Open Access Article
Open Access ArticleAnalysing micro- and nanoplastics with cutting-edge infrared spectroscopy techniques: a critical review
Junhao
Xie
 *a,
Aoife
Gowen
a,
Wei
Xu
b and
Junli
Xu
a
*a,
Aoife
Gowen
a,
Wei
Xu
b and
Junli
Xu
a
aSchool of Biosystems and Food Engineering, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: junhao.xie@ucdconnect.ie; Tel: +353 872315465
bDepartment of Life Sciences, Center for Coastal Studies, College of Sciences, Texas A&M University-Corpus Christi, USA
First published on 27th March 2024
Abstract
The escalating prominence of micro- and nanoplastics (MNPs) as emerging anthropogenic pollutants has sparked widespread scientific and public interest. These minuscule particles pervade the global environment, permeating drinking water and food sources, prompting concerns regarding their environmental impacts and potential risks to human health. In recent years, the field of MNP research has witnessed the development and application of cutting-edge infrared (IR) spectroscopic instruments. This review focuses on the recent application of advanced IR spectroscopic techniques and relevant instrumentation to analyse MNPs. A comprehensive literature search was conducted, encompassing articles published within the past three years. The findings revealed that Fourier transform infrared (FTIR) spectroscopy stands as the most used technique, with focal plane array FTIR (FPA-FTIR) representing the cutting edge in FTIR spectroscopy. The second most popular technique is quantum cascade laser infrared (QCL-IR) spectroscopy, which has facilitated rapid analysis of plastic particles. Following closely is optical photothermal infrared (O-PTIR) spectroscopy, which can furnish submicron spatial resolution. Subsequently, there is atomic force microscopy-based infrared (AFM-IR) spectroscopy, which has made it feasible to analyse MNPs at the nanoscale level. The most advanced IR instruments identified in articles covered in this review were compared. Comparison metrics encompass substrates/filters, data quality, spatial resolution, data acquisition speed, data processing and cost. The limitations of these IR instruments were identified, and recommendations to address these limitations were proposed. The findings of this review offer valuable guidance to MNP researchers in selecting suitable instrumentation for their research experiments, thereby facilitating advancements in research aimed at enhancing our understanding of the environmental and human health risks associated with MNPs.
1. Introduction
Microplastics (MPs) are commonly defined as plastics with the largest dimension of <5 mm. In contrast, there is no consensus on the definition of nanoplastics (NPs). Several studies define NPs as particles with a diameter of <1 μm.1,2 In this review, we will adopt this definition for NPs, and collectively refer to MPs and NPs as MNPs. MNPs, as emerging pollutants, are ubiquitously present in marine environments,3 terrestrial ecosystems,4 drinking water,5 and even human bodies.6 With the rapid growth of research on MNPs in recent years, the global distribution of them has become evident. While some studies have indicated that these minuscule plastic particles can have adverse effects on organisms,7 the knowledge regarding the impact on human health is still limited. To accurately assess the hazards posed by these emerging pollutants, reliable analysis of them is critical.Early analysis of plastic particles was often based on visual inspection (with the naked eye or a microscope), a method that is no longer accepted mainly due to its subjectivity in particle selection, lack of chemical specificity (or low chemical specificity when hot needles or Nile red dye was used), time-consuming and laborious nature, and inability to provide information on the chemical composition of particles.8 Consequently, this strategy has been largely replaced by methods that are convenient and could identify the chemical composition. Infrared (IR) spectroscopy is one of such methods and is advantageous compared to several other methods that could identify the polymer type. For instance, unlike pyrolysis-gas chromatography coupled to mass spectrometry, IR spectroscopy is non-destructive, hence the morphological information of plastic particles is not lost.8 Furthermore, in comparison to Raman spectroscopy, IR spectroscopy is rarely associated with fluorescence interference – a crucial attribute, especially when analysing weathered plastic particles or plastic particles with pigment additives.9
In recent years, with advancements in technology, IR spectroscopy has evolved into a broader category, encompassing not only the well-established Fourier transform infrared (FTIR) spectroscopy but also emerging techniques like quantum cascade laser infrared (QCL-IR) spectroscopy, atomic force microscopy-based infrared (AFM-IR) spectroscopy, and optical photothermal infrared (O-PTIR) spectroscopy. Among these IR spectroscopic techniques, FTIR spectroscopy was the earliest technique applied to the analysis of MPs. While standalone FTIR spectrometers might suffice for the analysis of large MPs (e.g., >500 μm), when dealing with small MPs (e.g., <500 μm), the use of a microscope, in addition to an FTIR spectrometer, becomes necessary. This combined approach involving both a microscope and an FTIR spectrometer is commonly referred to as micro-FTIR (μ-FTIR). With the application of focal plane array FTIR (FPA-FTIR), the data collection speed for MP analysis is significantly improved, and unbiased analysis could be achieved, since no manual presorting of particles is needed. Hence, FPA-FTIR has been considered to have tremendous potential in the automated analysis of MPs.10 Subsequently, advanced IR spectroscopy techniques, namely, QCL-IR spectroscopy, AFM-IR spectroscopy, and O-PTIR spectroscopy were applied for MNPs analysis,11–13 with each demonstrating great potentials. These IR spectroscopic techniques are based on distinct principles. A comprehensive understanding of the principles, advantages, and limitations of these IR spectroscopic techniques is crucial for conducting reliable MNP research with them. Therefore, a paper summarising the application of these IR spectroscopic techniques and their relevant instruments in MNP analysis would be of great interest to readers.
To this end, the objectives of this review article are (1) to investigate the application of the state-of-the-art IR techniques in MNP research over the past three years, (2) to compare instruments built from different IR spectroscopic techniques in terms of substrates/filters, data quality, spatial resolution/detection limit, analysis speed, data processing, cost, and etc., and (3) to identify limitations of certain IR instruments and provide recommendations for future applications.
1.1. Fundamentals of IR spectroscopic techniques
μ-FTIR can effectively analyse single MP particles smaller than 500 μm, a task that could be challenging to achieve using standalone FTIR spectrometers. Therefore, with the current trend of analysing smaller MPs, μ-FTIR might be preferred. In early (from 2004 to around 2012) detection of MPs, the μ-FTIR systems used were equipped with a single-element (IR) detector only, this means that only one FTIR spectrum could be obtained at a single point on the sample at a time. While using a single-element detector in combination with a motorised stage allows for the collection of spatial and spectral information (i.e., chemical imaging) of MP samples, the imaging speed is significantly slow. Subsequently, the introduction of line array detectors, where several single-element detectors are arranged in a line, has enabled the simultaneous collection of multiple spectra within a single line. By employing a line array detector and a motorised stage to collect data from multiple rows, chemical images could be rapidly generated. The most advanced detector for μ-FTIR is the FPA detector, consisting of an array of IR detectors arranged in a square pattern (e.g., 64 × 64 detectors, 128 × 128 detectors). An FPA detector allows for the simultaneous collection of spectral data from multiple points on a single plane. Consequently, when coupled with a motorised stage, the imaging speed is greatly enhanced. The amount of data that can be collected simultaneously depends on the size of the FPA. For instance, an FPA with a size of 64 × 64 can acquire 4096 (64 × 64 = 4096) spatially resolved spectra in a single acquisition. FPA-FTIR has demonstrated tremendous potential in the field of automated MP analysis.17
In MP analysis, the feature of tunability (of the IR frequency) of the QCL-IR technique allows the IR light to solely focus on the specific and optimal IR range and hence allows rapid identification of known types of MPs. Furthermore, this feature enables the direct generation of single frequency images. This is in stark contrast to FTIR spectroscopy, where the IR range cannot be manually adjusted, and therefore, acquiring single frequency images requires a significant amount of time to first acquire hyperspectral images.
Currently, two QCL-IR spectroscopy-based approaches have been employed in the field of MP research. One utilises a single-element detector in conjunction with a fully automated MP detection workflow, while the other employs a large FPA (480 × 480) detector.19 While the combination of an FPA detector and a QCL significantly improves the efficiency (due to super-fast imaging speed) in data collection,11 using an FPA detector in a QCL system may pose a risk of laser coherence artefacts in the acquired images or spectra.20
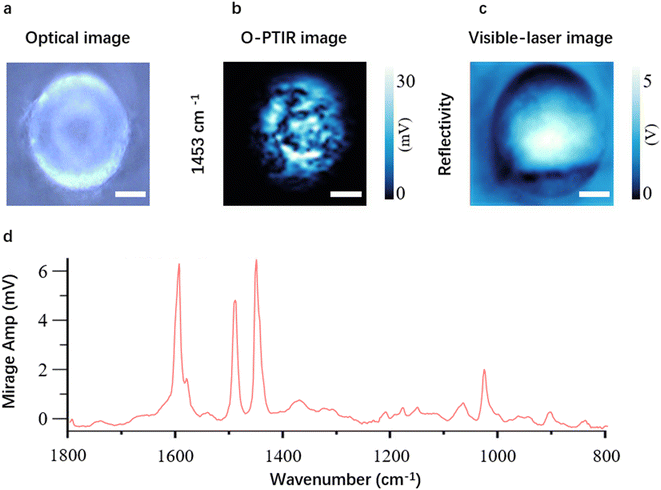
Currently, commercially available O-PTIR microscopes offer the capability to collect optical images (Fig. 2a), single frequency images (Fig. 2b), visible-laser images (Fig. 2c), point spectra (Fig. 2d) and hyperspectral images for MNP analysis, providing morphological and chemical information. However, a significant limitation exists in current O-PTIR microscopes, as they are equipped with a single-element detector only, which means that the imaging speed is greatly restricted.
It is worth mentioning that with the O-PTIR technique, it is possible to collect both O-PTIR spectra and Raman spectra at the same point and time. This feature can provide more reliable identification results for MNP research compared to obtaining IR or Raman spectra alone.23 But this review only focuses on IR techniques, so the capability of O-PTIR spectroscopy to acquire Raman spectra is not further discussed.
