Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of N-heterocyclic carbene gold(I) complexes from the marine betaine 1,3-dimethylimidazolium-4-carboxylate†
Seyedeh Mahbobeh
Mahdavi
a,
Dirk
Bockfeld
 a,
Rolf
Büssing
b,
Bianka
Karge
c,
Thomas
Bannenberg
a,
René
Frank
a,
Rolf
Büssing
b,
Bianka
Karge
c,
Thomas
Bannenberg
a,
René
Frank
 a,
Mark
Brönstrup
a,
Mark
Brönstrup
 c,
Ingo
Ott
b and
Matthias
Tamm
c,
Ingo
Ott
b and
Matthias
Tamm
 *a
*a
aInstitut für Anorganische und Analytische Chemie, Technische Universität Braunschweig, Hagenring30, 38106 Braunschweig, Germany. E-mail: m.tamm@tu-bs.de
bInstitute of Medicinal and Pharmaceutical Chemistry, Technische Universität Braunschweig, Beethovenstraße 55, 38106 Braunschweig, Germany
cDepartment of Chemical Biology, Helmholtz Centre for Infection Research GmbH, Inhoffenstrasse 7, 38124 Braunschweig, Germany
First published on 4th January 2024
Abstract
The marine natural product norzooanemonin (1,3-dimethylimidazolium-4-carboxylate) has been used to prepare a series of carboxyl- or carboxylate-functionalized N-heterocyclic carbene (NHC) gold(I) complexes from [(Me2S)AuCl] in the presence of potassium carbonate. The potential of the resulting mono- and dicarbene complexes to act as cytotoxic or antibacterial drugs was investigated.
Norzooanemonin (1,3-dimethylimidazolium-4-carboxylate, 1) is a naturally occurring betaine that has been isolated from various marine invertebrates.1 Its synthesis has been described several times,2 and it has also been observed as a by-product in the preparation of imidazolium-2-carboxylates.2b,3 Compounds of the latter type are usually referred to as “normal” N-heterocyclic carbene–carbon dioxide adducts (NHC·CO2) and have been used extensively, particularly as masked carbenes and carbene transfer reagents.4 In contrast, 1 has received little attention in NHC chemistry, although its potential to transfer the corresponding “abnormal” carbene, 1,3-dimethylimidazolin-4-ylidene, to organic substrates via decarboxylation has been demonstrated.2c In addition, there are a few reports describing the use of 1 as a zwitterionic carboxylate ligand in some cobalt, copper, and lanthanide complexes, in which 1 is bound through one or two oxygen atoms.5 Surprisingly, however, the possibility of using 1via its tautomeric N-heterocyclic carbene form 1′ (HOOCIMe)6 for the synthesis of carboxyl functionalized NHC metal complexes has, to the best of our knowledge, not yet been investigated (Scheme 1). According to our initial calculations, the tautomer 1′ is only slightly higher in energy than 1 (ΔG298 K = +4.0 kcal mol−1); given the strength of metal–NHC bonds,7 a significant stabilization of this tautomeric form by metal coordination can be expected (see ESI† for further details).
