Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multiphase photochemistry in flow mode via an integrated continuous stirred tank reactor (CSTR) approach†
Antonella Ilenia Alfano *a,
Megan Smyth
*a,
Megan Smyth b,
Scott Wharry
b,
Scott Wharry b,
Thomas S. Moody
b,
Thomas S. Moody bc,
Manuel Nuño
bc,
Manuel Nuño d,
Chris Buttersd and
Marcus Baumann
d,
Chris Buttersd and
Marcus Baumann *a
*a
aSchool of Chemistry, University College Dublin, Science Centre South, Dublin 4, Ireland. E-mail: marcus.baumann@ucd.ie
bAlmac Sciences, Technology Department, Craigavon BT63 5QD, UK
cArran Chemical Company, Monksland Industrial Estate, Roscommon N37 DN24, Ireland
dVapourtec, Fornham St Genevieve, Bury St Edmunds, Suffolk, IP28 6TS, UK
First published on 13th June 2024
Abstract
A new photochemical CSTR system capable of handling solids in scaled continuous processes is presented. High-power UV-LEDs are integrated in these CSTRs containing an insoluble base that aids in generating pyrazolines via cycloaddition between alkenes and in situ generated diazo species. Contrary to reported batch methods product degradation via ring contraction is suppressed whilst generating gram quantities of spirocyclic pyrazolines.
Photochemical reactions continue to be emerging transformations for the effective generation of target molecules that are difficult to access via ground state chemistry. The last two decades alone have witnessed numerous reports from chemists in academic and industrial labs outlining new and highly selective strategies employing both visible light1 and UV irradiation.2 Whilst small scale reactions are easily accommodated in batch-based systems, the use of continuous flow approaches in combination with reaction automation is the method of choice for scale-up and library synthesis.3 Flow processing additionally offers advantages in relation to better heat transfer, more uniform irradiation, and unique spatiotemporal control, which altogether allow better reaction performance independent of reaction scale.4 Moreover, continuous flow-based photochemistry is suitable for the safe and effective use of gases as reaction partners. This allows for safe process intensification by using pressurised gaseous hydrocarbons in tetra-n-butylammonium decatungstate (TBADT)-catalysed HAT reactions,5 as well as scaled photo-oxidation reactions using air or oxygen gas.6 Whilst these examples showcase the advantageous use of gaseous reaction partners for cost-effective photochemical transformations, the use of solids in photoreactions remains a major challenge that impedes on the development of equally attractive processes. The heterogeneous nature of such photochemical reactions thereby hinders light absorption and reproducibility for batch reactions, whereas continuous flow approaches will be challenging due to reactor fouling arising from blockages of narrow diameter channels.7 To date only a small number of bespoke solutions have been reported that accommodate solids in continuous photo-flow reactors. These include the HANU reactor which is an oscillatory flow reactor with static mixing elements capable of processing slurries. In a recent application, insoluble sodium carbonate was processed in a photo-catalysed reductive cross coupling reaction using the HANU reactor.8 An analogous heterogeneous photo-catalysed reaction was reported using a series of small CSTRs in combination with sodium carbonate that was introduced via a dedicated feeding pump.9 In addition, the use of ultrasonication has been demonstrated as part of a photo-catalysed triphasic oxidation reaction using benzylic alcohols, oxygen gas and insoluble titanium dioxide.10 A final concept study reports the use of a rotor-stator spinning disc reactor for the photochemical degradation of methylene blue using oxygen in the presence of titanium dioxide.11
As part of our endeavours to access underexplored scaffolds with potential applications in medicinal chemistry we recently embarked on a study towards spirocyclic pyrazolines exploiting flow processing. Crucially these entities can be accessed via a dipolar cycloaddition reaction between electron-deficient alkenes and diazo species. The latter arise photochemically from N-tosylhydrazones in the presence of inorganic bases as reported recently by George and König.12 However, a major limitation of this approach is the instability of the cycloadducts under the reaction conditions as they readily undergo ring contraction upon loss of nitrogen gas forming cyclopropanes instead (Scheme 1).