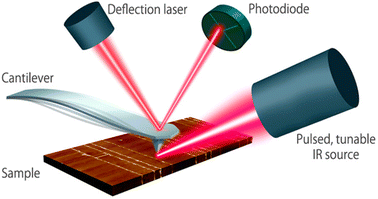 | ||
| Fig. 3 Schematic diagram of the AFM-IR technique. A pulsed tunable laser is directed towards the part of the sample close to the tip of an atomic force microscope. By tuning the laser to a specific absorbing wavelength of the sample, the IR radiation gets absorbed, leading to photothermal expansion in the absorbing regions of the sample. The tip of the cantilever serves as a detector to measure local IR absorption. Adapted with permission from Dazzi and Prater.25 Copyright 2023 American Chemical Society. | ||
1.2. MNP analysis with IR spectroscopic techniques
MNP samples from the environment are complex cocktails that not only contain MNPs but also various substances such as tissues, cellulose, chitin, silica, minerals. The presence of such organic and inorganic materials in the sample significantly affects the MNP analysis as the signal of these materials might overlay MNP spectra. Therefore, applying effective methods to extract MNPs from the environmental matrix before IR spectroscopic analysis is essential. The extraction of MNPs typically involves the digestion of organic materials and the removal of inorganic materials (usually by density separation). Examples of MNP extraction, as well as the major reagents/methods used are detailed in Section 3.1.1 of this article.The separation of MNPs from environmental matrices is often considered a necessary step. However, some researchers might apply an IR spectroscopic technique to directly image the sample matrix for the purpose of identifying MNPs mixed in it.26 In other words, depending on the research question of interest, the extraction of MNPs from a matrix may not be necessary.
If the research question of interest is to explore the effects of a treatment on MNPs, the MNPs might be intentionally treated (e.g., oxidised) before IR spectroscopic analysis.
Subsequently, the extracted MNPs, or samples containing MNPs, or treated MNPs are typically enriched on or transferred to a substrate/filter, getting ready for IR spectroscopic analysis.
For the detection of MNPs using IR spectroscopy, there are two widely adopted methods. The first one is hyperspectral imaging (Fig. 4a), where a spectrum for every “pixel” in the region of interest is collected. Individual pixels are then classified based on their spectral signatures. The second approach is particle-based analysis (Fig. 4b), where all particles in a region of interest are first located. Subsequently, the operator collects spectra for each located particle. Then the collected spectra are classified to distinguish which particles are MNPs and which are not. Particle location/recognition can be based on visible light (e.g., manually locating substances that appear as particles to the naked eye or under a microscope); it can also be based on single frequency IR light (see Section 3.1.6 for an example).
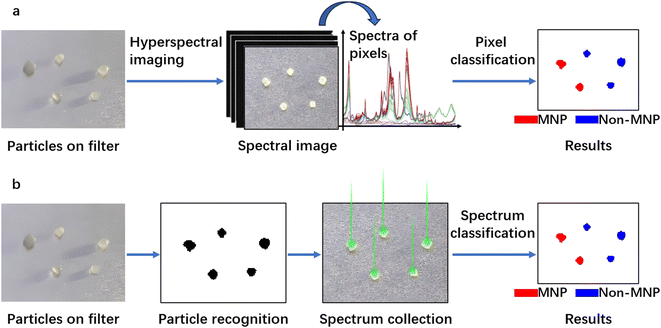 | ||
| Fig. 4 Detection of particles through hyperspectral imaging (a) and through particle-based analysis (b). | ||
A diverse array of algorithms has been developed to classify pixels or spectra, falling into two primary categories: instance-based and model-based machine learning approaches.27 In instance-based methods, reference data (i.e., instances) are directly employed to identify unknown spectra through similarity assessments. Various methods, such as Pearson correlation analysis and spectral angle mapper (SAM), can be used for similarity assessment. A notable advantage of instance-based approaches lies in their adaptability, as the spectroscopic reference data can be easily expanded or customised, for instance, by incorporating pertinent spectra into the existing spectral library.27 However, it should be noted that these methods can result in a significant computational burden and are time-consuming due to the necessity of computing similarities between each collected spectrum and every reference spectrum in the library. In contrast, model-based approaches rely on statistical models trained from spectroscopic reference data (e.g., partial least squares-discriminant analysis (PLS-DA) models, support vector machine (SVM) models, and random decision forest (RDF) classifiers), which are then applied to unknown spectra. These unknown spectra are then classified into predefined categories which may encompass polymer types and substances associated with environmental MNP samples. Notably, model-based techniques typically offer shorter analysis times, enabling a higher analytical throughput.28 Nonetheless, it is imperative to enhance the robustness of these models before their practical application can be fully realised.
Hyperspectral imaging is associated with larger file sizes and time demands (when an array detector is unavailable) since every pixel within the region of interest undergoes spectral acquisition, including pixels devoid of MNPs. Conversely, particle-based analysis selectively gathers spectra from particles only, resulting in reduced data storage space and time requirements (when the number of particles in the region of interest is relatively low). An inherent advantage of hyperspectral image analysis lies in its capacity to provide accurate particle characterisation including both chemical composition and physical properties such as size and shape. In contrast, particle-based analysis often involves collecting one to several spectra from a particle solely for identification purposes. If precise information on particle size and shape is desired, complementary image analysis methods need to be employed. Both approaches ensure the chemical identification of observed particles, thereby providing reliable outcomes. Without the utilisation of these two approaches (or other effective approaches) for chemical identification, the quantification of MNP particles would yield inaccurate results. For instance, Hernandez et al.29 directly labelled particle-like substances observed under a scanning electron microscope as MNPs, without subjecting them to proper chemical identification. As a consequence, their results have faced criticism.30
2. Literature review methodology
A literature search on Scopus (https://www.scopus.com) was conducted to gather information about the IR techniques and instruments used in recent years in MNP research. The keywords used for the search were “IR OR infrared” and “microplastic*”, and these were searched within the Article title, Abstract, and Keywords. The search was conducted on April 13, 2023. The results obtained were first filtered on the Scopus website using the following criteria: the year of publication was limited to “2023”, “2022”, and “2021”; the document type was limited to “Article”; the publication stage was limited to “Final”; and the language was limited to “English”. A database was then formed, containing the retained articles, one paper published in 2020 that describes the combination of QCL-IR spectroscopy with a large FPA detector for MP monitoring, and three studies related to O-PTIR spectroscopy for MNP research but unavailable on Scopus.The published work in the database was then accessed one by one. Through reading the titles, abstracts, and parts of the text, the literature was further screened according to the following exclusion criteria: articles that could not be accessed, research that did not focused on MP (e.g., focused on bulk plastics), research that did not use IR spectroscopy/instruments and research that did not specify either the brand or the model of the IR instrument used.
3. Results from the literature review
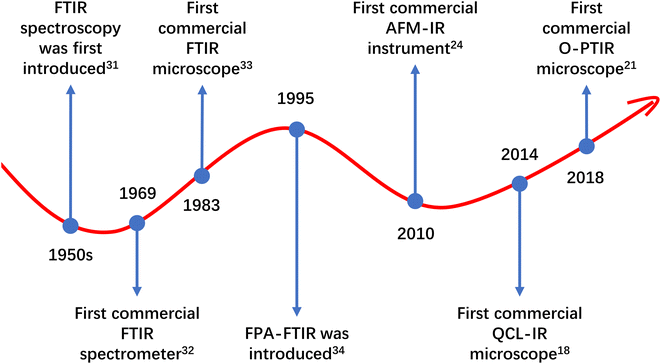
The literature search on Scopus initially yielded 1987 sources. After the first round of screening, 1178 articles were deemed relevant and retained. These 1178 retained articles, along with an additional four relevant articles identified through other sources, were amalgamated to form a database comprising a total of 1182 articles. A second round of screening was then conducted on the database, resulting in a final selection of 988 articles for further analysis.The findings from the studies covered in this review indicate that FTIR spectroscopy is the most extensively utilised technique in MP research, with 953 studies employing it. Following FTIR spectroscopy, QCL-IR spectroscopy was used in 35 studies, and O-PTIR spectroscopy in six studies. AFM-IR spectroscopy was the least used technique, with only three publications incorporating it. Among these IR techniques, FTIR spectroscopy stands as the oldest technique and has been continuously developed since its introduction in the 1950s.31–34 The prevalent use of FTIR spectroscopy in MP research can be attributed to its capability to deliver reliable qualitative and quantitative results (and some surface characterisation results through hyperspectral imaging). Additionally, its low costs (compared to instruments based on the newer IR spectroscopic techniques) and early commercial availability (the first commercial FTIR instrument introduced as early as 1969),32 might have contributed to its widespread adoption. The developmental timeline of FTIR spectroscopy and the introduction of instruments based on the newer IR techniques can be observed in Fig. 5. As seen, instruments based on newer IR spectroscopic techniques have become commercially available only in the last decade. Despite these new IR spectroscopic techniques having demonstrated greater potential than FTIR spectroscopy in MP research,11–13 they might not have had sufficient time to become as popular as FTIR spectroscopy. Among the newer IR spectroscopic techniques, O-PTIR spectroscopy and QCL-IR spectroscopy seem to be more favoured by MNP researchers, despite their relevant instrumentation becoming commercially available later than AFM-IR spectroscopy-based instrumentation.