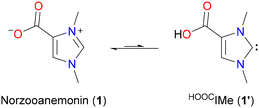 | ||
| Scheme 1 Norzooanemonin (1,3-dimethylimidazolium-4-carboxylate, 1) and its tautomeric N-heterocyclic carbene (NHC) form HOOCIMe (1′);6 the energy difference between the tautomers is ΔG298 K = +4.0 kcal mol−1 (see the ESI† for details). | ||
The use of norzooanemonin (1) as an NHC precursor would provide direct access to carboxyl- or carboxylate-functionalized NHC metal complexes, since the few existing examples of NHC metal complexes with COO or COOH groups directly attached to the 4-position of the N-heterocycle were prepared by template synthesis through “post-coordination backbone functionalization”.8 Thus, the manganese(I) complex [Cp(IMes)Mn(CO)2] (IMes = 1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene, Cp = η5-C5H5) was treated with n-butyl lithium (n-BuLi) followed by CO2 and HCl to afford [Cp(HOOCIMes)Mn(CO)2]. This complex was further used to prepare polymetallic complexes containing the corresponding ambidentate NHC-carboxylate ligand OOCIMes.8 Another approach to site-specific carboxylation of NHCs was realized by deprotonation of NHCs such as IDipp (1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene) with n-BuLi. The resulting lithium salt Li(OOCIDipp) could serve as a carbene transfer reagent, but this has not been further investigated.9 The latter approach corresponds in principle to the construction of anionic N-heterocyclic carbenes with a fluoroborate unit in the 4-position, which have found widespread application in transition metal and main group element chemistry as well in homogeneous catalysis.10
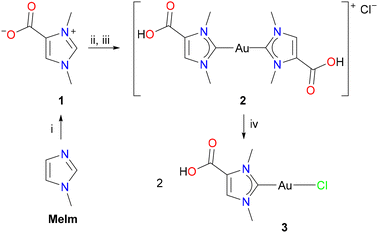
Norzooanemonin (1) was prepared from 1-methylimidazole (MeIm) and dimethyl carbonate according to a slightly modified version of a previously reported protocol (Scheme 2).2b The white solid (m.p. = 235.5 °C) crystallized as the mono- and dihydrates 1·H2O and 1·2H2O from aqueous solutions or as the solvate 1·CH3OH from methanol, and their crystal structures were determined by X-ray diffraction analysis (see Fig. S15, S16, Tables S1 and S2, ESI†).11 Since gold NHC complexes are important for both catalytic and medical applications,12 our initial goal was to prepare a monocarbene gold(I) complex derived from 1. Among many other approaches toward the synthesis of late transition metal NHC complexes,13 [(NHC)AuCl] complexes were recently obtained by reliable and scalable protocols from imidazolium salts with [(Me2S)AuCl] in the presence of potassium carbonate as a suitable base.14 However, our initial efforts to prepare the monocarbene complex [(HOOCIMe)AuCl] (3) failed, and the reactions between 1, K2CO3, and [(Me2S)AuCl] in methanol yielded only mixtures of mono- and dicarbene species. Similar observations have also been made for water-soluble gold(I) NHC complexes with pendant sulfonate groups.15 Therefore, we first turned our attention to the synthesis of dicarbene complexes, and [(Me2S)AuCl] was treated with two equivalents each of 1 and K2CO3 in methanol (Scheme 2). After evaporation, the resulting white powder proved to be very soluble in water, which prevented the separation of the anionic dicarbene complex K[(OOCIMe)2Au] (5K, see below, Scheme 3) from potassium chloride and bicarbonate. For this purpose, an aqueous solution of the product mixture was treated with hydrochloric acid (2 N HCl) until a pH of 1–2 was reached, resulting in the precipitation of the cationic dicarbene complex [(HOOCIMe)2Au]Cl (2) as a white solid, which was isolated in 60% yield by filtration. The monocarbene complex 3 was then prepared by a comproportionation reaction of 2 with another equivalent of [(Me2S)AuCl] in methanol, affording [(HOOCIMe)AuCl] (3) as a white solid in 85% yield (step iv, Scheme 2).