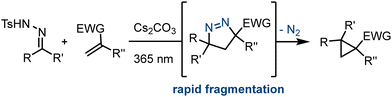 | ||
| Scheme 1 Photochemical batch route to cyclopropanes via labile spirocyclic pyrazoline intermediates. | ||
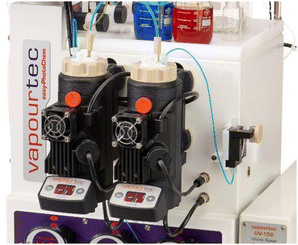
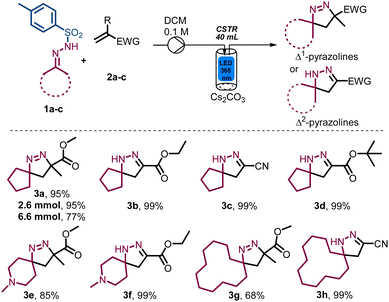
Whilst we surmised that superior spatiotemporal control provided by continuous flow processing would negate the fragmentation process, a remaining challenge concerned the necessary use of insoluble inorganic bases such as caesium carbonate. To overcome this issue, we turned our attention to a newly developed photochemical CSTR module that is compatible with standardised commercial flow reactors such as the Vapourtec E-Series and R-Series flow systems. These CSTRs are made of glass (volume 50 mL) and integrate adjustable magnetic stirring, temperature control via compressed air cooling as well as an exchangeable high-intensity LED panel that can accommodate different wavelengths (i.e., 365 nm vs. 420 nm, up to 70 W). As shown in Fig. 1, these photo-CSTRs can be used as single reactors or as part of a cascade whereby the liquid delivery is achieved via the pumps of the Vapourtec system (i.e., piston or peristaltic pumps).
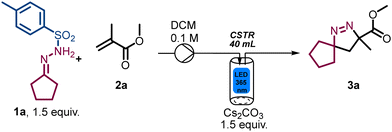
To validate the photo-CSTR module for multiphasic transformations, we targeted the synthesis of Δ1-pyrazoline and Δ2-pyrazoline systems from bench stable N-tosylhydrazones and electron-deficient alkenes. Specifically, methyl methacrylate (2a) and N′-cyclopentylidene-4-methylbenzene-sulfonylhydrazide (1a) were used as reaction partners to generate methyl 3-methyl-1,2-diazaspiro[4.4]non-1-ene-3-carboxylate (3a). Initially, the mixture of reactants was pumped into the CSTR module in a semi batch fashion (i.e., without simultaneous removal of the reaction mixture) to evaluate both reactivity and product stability. As summarised in Table 1, the exploration of key parameters quickly allowed the identification of the optimum residence time, flow rate, concentration, stirring rate and light intensity (i.e., wattage). The best conditions were found when using 1.5 equiv. N-tosylhydrazone, 1.5 equiv. of Cs2CO3, a substrate concentration of 0.1 M in DCM, light of 365 nm (36.7 W input power) and a residence time of 20 min (Table 1, entry 1), affording 95% yield of product 3a under steady state conditions. When the substrate concentration was increased to 0.2 M employing 22 W of input power, a drop in the yield was noticed (Table 1, entry 2). A lower concentration of 0.05 M or a shorter residence time of 15 min, did not improve the results as incomplete substrate conversion was observed (Table 1, entries 3 and 4). The yield decreased under both lower input power due to incomplete conversion (Table 1, entry 5) and higher input power due to the degradation into the anticipated spirocyclopropane product via nitrogen extrusion (Table 1, entry 6). Reducing the stirring rate to 300 rpm also gave a lower yield (Table 1, entry 7). Lastly, control experiments demonstrated that the light is essential for the observed reactivity (Table 1, entry 8).