FTIR spectroscopy is widely employed for the analysis of MPs, and it can be implemented using either FTIR spectrometers or μ-FTIR systems. FTIR spectrometers employing ATR have been commonly used for the identification of MPs >500 μm, while μ-FTIR systems are more suitable for identifying MPs <500 μm. Currently, there are numerous models of FTIR instruments used in MP research. The most frequently utilised FTIR instruments in the last three years are listed in Table 1. Instruments that have been used in fewer than 8 studies are not included in this table. Additionally, it is indicated in the table that whether the listed instrument is equipped with a line array detector or an FPA detector, and the number of times these array detectors were used for imaging (instruments equipped with an array detector is also equipped with a single-element detector, allowing users to collect point spectra when analysing MPs, rather than necessarily performing imaging). As evident from Table 1, the most popular FTIR spectrometer is the Nicolet 6700 (Thermo Scientific), used in 44 studies, while the Nicolet iN10 (Thermo Scientific) is the preferred μ-FTIR system, employed in 70 studies. FTIR spectrometers have been used in 508 studies, while μ-FTIR has been used in 255 studies. This observation might suggest that in the past three years, research on MPs >500 μm has been approximately twice as prevalent as research on MPs <500 μm. In addition, the ownership rate of IR instruments equipped with an array detector among MP research teams seems to be low, possibly because instruments with an array detector are more expensive than those with a single-element detector only. Furthermore, even one has an FTIR instrument equipped with an array detector, it does not necessarily mean they need to use the array detector for chemical imaging, as revealed by the data, the usage rate for line array detectors is only 8%, and for FPA detectors, it is 67%. Indeed, if a single-element detector can quickly complete an MP detection task, chemical imaging may not be necessary. For instance, when the number of particles in a region of interest is low, the operator can directly click on each particle and collect spectra without the need for imaging the entire region of interest, which produces a huge amount of data that requires significant computing power and time to process.35
| Instrument and brand | Is μ-FTIR? | Frequencyb |
|---|---|---|
| a The FTIR microscopes listed in this table may be coupled to the FTIR spectrometers also listed in this table. In such cases, the FTIR microscope, when coupled to the FTIR spectrometer, is considered as a single entity of the of FTIR microscope, and its usage is not counted again as the FTIR spectrometer. b In this column, if the frequency (number) listed is followed by a sentence, it indicates the instrument is equipped with an FPA detector or a line array detector, and the number of studies (specified in the sentence) these array detectors were used in. | ||
| Nicolet iN10, Thermo Scientific | Yes | 70 |
| Nicolet iN10 MX, Thermo Scientific | Yes | 45, in which 8 used the line array detector |
| Nicolet 6700, Thermo Scientific | No | 44 |
| Spotlight 400 microscope coupled to a FTIR spectrometer, PerkinElmer | Yes | 43, in which 15 used the line array detector |
| Nicolet iS50, Thermo Scientific | No | 42 |
| Spotlight 200 microscope coupled to a FTIR spectrometer, PerkinElmer | Yes | 40 |
| Spectrum Two, PerkinElmer | No | 40 |
| Vertex70, Bruker | No | 39 |
| Cary 630, Agilent | No | 39 |
| Nicolet iS5, Thermo Scientific | No | 37 |
| Nicolet iS10, Thermo Scientific | No | 37 |
| ALPHA, Bruker | No | 36 |
| Tensor 27, Bruker | No | 29 |
| Spectrum 100, PerkinElmer | No | 24 |
| Cary 620 microscope coupled to a FTIR spectrometer, Agilent | Yes | 21, in which 17 used the 64 × 64 FPA detector |
| FT/IR 6000, JASCO | No | 19 |
| IRTracer-100, Shimadzu | No | 18 |
| IRAffinity-1/S, Shimadzu | No | 16 |
| IR Prestige-21, Shimadzu | No | 14 |
| Frontier, PerkinElmer | No | 14 |
| HYPERION 3000, Bruker | Yes | 14, in which 7 used the 64 × 64 FPA detector |
| HYPERION 2000, Bruker | Yes | 14 |
| ALPHA II, Bruker | No | 10 |
| Nexus 670, Thermo Scientific | No | 9 |
| TENSOR II, Bruker | No | 9 |
| Nicolet iS20, Thermo Scientific | No | 8 |
| FT/IR 4000, JASCO | No | 8 |
| Spectrum ONE, PerkinElmer | No | 8 |
| VERTEX 70v, Bruker | No | 8 |
| LUMOS II, Bruker | Yes | 8, in which 5 used the 32 × 32 FPA detector |
In comparison to the numerous models of FTIR instruments used in MP research, there are relatively fewer instrument models available for the other three IR techniques. This review identified two QCL-IR instruments used for MP analysis: the 8700 LDIR (Agilent), employed in 34 studies, and the SPERO microscope (Daylight Solutions), used in one study. The 8700 LDIR is equipped with a single-element detector and comes with specialised software Clarity (Agilent) designed for automated MP analysis. The SPERO microscope is equipped with a large 480 × 480 FPA detector. Under low magnification (4×), it covers a field of view of 2000 μm × 2000 μm and produces a projected pixel size of 4.25 μm. Under high magnification (12.5×), it covers a field of view of 650 μm × 650 μm and generates a projected pixel size of 1.36 μm. For O-PTIR instruments, we found two models: the mIRage microscope (Photothermal Spectroscopy Corp) and the mIRage+R microscope (Photothermal Spectroscopy Corp). These were used in four and two studies, respectively, and both have a single-element detector. For AFM-IR instruments, we identified the Nano IR2 (Anasys Instruments Inc), which was used in three studies.
3.1. Comparisons of IR instruments
In the following section, we will conduct a comprehensive comparison of several cutting-edge IR instruments that have been identified in the studies covered by this review. FPA-FTIR stands out as the most advanced form within the FTIR category, thereby warranting the inclusion of all FPA-FTIR models, namely the Cary 620, the HYPERION 3000, and the LUMOS II, in our comparison. Additionally, the 8700 LDIR and the SPERO microscope, both QCL-IR instruments, as well as the mIRage microscope and the mIRage+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R microscope, which are O-PTIR microscopes, and the Nano IR2, an AFM-IR instrument, will also be included in the comparison.
R microscope, which are O-PTIR microscopes, and the Nano IR2, an AFM-IR instrument, will also be included in the comparison.
| IR instrument | Sample types | Pretreatmentb | Size, % recovery, and particle type | Reference |
|---|---|---|---|---|
| a MP = microplastic, WWTP = wastewater treatment plant, GIT = gastrointestinal tract, ZnCl2 = zinc chloride, SPT = sodium polytungstate, NaCl = sodium chloride, H2O2 = hydrogen peroxide, LMT = lithium metatungstate, LST = lithium heteropolytungstate, KOH = potassium hydroxide, NaOH = sodium hydroxide, CsCl = caesium chloride, HNO3 = nitric acid, NaI = sodium iodide, CaCl2 = calcium chloride, TIO2 = titanium dioxide, PP = polypropylene, PS = polystyrene, PE = polyethylene, PC = polycarbonate, PET = polyethylene terephthalate, PU = polyurethane, PVC = polyvinyl chloride, PA = polyamide (nylon), CPE = chlorinated polyethylene, LDPE = low density polyethylene, HDPE = high density polyethylene, UEPP = Universal Enzymatic Purification Protocol. b D = digestion, DS = density separation. | ||||
| Cary 620 | Tap water | Acetic acid to dissolve inorganics | NA | 36 |
| Bottled water | DS: ZnCl2 | 95–145 μm, 63 ± 8%, PS | 37 | |
| Citric acid to dissolve remaining inorganics | ||||
| Water from drinking water distribution pipes | DS: SPT | NA | 38 | |
| Sea water | D: protease, cellulase, Viscozyme, Fenton's reagent | 90–106 μm, 90.3 ± 1.1%, PE | 39 | |
| DS: SPT | ||||
| Sea water | NA | NA | 40 | |
| Sea water | Modified UEPP | 90 μm, 75%, PS | 41 | |
| Sea water | D: protease, cellulase, Viscozyme, Fenton's reagent | NA | 42 | |
| DS: SPT | ||||
| Sand | DS: NaCl | NA | 43 | |
| Sediment | D: H2O2 | 90–180 μm, 42.3 ± 4.4%, PE | 44 | |
| DS: ZnCl2 | 100–500 μm, 78.9 ± 6.2%, PS | |||
| 100–500 μm, 77.6 ± 6.5%, PC | ||||
| Sediment | D: H2O2 | 250–500 μm, 98%, PET | 45 | |
| DS: LST | 250 μm, 89%, HDPE | |||
| Sediment | DS: NaCl | 85 ± 3%, PE, PP, PS, PU | 46 | |
| 67 ± 3%, PVC, PET | ||||
| Soil | DS: NaCl, ZnCl2 | NA | 47 | |
| Fatty slurries, grease, sewage sludge, digested sludge from WWTP digesters | D: lipase, cellulase, protease, H2O2, Fenton's reagent | NA | 48 | |
| DS: SPT | ||||
| GITs of fish | UEPP | 90 μm, 75.0 ± 10.0%, PS | 49 | |
| GITs of fish | NA | NA | 50 | |
| Invertebrates | D: H2O2, chitinase, protease | 90–180 μm, 50.0 ± 4.4%, PE | 44 | |
| 100–500 μm, 85.3 ± 5.7%, PS | ||||
| 100–500 μm, 81.3 ± 4.2%, PC | ||||
| Antarctic krill and salps | Modified UEPP | NA | 51 | |
| Material from bar screens, influent wastewater, digested sludge, and effluent of a WWTP | D: alcalase, cellulase, Fenton's reagent | NA | 52 | |
| DS: ZnCl2 | ||||
| HYPERION 3000 | Samples collected from the air | D: Fenton's reagent, protease, cellulase, chitinase | NA | 53 |
| DS: ZnCl2 | ||||
| Drinking water treatment plant treated water | NA | NA | 54 | |
| River/lake water | D: H2O2 | NA | 54 | |
| DS: ZnCl2 | ||||
| Estuary water | Modified UEPP | NA | 55 | |
| Effluent of WWTPs | Modified UEPP | NA | 56 | |
| Mussel | Modified UEPP | NA | 57 | |