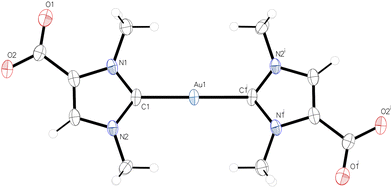
The NMR spectra of 2 and 3 were recorded in CD3OD. Three 1H NMR signals are observed at 8.01, 4.17, and 3.95 ppm for 2 and at 7.92, 4.07, and 3.84 ppm for 3. These signals can be assigned to the CH and the two CH3 groups, respectively, while the COOH proton is exchanged in methanol solution. The 13C{1H} NMR spectra show low-field signals at 189.7 ppm (2) and 176.1 ppm for the carbene carbon atoms, which are in the expected ranges for gold(I) NHC complexes.16 The 13C{1H} NMR signals for the COOH group appear at 161.3 ppm (2) and 161.9 ppm (3). The molecular structures of 2 and 3 were determined by X-ray diffraction analysis; the structure of the monocarbene complex 3 is shown in Fig. 1, while the structure of the dicarbene complex 2 is discussed in a later section. 3 crystallizes in the monoclinic space group P21/c with one molecule 3 in the asymmetric unit. The C1–Au–Cl angle of 176.50(4)° is consistent with the expected nearly linear two-coordinate environment around the gold atom, and the Au–C1 and Au–Cl bond lengths of 1.9849(12) Å and 2.2908(3) Å are in the range reported for other [(NHC)AuCl] complexes.173 forms centrosymmetric dimers in the solid with an Au–Au′ distance of 3.23241(11) Å, which is well within the typical range expected for aurophilic interactions (ca. 2.8–3.50 Å).18 The dimeric units are in turn linked to form chains by hydrogen bonding between the carboxyl groups, with the typical pattern of two O–H⋯O hydrogen bonds between each pair of COOH groups, see Fig. S17 (ESI†) for a packing diagram. Accordingly, 3 represents a nice example of complementarity between aurophilic and conventional hydrogen bonding interactions in the supramolecular assembly of gold(I) compounds.19
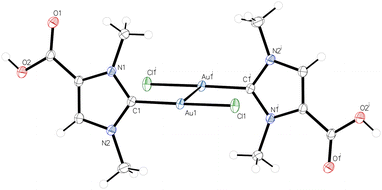 | ||
| Fig. 1 Molecular structure of 3; a dimeric centrosymmetric unit is shown with an Au–Au′ distance of 3.23241(11) Å. | ||
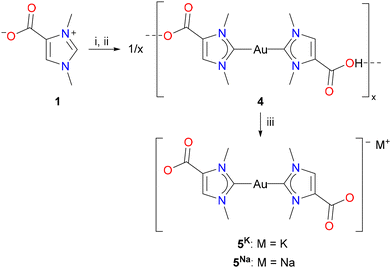
The successful isolation of the cationic dicarboxyl-dicarbene complex [(HOOCIMe)2Au] (2) raised the question of whether this complex could be used as a starting material for the synthesis of the corresponding anionic dicarboxylate-dicarbene complexes M[(OOCIMe)2Au] (5K, M = K; 5Na, M = Na) by treatment with potassium or sodium hydroxide. However, their high solubility in water, as described above for 5K (Scheme 2), would probably again prevent their isolation and separation from KCl or NaCl. Considering the better solubility of the corresponding iodides, KI and NaI, in organic solvents, we adapted the protocol for the synthesis of dicarbene–gold(I) complexes from 1, and the resulting mixture of 5K, KCl, and KHCO3 was treated with hydroiodic acid (2 N HI). We found that a white solid precipitated already at pH of 6–7, and the neutral complex [(HOOCIMe)Au(OOCNHC)] (4) was isolated in 52% yield by filtration (Scheme 3). Interestingly, complex 4 did not precipitate upon treatment with HCl, although both HCl and HI are strong acids in aqueous media. Treatment of this complex 4 with one equivalent of either potassium or sodium hydroxide afforded the desired dicarboxylate-dicarbene complexes 5K and 5Na in >90% yield after washing with acetone to remove any traces of KI or NaI. Both complexes are highly water soluble, and their NMR spectra were recorded in D2O. In the 1H NMR spectra, the three CH and CH3 signals are all shifted to slightly lower field compared to 2 (in CD3OD); the 13C{1H} NMR exhibit signals at ca. 189 and 167 ppm for the carbene and carboxylate carbon atoms, respectively.
