alkyne ligands with conjugation properties can also act as excellent materials for optical limiting applications.33–35 Consequently, in this work, we have endeavored to utilize such Ti12-oxo shells to protect silver nanoclusters while simultaneously introducing alkynyl ligands to modulate the structure and optical properties of the obtained compounds.
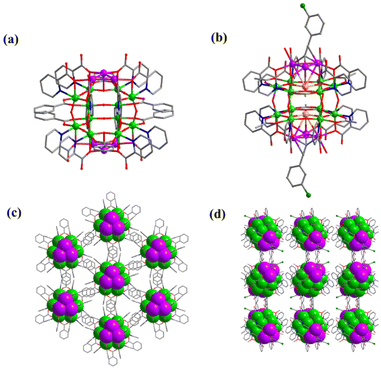
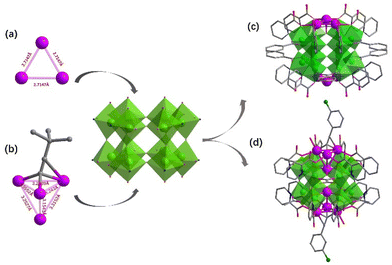
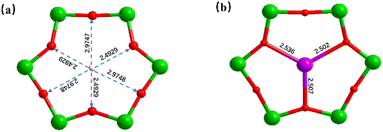
2-Picolinicate exhibits diversified coordination models and high affinity towards transition metal ions. Therefore, in this study, we selected 2-picolinicate as the primary ligand, along with benzoate and ethanoate as auxiliary ligands, to stabilize the outer Ti–O shell. Furthermore, we have employed alkynyl ligands to further control and stabilize the silver cluster cores. The initially synthesized Ag6Ti12(μ3-O)6(μ2-O)12(BA)6(L)12 (PTC-349: L = 2-picolinicate, BA = benzoate) has an Ag6Ti12 core as its structural center, which can be divided into two Ag3Ti6 segments. Although the introduced small amount of 1-hexyne was not presented in the final cluster structure of PTC-349, it is still essential for the synthesis of PTC-349 which may participate in the aggregation of silver ions. Two Ti6 rings in PTC-349 are stacked with a 120° rotation, connected by six μ2-O bridges, and each Ti2 pair is linked through benzoic acid. The hydroxy oxygen of 2-picolinicate on the Ti12-oxo shell is employed to draw silver ions into the Ti12-oxo cavity, providing protection to the silver ions within the cavity. From a side view (Fig. 1a), 2-picolinicate acts as a bidentate ligand, with its nitrogen in the pyridine ring coordinating with titanium atoms and its hydroxy oxygen linked to the pyridine ring coordinating with silver ions. Two 2-picolinicates are accompanied by benzoate, collectively providing protection for a single silver ion, resulting in PTC-349. As shown in Fig. 2c, the cluster structure of PTC-349 can be divided into two parts: an inner core consisting of two Ag3 moieties and an outer layer comprising a Ti12 ring and surrounding ligands. The distance between two Ag+ ions within each Ag3 core is 3.079 Å, which is shorter than the sum of their van der Waals radii (3.44 Å), indicating strong Ag⋯Ag interactions (Fig. 2a). Each of the six bridging oxygen atoms in the Ti6 ring has an average distance of about 2.73 Å to the center of the Ti6 ring, which is larger than the van der Waals radius of Ag+, suggesting the possibility of embedding a further Ag+ ion within the center of the Ti6 ring (Fig. 3a).
Building upon this, we replaced 1-hexyne with 3-chlorophenylacetylene (CPA) and removed the auxiliary benzoic acid, leading to the formation of Ag8Ti12(μ3-O)6(μ2-O)12(Ac)6(L)12(CPA)2(H2O)2 (PTC-350). Thereinto, each 3-chlorophenylacetylene ligand connects four silver ions arranged in a triangular pyramid configuration within the Ti6 ring. The average distance between Ag4 at the apex and the other three bottom Ag ions is 3.2643 Å, longer than the average Ag–Ag distance within the three bottom Ag atoms (3.0569 Å) (Fig. 2b). Ag4 at the apex is connected to μ3-O in the Ti6 ring (with an average Ag–O bond distance of 2.515 Å) (Fig. 3b), while the three bottom Ag ions are linked to titanium atoms through the hydroxy oxygen of 2-pyridinecarboxylate ligands and encapsulated by 3-chlorophenylacetylene (Fig. 2d).