| Sediment | D: Fenton's reagent | NA | 58 | |
| DS: ZnCl2 | ||||
| Zooplankton | D: H2O2 or HNO3 | 15 μm, 54%, PS | 59 | |
| DS: NaCl | ||||
| LUMOS II | Ground water | D: H2O2 | 60 | |
| DS: LMT | ||||
| Clam | D: KOH | NA | 61 | |
| DS: ZnCl2 | ||||
| Soil | DS: CsCl | 140–954 μm, 94–99%, PA | 62 | |
| 128–1636 μm, 86–98%, PET | ||||
| Soil | D: H2O2 | NA | 9 | |
| DS: ZnCl2 | ||||
| Pristine MPs | NA | NA | 9 | |
| Corn flour | NA | NA | 26 | |
| 8700 LDIR | Samples collected from the air | D: HNO3 | NA | 63 |
| Atmospheric dustfall | D: H2O2 | NA | 64 | |
| DS: ZnCl2 | ||||
| Indoor house dust | NA | NA | 65 | |
| Gel tape | D: glacial acetic acid, H2O2 | NA | 66 | |
| DS: NaI | ||||
| Bottled water and air deposition | NA | NA | 67 | |
| Bottled water | NA | NA | 68 and 69 | |
| Groundwater | D: H2O2 | NA | 70 | |
| DS: ZnCl2 | ||||
| Groundwater | D: H2O2 | 75–90 μm, 70%, PE | 71 | |
| DS: CaCl2 | 250–300 μm, 68%, PE | |||
| Water from reservoir, dune filtrate, riverbank, and groundwater | D: KOH, H2O2 | 100 μm, 65–80%, PE | 72 | |
| DS: ZnCl2 | ||||
| Surface water, groundwater, and water from different stages of drinking water treatment | D: KOH, H2O2 | NA | 73 | |
| DS: ZnCl2 | ||||
| Surface water of a river | D: H2O2 | NA | 74 | |
| DS: ZnCl2 | ||||
| Surface water of a river | D: H2O2 | 100–200 μm, 96 ± 2.83%, PE | 75 | |
| DS: NaCl | ||||
| Surface water of a river | D: H2O2 | NA | 76 | |
| Surface water of a river | D: Fenton's reagent | 125–250 μm, 88.3 ± 1.2% | 77 | |
| Surface water and bottom water of a river | D: Fenton's reagent | 96%, PE; 94%, PP; 96%, PS | 78 | |
| DS: ZnCl2 | 92%, PP–PE copolymer | |||
| 90%, PVC; 94%, PET | ||||
| Fjord water | D: Fenton's reagent | NA | 79 | |
| DS: NaCl | ||||
| Influents and effluents from WWTPs | D: H2O2 | NA | 80 | |
| DS: ZnCl2 | ||||
| Effluents from a WWTP | D: H2O2 | 100 μm, 90%, PE | 81 | |
| DS: ZnCl2 | ||||
| Influent and effluent of a petrochemical WWTP | D: H2O2 | 90.0%, PE; 76.0%, PP | 82 | |
| DS: ZnCl2 | 90.9%, resin; 100%, PU | |||
| 85.4%, PVC; 87.0%, CPE | ||||
| Sea water | D: HNO3 | 200–500 μm, 115 ± 30%, PP | 83 | |
| DS: NaCl | 83 ± 10%, PE; 100 ± 15%, PS | |||
| 148 ± 49%, PVC; 115 ± 35%, PET | ||||
| Sea water | D: proteinase, H2O2, Fenton's reagent, chitinase (the D was microwave-assisted) | 20–63 μm, 98.3 ± 0.3%, PET | 84 | |
| DS: ZnCl2 | 95 ± 4%, HDPE | |||
| Sand | DS: NaCl | 20–160 μm, 98%, PP | 85 | |
| Sediment | D: H2O2 | NA | 76 | |
| DS: ZnCl2 | ||||
| Sediment | DS: NaCl | 200–500 μm, 93 ± 4%, PP | 83 | |
| 98 ± 17%, PE; 107 ± 43%, PS | ||||
| 153 ± 65%, PVC; 93 ± 20%, PET | ||||
| Sediment and scum of a septic tank | D: Fenton's reagent, HNO3 | NA | 86 | |
| DS: NaCl, NaI | ||||
| Soil | D: H2O2 | NA | 9 and 63 | |
| DS: ZnCl2 | ||||
| Soil | D: Fenton's reagent, H2O2 | 100 μm, 83.9 ± 5.5%, PVC | 87 | |
| DS: NaCl, NaI | 0.5–5 mm, 91.1 ± 6.7%, PE | |||
| Soil | D: H2O2 | NA | 88 | |
| DS: NaCl | ||||
| Sludge of a petrochemical WWTP | D: Fenton's reagent, H2O2 | 87.6%, PE; 78.3%, PP | 82 | |
| DS: ZnCl2 | 88.7%, resin; 95.3%, PU | |||
| 83.2%, PVC; 88.6%, CPE | ||||
| Mineralized refuse and leachate samples of a landfill | D: H2O2 | 95.8 ± 3.5%, PET; 88.3 ± 4.7%, PC; 95.5% ± 0.7%, PP; 97.0 ± 1.4%, PE | 89 | |
| DS: ZnCl2 | ||||
| GITs of fish | D: KOH, H2O2 | 200–300 μm, 88 ± 4%, PS, PP, PVC, PET, PE; 1–5 mm, 76 ± 7%, PET fibre | 90 | |
| Mussel | D: HNO3 | 200–500 μm, 69 ± 17%, PP | 83 | |
| DS: NaCl | 78 ± 7%, PE; 101 ± 9%, PS | |||
| 143 ± 35%, PVC; 81 ± 22%, PET | ||||
| Snail | D: KOH, H2O2 | 100–200 μm, 90 ± 1.25%, PE | 75 | |
| DS: NaCl | ||||
| Human intestinal and nasal secretions | D: HNO3 | NA | 63 | |
| Human intestinal and nasal secretions | NA | NA | 91 | |
| Human placenta | D: KOH | 50–210 μm and 60–330 μm | 92 | |
| 88.00 ± 10.58% | ||||
| Human placentas, meconium, infant feces, breast milk and infant formula | D: HNO3 | 96%, PP; 97%, PE; 96%, PS | 93 | |
| 91%, PET; 87%, PVC; 84%, PU | ||||
| Human respiratory tract | D: HNO3 | 92%, PS; 89%, PP; 78%, PE | 94 | |
| Human sputum | D: HNO3, NaOH | <333 μm, 96%, PP; 97%, PE | 95 | |
| DS: ZnCl2 | 96%, PS; 91%, PET; 93%, rubber | |||
| 87%, PVC; 84%, PU; 6%, PA | ||||
| SPERO microscope | Treated wastewater, marine surface water, marine sediment, snow samples, pristine MPs | NA | NA | 11 |
| mIRage microscope | Baby teat | NA | NA | 13 |
| Corn flour | NA | NA | 26 | |
| Sea water | MP concentrator | >90% | 96 | |
| Plastic food container | MP concentrator | >90% | 96 | |
| Deep-sea sediment | DS: NaCl | >90% | 96 | |
| MP concentrator | ||||
| Fish tissue spiked with MPs | D: HNO3, H2O2 | <60 μm, 87–97% | 97 | |
| 60–100 μm, 87–100% | ||||
| 100–200 μm, 87–107% | ||||
| 200–500 μm, 90–110% | ||||
| mIRage+R microscope | Soil | D: H2O2 | NA | 9 |
| DS: ZnCl2 | ||||
| Pristine MPs | NA | NA | 9 | |
| Nano IR2 | Masterbatches (composed of pigment red and LDPE) | NA | NA | 98 |
| PP film (largest dimension <5 mm) | NA | NA | 12 | |
| TiO2 coated PP particles | NA | NA | 99 | |
In a study using near-field molecular spectral imaging to detect MPs in corn flour, the corn flour was intentionally spiked with MPs and the MPs were not subsequently extracted.26 This is because the primary research objective of this study was to directly detect MPs in corn flour. To explore the self-assembly of extracellular polymeric substances (EPS) on the surface of MPs, MPs were deliberately immersed in an EPS solution and then were lyophilised.12 To study the changes in the microstructure of the MP surface, the MPs were artificially oxidised,98 or coated.99
As the last step before IR spectroscopic analysis, the extracted or intentionally treated MNPs or samples spiked with MNPs were typically deposited on or transferred to a substrate.
For FTIR microscopes, the working mode (i.e., transmission, reflection/transflectance) decides the type of substrates/filters needed. In transmission mode, IR radiation that passes through the sample is detected. Therefore, IR-transparent/weak-IR-absorbent substrates/filters are required for this mode. Commonly used IR-transparent/weak-IR-absorbent substrates/filters include zinc selenide windows, aluminium oxide filters, polycarbonate filters, calcium fluoride windows, and barium fluoride windows. In reflection mode, the IR beam that passes through the sample, reflects off a substrate/filter, and travels back through the sample a second time is measured. Hence, IR-refractive substrates/filters are required. Examples of IR-refractive substrates/filters include the MirrIR Low-E slides, silver membranes, and gold-coated filters.
For the SPERO microscope, the choice of substrate/filter also depends on whether the transmission or reflection mode is employed. In transmission mode, IR transparent substrates/filters are required, while in reflection mode, IR reflective substrates/filters are needed. In the only identified article that used the SPERO microscope for MP analysis, the researchers analysed MPs placed on barium fluoride windows and on aluminium oxide filters (placed on barium fluoride windows) in transmission mode.11 The 8700 LDIR typical works in transflectance mode for MP analysis and only accepts IR reflective glass slides for loading samples. Among the 34 publications that utilised the 8700 LDIR, 11 of them used Kevley/MirrIR IR reflective slides, and 16 used highly refractive slides (although the slide manufacturer was not explicitly specified), while the remaining publications did not specify what slides were used. For those users of the 8700 LDIR using filters to concentrate MPs, they needed to transfer the MPs from the filters to IR refractive glass slides. To achieve this, the filter containing MPs was shaken and sonicated in an ethanol solution to dislodge the MPs. The ethanol solution was then concentrated and dripped onto the slide. After the ethanol evaporated, the MPs on the glass slide were ready for analysis. However, the practice of transferring MPs from filters to IR reflective slides has faced criticism as it might cause particle loss/aggregation. As an alternative, the direct placement of an IR transparent filter containing MPs onto an IR reflective slide has been proposed.83
Rather than measuring residual IR radiation, O-PTIR microscopes measure the sample's intrinsic IR absorption by monitoring changes in the intensity of the visible laser probe. In principle, in the reflection mode, as long as the visible light (the probe) can escape from the sample surface and reach the detector, O-PTIR spectra can be obtained. This process is not significantly influenced by the IR transmissivity or reflectivity of the substrate/filter. Therefore, both IR transparent and IR reflective substrate/filters have been used in MNP studies employing O-PTIR microscopes (in the reflection mode). It is noteworthy that the mIRage microscope operates in reflection mode only, while the mIRage+R microscope can work in transmission mode, where the intensity of the transmitted probe beam is monitored. Currently, the transmission mode of the mIRage+R microscope has not been used in MNP research. Suitable substrates/filters for the transmission mode should allow the probe beam to pass through without altering its intensity. A calcium fluoride window is one of the suitable substrates for transmission O-PTIR spectroscopy;103 however, transferring MPs from filters remains problematic.