The molecular structures of the dicarbene complexes 2, 4, and 5K were confirmed by X-ray diffraction analysis. Complex 2, as well as the complexes 4 and 5K crystallized as solvates, namely 2·H2O, 4·6H2O, 5K·4H2O and 5K·2H2O·MeOH, the latter structure suffering from poor data quality. 5K·4H2O and 5K·2H2O·MeOH are isostructural and crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with one centrosymmetric molecule of 5K in the unit cell, and both structures differ significantly only in the arrangement of the solvate molecules. Therefore, the following discussion is limited to the structures of 2·H2O, 4·6H2O, and 5K·4H2O. As observed for 5K, 2 and 4 are centrosymmetric and crystallize with one half molecule in the asymmetric unit in the monoclinic space groups P21/c (2·H2O) and P21/n (4·6H2O), respectively. The molecular structure of the anion [(OOCIMe)2Au]− in 5K·4H2O is shown in Fig. 2, while the structures of 2·H2O and 4·6H2O are presented in the ESI section.† The gold atoms in all structures exhibit perfectly linear coordination spheres with C1–Au–C1′ angles of 180°, resulting from the fact that the gold atoms in all structures are located on inversion centers. The Au–C1 bond lengths are 2.010(10) (2·H2O), 2.027(8) (4·6H2O), and 2.027(7) Å (5K·4H2O) and are within the range observed for related dicarbene gold(I) complexes containing [(NHC)2Au]+ cations (Table 1).20
with one centrosymmetric molecule of 5K in the unit cell, and both structures differ significantly only in the arrangement of the solvate molecules. Therefore, the following discussion is limited to the structures of 2·H2O, 4·6H2O, and 5K·4H2O. As observed for 5K, 2 and 4 are centrosymmetric and crystallize with one half molecule in the asymmetric unit in the monoclinic space groups P21/c (2·H2O) and P21/n (4·6H2O), respectively. The molecular structure of the anion [(OOCIMe)2Au]− in 5K·4H2O is shown in Fig. 2, while the structures of 2·H2O and 4·6H2O are presented in the ESI section.† The gold atoms in all structures exhibit perfectly linear coordination spheres with C1–Au–C1′ angles of 180°, resulting from the fact that the gold atoms in all structures are located on inversion centers. The Au–C1 bond lengths are 2.010(10) (2·H2O), 2.027(8) (4·6H2O), and 2.027(7) Å (5K·4H2O) and are within the range observed for related dicarbene gold(I) complexes containing [(NHC)2Au]+ cations (Table 1).20
| Complex | Au–C1 | C1–Au–C1′/Cl | N1–C1–N2 |
|---|---|---|---|
| 2 | 2.010(10) | 180.0 | 104.5(8) |
| 3 | 1.9849(12) | 176.50(4) | 105.95(11) |
| 4·6H2O | 2.027(8) | 180.0(10) | 105.6(7) |
| 5K·4H2O | 2.021(7) | 180.0(2) | 105.0(6) |
In contrast to the hydrogen bonding pattern observed between the carboxyl groups in the monocarbene complex 3, the OH groups in the dicarbene complex 2 in 2·H2O form hydrogen bonds with the chloride counterions with OH⋯Cl contacts of 2.102(15) Å. The chloride ion again forms a hydrogen bond with the water molecule (Cl⋯HOH 2.167(15) Å). Both the chloride ion and the water molecule reside in approximately the same position, each 50% occupied, and are connected by an inversion center. The water molecule forms another hydrogen bond to the OH groups in the neighbouring dicarbene complex (O⋯HO 1.87(4) Å). The [(HOOCIMe)2Au]+ cations stack along the a axis, resulting in Au–Au distances of 3.6946(5) Å, just outside the range expected for unambiguous aurophilic interactions.18 In 4·6H2O, both the gold and the hydrogen atoms at the carboxyl groups are located on inversion centers, linking the neutral dicarbene gold units [(HOOCIMe)Au(OOCIMe)] to chains parallel to the ac plane. These chains are further connected through hydrogen bonds with the solvate water molecules. The anionic dicarbene gold units [(OOCIMe)2Au] in 5K·4H2O are connected via contacts between the carboxylate groups and the potassium atoms, which suffer from disorder and are located above and below the NHC planes, resulting in a corrugated plane parallel to the a axis and to a diagonal of the bc plane (see ESI† for further details and presentation of packing diagrams for all gold complexes).