Optical limiting (OL) materials have garnered widespread attention due to their potential applications in optical systems for protecting, modulating, and controlling optical signals.36–39 Heavy metal atoms like silver have been shown to exhibit optical limiting effects, where their electrons can transition to higher energy levels by absorbing multiple photons, resulting in optical limiting properties.35,40,41 Alkynyl ligands are characterized by their conjugated structures, where carbon atoms are connected by π bonds to form an extended π electron system, displaying strong excited-state absorption and efficient charge transfer to enhance the response of optical limiting performance.42–44 Therefore, we hypothesize that despite the similar cluster structures of PTC-349 and PTC-350, the increased Ag core and the introduction of alkynyl ligands in PTC-350 may lead to an enhancement in the optical limiting performance.
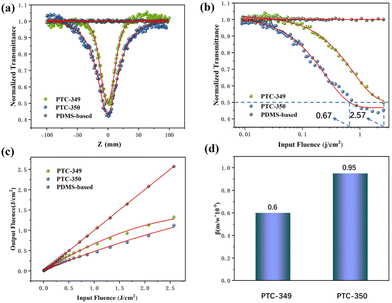
We conducted measurements using a method of solid particle composite films containing selected crystals in the size range of 80 mesh (180 μm) to 60 mesh (250 μm) through crystal sieves. PDMS is a chemically inert material with excellent flexibility and transparency, and it does not affect the optical limiting performance. Therefore, we chose PDMS as the solid dispersion base for our samples. We fabricated a series of flexible AgTiOC@PDMS films containing PTC-349 and PTC-350 with a diameter of 30 mm. The optical limiting properties of the fabricated AgTiOC@PDMS films were studied using an open-aperture (OA) Z-scan technique with an input laser pulse energy of 120 μJ, a pulse width of 5 ns, and a wavelength of 532 nm. To compare the OL effects, all sample films had similar thicknesses and linear transmittance (Table S4†). The measurement results in Fig. 4a reveal that, compared to the original PDMS film without nonlinear optical properties, PTC-349 and PTC-350 exhibit significant reverse saturable absorption (RSA) responses in their OA Z-scan valley curves. As expected, the samples show the following order of minimum normalized transmittance (Tmin) at the focus (Z = 0): PTC-349 > PTC-350. In Fig. 4c, we can observe that the output flux increases linearly at low incident flux and deviates from linearity at high incident flux, showing the characteristic of an optical limiting response. Furthermore, for PTC-349 and PTC-350, the optical limiting thresholds can be as low as 0.67 and 2.57 J cm−2 (Fig. 4b and Table S4†). The corresponding nonlinear absorption coefficients β of PTC-349 and PTC-350 are 0.6 × 10–9 m W−1 and 0.95 × 10–9 m W−1, respectively (Fig. 4d). The research results indicate that PTC-349 and PTC-350 exhibit good optical limiting performance. Despite the similar cluster core structures of PTC-349 and PTC-350, the increased cluster core in PTC-350 and the introduction of alkynyl ligands indeed contribute to an improvement in the optical limiting effects of PTC-350.
In summary, this work demonstrates an efficient Ti12-oxo module for protecting silver nanoclusters. Two heterometallic Ag6@Ti12 and Ag8@Ti12 oxo clusters have been successfully constructed, with structural differences originating from the addition of alkyne ligands. Moreover, both clusters exhibited excellent optical limiting performance, which benefited from the increase in cluster size and the introduction of alkyne ligands. These results provide new insights for the synthesis of heterometallic clusters and the design of efficient optical limiting materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Natural Science Foundation of Fujian Province (2022J02013).References
- Y.-M. Su, B.-Q. Ji, Z. Wang, S.-S. Zhang, L. Feng, Z.-Y. Gao, Y.-W. Li, C.-H. Tung, D. Sun and L.-S. Zheng, Sci. China: Chem., 2021, 64, 1482–1486 CrossRef CAS.