An ideal substrate/filter for the AFM-IR technique to detect MNPs should meet the criteria of both AFM and IR: the surface of the substrate/filter should be smooth, and the IR background should be low. Additionally, substrate metallisation has been suggested to play a role in improving the quality of AFM-IR spectra.104 These features of a substrate/filter have a significant impact on the analysis of thin objects; however, their effects would be minimised when analysing relatively thick (>500 nm) objects.105 Among the three studies that used the AFM-IR technique for MNP analysis, Xu et al.12 investigated a 3 mm × 3 mm plastic film, but the thickness of the film and the type of substrate used were not reported; Luo et al.98 studied MPs captured on a filter with a pore size of 0.45 μm, but the filter type was not specified; in another study by Luo et al.,99 plastic films with an equivalent diameter of 3–5 mm and a thickness of about 1 mm were analysed using a silicon wafer as the substrate. However, the effects of substrates/filters were not reported in any of these studies.
Spectral resolution is an important metric for evaluating spectral quality and refers to the ability of an IR instrument to distinguish between bands that are in close proximity. Typically, the spectral resolution can be set to values such as 16 cm−1, 8 cm−1, 4 cm−1, or 2 cm−1. Generally, a higher spectral resolution allows for better representation of small IR bands. However, acquiring high-resolution spectra may lead to longer data acquisition time and larger file sizes. Studies have suggested that a resolution of 8 cm−1 is optimal for MP research using FTIR spectroscopy,106 as it provides the best balance between spectral quality and data acquisition time. Engaging in instrument comparison based on the spectral resolution of spectra generated by different instruments is not applicable. This is because spectral resolution is typically user-defined rather than an inherent instrument attribute.
SNR is also an important metric for evaluating spectral quality. High SNR spectra have clear signals and are easier to interpret. Increasing the number of co-added scans (e.g., 6 scans) has been reported to significantly improve the SNR of the spectrum,106 but this also increases the data collection time. Under equivalent scan numbers, instruments equipped with QCLs exhibit higher SNR compared to the FTIR microscopes. This advantage can be attributed to the inherent high photon flux of QCLs.18 For users of O-PTIR microscopes, the improvement of SNR can be achieved not only by increasing the number of co-added scans but also by elevating the power of the pump (IR) laser and/or the power of the probe laser. However, it is important to be careful when increasing the power of the lasers, as excessive power levels may risk damaging the sample. The SNR of the Nano IR2, which employs an OPO laser as the IR source, is comparatively subdued due to the low repetition rate of the OPO laser.25
The presence of spectral artefacts has been considered a key indicator for evaluating the quality of spectra. When investigating minute substances such as MP particles using the FTIR or QCL-IR technique, the acquired spectra often encompass a diverse range of spectral artefacts. Among these, the most prominent and challenging artefact is the so-called dispersion artefact, primarily associated with resonant Mie scattering.107 This dispersion artefact arises when the size of the sample is comparable to the wavelength of the incident IR light. It compromises the quality of spectra and has two main manifestations: firstly, it results in a broad sinusoidal oscillation in the spectrum's baseline, leading to distortions in both the position and intensity of absorption bands. Secondly, it induces considerable distortions in the shape of spectral bands, notably a derivative-like distortion on the high-wavenumber side of the amide I band. To mitigate the impact of resonant Mie scattering or dispersion artefact, several computational algorithms have been proposed.107,108 Other notable spectral artefacts associated with FTIR spectroscopy or QCL-IR spectroscopy include artefacts arising from the morphology of the sample. Briefly, when analysing samples with rough surfaces in transflectance mode, strong light scattering (due to the rough surface) would occur and disrupt the reflected signal, ultimately resulting in compromised spectra.
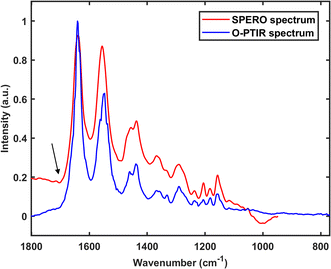
The fundamental cause of these spectral artefacts is the measurement of residual IR radiation by FTIR and QCL-IR instruments, and the residual IR radiation can be interfered with by phenomena such as resonant Mie scattering. On the contrary, the O-PTIR technique and the AFM-IR technique do not measure residual IR radiation, making them immune to such spectral artefacts. Fig. 6 presents an O-PTIR spectrum and a SPERO transmission spectrum (i.e., one collected using the SPERO microscope in transmission mode) of a 10 μm nylon particle. It can be seen that the SPERO spectrum exhibits traces of dispersion artefact, indicated by the presence of a first derivative-like line shape in the vicinity of the carbonyl stretching mode (as indicated by the black arrow in the figure). Additionally, a subtle oscillation of the baseline is discernible. In contrast, the region of the carbonyl stretching mode in the O-PTIR spectrum remains unaffected, and the baseline of the spectrum appears comparatively flat, indicating the immunity of the O-PTIR technique against dispersion artefact.
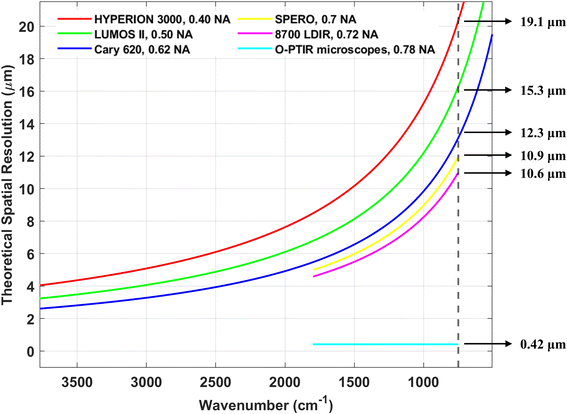
Based on our findings, the reported detection limit for FPA-FTIR is typically in the range of 10 to 20 μm. For FPA detectors with a projected pixel size smaller than the detection limit, “particles” smaller than the detection limit (i.e., particles occupying only one pixel) might be detected. Nevertheless, such “particles” should be treated with caution or excluded from the analysis since they could be false positives. To address this issue, binning is commonly employed by FPA-FTIR users to increase the pixel size to a value greater than the diffraction limit, effectively eliminating these false positives. With the 8700 LDIR, users can set the detection limit themselves. Most users set the detection limit at 20 μm, while a minority set it at 10 μm. The official documentation for the 8700 LDIR states that analysing MPs larger than 20 μm tends to yield relatively reliable results, while the reliability decreases for MPs in the 10 to 20 μm range. However, a recent study has shown that the 8700 LDIR might miss or overlook particles smaller than 60 μm.9 Regarding the SPERO microscope, Primpke et al.11 reported detection of MPs with a size of 4.2 μm (at low magnification) and 1.4 μm (at high magnification), which corresponds to the projected sizes of the FPA detector of the SPERO microscope. However, it is important to note that such small-sized MPs should not be considered reliable results unless tested against binned measurements.11 This is because the fundamental detection limit of the SPERO microscope is 10.9 μm (Fig. 7) due to the diffraction limit of light. In studies using the O-PTIR microscopes, the smallest detected MNP particle size was reported to be 600 nm,13 suggesting that the detection limit of the mIRage/mIRage+R microscope with a 532 nm probe laser is at least 600 nm. As for the Nano IR2, though the theoretical detection limit can reach around 20 nm, the actual detection limit was not explored in the studies covered in this review, as the focus of these studies was on the surface/physical characteristics of relatively large MNPs.
Imaging speed is defined here as the time required to perform hyperspectral imaging on a certain area. The imaging speed of instruments equipped with an FPA detector primarily depends on factors such as spatial resolution, FPA size (e.g., 32 × 32, 64 × 64, 128 × 128), number of scans, and the IR source used. The SPERO microscope benefits from having a large FPA detector (480 × 480) and a QCL, making it the fastest in terms of imaging. It has been reported that the SPERO microscope could image a 144 mm2 area in just 36 minutes with a pixel resolution of 4.2 μm.11 The imaging speeds of the Cary 620 and HYPERION 3000 are slower than the SPERO microscope because they use thermal Globar as the IR radiation source, and their FPA size is only 64 × 64. Following them is the LUMOS II, which has an FPA size of 32 × 32. Dong et al.9 reported that the LUMOS II achieved an imaging speed of 9 minutes per mm2 (spectral resolution of 8 cm−1, no binning, 2 scans). For instruments without an FPA detector, imaging relies on a single-element detector and a motorised stage, resulting in significantly slow speeds. For example, our in-house mIRage O-PTIR microscope requires approximately two full weeks to perform hyperspectral imaging on a 480 μm × 640 μm area (spectral range: 1801–769 cm−1, spectral resolution: 2 cm−1, spatial resolution: 2 μm, number of scans: 5).
Our findings reveal that over half of the FPA-FTIR users used the software siMPle (available for free at https://simple-plastics.eu/) for processing hyperspectral data and polymer identification. siMPle is software designed for automated MP analysis and it is an instance-based machine learning approach.27 This software uses Pearson's correlation coefficient as a metric to gauge the degree of correlation between each sample spectrum and reference spectra. Importantly, there exist three distinct Pearson's correlation coefficients, to which the user assigns global weights (weight raw/weight 1st/weight 2nd). In addition, siMPle is capable of providing information concerning the size of particles. Users could not only fine-tune the global weights to suit their needs but also set thresholds for the identification of particles. Furthermore, the software affords users the flexibility to incorporate external reference spectra into the built-in spectral library. FPA-FTIR users who did not use siMPle for data analysis employed alternative software tools such as ImageLab (Epina GmbH, Austria) in conjunction with a non-commercial, custom-made software tool based on random forest decision classifiers,53,57,62 or Purency Microplastics Finder.9 A few users did not provide detailed descriptions of how they processed the hyperspectral data. The hyperspectral data generated by the SPERO microscope was processed using a custom-written Python script.11
The 8700 LDIR mainly collects point spectra. Users of the 8700 LDIR typically employed the Clarity software (Agilent) for MP identification and characterisation. Clarity is a software tool tied to the 8700 LDIR, and it has an automated particle analysis workflow specifically tailored for MP analysis. In this workflow, a single frequency chemical image at 1800 cm−1 is initially generated, and Clarity counts and measures these particles. Subsequently, the spectra of all particles in this chemical image are obtained and compared to the spectral library automatically. For the library search, Clarity utilises derivative spectral treatment. The software comes with a built-in polymer reference library, and users have the option to import external reference spectra.