All gold complexes were tested for cytotoxicity and antibacterial activity. The minimum inhibitory concentrations (MICs) against six pathogenic bacterial strains, two Gram-positive (Enterococcus faecium, methicillin-resistant Staphylococcus aureus (MRSA)) and four Gram-negative (Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa) were determined by a curve-fitting method. In general, the NHC–Au(I) complexes displayed moderate to low activities (Table 2 and ESI†). Complex 4 was the most potent analog, with activity against A. baumannii (MIC = 16 μM), E. coli (MIC = 32 μM), K. pneumoniae (MIC = 32 μM), P. aeruginosa (MIC = 32 μM), while it was poorly active against MRSA (MIC = 65 μM) and inactive against E. faecium (MIC > 100 μM). Inhibition of thioredoxin reductase has been reported as the antibacterial mechanism of the gold lead compound auranofin as well as gold mono- and bis-NHC complexes.21 In fact, complex 3 showed good activity in an inhibition assay of the TrxR from E. coli, with IC50 values of 0.786 ± 0.118 [μM] (Table 3), while complexes 2, 4 and 5 were inactive (IC50 values > 50 μM). The observed pattern of activity (high TrxR inhibition by the monocarbene complex 3 and low TrxR inhibition by the dicarbene complexes 2, 4 and 5) is in excellent agreement with our previous reports.21b,c Preliminary cytotoxicity experiments in several cancer cell lines revealed very low antiproliferative effects for 3 (Table 3), while 2, 4 and 5 were inactive (IC50 values >100 μM). It can be concluded that while the overall cellular potency of the gold–NHC compounds is moderate, the preference for Gram-negative bacteria is surprising and warrants further investigation. To this end, the possibility of specifically functionalizing the novel gold complexes via the carboxyl function offers an interesting opportunity to optimize their activity. Furthermore, we see the potential to use these gold complexes for the synthesis of water-soluble nanoparticles22 or as building blocks in supramolecular chemistry,19 which seems very promising due to their easy accessibility from the marine natural product norzooanemonin (1), as described above.
| Compound | E.f. | MRSA | A.b. | E.c. | K.p. | P.a. |
|---|---|---|---|---|---|---|
| Minimal inhibitory concentrations (MIC) are given in μM. E.f. = Enterococcus faecium DSM20477, MRSA = methicillin-resistant Staphylococcus aureus DSM 11822, A.b. = Acinetobacter baumannii DSM30007, E.c. = Escherichia coli, K.p. = Klebsiella pneumoniae DSM111678, P.a. = Pseudomonas aeruginosa DSM 24068.a As positive control antibiotics, amikacin (P.a.), linezolid (MRSA) and ciprofloxacin (all other strains) have been used. | ||||||
| 2 | >100 | >100 | 31 | 62 | 62 | 62 |
| 3 | >100 | >100 | 43 | 86 | 43 | >100 |
| 4 | >100 | 64 | 16 | 32 | 32 | 32 |
| 5K | >100 | >100 | >100 | >100 | >100 | >100 |
| 5Na | >100 | >100 | >100 | >100 | >100 | >100 |
| Auranofin | 0.3 | 0.4 | 55 | 46 | 81 | >100 |
| Antibiotica | 9.5 | 4.7 | 1 | 0.1 | 0.2 | 7 |
| Compound | A549 | HT-29 | MCF-7 | MDA-MB-231 | TrxR (E. coli) |