- L. Qin, F. Sun, X. Ma, G. Ma, Y. Tang, L. Wang, Q. Tang, R. Jin and Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 26136–26141 CrossRef CAS PubMed.
- B. L. Han, Z. Wang, R. K. Gupta, L. Feng, S. Wang, M. Kurmoo, Z. Y. Gao, S. Schein, C. H. Tung and D. Sun, ACS Nano, 2021, 15, 8733–8741 CrossRef CAS PubMed.
- X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- C. Deng, C. Sun, Z. Wang, Y. Tao, Y. Chen, J. Lin, G. Luo, B. Lin, D. Sun and L. Zheng, Angew. Chem., Int. Ed., 2020, 59, 12659–12663 CrossRef CAS PubMed.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Acc. Chem. Res., 2018, 51, 1338–1348 CrossRef CAS PubMed.
- S. Q. Li, L. F. Dai, Y. Q. Tian, Y. X. Yi, J. Yan and C. Liu, Chem. Commun., 2023, 59, 575–578 RSC.
- Z. Wang, H. F. Su, M. Kurmoo, C. H. Tung, D. Sun and L. S. Zheng, Nat. Commun., 2018, 9, 2094 CrossRef PubMed.
- B. Bhattarai, Y. Zaker, A. Atnagulov, B. Yoon, U. Landman and T. P. Bigioni, Acc. Chem. Res., 2018, 51, 3104–3113 CrossRef CAS.
- O. Fuhr, S. Dehnen and D. Fenske, Chem. Soc. Rev., 2013, 42, 1871–1906 RSC.
- Y.-Q. Tian, W.-L. Mu, L.-L. Wu, X.-Y. Yi, J. Yan and C. Liu, Chem. Sci., 2023, 14, 10212–10218 RSC.
- J.-P. Gao, Z. Qi, F.-Q. Zhang and X.-M. Zhang, Nanoscale, 2022, 14, 4469–4473 RSC.
- Y. Niihori, Y. Wada and M. Mitsui, Angew. Chem., Int. Ed., 2021, 60, 2822–2827 CrossRef CAS PubMed.
- S. S. Zhang, F. Alkan, H. F. Su, C. M. Aikens, C. H. Tung and D. Sun, J. Am. Chem. Soc., 2019, 141, 4460–4467 CrossRef CAS PubMed.
- S. Li, X. S. Du, B. Li, J. Y. Wang, G. P. Li, G. G. Gao and S. Q. Zang, J. Am. Chem. Soc., 2018, 140, 594–597 CrossRef CAS PubMed.
- R. W. Huang, Y. S. Wei, X. Y. Dong, X. H. Wu, C. X. Du, S. Q. Zang and T. C. W. Mak, Nat. Commun., 2017, 9, 689–697 CAS.
- K. G. Liu, S. K. Chen, Y. M. Lin and Q. M. Wang, Chem. Commun., 2015, 51, 9896–9898 RSC.
- X. Fan, S. Chen, L. Zhang and J. Zhang, Chem. – Eur. J., 2021, 27, 15563–15570 CrossRef CAS PubMed.
- S. Chen, W. H. Fang, L. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2018, 57, 11252–11256 CrossRef CAS PubMed.
- K. Chintakrinda, N. Narayanam, Y.-Z. Li, F. Wang, C. Kashi, Q.-H. Li, G. Xu, L. Zhang and J. Zhang, CCS Chem., 2020, 2, 209–215 CrossRef CAS.
- L. Zhang, X. Fan, X. F. Yi, X. Lin and J. Zhang, Acc. Chem. Res., 2022, 55, 3150–3161 CrossRef CAS PubMed.
- C. Zhao, Y. Z. Han, S. Dai, X. Chen, J. Yan, W. Zhang, H. Su, S. Lin, Z. Tang, B. K. Teo and N. Zheng, Angew. Chem., Int. Ed., 2017, 56, 16252–16256 CrossRef CAS PubMed.
- C. Artner, M. Czakler and U. Schubert, Chem. – Eur. J., 2014, 20, 493–498 CrossRef CAS.
- Y. J. Liu, P. Shao, M. Y. Gao, W. H. Fang and J. Zhang, Inorg. Chem., 2020, 59, 11442–11448 CrossRef CAS PubMed.
- C. Gao, D. Wang, Y. S. Liu, G. Y. Zhang, C. Y. Liu, A. Said, H. H. Niu, G. Wang, C. H. Tung and Y. F. Wang, Dalton Trans., 2022, 51, 15385 RSC.
- J. L. Zeng, Z. J. Guan, Y. Du, Z. A. Nan, Y. M. Lin and Q. M. Wang, J. Am. Chem. Soc., 2016, 138, 7848–7851 CrossRef CAS PubMed.
- S.-S. Zhang, H.-F. Su, G.-L. Zhuang, X.-P. Wang, C.-H. Tung, D. Sun and L.-S. Zheng, Chem. Commun., 2018, 54, 11905–11908 RSC.
- J. Yan, B. K. Teo and N. Zheng, Acc. Chem. Res., 2018, 51, 3084–3093 CrossRef CAS PubMed.
- Z. Wang, H. F. Su, X. P. Wang, Q. Q. Zhao, C. H. Tung, D. Sun and L. S. Zheng, Chem. – Eur. J., 2018, 24, 1640–1650 CrossRef CAS PubMed.
- Y. Wang, H. Su, C. Xu, G. Li, L. Gell, S. Lin, Z. Tang, H. Hakkinen and N. Zheng, J. Am. Chem. Soc., 2015, 137, 4324–4327 CrossRef CAS PubMed.
- M.-M. Zhang, X.-Y. Dong, Y.-J. Wang, S.-Q. Zang and T. C. W. Mak, Coord. Chem. Rev., 2022, 453, 214315 CrossRef CAS.
- A. K. Gupta and A. Orthaber, Chem. – Eur. J., 2018, 24, 7536–7559 CrossRef CAS PubMed.
- N. R. Champness, Dalton Trans., 2011, 40, 10311–10315 RSC.
- M. de Wergifosse and B. Champagne, J. Chem. Phys., 2011, 134, 074113 CrossRef PubMed.
- K. Zhou, L. K. Yan, Y. Geng, J. Y. Ji, X. L. Wang, Z. M. Su and Z. G. Xiao, RSC Adv., 2021, 11, 38814–38819 RSC.
- S. T. Wang, Y. J. Liu, C. C. Feng, W. H. Fang and J. Zhang, Aggregate, 2022, 4, e264 CrossRef.
- M. Y. Gao, Z. Wang, Q. H. Li, D. Li, Y. Sun, Y. H. Andaloussi, C. Ma, C. Deng, J. Zhang and L. Zhang, J. Am. Chem. Soc., 2022, 144, 8153–8161 CrossRef CAS PubMed.
- D. J. Li, Q. H. Li, Z. R. Wang, Z. Z. Ma, Z. G. Gu and J. Zhang, J. Am. Chem. Soc., 2021, 143, 17162–17169 CrossRef CAS PubMed.
- Y. P. He, G. H. Chen, D. J. Li, Q. H. Li, L. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2021, 60, 2920–2923 CrossRef CAS PubMed.
- H. Nie, W. Duan, J. Liu, H. Xia, K. Yang, F. Wang, B. Zhang and J. He, Nanophotonics, 2020, 9, 2537–2548 CrossRef CAS.
- M. Nidya, M. Umadevi, P. Sankar, R. Philip and B. J. M. Rajkumar, Opt. Mater., 2015, 42, 152–159 CrossRef CAS.
- G. J. Zhou and W. Y. Wong, Chem. Soc. Rev., 2011, 40, 2541–2566 RSC.
- D. Dini, M. J. Calvete and M. Hanack, Chem. Rev., 2016, 116, 13043–13233 CrossRef CAS PubMed.
- V. Singh and P. Aghamkar, Appl. Phys. Lett., 2014, 104, 111112 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, additional figures and general characterization. CCDC 2309533 (PTC-349) and 2309434 (PTC-350). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt03941b |
| This journal is © The Royal Society of Chemistry 2024 |