Unlike FPA-FTIR or 8700 LDIR users, O-PTIR microscope users do not follow a fixed data processing approach. For point spectra collected by O-PTIR microscopes, users might choose to perform Savitzky–Golay smoothing using the software PTIR studio (Photothermal Spectroscopy Corp)96 before comparing the spectra with spectral libraries for identification purposes. Hyperspectral data acquired using an O-PTIR microscope was processed using an in-house script, which included SNV, correlation coefficient calculation, and independent component analysis, to visualise the distribution of MPs within the sample matrix.26
Users of the Nano IR2 collected point spectra and single frequency images of MPs before and after treatment to elucidate the alterations in the nanoscale IR, thermal, and mechanical properties of the MPs analysed. This process solely involved examining the changes in spectra and single frequency images before and after treatment. Therefore, only basic data processing such as Savitzky–Golay smoothing might be employed.98
The results of the comparison of the IR instruments conducted in this section are summarised in Table 3. In MNP analysis, the distinctive merits of the Cary 620, the HYPERION 3000, and the LUMOS II lie in their ability to accommodate an FPA detector, coupled with their cost-effectiveness, albeit with constraints in spectral quality (low SNR and susceptible to spectral artefacts) and analysis of smaller MPs or NPs. The SPERO microscope shines in its exceptional imaging speed, yet it is associated with a higher price tag, a limited spectral range, and compromises in imaging quality (for details see Section 4). A standout feature of the 8700 LDIR is its intelligent software Clarity, which empowers rapid and automated analysis of MPs. Nonetheless, it is important to note its narrow spectral range, and concerns have arisen regarding the accuracy of this instrument when it is used to analyse MPs smaller than 60 μm.9 The notable feature of the O-PTIR microscopes is the capability to analyse MNP sized down to around 0.5 μm. However, it is essential to recognise that these microscopes are paired with a single-element detector, resulting in a sluggish imaging speed, and they belong to a higher price range. The Nano IR2 can provide a nano-level imaging resolution. Nevertheless, its SNR is low and imaging speed is limited.
| Technique | Instrument | Spectral rangea | Spectral quality | Imaging speedc | Theoretical detection limit | Size of the smallest MNP detectedf | Cost |
|---|---|---|---|---|---|---|---|
| a “Broad” represents a spectral range of ∼3800 cm−1 to ∼900 cm−1, while “Narrow” represents a range of ∼1800 cm−1 to ∼800 cm−1. b The spectral range of an O-PTIR microscope could be extended by either changing the IR source or adding an additional IR source. c Imaging speed is the speed of hyperspectral imaging. d The theoretical detection limit depends on the wavelength of the probe. 0.42 μm corresponds to the use of a 532 nm probe. e The theoretical detection limit depends on the size of the probe tip and is roughly the size of the probe tip. f Including environmental MNPs, and these results are based on the studies covered in this review. | |||||||
| FPA-FTIR | Cary 620 | Broad | Low SNR, susceptible to spectral artefacts | Fast | 12.3 μm | 10–20 μm | ∼$300k |
| HYPERION 3000 | Broad | Low SNR, susceptible to spectral artefacts | Fast | 19.1 μm | 10–20 μm | ∼$350k | |
| LUMOS II | Broad | Low SNR, susceptible to spectral artefacts | Fast | 15.3 μm | 10–20 μm | ∼$200k | |
| QCL-IR | SPERO microscope | Narrow | High SNR, susceptible to spectral artefacts | Very fast | 10.9 μm | 1.4 μm | ∼$500k |
| 8700 LDIR | Narrow | High SNR, susceptible to spectral artefacts | Slow | 10.6 μm | 10–20 μm | ∼$350k | |
| O-PTIR | mIRage microscope | Narrowb | High SNR, immune to spectral artefacts | Slow | 0.42 μmd | 0.6 μm | ∼$500k |
| mIRage+R microscope | Narrowb | High SNR, immune to spectral artefacts | Slow | 0.42 μmd | ∼0.6 μm | ∼$600k | |
| AFM-IR | Nano IR2 | Broad | Low SNR, immune to spectral artefacts | Slow | ∼20 nme | NA | NA |
4. Challenges and recommendations
The monitoring of MNPs in environmental contexts, water supplies, and food safety holds significant importance for both consumers and governments.11 Initially, the analysis of MNPs was inaccurate and labour-intensive. However, with advancements in IR instruments (as well as in other kinds of instruments such as Raman microscopes), MNP analysis has been made easier and even automated. FPA-FTIR microscopes, the SPERO microscope, and the 8700 LDIR are effective in the automated analysis of MPs, with the SPERO microscope and the 8700 LDIR being faster than FPA-FTIR microscopes. Nevertheless, these instruments are affected by the diffraction of light, which means they cannot reliably analyse MPs smaller than 20 μm or 10 μm. To address this challenge, increasing the NA of the IR objective is one approach.Undoubtedly, using a QCL as the IR source, and being equipped with a large FPA detector, the SPERO microscope is super-fast in imaging MP samples. However, it is essential to recognise that the high degree of coherence of the QCL can unavoidably lead to severe image distortions, such as fringes and speckles,20 hindering a meaningful interpretation of hyperspectral images and thereby affecting the reliable analysis of MPs. To mitigate the impact of spatial coherence phenomena, Schönhals et al.20 proposed the use of both a moving and a stationary scatterer/diffuser to reduce the time-averaged spatiotemporal coherence properties of the illumination.
An additional challenge concerning the image quality of the SPERO microscope pertains to the noticeable artefacts at the junctions of hyperspectral tiles, as illustrated in Fig. 8. This figure is composed of the upper hyperspectral tile and the lower hyperspectral tile, and the horizontal midline of the image is where the two tiles meet. It can be seen that the upper hyperspectral tile and the lower hyperspectral tile are not aligned correctly. This misalignment may be attributed to inaccuracies in the motorised stage. This issue can be addressed by manually adjusting the relative positions of the hyperspectral tiles or by fine-tuning the motorised stage. Additionally, from Fig. 8, it is evident that the brightness of the lower edge of the upper hyperspectral tile and the upper edge of the lower hyperspectral tile is inconsistent. The underlying cause of this occurrence could be attributed to the uneven illumination of the FPA, resulting in inconsistent spectral sensitivity across the entire FPA. To rectify this artefact, it is imperative to ensure homogeneous illumination across the FPA.
The O-PTIR microscopes provide submicron-level resolution in the analysis of MNPs, enabling the identification and characterisation of smaller plastic particles. However, their potential for automated analysis of MNPs is limited. Future improvements could involve designing software for the O-PTIR microscopes similar to Clarity.9 Another opportunity for improvement is the development of a large FPA detector for the O-PTIR microscopes.
5. Conclusion
The globally widespread distribution of MNPs is well-documented, encompassing their presence in the air, oceans, organisms, flora, and even within the human body. To comprehensively assess the hazards posed by these emerging pollutants, reliable analytical tools are essential. FTIR spectroscopy, QCL-IR spectroscopy, O-PTIR spectroscopy, and AFM-IR spectroscopy are such reliable analytical tools that can provide precise identification and characterisation of MNP particles. The principles of these IR spectroscopic techniques, the advantages, and disadvantages of relevant instruments in MNP analysis, and recommendations for addressing the limitations of some instruments have been discussed in this review article.It is imperative to acknowledge that the summation of the most advanced IR instruments presented in this review is predicated upon studies published between 2021 and 2023. This snapshot may not holistically encapsulate the latest advancements in the field, as some cutting-edge IR instruments might not have been featured in studies during this specific timeframe.
By gaining an in-depth understanding of the underlying principles of various IR spectroscopic techniques expounded in this review and delineating the merits and limitations of instruments based on these techniques, researchers and practitioners can derive invaluable insights to guide their selection or procurement of IR instruments for MNP research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding for this research was provided by the Science Foundation Ireland (SFI)-Irish Research Council Pathway Programme Proposal ID 21/PATH-S/9290.References
- L. R. Arenas, S. R. Gentile, S. Zimmermann and S. Stoll, Sci. Total Environ., 2021, 791, 148175 CrossRef PubMed.
- L. P. Domínguez-Jaimes, E. I. Cedillo-González, E. Luévano-Hipólito, J. D. Acuña-Bedoya and J. M. Hernández-López, J. Hazard. Mater., 2021, 413, 125452 CrossRef PubMed.
- S. Manzo and S. Schiavo, Sci. Total Environ., 2022, 808, 152105 CrossRef CAS PubMed.
- R. Ullah, M. T.-K. Tsui, A. Chow, H. Chen, C. Williams and A. Ligaba-Osena, Environ. Monit. Assess., 2023, 195, 252 CrossRef CAS PubMed.
- N. P. Mortensen, T. R. Fennell and L. M. Johnson, NanoImpact, 2021, 21, 100302 CrossRef CAS PubMed.
- H. A. Leslie, M. J. Van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Environ. Int., 2022, 163, 107199 CrossRef CAS PubMed.
- Y. Deng, Y. Zhang, B. Lemos and H. Ren, Sci. Rep., 2017, 7, 46687 CrossRef PubMed.
- A. Faltynkova, G. Johnsen and M. Wagner, Microplastics and Nanoplastics, 2021, 1, 1–19 CrossRef.
- M. Dong, Z. She, X. Xiong, G. Ouyang and Z. Luo, Anal. Bioanal. Chem., 2022, 414, 3359–3372 CrossRef CAS PubMed.
- Y. Chen, D. Wen, J. Pei, Y. Fei, D. Ouyang, H. Zhang and Y. Luo, Current Opinion in Environmental Science & Health, 2020, 18, 14–19 Search PubMed.
- S. Primpke, M. Godejohann and G. Gerdts, Environ. Sci. Technol., 2020, 54, 15893–15903 CrossRef CAS PubMed.
- S. Xu, C. Wang, P. Zhu, D. Zhang and X. Pan, J. Hazard. Mater., 2022, 440, 129773 CrossRef CAS PubMed.