|---|---|---|---|---|---|
| Half maximal inhibitory concentration IC50 values are given in μM (n = 3).a Half maximal inhibitory concentration IC50 values are given in μM (n = 1). For experimental procedures see ref. 21a. | |||||
| Auranofin | 4.24 ± 0.61 | 2.71 ± 0.29 | 2.13 ± 0.47 | 1.21 ± 0.34 | 0.210 ± 0.30 |
| 3 | 60.0a | 63.9a | 53.4a | 76.1a | 0.786 ± 0.118 |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by the Lower Saxony Ministry for Science and Culture for the doctoral program “Drug Discovery and Cheminformatics for New Anti-Infectives (iCA)” and technical support by Petra Lippmann are gratefully acknowledged.References
- (a) A. J. Weinheimer, E. K. Metzner, M. L. Mole Jr. and M. L. Mole, Tetrahedron, 1973, 29, 3135 CrossRef; (b) K. C. Gupta, R. L. Miller, J. R. Williams and J. F. Blount, Experientia, 1977, 33, 1556 CrossRef CAS; (c) T. Jahn, G. M. König, A. D. Wright, G. Wörheide and J. Reitner, Tetrahedron Lett., 1997, 38, 3883 CrossRef CAS; (d) R. J. Capon, D. Vuong, M. McNally, T. Peterle, N. Trotter, E. Lacey and J. H. Gill, Org. Biomol. Chem., 2005, 3, 118 RSC; (e) P. Sauleau, C. Moriou and A. Al Mourabit, Nat. Prod. Res., 2017, 31, 1625 CrossRef CAS PubMed.
- (a) M. Berezin and S. Achilefu, Tetrahedron Lett., 2007, 48, 1195 CrossRef CAS PubMed; (b) A. M. Voutchkova, M. Feliz, E. Clot, O. Eisenstein and R. H. Crabtree, J. Am. Chem. Soc., 2007, 129, 12834 CrossRef CAS PubMed; (c) A. Schmidt, A. Beutler, M. Albrecht, B. Snovydovych and F. J. Ramírez, Org. Biomol. Chem., 2008, 6, 287 RSC.
- (a) I. Tommasi and F. Sorrentino, Tetrahedron Lett., 2006, 47, 6453 CrossRef CAS; (b) M. Smiglak, J. D. Holbrey, S. T. Griffin, W. M. Reichert, R. P. Swatloski, A. R. Katritzky, H. Yang, D. Zhang, K. Kirichenko and R. D. Rogers, Green Chem., 2007, 9, 90 RSC; (c) J. A. Stewart, R. Drexel, B. Arstad, E. Reubsaet, B. M. Weckhuysen and P. C. A. Bruijnincx, Green Chem., 2016, 18, 1605 RSC.
- (a) L. Delaude, Eur. J. Inorg. Chem., 2009, 2009, 1681 CrossRef; (b) L. Delaude, Adv. Synth. Catal., 2020, 362, 3259 CrossRef CAS.
- (a) S. S. Shapovalov, A. V. Kolos, A. P. Makhin, I. V. Skabitskii, N. P. Simonenko and V. V. Minin, J. Struct. Chem., 2019, 60, 1648 CrossRef; (b) S. S. Shapovalov, O. G. Tikhonova, M. O. Grigor'eva, I. V. Skabitskii and N. P. Simonenko, Russ. J. Coord. Chem., 2019, 45, 799 CrossRef CAS.
- A. Doddi, M. Peters and M. Tamm, Chem. Rev., 2019, 119, 6994 CrossRef CAS PubMed.
- H. Jacobsen, A. Correa, A. Poater, C. Costabile and L. Cavallo, Coord. Chem. Rev., 2009, 253, 687 CrossRef CAS.
- D. A. Valyaev, M. A. Uvarova, A. A. Grineva, V. César, S. N. Nefedov and N. Lugan, Dalton Trans., 2016, 45, 11953 RSC.
- M. Vogt, C. Wu, A. G. Oliver, C. J. Meyer, W. F. Schneider and B. L. Ashfeld, Chem. Commun., 2013, 49, 11527 RSC.
- L. P. Ho and M. Tamm, Chem. – Eur. J., 2022, 28, e202200530 CrossRef CAS PubMed.
- The structure of 1·H2O can also be found in the CCDC database: R. Rogers, 2020, DOI:10.5517/ccdc.csd.cc26wmxn, CCDC 2052352,† experimental crystal structure determination.