| Entry | Deviations from above conditions | Yielda % 3a |
|---|---|---|
| Reaction conditions: N-tosylhydrazone 1a (3 mmol), methyl methacrylate 2a (2 mmol), Cs2CO3 (3 mmol), DCM 0.1 M (20 mL), CSTR (λ = 365 nm, 36.7 W, 500 rpm), 35 °C, 20 min.a Yields of 3a are calculated by 1H NMR using trichloroethylene as internal standard. | ||
| 1 | None | 95 |
| 2 | 0.2 M, 22 W | 47 |
| 3 | 0.05 M | 58 |
| 4 | 15 min | 59 |
| 5 | 29 W | 75 |
| 6 | 44 W | 80 |
| 7 | 300 rpm | 75 |
| 8 | Dark | 0 |
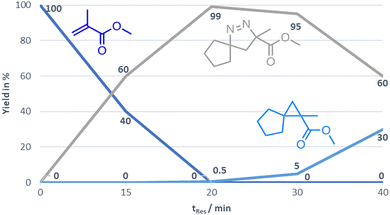
Furthermore, a kinetic study was performed for the generation of compound 3a revealing that the optimal residence time in the CSTR set-up is 20 min. At this point full conversion of the substrate is observed, whereas longer residence times show the onset of cyclopropane formation via nitrogen extrusion (Fig. 2).
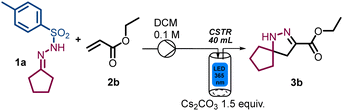
When evaluating the scope of this process using ethyl acrylate as acceptor, modest results were initially obtained due to incomplete substrate conversion (Table 2, entry 1). Notably, this changed when performing the reaction at higher input power (50 W), whilst simultaneously increasing the residence time to 30 min and reducing the amounts of 1a and Cs2CO3 to 1.2 (Table 2, entry 2). Whereas the input power minimally affected the reaction outcome (Table 2, entry 3), extended irradiation time resulted in partial product decomposition (Table 2, entry 4). Reducing the amount of 1a resulted in incomplete substrate conversion and a product yield of 65% (Table 2, entry 5).
| Entry | Deviations from above conditions | Yielda % 3b |
|---|---|---|
| Reaction conditions: N-tosylhydrazone 1a (3 mmol), ethyl acrylate 2b (2 mmol), Cs2CO3 (3 mmol), DCM 0.1 M (20 mL), CSTR (λ = 365 nm, 36.7 W, 500 rpm), 35 °C, 20 min.a Yields of 3b are calculated by 1H NMR using trichloroethylene as internal standard. | ||
| 1 | None | 40 |
| 2 | 50 W, 30 min, 1.2 equiv. of 1a and Cs2CO3 | 99 |
| 3 | 36.7 W, 30 min | 90 |
| 4 | 36.7 W, 1 h | 60 |
| 5 | 1.0 equiv. of 1a | 65 |
Next, the model reaction giving product 3a was performed at slightly larger scales (2.6 and 6.6 mmol, Scheme 2), giving a yield of 95% at the smaller scale, whereas a lower yield of 77% together with ca. 20% remaining substrate was obtained when increasing the scale. This outcome likely points towards an inconsistent residence time distribution as the reaction scale is increased and suggests that a cascade of photo-CSTRs should be used when the process is further scaled (vide infra).
After the initial reaction optimisation, the resulting photochemical process was explored across a small set of N-tosylhydrazones whilst simultaneously varying the alkene component. As summarised in Scheme 2, this study successfully yielded a selection of interesting Δ1-pyrazolines and Δ2-pyrazolines and shows that acrylonitrile and t-butyl acrylate are suitable replacements as acceptor components giving the corresponding products in quantitative yields (3c and 3d). When employing two different alkenes in combination with 4-methyl-N′-(1-methylpiperidin-4-ylidene)benzenesulfonylhydrazide as reaction partner the desired spirocyclic products 3e and 3f were again obtained in excellent yields without competing degradation. Additionally, it was found that slightly higher yields are obtained in the absence of an α-substituent adjacent to the electron-withdrawing group. The same trend was observed when using N'-cyclododecylidene-4-methylbenzene-sulfonylhydrazide as a larger cyclic fragment, furnishing the two spirocyclic products in 68% and 99% yield (3g, 3h). Notably, no purification was required for Δ2-pyrazoline products (i.e., 3b–d, 3f and 3h), rendering this an attractive method to generate these scaffolds.
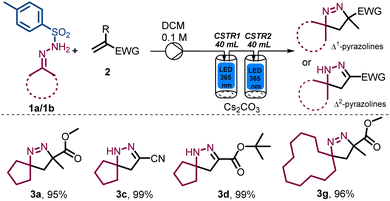
Having accomplished the selective synthesis of different Δ1-pyrazoline and Δ2-pyrazoline products in high yields, we targeted the preparation of selected examples at larger scale. As indicated for product 3a (Scheme 2) a drop in conversion was noted during an initial scale-up attempt which was ascribed to a wider residence time distribution (RTD) in the CSTR.13 To overcome this problem, we opted to use two photo-CSTR modules in sequence, each being fitted with 365 nm LEDs. When CSTRs are connected in series, their RTD is sufficiently narrowed to become quasi plug flow. Building upon the established optimal conditions with this improved set-up, the scale-up of this transformation was successful (at 10 mmol and 15 mmol scale), furnishing yields greater than 95% for the Δ1- and Δ2 pyrazolines targeted (Scheme 3).
To accommodate the increased heat generated from both photo-CSTRs that was anticipated to have a negative effect on the product stability, a stream of chilled air was used to regulate the CSTR temperature to ca. 35 °C. These adjustments were successful affording excellent yields for these products independent of the reaction scale. Overall, this approach shows reaction robustness and the importance of RTD in this application which reached a final throughput of 5 mmol h−1.
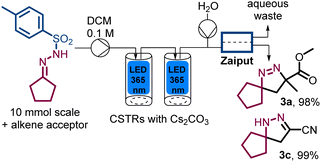
A final aspect of our study concerned the integration of an extractive in-line work-up that would remove traces of inorganic materials and ensure isolation of clean pyrazoline products upon solvent evaporation only. To achieve this the solution exiting the second photo-CSTR was mixed with a stream of water before passing a Zaiput membrane separator.14 As summarised in Scheme 4, this approach worked well for the products tested and provided for a streamlined entry to pure products with minimal manual downstream processing (see ESI† for full details).
In conclusion, we have developed a robust continuous approach for the photochemical generation of Δ1-pyrazolines and Δ2-pyrazolines from readily available N-tosylhydrazones and electron-deficient alkenes. Key to the success of this method is the use of a new photo-CSTR module containing solid Cs2CO3 as base which allows for the uniform irradiation of the resulting suspension via in-built UV-LEDs (365 nm). With reaction stirring, temperature control and light intensity being adjustable, this approach is highly effective in handling the heterogeneous reaction mixture without clogging as the solids are retained in the CSTR. Compared to prior batch-mode studies, our application harnesses superior spatiotemporal and thermal control which is crucial for realising selectivity in the generation of Δ1-pyrazoline and Δ2-pyrazoline products without competitive ring contraction to generate more stable cyclopropanes. Moreover, as prior batch studies were limited to small scale (i.e., 0.2 mmol) we demonstrate reaction scalability towards gram quantities of products using a photo-CSTR cascade that ensures narrow residence time distribution and therefore consistently high substrate conversion. The integration of an extractive in-line work-up using a Zaiput membrane separator renders this approach an attractive strategy towards underexplored spirocyclic pyrazoline scaffolds.
We are grateful to Science Foundation Ireland (SFI) for providing funding for this research through a Future Frontiers Project grant (20/FFP-P/8712, M. B.). The School of Chemistry at UCD is acknowledged for supporting our research program and providing access to all spectroscopic facilities. We thank Duncan Guthrie (Vapourtec) for the provision of the photo-CSTR units, and Andrea Adamo (Zaiput Flow Technologies) for fruitful discussions regarding the Zaiput membrane extractor.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) K. P. S. C. Cheung, S. Sarkar and V. Gevorgyan, Chem. Rev., 2022, 122, 1543 CrossRef; (b) J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 52 CrossRef CAS; (c) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS.
- (a) G. Goti, K. Manal, J. Sivaguru and L. Dell'Amico, Nat. Chem., 2024, 16, 684 CrossRef CAS; (b) T. Bach and J. P. Hehn, Angew. Chem., Int. Ed., 2011, 50, 1000 CrossRef CAS.
- (a) L. Buglioni, F. Raymenants, A. Slattery, S. D. A. Zondag and T. Noël, Chem. Rev., 2022, 122, 2752 CrossRef CAS; (b) A. Gioiello, A. Piccinno, A. M. Lozza and B. Cerra, J. Med. Chem., 2020, 63, 6624 CrossRef CAS; (c) M. Baumann, T. S. Moody, M. Smyth and S. Wharry, Eur. J. Org. Chem., 2020, 7398 CrossRef CAS.
- (a) K. Donnelly and M. Baumann, J. Flow Chem., 2021, 11, 223 CrossRef CAS; (b) S. D. A. Zondag, D. Mazzarella and T. Noël, Annu. Rev. Chem. Biomol. Eng., 2023, 14, 283 CrossRef CAS.
- G. Laudadio, Y. Deng, K. van der Wal, D. Ravelli, M. Nuño, M. Fagnoni, D. Guthrie, Y. Sun and T. Noël, Science, 2020, 336, 92 CrossRef.
- For selected examples, please see: (a) G. Laudadio, S. Govaerts, Y. Wang, D. Ravelli, H. F. Koolman, M. Fagnoni, S. W. Djuric and T. Noël, Angew. Chem., Int. Ed., 2018, 57, 4078 CrossRef CAS; (b) D. M. Schultz, F. Lévesque, D. A. DiRocco, M. Reibarkh, Y. Ji, L. A. Joyce, J. F. Dropinski, H. Sheng, B. D. Sherry and I. W. Davies, Angew. Chem., Int. Ed., 2017, 129, 15476 CrossRef.
- (a) H. L. D. Hayes and C. J. Mallia, Org. Process Res. Dev., 2024, 28, 1327 CrossRef CAS; (b) R. L. Hartman, Org. Process Res. Dev., 2012, 16, 870 CrossRef CAS.
- W. Debrouwer, W. Kimpe, R. Dangreau, K. Huvaere, H. P. L. Gemoets, M. Mottaghi, S. Kuhn and K. Van Aken, Org. Process Res. Dev., 2020, 24, 2319 CrossRef CAS.
- A. Pomberger, Y. Mo, K. Y. Nandiwale, V. L. Schultz, R. Duvadie, R. I. Robinson, E. I. Altinoglu and K. F. Jensen, Org. Process Res. Dev., 2019, 23, 2699 CrossRef CAS.
- Z. Dong, S. D. A. Zondag, M. Schmid, Z. Wen and T. Noël, Chem. Eng. J., 2022, 428, 130968 CrossRef CAS.
- A. Chaudhuri, S. D. A. Zondag, J. H. A. Schuurmans, J. van der Schaaf and T. Noël, Org. Process Res. Dev., 2022, 26, 1279 CrossRef CAS.
- V. George and B. König, Chem. Commun., 2023, 59, 11835 RSC.
- (a) N. Cherkasov, S. J. Adams, E. G. A. Bainbridge and J. A. M. Thornton, React. Chem. Eng., 2023, 8, 266 RSC; (b) M. R. Chapman, M. H. T. Kwan, G. King, K. E. Jolley, M. Hussain, S. Hussain, I. E. Salama, C. González Niño, L. A. Thompson, M. E. Bayana, A. D. Clayton, B. N. Nguyen, N. J. Turner, N. Kapur and A. J. Blacker, Org. Process Res. Dev., 2017, 21, 1294 CrossRef CAS; (c) K. Y. Nandiwale, T. Hart, A. F. Zahrt, A. M. K. Nambiar, P. T. Mahesh, Y. Mo, M. J. Nieves-Remacha, M. D. Johnson, P. García-Losada, C. Mateos, J. A. Rincón and K. F. Jensen, React. Chem. Eng., 2022, 7, 1315 RSC; (d) D. Francis, A. J. Blacker, N. Kapur and S. P. Marsden, Org. Process Res. Dev., 2022, 26, 215 CrossRef CAS.
- (a) N. Weeranoppanant and A. Adamo, ACS Med. Chem. Lett., 2020, 11, 9 CrossRef CAS; (b) J. Garcia-Lacuna and M. Baumann, Beilstein J. Org. Chem., 2022, 18, 1720 CrossRef CAS; (c) Z. Lei, H. Ting Ang and J. Wu, Org. Process Res. Dev., 2024, 28, 1355 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc02477j |
| This journal is © The Royal Society of Chemistry 2024 |