- Y. Su, X. Hu, H. Tang, K. Lu, H. Li, S. Liu, B. Xing and R. Ji, Nat. Nanotechnol., 2022, 17, 76–85 CrossRef CAS PubMed.
- G. Renner, T. C. Schmidt and J. Schram, in Comprehensive Analytical Chemistry, Elsevier, 2017, vol. 75, pp. 67–118 Search PubMed.
- B. Man Thaiba, T. Sedai, S. Bastakoti, A. Karki, K. C. Anuradha, G. Khadka, S. Acharya, B. Kandel, B. Giri and B. Bhakta Neupane, Arabian J. Chem., 2023, 16, 104686 CrossRef CAS.
- J.-L. Xu, K. V. Thomas, Z. Luo and A. A. Gowen, TrAC, Trends Anal. Chem., 2019, 119, 115629 CrossRef CAS.
- S. Primpke, C. Lorenz, R. Rascher-Friesenhausen and G. Gerdts, Anal. Methods, 2017, 9, 1499–1511 RSC.
- A. Ogunleke, V. Bobroff, H.-H. Chen, J. Rowlette, M. Delugin, B. Recur, Y. Hwu and C. Petibois, TrAC, Trends Anal. Chem., 2017, 89, 190–196 CrossRef CAS.
- C. Krafft, in Molecular and Laser Spectroscopy, ed. V. P. Gupta, Elsevier, 2022, pp. 305–336, DOI:10.1016/B978-0-323-91249-5.00007-7.
- A. Schönhals, N. Kröger-Lui, A. Pucci and W. Petrich, J. Biophot., 2018, 11, e201800015 CrossRef PubMed.
- J. Reffner, Spectroscopy, 2018, 33, 12–17 CAS.
- M. Kansiz, C. Prater, E. Dillon, M. Lo, J. Anderson, C. Marcott, A. Demissie, Y. Chen and G. Kunkel, Microsc. Today, 2020, 28, 26–36 CrossRef CAS PubMed.
- J. S. Böke, J. Popp and C. Krafft, Sci. Rep., 2022, 12, 18785 CrossRef PubMed.
- J. R. Felts, K. Kjoller, M. Lo, C. B. Prater and W. P. King, ACS Nano, 2012, 6, 8015–8021 CrossRef CAS PubMed.
- A. Dazzi and C. B. Prater, Chem. Rev., 2017, 117, 5146–5173 CrossRef CAS PubMed.
- Y. Shi, L. Yi, G. Du, X. Hu and Y. Huang, Sci. Total Environ., 2023, 862, 160714 CrossRef CAS PubMed.
- S. R. Moses, L. Roscher, S. Primpke, B. Hufnagl, M. G. Löder, G. Gerdts and C. Laforsch, Anal. Bioanal. Chem., 2023, 1–13 Search PubMed.
- B. Hufnagl, M. Stibi, H. Martirosyan, U. Wilczek, J. N. Möller, M. G. Löder, C. Laforsch and H. Lohninger, Environ. Sci. Technol. Lett., 2021, 9, 90–95 CrossRef PubMed.
- L. M. Hernandez, E. G. Xu, H. C. Larsson, R. Tahara, V. B. Maisuria and N. Tufenkji, Environ. Sci. Technol., 2019, 53, 12300–12310 CrossRef CAS PubMed.
- K. Busse, I. Ebner, H.-U. Humpf, N. Ivleva, A. Kaeppler, B. E. Oßmann and D. Schymanski, Environ. Sci. Technol., 2020, 54, 14134–14135 CrossRef CAS PubMed.
- N. Rakovitsky, S. Frenk, H. Kon, D. Schwartz, E. Temkin, E. Solter, S. Paikin, R. Cohen, M. J. Schwaber and Y. Carmeli, J. Clin. Microbiol., 2020, 58(5), e00098 CrossRef CAS PubMed.
- R. Salzer, Anal. Bioanal. Chem., 2008, 391, 2379–2380 CrossRef CAS.
- J. A. Reffner, Microsc. Today, 1993, 1, 6–7 CrossRef.
- E. N. Lewis, P. J. Treado, R. C. Reeder, G. M. Story, A. E. Dowrey, C. Marcott and I. W. Levin, Anal. Chem., 1995, 67, 3377–3381 CrossRef CAS PubMed.
- S. M. Mintenig, I. Int-Veen, M. G. Löder, S. Primpke and G. Gerdts, Water Res., 2017, 108, 365–372 CrossRef CAS PubMed.
- L. Feld, V. H. d. Silva, F. Murphy, N. B. Hartmann and J. Strand, Water, 2021, 13, 2097 CrossRef CAS.
- J. Weisser, I. Beer, B. Hufnagl, T. Hofmann, H. Lohninger, N. P. Ivleva and K. Glas, Water, 2021, 13, 841 CrossRef CAS.
- I. V. Kirstein, F. Hensel, A. Gomiero, L. Iordachescu, A. Vianello, H. B. Wittgren and J. Vollertsen, Water Res., 2021, 188, 116519 CrossRef CAS PubMed.
- Y. Liu, C. Lorenz, A. Vianello, K. Syberg, A. H. Nielsen, T. G. Nielsen and J. Vollertsen, Sci. Total Environ., 2023, 865, 161255 CrossRef CAS PubMed.
- A. F. dos Santos Queiroz, A. S. da Conceição, D. Chelazzi, M. Rollnic, A. Cincinelli, T. Giarrizzo and J. E. Martinelli Filho, Sci. Total Environ., 2022, 839, 156259 CrossRef PubMed.
- E. Uurasjärvi, M. Pääkkönen, O. Setälä, A. Koistinen and M. Lehtiniemi, Environ. Pollut., 2021, 268, 115700 CrossRef PubMed.
- K. Gunaalan, R. Almeda, C. Lorenz, A. Vianello, L. Iordachescu, K. Papacharalampos, C. M. R. Kiær, J. Vollertsen and T. G. Nielsen, Environ. Pollut., 2023, 318, 120853 CrossRef CAS PubMed.
- C. Scopetani, D. Chelazzi, T. Martellini, J. Pellinen, A. Ugolini, C. Sarti and A. Cincinelli, Mar. Pollut. Bull., 2021, 171, 112712 CrossRef CAS PubMed.
- C.-G. Pan, S. M. Mintenig, P. E. Redondo-Hasselerharm, P. H. Neijenhuis, K.-F. Yu, Y.-H. Wang and A. A. Koelmans, Environ. Sci. Technol., 2021, 55, 9916–9925 CrossRef CAS PubMed.
- S. Saarni, S. Hartikainen, S. Meronen, E. Uurasjärvi, M. Kalliokoski and A. Koistinen, Environ. Pollut., 2021, 274, 116568 CrossRef CAS PubMed.
- A. Cincinelli, C. Scopetani, D. Chelazzi, T. Martellini, M. Pogojeva and J. Slobodnik, Sci. Total Environ., 2021, 760, 143898 CrossRef CAS PubMed.
- F. Corradini, F. Casado, V. Leiva, E. Huerta-Lwanga and V. Geissen, Sci. Total Environ., 2021, 752, 141917 CrossRef CAS PubMed.
- R. Chand, L. A. Rasmussen, S. Tumlin and J. Vollertsen, Sci. Total Environ., 2021, 798, 149287 CrossRef CAS PubMed.
- E. Uurasjärvi, E. Sainio, O. Setälä, M. Lehtiniemi and A. Koistinen, Environ. Pollut., 2021, 288, 117780 CrossRef PubMed.
- T. Pegado, L. Brabo, K. Schmid, F. Sarti, T. T. Gava, J. Nunes, D. Chelazzi, A. Cincinelli and T. Giarrizzo, Mar. Pollut. Bull., 2021, 162, 111799 CrossRef CAS PubMed.
- L. Wilkie Johnston, E. Bergami, E. Rowlands and C. Manno, R. Soc. Open Sci., 2023, 10, 221421 CrossRef PubMed.
- L. A. Rasmussen, L. Iordachescu, S. Tumlin and J. Vollertsen, Water Res., 2021, 201, 117307 CrossRef CAS PubMed.
- S. Kernchen, M. G. Löder, F. Fischer, D. Fischer, S. R. Moses, C. Georgi, A. C. Nölscher, A. Held and C. Laforsch, Sci. Total Environ., 2022, 818, 151812 CrossRef CAS PubMed.
- J.-W. Jung, S. Kim, Y.-S. Kim, S. Jeong and J. Lee, Sci. Total Environ., 2022, 825, 154015 CrossRef CAS PubMed.
- L. Roscher, A. Fehres, L. Reisel, M. Halbach, B. Scholz-Böttcher, M. Gerriets, T. H. Badewien, G. Shiravani, A. Wurpts and S. Primpke, Environ. Pollut., 2021, 288, 117681 CrossRef CAS PubMed.
- L. Roscher, M. Halbach, M. T. Nguyen, M. Hebeler, F. Luschtinetz, B. M. Scholz-Böttcher, S. Primpke and G. Gerdts, Sci. Total Environ., 2022, 817, 152619 CrossRef CAS PubMed.
- B. V. Kumar, L. A. Löschel, H. K. Imhof, M. G. Löder and C. Laforsch, Environ. Pollut., 2021, 269, 116147 CrossRef PubMed.
- S. M. Abel, S. Primpke, I. Int-Veen, A. Brandt and G. Gerdts, Environ. Pollut., 2021, 269, 116095 CrossRef CAS PubMed.
- K. Sipps, G. Arbuckle-Keil, R. Chant, N. Fahrenfeld, L. Garzio, K. Walsh and G. Saba, Sci. Total Environ., 2022, 817, 152812 CrossRef CAS PubMed.
- Y.-I. Kim, E. Jeong, J.-Y. Lee, R. W. Chia and M. Raza, Environ. Res., 2023, 226, 115682 CrossRef CAS PubMed.
- M. K. de Guzman, M. Andjelković, V. Jovanović, J. Jung, J. Kim, L. A. Dailey, A. Rajković, B. De Meulenaer and T. Ć. Veličković, Mar. Pollut. Bull., 2022, 181, 113846 CrossRef CAS PubMed.