- (a) L. Oehninger, R. Rubbiani and I. Ott, Dalton Trans., 2013, 42, 3269 RSC; (b) I. Ott, in Adv. Inorg. Chem, 2020, vol. 75, pp. 121–148 Search PubMed; (c) B. Dominelli, C. H. G. Jakob, J. Oberkofler, P. J. Fischer, E.-M. Esslinger, R. M. Reich, F. Marques, T. Pinheiro, J. D. G. Correia and F. E. Kühn, Eur. J. Med. Chem., 2020, 203, 112576 CrossRef CAS PubMed; (d) G. A. Fernández, M. S. V. Gurovic, N. L. Olivera, A. B. Chopa and G. F. Silbestri, J. Inorg. Biochem., 2014, 135, 54 CrossRef PubMed.
- T. Scattolin and S. P. Nolan, Trends Chem., 2020, 2, 721 CrossRef CAS.
- (a) A. Collado, A. Gómez-Suárez, A. R. Martin, A. M. Z. Slawin and S. P. Nolan, Chem. Commun., 2013, 49, 5541 RSC; (b) F. Nahra, N. V. Tzouras, A. Collado and S. P. Nolan, Nat. Protoc., 2021, 16, 1476 CrossRef CAS PubMed; (c) E. A. Martynova, N. V. Tzouras, G. Pisanò, C. S. J. Cazin and S. P. Nolan, Chem. Commun., 2021, 57, 3836 RSC.
- G. A. Fernández, A. S. Picco, M. R. Ceolín, A. B. Chopa and G. F. Silbestri, Organometallics, 2013, 32, 6315 CrossRef.
- D. Tapu, D. A. Dixon and C. Roe, Chem. Rev., 2009, 109, 3385 CrossRef CAS PubMed.
- P. de Frémont, N. M. Scott, E. D. Stevens and S. P. Nolan, Organometallics, 2005, 24, 2411 CrossRef.
- (a) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931 RSC; (b) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC; (c) J. Muñiz, C. Wang and P. Pyykkö, Chem. – Eur. J., 2011, 17, 368 CrossRef PubMed.
- E. R. T. Tiekink, Coord. Chem. Rev., 2014, 275, 130 CrossRef CAS.
- (a) M. V. Baker, P. J. Barnard, S. J. Berners-Price, S. K. Brayshaw, J. L. Hickey, B. W. Skelton and A. H. White, Dalton Trans., 2006, 3708 RSC; (b) Y. Chen, G. Cheng, K. Li, D. P. Shelar, W. Lu and C.-M. Che, Chem. Sci., 2014, 5, 1348 RSC; (c) Q. Liu, M. Xie, X. Chang, S. Cao, C. Zou, W.-F. Fu, C.-M. Che, Y. Chen and W. Lu, Angew. Chem., Int. Ed., 2018, 57, 6279 CrossRef CAS PubMed; (d) Di Zhang, S. Suzuki and T. Naota, Angew. Chem., Int. Ed., 2021, 60, 19701 CrossRef CAS PubMed.
- (a) R. Büssing, B. Karge, P. Lippmann, P. G. Jones, M. Brönstrup and I. Ott, ChemMedChem, 2021, 16, 3402 CrossRef PubMed; (b) C. Schmidt, B. Karge, R. Misgeld, A. Prokop, R. Franke, M. Brönstrup and I. Ott, Chem. – Eur. J., 2017, 23, 1869 CrossRef CAS PubMed; (c) C. Schmidt, B. Karge, R. Misgeld, A. Prokop, M. Brönstrup and I. Ott, MedChemComm, 2017, 8, 1681 RSC.
- K. Salorinne, R. W. Y. Man, C.-H. Li, M. Taki, M. Nambo and C. M. Crudden, Angew. Chem., Int. Ed., 2017, 56, 6198 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2310639–2310645. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt04135b |
| This journal is © The Royal Society of Chemistry 2024 |