- A. Jakobs, E. Gürkal, J. N. Möller, M. G. Löder, C. Laforsch and T. Lueders, Sci. Total Environ., 2023, 857, 159610 CrossRef CAS PubMed.
- X. Zhang, H. Wang, S. Peng, J. Kang, Z. Xie, R. Tang, Y. Xing, Y. He, H. Yuan, C. Xie and Y. Liu, Front. Public Health, 2022, 10, 1005535 CrossRef PubMed.
- P. Liu, L. Shao, Y. Li, T. Jones, Y. Cao, C. X. Yang, M. Zhang, M. Santosh, X. Feng and K. BéruBé, Sci. Total Environ., 2022, 838, 155989 CrossRef CAS PubMed.
- E. Lim, H. Tanaka, Y. Ni, Y. Bai and K. Ito, Japan Architectural Review, 2022, 5, 682–690 CrossRef.
- N. A. Forster, S. C. Wilson and M. K. Tighe, J. Environ. Manage., 2023, 331, 117304 CrossRef CAS PubMed.
- J. Nizamali, S. M. Mintenig and A. A. Koelmans, J. Hazard. Mater., 2023, 441, 129942 CrossRef CAS.
- L. Huan, Z. Long, M. Mindong, W. Haiwen, A. Lihui and Y. Zhanhong, Sci. Total Environ., 2023, 867, 161553 CrossRef PubMed.
- S. Samandra, O. J. Mescall, K. Plaisted, B. Symons, S. Xie, A. V. Ellis and B. O. Clarke, Sci. Total Environ., 2022, 837, 155329 CrossRef CAS PubMed.
- H. Mu, Y. Wang, H. Zhang, F. Guo, A. Li, S. Zhang, S. Liu and T. Liu, Sci. Total Environ., 2022, 839, 156318 CrossRef CAS PubMed.
- S. Samandra, J. M. Johnston, J. E. Jaeger, B. Symons, S. Xie, M. Currell, A. V. Ellis and B. O. Clarke, Sci. Total Environ., 2022, 802, 149727 CrossRef CAS PubMed.
- P. S. Bäuerlein, R. C. H. M. Hofman-Caris, E. N. Pieke and T. L. ter Laak, Water Res., 2022, 221, 118790 CrossRef PubMed.
- X. Tian, F. Beén and P. S. Bäuerlein, Environ. Res., 2022, 212, 113569 CrossRef CAS PubMed.
- L. Mughini-Gras, R. Q. J. van der Plaats, P. W. J. J. van der Wielen, P. S. Bauerlein and A. M. de Roda Husman, Water Res., 2021, 192, 116852 CrossRef CAS PubMed.
- L. An, T. Cui, Y. Zhang and H. Liu, Sci. Total Environ., 2022, 847, 157461 CrossRef CAS PubMed.
- J. Wu, Q. Ye, L. Sun, J. Liu, M. Huang, T. Wang, P. Wu and N. Zhu, Sci. Total Environ., 2023, 879, 163066 CrossRef CAS PubMed.
- Q. T. Whiting, K. F. O'Connor, P. M. Potter and S. R. Al-Abed, Anal. Bioanal. Chem., 2022, 414, 8353–8364 CrossRef CAS PubMed.
- Y. Fan, J. Zheng, L. Deng, W. Rao, Q. Zhang, T. Liu and X. Qian, Water Res., 2022, 212, 118116 CrossRef CAS PubMed.
- M. Bao, Q. Huang, Z. Lu, F. Collard, M. Cai, P. Huang, Y. Yu, S. Cheng, L. An, A. Wold and G. W. Gabrielsen, Environ. Sci. Pollut. Res., 2022, 29, 56525–56534 CrossRef CAS PubMed.
- Y. Tian, Z. Chen, J. Zhang, Z. Wang, Y. Zhu, P. Wang, T. Zhang, J. Pu, H. Sun and L. Wang, J. Hazard. Mater., 2021, 407, 124861 CrossRef CAS PubMed.
- P. S. Bäuerlein, E. N. Pieke, F. I. H. M. Oesterholt, T. Ter Laak and S. A. E. Kools, Water Sci. Technol., 2023, 87, 39–56 CrossRef PubMed.
- L. Deng, H. Xi, C. Wan, L. Fu, Y. Wang and C. Wu, J. Hazard. Mater., 2023, 451, 131199 CrossRef CAS PubMed.
- M. Ourgaud, N. N. Phuong, L. Papillon, C. Panagiotopoulos, F. Galgani, N. Schmidt, V. Fauvelle, C. Brach-Papa and R. Sempéré, Environ. Sci. Technol., 2022, 56, 9999–10009 CrossRef CAS PubMed.
- L. Hildebrandt, F. El Gareb, T. Zimmermann, O. Klein, A. Kerstan, K. C. Emeis and D. Pröfrock, Environ. Pollut., 2022, 307, 119547 CrossRef CAS PubMed.
- X. Lu, H. He, Y. Wang, Y. Guo and X. Fei, J. Hazard. Mater., 2023, 445, 130542 CrossRef CAS PubMed.
- N. Liu, S. Cheng, X. Wang, Z. Li, L. Zheng, Y. Lyu, X. Ao and H. Wu, Water Res., 2022, 226, 119293 CrossRef CAS PubMed.
- W. Jia, A. Karapetrova, M. Zhang, L. Xu, K. Li, M. Huang, J. Wang and Y. Huang, Sci. Total Environ., 2022, 844, 156853 CrossRef CAS PubMed.
- Q. Li, A. Zeng, X. Jiang and X. Gu, J. Hazard. Mater., 2021, 412, 125164 CrossRef CAS PubMed.
- Y. Zhang, Y. Peng, C. Peng, P. Wang, Y. Lu, X. He and L. Wang, Environ. Sci. Technol., 2021, 55, 13802–13811 CrossRef CAS PubMed.
- A. López-Rosales, J. Andrade, V. Fernández-González, P. López-Mahía and S. Muniategui-Lorenzo, Mar. Pollut. Bull., 2022, 178, 113591 CrossRef PubMed.
- X. Zhang, Y. He, Z. Xie, S. Peng, C. Xie, H. Wang, L. Liu, J. Kang, H. Yuan and Y. Liu, Medicine, 2022, 101, E30215 CrossRef CAS PubMed.
- L. Zhu, J. Zhu, R. Zuo, Q. Xu, Y. Qian and L. An, Sci. Total Environ., 2023, 856, 159060 CrossRef CAS PubMed.
- S. Liu, G. Lin, X. Liu, R. Yang, H. Wang, Y. Sun, B. Chen and R. Dong, Sci. Total Environ., 2023, 854, 158699 CrossRef CAS PubMed.
- L. Qiu, W. Lu, C. Tu, X. Li, H. Zhang, S. Wang, M. Chen, X. Zheng, Z. Wang, M. Lin, Y. Zhang, C. Zhong, S. Li, Y. Liu, J. Liu and Y. Zhou, Environ. Sci. Technol., 2023, 57, 2435–2444 CrossRef CAS PubMed.
- S. Huang, X. Huang, R. Bi, Q. Guo, X. Yu, Q. Zeng, Z. Huang, T. Liu, H. Wu, Y. Chen, J. Xu, Y. Wu and P. Guo, Environ. Sci. Technol., 2022, 56, 2476–2486 CrossRef CAS PubMed.
- C. K. Chen, J. Zhang, A. Bhingarde, T. Matotek, J. Barrett, B. D. Hardesty, M. M. Banaszak Holl and B. L. Khoo, Chem. Eng. J., 2022, 428, 132614 CrossRef CAS.
- F. Yan, X. Wang, H. Sun, Z. Zhu, W. Sun, X. Shi, J. Zhang, L. Zhang, X. Wang, M. Liu, M. Cai and Y. Zhang, Front. Mar. Sci., 2022, 9, 845062 CrossRef.
- H. Luo, Y. Zeng, Y. Zhao, Y. Xiang, Y. Li and X. Pan, J. Hazard. Mater., 2021, 413, 125342 CrossRef CAS PubMed.
- H. Luo, Y. Xiang, Y. Li, Y. Zhao and X. Pan, J. Hazard. Mater., 2021, 404, 124159 CrossRef CAS PubMed.
- M. G. Löder, H. K. Imhof, M. Ladehoff, L. A. Löschel, C. Lorenz, S. Mintenig, S. Piehl, S. Primpke, I. Schrank and C. Laforsch, Environ. Sci. Technol., 2017, 51, 14283–14292 CrossRef PubMed.
- S. Wang, X. Tan, Y. Wu, J. Zhang, Z. Tian and J. Ma, J. Hazard. Mater., 2024, 463, 132840 CrossRef CAS PubMed.
- M. Kohantorabi, G. Moussavi and S. Giannakis, Chem. Eng. J., 2021, 411, 127957 CrossRef CAS.
- A. Spadea, J. Denbigh, M. J. Lawrence, M. Kansiz and P. Gardner, Anal. Chem., 2021, 93, 3938–3950 CrossRef CAS PubMed.
- J. Chae, S. An, G. Ramer, V. Stavila, G. Holland, Y. Yoon, A. A. Talin, M. Allendorf, V. A. Aksyuk and A. Centrone, Nano Lett., 2017, 17, 5587–5594 CrossRef CAS PubMed.
- S. Rizevsky, K. Zhaliazka, T. Dou, M. Matveyenka and D. Kurouski, J. Phys. Chem. C, 2022, 126, 4157–4162 CrossRef CAS PubMed.
- M. G. J. Löder, M. Kuczera, S. Mintenig, C. Lorenz and G. Gerdts, Environ. Chem., 2015, 12, 563–581 CrossRef.
- P. Bassan, H. J. Byrne, F. Bonnier, J. Lee, P. Dumas and P. Gardner, Analyst, 2009, 134, 1586–1593 RSC.
- A. Kohler, J. Sule-Suso, G. Sockalingum, M. Tobin, F. Bahrami, Y. Yang, J. Pijanka, P. Dumas, M. Cotte and D. Van Pittius, Appl. Spectrosc., 2008, 62, 259–266 CrossRef CAS PubMed.
- S. Primpke, R. K. Cross, S. M. Mintenig, M. Simon, A. Vianello, G. Gerdts and J. Vollertsen, Appl. Spectrosc., 2020, 74, 1127–1138 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |