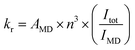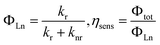Thermally stable and strongly emitted CPL in Eu(D-facam)3 hybrid solids with an alkylammonium salt†
Ziying
Li
 ,
Kazuki
Nakamura
,
Kazuki
Nakamura
 and
Norihisa
Kobayashi
and
Norihisa
Kobayashi
 *
*
Graduate School of Engineering, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba 263-8522, Japan. E-mail: koban@faculty.chiba-u.jp
First published on 29th November 2022
Abstract
A novel europium-based hybrid material, Eu(D-facam)3-TMAOAc (tetramethylammonium acetate), with ultra-high luminescence, excellent circular polarization and remarkable thermostability was prepared. Its photophysical performance was studied based on the luminescence properties and energy transfer process. Compared to Eu(D-facam)3, Eu(D-facam)3-TMAOAc exhibited much brighter luminescence and stronger circular polarization. Additionally, Eu(D-facam)3-TMAOAc well retained its structure and luminescence properties even after heat treatment at 200 °C for 24 hours, whereas Eu(D-facam)3 rapidly decomposed. Eu(D-facam)3-TMAOAc was characterized by TG analysis, elemental analysis, ESI-mass spectrometry, PXRD, and FT-IR spectroscopy. It was found that TMAOAc acted as a bidentate bridge linker with Eu(D-facam)3 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. This coordination structure contributed to the excellent photophysical properties and thermal stability of Eu(D-facam)3-TMAOAc. Furthermore, Eu(D-facam)3-TMAOAc showed a high solubility in common organic solvents, and it could maintain its outstanding luminescence properties in solid as well as solution states.
1 ratio. This coordination structure contributed to the excellent photophysical properties and thermal stability of Eu(D-facam)3-TMAOAc. Furthermore, Eu(D-facam)3-TMAOAc showed a high solubility in common organic solvents, and it could maintain its outstanding luminescence properties in solid as well as solution states.
Introduction
Trivalent lanthanide complexes have attracted considerable attention owing to their characteristic luminescence properties including sharp and abundant emission lines, long emission lifetime, and large pseudo-Stokes shifts for high transparency in the visible light range. They are promising materials in widespread applications such as bio-probing, bio-sensing1–6 and electroluminescent devices.7–10 These complexes are also expected to act as potential circularly polarized luminescence (CPL) luminophores. CPL spectroscopy measures the differential emission intensity of the right and left circularly polarized light, and thus reflects the excited state of chiral luminophores.11 At a certain particular emission wavelength, the dissymmetry factor of CPL (glum) is defined as 2(IL − IR)/(IL + IR), where IL (IR) is the intensity of the left (right) circularly polarized luminescence. Chiral lanthanide complexes are potential candidates for CPL luminophores because they show high values of glum, two orders of magnitude greater than those observed in organic luminophores.11 The interest in CPL has significantly increased with the development of highly sensitive bioassays12,13 and high-resolution 3D displays.9,14,15 Despite most of these applications preferring solid-state luminophores with high CPL activities, there are few solid-state complexes with CPL reported16,17 (S1, ESI†). In addition, materials with both high luminescence and circular polarization are rarely reported even in the solution state. Moreover, the poor thermal stability of lanthanide complexes also significantly limits their practical applications.18,19 The preparation of a highly luminescent material with effective CPL and outstanding thermostability is therefore of primary importance.In recent decades, hybrid lanthanide materials such as coordination polymers,20,21 metal–organic frameworks,22–24 and nanocomposites,25,26 have become a focus of considerable research interest. This is because lanthanide-based hybrid materials have a better emission output and thermal stability than single lanthanide complexes owing to their hybridized structures.18 In these lanthanide hybrid systems, carboxylate compounds are widely used as a linkage between lanthanide ions,5,18,21,22 because lanthanide ions are hard Lewis acids and tend to interact with hard anions such as carbonate and carboxylate ions.27
In our previous study, the Eu(III) complex (Eu(D-facam)3) was observed to exhibit outstanding luminescence performance in the presence of tetramethylammonium acetate (TMAOAc) in alcohol.28,29 It was found that the interaction between Eu(D-facam)3 and TMAOAc contributed to a significant improvement in the luminescence properties of Eu(D-facam)3 in solution. However, solid systems which toward device fabrication and practical applications of this excellent circularly polarized luminescent Eu(III) material have not been investigated yet. In this study, a new Eu(III) hybrid material, Eu(D-facam)3-TMAOAc, was successfully obtained in a solid state from Eu(D-facam)3/TMAOAc mixed solutions. It was revealed that TMAOAc acts as a bidentate bridge with a function of linking Eu3+ ions, leading to a chain structure similar to that of coordination polymers. The photophysical properties, thermal behaviours, and coordination structures of Eu(D-facam)3-TMAOAc were investigated in detail. This Eu(III) hybrid material exhibited outstanding luminescence properties, remarkable superiority in CPL activity and thermal stability in the solid state, showing its considerable potential for practical CPL applications.
Experimental section
Materials
All chemicals were commercially available and were used as received. Europium tris[3-(trifluoromethylhydroxymethylene)-(+)-camphorate] (Eu(D-facam)3) and tetramethylammonium acetate (TMAOAc) were purchased from Sigma-Aldrich, Japan. The solvents, 1-butanol and acetone, were purchased from Tokyo Chemical Industry Co. Ltd, Japan.Measurement and characterization
Oxygen dissolved in the Eu(III) compound solution was removed by bubbling nitrogen gas through the solution before carrying out optical measurements. The absorption and reflectance spectra were acquired using a UV-visible/NIR spectrophotometer (V-770, JASCO Corporation, Japan). The absorption and circular dichroism (CD) spectra of Eu(D-facam)3-TMAOAc solutions were acquired using a circular dichroism spectrometer (J-1100, JASCO Corporation, Japan). The photoluminescence spectra were acquired using a spectrofluorometer (FP-6800, JASCO Corporation, Japan). The circularly polarized luminescence (CPL) measurements were conducted using a previously reported system.30 This system consists of the following components: a 375 nm LED (M365L2, Thorlabs Japan Inc., Japan), an LED driver (DC2100, Thorlabs Japan Inc., Japan), a photoelastic modulator (PEM-90, Hinds Instruments Inc., United States), a photomultiplier tube (H7732-10, Hamamatsu Photonics K. K., Japan), a linearly polarized cubic prism (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), a photomultiplier tube (H7732-10, Hamamatsu Photonics K. K., Japan), and a dual-phase DSP (digital signal processing) lock-in amplifier (7265, Signal Recovery Ltd, United Kingdom). The appropriate detection wavelengths of the monochromator and the PEM (photoelastic modulator) were controlled using a PC (Dell D11M). Thermogravimetric analysis (TG) spectra were acquired using a thermogravimetry/differential thermal analyzer (TG/DTA6300, Seiko Instruments Inc., Japan). Elemental analysis was performed using a CHN/O/S elemental analyzer (CE-440F, Exeter Analytical, Inc., United States). Electrospray ionization (ESI) mass spectra were acquired using a hybrid ion trap mass spectrometer (LTQ Orbital XL, Thermo Fisher Scientific, United States). Fourier-transform infrared (FT-IR) spectra were obtained using a FT-IR spectrometer (FT/IR 680, JASCO Corporation, Japan). Powder diffraction (PXRD) spectra were acquired using a powder X-ray diffractometer (D8 ADVANCE, Bruker AXS, United States).
1), a photomultiplier tube (H7732-10, Hamamatsu Photonics K. K., Japan), and a dual-phase DSP (digital signal processing) lock-in amplifier (7265, Signal Recovery Ltd, United Kingdom). The appropriate detection wavelengths of the monochromator and the PEM (photoelastic modulator) were controlled using a PC (Dell D11M). Thermogravimetric analysis (TG) spectra were acquired using a thermogravimetry/differential thermal analyzer (TG/DTA6300, Seiko Instruments Inc., Japan). Elemental analysis was performed using a CHN/O/S elemental analyzer (CE-440F, Exeter Analytical, Inc., United States). Electrospray ionization (ESI) mass spectra were acquired using a hybrid ion trap mass spectrometer (LTQ Orbital XL, Thermo Fisher Scientific, United States). Fourier-transform infrared (FT-IR) spectra were obtained using a FT-IR spectrometer (FT/IR 680, JASCO Corporation, Japan). Powder diffraction (PXRD) spectra were acquired using a powder X-ray diffractometer (D8 ADVANCE, Bruker AXS, United States).
Preparation of Eu(D-facam)3-TMAOAc
A solution of Eu(D-facam)3 (89.37 mg, 0.1 mmol) in 1-butanol (10 ml, 10 mmol L−1) was added to a solution of TMAOAc (66.60 mg, 0.5 mmol) in 1-butanol (10 ml, 50 mmol L−1). Eu(D-facam)3 is a yellow powder, and its highly concentrated (>5 mmol L−1) 1-butanol solution shows a pale-yellow colour. By addition of TMAOAc solution, the yellow colour of Eu(D-facam)3 solution quickly disappeared and the solution became transparent. A white precipitate was observed in the transparent mixed solution only within 1 h after mixing. The mixture solution without stirring was set aside for 48 h, and the white precipitate was filtered, thoroughly washed with deionized water, and allowed to dry in vacuo.Results and discussion
Luminescence performance of Eu(D-facam)3-TMAOAc
The absorption spectra of Eu(D-facam)3 and the Eu(D-facam)3/TMAOAc mixed solution were recorded, and they are shown in Fig. 1. The concentration of Eu(D-facam)3 was fixed at 0.5 mmol L−1 for fair comparison. The absorbance peaks at approximately 300 nm were ascribed to π–π* transition of ligands and were not affected by adding TMAOAc. At high concentration (10 mmol L−1) of Eu(III) compounds, colour change of solution was directly observed on addition of TMAOAc. As shown in the Fig. 1 inset, a tiny peak in both solutions at 465 nm was derived from 5D2 ← 7F0 transition.31 In the spectra of Eu(D-facam)3 solution, a broad band appeared around 400 nm∼ owing to the observed yellow colour. On the other hand, in the spectra of the Eu(D-facam)3/TMAOAc mixed solution, a lower absorption band was observed at the same wavelength, indicating that the solution was colourless and transparent. The colour bleaching that occurred in the solution suggested a rapid interaction between Eu(D-facam)3 and TMAOAc in 1-butanol. The alteration of colour in both solution and solid systems confirmed the successful fabrication of the new Eu(III) hybrid material, Eu(D-facam)3-TMAOAc.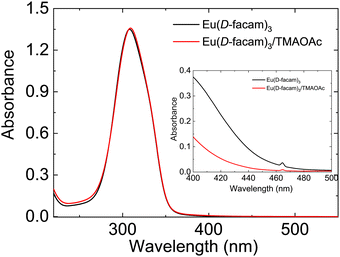 | ||
| Fig. 1 Absorption spectra of Eu(D-facam)3 and the Eu(D-facam)3/TMAOAc mixed solution in 1-butanol. The concentration was fixed to 0.5 mmol L−1 and 10 mmol L−1 (inset). | ||
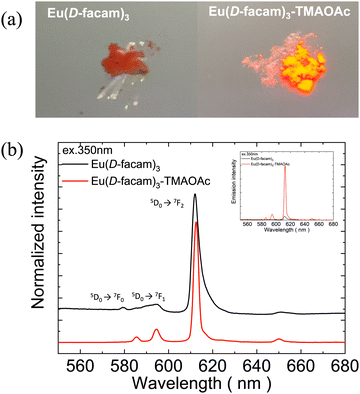
Fig. 2(a) shows digital photographs of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc upon irradiation with a UV lamp (λ = 365 nm). Clearly, Eu(D-facam)3-TMAOAc exhibited a much stronger red luminescence than that of Eu(D-facam)3. In order to characterize the luminescence properties of Eu(D-facam)3-TMAOAc, normalized emission spectra of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc in the solid state were obtained (Fig. 2(b)). The original emission spectra (inset in Fig. 2(b)) of Eu(D-facam)3-TMAOAc exhibited a dramatic increase compared to Eu(D-facam)3 spectra, which is in agreement with the visually observed luminescence shown in Fig. 2(a). The characteristic bands of the Eu(III) complex corresponding to the 5D0 → 7FJ (J = 0–3) transitions are observed at approximately 575, 595, 612 and 650 nm. 5D0 → 7F0 transition was reported as an indicator for Eu(III) complexes in specific symmetry systems.32–34 The emission peak derived from 5D0 → 7F0 transition in Eu(D-facam)3 cannot be observed when Eu(D-facam)3 is coordinated with TMAOAc, indicating that Eu(D-facam)3-TMAOAc has a different symmetry structure from Eu(D-facam)3. 5D0 → 7F1 is a magnetic dipole (MD) transition, and its radiative rate is independent of the environment around Eu3+ ions, and the shape of the emission band directly reflects the crystal-field splitting of the 7F1 level.31 An obvious split of the emission peak related to the 5D0 → 7F1 transitions in Eu(D-facam)3-TMAOAc was observed, suggesting that TMAOAc perturbed the crystal fields of Eu(III) complexes. In addition, 5D0 → 7F2 transitions are known to be typical electric dipole (ED) transitions that are highly sensitive to the environment around Eu3+ ion and the nature of ligands. The dominant emission peaks derived from 5D0 → 7F2 transitions became narrower and sharper in Eu(D-facam)3-TMAOAc than in Eu(D-facam)3, accounting for the changed coordination structure of Eu(III) complexes caused by interactions with TMAOAc. According to the Judd–Ofelt theory, the hypersensitive 5D0 → 7F2 transition is strictly forbidden for Eu3+ ions with a centre of symmetry, and, thus, a low-site symmetry around Eu3+ ions typically exhibits a more intense emission peak derived from 5D0 → 7F2 transition.31 Moreover, the emission intensity from the 5D0 → 7F1 transition remains constant in the total integrated intensity and normally it is used as an ‘internal reference’.35,36 The site symmetry can thus be evaluated from the intensity ratio (Irel) of the integrated intensities from 5D0 → 7F2 to 5D0 → 7F1 transition.31 The values of Irel for Eu(D-facam)3 and Eu(D-facam)3-TMAOAc were calculated to be 10.58 and 6.88, respectively, implying that the interaction between the Eu(III) complex and TMAOAc contributed to a higher site symmetry around Eu3+ ions in this new Eu(III) hybrid material. These changes in emission spectra could be attributed to the rearrangement of the coordination geometry in Eu(III) complexes.
The circularly polarized luminescence (CPL) of Eu(III) complexes is largely influenced by their coordination geometries. CPL measurements of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc were performed in a solid state (in KBr pellets). The dissymmetry factor, glum, was used to quantitatively evaluate the CPL activity of both Eu(III) compounds as shown in Fig. 3. The value of glum was calculated from 2(IL − IR)/(IL + IR), where IL (IR) is the intensity of the left (right) circularly polarized luminescence. Therefore, glum can be used to evaluate the emission ratio of left or right CPL. Characteristic CPL signals were observed at approximately 585–605 and 610–620 nm, corresponding to the emissions from 5D0 → 7F1 and 5D0 → 7F2 transition, respectively. For 5D0 → 7F1 MD transition, a high glum of −0.21 from Eu(D-facam)3-TMAOAc was observed, which was more than four times higher than that of Eu(D-facam)3 (glum = −0.05). The CPL properties of Eu(D-facam)3 in KBr pellets, as observed in this study, agreed well with those in previous reports.17 For the 5D0 → 7F2 electronic transition, a CPL signal of Eu(D-facam)3-TMAOAc (glum = +0.03) was observed, whereas no CPL was observed in Eu(D-facam)3 (glum = 0). In this novel Eu(III) hybrid material, the right CPL from the 5D0 → 7F1 transition was obviously enhanced (glum = −0.05 → −0.21) and left CPL from the 5D0 → 7F2 transition was induced (glum = 0 → −0.03). The excellent CPL activity indicated that the chiral environment and coordination structure of Eu(D-facam)3 were obviously affected because of the interaction with TMAOAc.
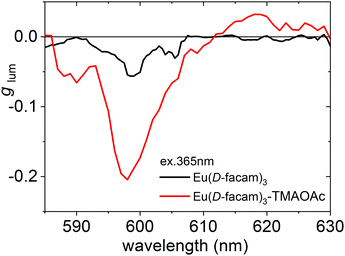 | ||
| Fig. 3 g lum spectra of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc in KBr pellets. The excitation wavelength was 365 nm. | ||
Furthermore, the differential absorption intensity of the light and left circularly polarized light was measured by circular dichroism (CD) spectroscopy. CD is typically used to investigate the chiral structure of materials as a complementary tool to CPL. The absorption and CD spectra of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc were then studied in KBr pelleta (Fig. S2, ESI†). Reproducibility of the CD signal from the film samples was validated by rotating the samples to avoid the artefacts. For both Eu(III) complexes, a broad absorption band covering 300–350 nm, attributed to π–π* transition from β-diketonate ligands, was observed. In the CD measurement, Eu(D-facam)3 did not exhibit any significant peaks and the lack of CD is probably responsible for a weak CPL signal.37 An obvious positive CD peak at approximately 350 nm was observed for Eu(D-facam)3-TMAOAc, indicating the changed spatial disposition of multi-chromophores of β-diketonate ligands. This change was probably ascribed to the interaction with TMAOAc, which presumably could distort the geometric structure of ligands in this Eu(III) hybrid material. The finding in chiroptical properties (CD and CPL) revealed that the interaction between Eu(D-facam)3 and TMAOAc showed profound effects on the chirality of β-diketonate ligands and coordination geometry of Eu(III) complexes. These structural changes could have probably led to the altered electronic structure of Eu(III) complexes owing to TMAOAc hybridization, which can also be observed from the affected diffuse reflectance spectra of Eu(III) complexes.
In accordance with absorption spectra, diffuse reflectance spectra of both Eu(D-facam)3 and Eu(D-facam)3-TMAOAc in the solid state also exhibited broad peaks at approximately 350 nm from β-diketonate ligands (Fig. S3, ESI†). The variation in the shape of both Eu(III) compounds was observed, suggesting that TMAOAc affected the electrical transition of ligands in this new Eu(III) hybrid material.
Eu(D-facam)3-TMAOAc presented much better luminescence performance than Eu(D-facam)3 even under the condition of higher symmetry. Higher symmetry around Eu3+ contributed to a low radiative rate constant, leading to a low emission intensity.38 To further elucidate the photophysical properties of Eu(III) complexes, their energy transfer process was precisely analysed. Firstly, the time-resolved emission profiles (Fig. 4) and the quantum yield of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc in the solid state were measured (Table 1). The values of lifetime (τ) and quantum yield (Φtot) for Eu(D-facam)3 were 278.5 μs and 1.6%, respectively. A significant enhancement was obtained in Eu(D-facam)3-TMAOAc with values 692.1 μs and 21.5% for τ and Φtot, respectively, which was consistent with the photographs of the bright luminescence from Eu(D-facam)3-TMAOAc as seen in Fig. 1(a).
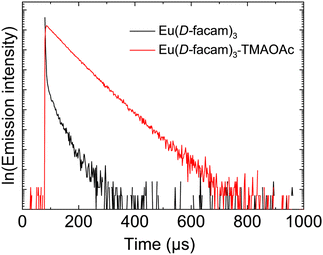 | ||
| Fig. 4 Emission decay profiles of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc in the solid state. The excitation wavelength was 350 nm. | ||
| I tot/IMD | τ (μs) | Φ tot (%) | Φ Ln (%) | k r (s−1) | k nr (s−1) | η sens (%) | |
|---|---|---|---|---|---|---|---|
| Eu(D-facam)3 | 11.9 | 278.5 | 1.6 | 16.4 | 588.4 | 3002.3 | 9.7 |
| Eu(D-facam)3-TMAOAc | 7.7 | 692.1 | 21.5 | 26.3 | 380.7 | 1064.2 | 81.6 |
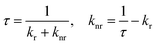
A series of key photophysical parameters were estimated and the values are shown in Table 1. The radiative rate constant (kr) and non-radiative rate constant (knr) were calculated as follows:
where AMD,0 is the spontaneous emission probability for the 5D0 → 7F1 magnetic dipole transition in vacuo (14.65 s−1), n is the refractive index of the medium (1.5 for solid) and Itot/IMD is the ratio of the total integrated area of the emission spectrum to the area of the 5D0 → 7F1 band.
The intrinsic quantum yield (ΦLn) and sensitization efficiency of the lanthanide luminescence by the ligands (ηsens) was then evaluated as follows:
Compared to Eu(D-facam)3, a decrease in the radiative rate constant for Eu(D-facam)3-TMAOAc was observed, corresponding to its higher symmetry. This is because, in the Eu(III) complex with higher symmetry, the forbidden electric dipole transitions in the Judd–Ofelt theory would be more strictly forbidden and thus leading to a lower radiative rate constant.31 In addition, the non-radiative rate constant for Eu(D-facam)3-TMAOAc was significantly suppressed (3002.3 s−1 → 1064.2 s−1). Under this condition, the competition ability of the radiative process for Eu(D-facam)3-TMAOAc is stronger than Eu(D-facam)3 in emission pathways, so a higher intrinsic quantum yield in Eu(D-facam)3-TMAOAc was obtained. Therefore, superior photophysical properties were observed in Eu(D-facam)3-TMAOAc even with a higher symmetry. Based on these variations, the sensitization efficiency of Eu(D-facam)3-TMAOAc was significantly improved to 81.6%, obviously higher than that of Eu(D-facam)3 (9.7%). Compared to an improved sensitization efficiency of 28% in the Eu(D-facam)3/TMAOAc mixed solution previously reported,28 the new Eu(III) hybrid material in this study showed a significant progress in the sensitization performance of the original Eu(D-facam)3. This was likely because the interaction between Eu(III) complexes and TMAOAc decreases the distance between the Eu3+ ion and the β-diketonate ligands, and the shorter donor–acceptor distance can accelerate the energy transfer rate for a higher sensitization efficiency.39
Thermostability and interaction ratio of Eu(D-facam)3-TMAOAc
It is typically difficult for lanthanide complexes to maintain a stable structure and luminescence at a high temperature for a long time, although many applications require thermal stability. Therefore, the thermal stability for the luminescence and structure of both Eu(III) compounds was investigated after a long-time heat treatment and cooling to room temperature. Emission spectra (Fig. S4, ESI†) and emission decay profiles of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc were obtained at room temperature before and after the heat treatment at 150 °C for 24 h. The emission peaks of both Eu(III) compounds were not changed, suggesting their stable coordination structures after 150 °C heat treatment. In addition, the decrease in the emission lifetime was 9.8% for Eu(D-facam)3 and 0.8% for Eu(D-facam)3-TMAOAc. Therefore, both Eu(III) compounds maintained their luminescence performance after the 150 °C heat-treatment. On the other hand, a notable difference in the physical state and luminescence performance between Eu(D-facam)3 and Eu(D-facam)3-TMAOAc was observed upon heat treatment at 200 °C for 24 h. Eu(D-facam)3 quickly melted and turned into a dark adhesive through heating at 200 °C. This could be explained by the fact that Eu(D-facam)3 lost its complex structure and luminescence, which was invisible to the naked eye, upon UV irradiation (Fig. S5(a), ESI†). In contrast, Eu(D-facam)3-TMAOAc well maintained its solid state and bright luminescence even after 24 h of the heat treatment at 200 °C (Fig. S5(b), ESI†). As seen in Table 2, the emission lifetime of melted Eu(D-facam)3 and well maintained solid-state Eu(D-facam)3-TMAOAc was 42.3 μs and 682.9 μs, respectively. Eu(D-facam)3-TMAOAc retained its high luminescence properties with a slight loss of 1.3% in the emission lifetime, whereas the emission lifetime of Eu(D-facam)3 was reduced by 84.8%. Fourier-transform infrared spectra (FT-IR) of Eu(D-facam)3-TMAOAc before and after 24 h of heat treatment at 200 °C were obtained (Fig. S6, ESI†). There was no change in FT-IR spectra before and after heat treatment, confirming the high thermal stability of the structure and physical state of Eu(D-facam)3-TMAOAc, consistent with the above results. Actually, TMAOAc itself is very unstable at high temperatures above 150 °C. The improved thermal stability for both Eu(D-facam)3 and TMAOAc in this new hybrid material could be ascribed to their stable interactions.| τ (μs) before heat-treatment | τ (μs) after heat-treatment for 24 hours | ||
|---|---|---|---|
| 150 °C | 200 °C | ||
| Eu(D-facam)3 | 278.5 | 251.2 | 42.3 |
| Eu(D-facam)3-TMAOAc | 692.1 | 686.6 | 682.9 |
Furthermore, the red luminescence of Eu(D-facam)3-TMAOAc was maintained even at high temperatures. As shown in Fig. 5(a), sharp and intense emission peaks of Eu(D-facam)3-TMAOAc were observed even at 100 °C. The shape of emission peaks was consistent with that obtained from room-temperature measurements, suggesting the stable luminescence properties and coordination structure of Eu(D-facam)3-TMAOAc at 100 °C. On the contrary, the luminescence of Eu(D-facam)3 was totally quenched with no emission peaks at 100 °C (Fig. 5(b)). During the energy transfer process of Eu(III) complexes, the radiative rate constant (kr) is independent of temperature, and the non-radiative rate constant (knr) is known as a temperature-dependent parameter.40 The intrinsic quantum yield (ΦLn) can be calculated as kr/(kr + knr). In the case of Eu(D-facam)3, the enhanced knr at 100 °C gave rise to a significant increase in the denominator, making ΦLn extremely close to zero. The luminescence of Eu(D-facam)3 at 100 °C was thus quenched. Conversely, Eu(D-facam)3-TMAOAc exhibited a much lower knr (1064.2 s−1 at room temperature) than Eu(D-facam)3 (3002.3 s−1 at room temperature), so the denominator, (kr + knr), at 100 °C for Eu(D-facam)3-TMAOAc was much smaller than that for Eu(D-facam)3. Therefore, ΦLn of Eu(D-facam)3-TMAOAc at 100 °C maintained a relatively substantial value, accounting for the red luminescence even at a high temperature.
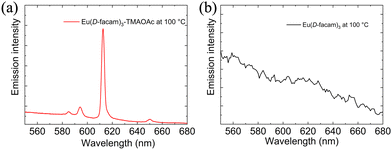 | ||
| Fig. 5 Emission spectra of (a) Eu(D-facam)3-TMAOAc and (b) Eu(D-facam)3 at 100 °C in the solid state. | ||
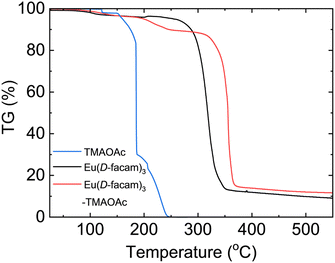
The thermal properties of Eu(D-facam)3-TMAOAc were investigated by thermogravimetric (TG) analysis. Fig. 6 shows the weight-loss process of Eu(D-facam)3, TMAOAc, and Eu(D-facam)3-TMAOAc at 20–500 °C. Eu(D-facam)3-TMAOAc showed improved thermal stability up to 320 °C, whereas a successive mass loss of Eu(D-facam)3 started at 270 °C. In the case of TMAOAc, two decomposition steps were observed for TMA+ and OAc−, respectively. A complete thermal decomposition of TMAOAc occurred at 250 °C, accompanied by a mass loss of 100%. Interestingly, Eu(D-facam)3-TMAOAc could still exhibit a brilliant luminescence after 24 h of heat treatment at 250 °C. The excellent thermal stability was ascribed to the stable structure owing to the interaction between Eu(D-facam)3 and TMAOAc.
To investigate the interaction ratio of Eu(D-facam)3 to TMAOAc in this new hybrid Eu(III) material, elemental analysis (for C, H, and N) was conducted. The calculated and experimental elemental compositions are shown in Table 3. The calculated values based on the interaction ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 matched well with the experimental values, and thereby the interaction ratio of Eu(D-facam)3 to TMAOAc was estimated to be 1
1 matched well with the experimental values, and thereby the interaction ratio of Eu(D-facam)3 to TMAOAc was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| C (%) | H (%) | N (%) | |||
|---|---|---|---|---|---|
| Calcd | Found | Calcd | Found | Calcd | Found |
| 49.13 | 49.50 | 5.60 | 5.59 | 1.36 | 1.33 |
Moreover, Eu(D-facam)3-TMAOAc, precipitated from 1-butanol with high concentrations of both starting materials, is soluble in 1-butanol at a relatively low concentration (0.2 mmol L−1). Based on our previous report, a higher concentration of TMAOAc could lead to an increase in the site symmetry around Eu3+ ions (decreased Irel) and a change in the emission peaks.28 The emission spectra of Eu(D-facam)3-TMAOAc and a mixed solution of Eu(D-facam)3 and TMAOAc with a concentration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 1-butanol were measured (Fig. S7, ESI†). The perfectly matched emission spectra and the same site symmetry (Irel = 6.45) indicated that their identical coordination structures still maintained even in the solution state, further supporting the 1
1 in 1-butanol were measured (Fig. S7, ESI†). The perfectly matched emission spectra and the same site symmetry (Irel = 6.45) indicated that their identical coordination structures still maintained even in the solution state, further supporting the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 interaction ratio between TMAOAc and Eu(D-facam)3 in Eu(D-facam)3-TMAOAc.
1 interaction ratio between TMAOAc and Eu(D-facam)3 in Eu(D-facam)3-TMAOAc.
Based on the confirmed interaction ratio, the experimental and corresponding theoretical thermoanalytical data of Eu(D-facam)3-TMAOAc are shown in Table 4. The first mass loss stage in Eu(D-facam)3-TMAOAc was observed from 100 to 320 °C, which was a gradual mass loss, likely, from TMAOAc. The total loss during the first stage was 12.90%, which was close to the calculated loss percentage (12.89%), further verifying the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 interaction ratio between Eu(D-facam)3 and TMAOAc. The second main successive mass loss stage was from 320 to 370 °C, and the corresponding mass loss of 72.48% was attributed to the loss of (D-facam) ligands. The final residue was predicted to be Eu2O3, which was also consistent with the theoretical result. In addition, one thermal decomposition step of the ligand loss in Eu(D-facam)3 occurred at 270 °C. Therefore, Eu(D-facam)3-TMAOAc exhibited better thermal stability than Eu(D-facam)3. The interaction between Eu(D-facam)3 and TMAOAc at a 1
1 interaction ratio between Eu(D-facam)3 and TMAOAc. The second main successive mass loss stage was from 320 to 370 °C, and the corresponding mass loss of 72.48% was attributed to the loss of (D-facam) ligands. The final residue was predicted to be Eu2O3, which was also consistent with the theoretical result. In addition, one thermal decomposition step of the ligand loss in Eu(D-facam)3 occurred at 270 °C. Therefore, Eu(D-facam)3-TMAOAc exhibited better thermal stability than Eu(D-facam)3. The interaction between Eu(D-facam)3 and TMAOAc at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio significantly contributed to both the outstanding luminescence performance and thermal stability in Eu(D-facam)3-TMAOAc.
1 ratio significantly contributed to both the outstanding luminescence performance and thermal stability in Eu(D-facam)3-TMAOAc.
| Temperature range (°C) | Mass loss (%) found (calcd) | Probable lost molecules | Residue mass (%) found (calcd) | Composition of the residue |
|---|---|---|---|---|
| 100–320 | 12.90 (12.89) | TMAOAc | ||
| 320–370 | 72.48 (72.35) | D-facam | 14.62(14.76) | Eu2O3 |
Structure analysis of Eu(D-facam)3-TMAOAc
To elucidate the impressive improvement in optical behaviours and thermal stability of this Eu(III) hybrid material, the structures and configurations were studied. Electrospray ionization (ESI) mass spectrometry of Eu(D-facam)3-TMAOAc was first conducted. A series of experimental and corresponding calculated mass signals identifying the coordination structure of this Eu(III) hybrid material were obtained. All the experimental mass spectra were highly consistent with the calculated signals, ensuring the reliability of the following fragments. The fragments [Eu(D-facam)3·TMA]+, [Eu(D-facam)3·OAc]−, [Eu(D-facam)3·TMA·2OAc]−, and [Eu(D-facam)3·2TMA·OAc]+ (Fig. S8, ESI†) indicated the interaction between single Eu(D-facam)3 and TMAOAc. [2Eu(D-facam)3·TMA]+, [2Eu(D-facam)3·OAc]−, [2Eu(D-facam)3·TMA·2OAc]−, and [2Eu(D-facam)3·2TMA·OAc]+ (Fig. S9, ESI†) demonstrated that multi-Eu(III) complexes were associated via TMAOAc.It was reported that acetates could act as a bidentate ligand binding to lanthanide ions.41 In our study, acetates probably play a similar role as a bidentate bridge to link multiple Eu3+ ions and form a chain structure of Eu(III) complexes. As many studies have reported, carboxylate compounds generally act as a linkage to form a lanthanide polymer with remarkable luminescence and stability.5,18,21,22 In such a chain structure, the distance between a donor and an accepter (ligand-Eu3+) in the Eu(III) hybrid materials is typically shorter than that in a single complex. Therefore, Eu(D-facam)3-TMAOAc was endowed with a significantly accelerated transfer energy (high sensitization efficiency) and enhanced emission intensity. Moreover, the Eu(III) coordination polymers are known to decrease vibrational quenching because of their chain-structural rigidness.42 Thus, the non-radiative rate was significantly suppressed (3002.3 s−1 → 1064.21 s−1) in Eu(D-facam)3-TMAOAc with its rigid chain structures.
In our previous study concerning the solution state, the effect of a series of ammonium salts with various cations and anions on Eu(III) complexes was investigated. The results demonstrated the irreplaceability of both TMA+ and OAc− in the photoluminescence improvement of Eu(D-facam)3. In this study, the fragments were also indicative of the importance of both TMA+ and OAc− in this novel Eu(III) hybrid material. TMAOAc was indispensable for the improvement of luminescence properties of Eu(D-facam)3 in both solution and solid states.
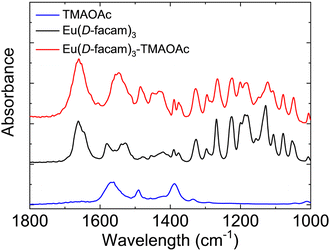
Fig. 7 shows the FT-IR spectra of Eu(D-facam)3, TMAOAc, and Eu(D-facam)3-TMAOAc. For Eu(D-facam)3, two peaks shown in the range 1500–1600 cm−1 suggested the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the β-diketonate moiety in Eu(D-facam)3.43 The double peaks merged into one peak at around 1550 cm−1 in Eu(D-facam)3-TMAOAc, indicating a more equivalent C
C stretching vibration of the β-diketonate moiety in Eu(D-facam)3.43 The double peaks merged into one peak at around 1550 cm−1 in Eu(D-facam)3-TMAOAc, indicating a more equivalent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonding. In TMAOAc, the peaks at around 1560 and 1390 cm−1 were attributed to the antisymmetric and symmetric stretching vibration of COO−, respectively.44 With respect to Eu(D-facam)3-TMAOAc, the COO− antisymmetric stretching vibration shifted from 1560 to 1550 cm−1, and signals at around 1420 cm−1 were slightly enhanced due to the COO− symmetric stretching vibration. Nakamoto reported that the difference (delta value) between the COO− antisymmetric and symmetric stretching frequencies could be used to determine the bonding mode of acetate groups.45 The ionic mode (TMAOAc itself) and bridging mode (Eu(D-facam)3-TMAOAc) both showed a delta value of 140–170 cm−1, whereas the unidentate mode had a much larger delta value of 200–300 cm−1. In Eu(D-facam)3-TMAOAc, a delta value of 160 cm−1 was clear evidence for the bidentate-bridging role of OAc− and its chain structure.
C bonding. In TMAOAc, the peaks at around 1560 and 1390 cm−1 were attributed to the antisymmetric and symmetric stretching vibration of COO−, respectively.44 With respect to Eu(D-facam)3-TMAOAc, the COO− antisymmetric stretching vibration shifted from 1560 to 1550 cm−1, and signals at around 1420 cm−1 were slightly enhanced due to the COO− symmetric stretching vibration. Nakamoto reported that the difference (delta value) between the COO− antisymmetric and symmetric stretching frequencies could be used to determine the bonding mode of acetate groups.45 The ionic mode (TMAOAc itself) and bridging mode (Eu(D-facam)3-TMAOAc) both showed a delta value of 140–170 cm−1, whereas the unidentate mode had a much larger delta value of 200–300 cm−1. In Eu(D-facam)3-TMAOAc, a delta value of 160 cm−1 was clear evidence for the bidentate-bridging role of OAc− and its chain structure.
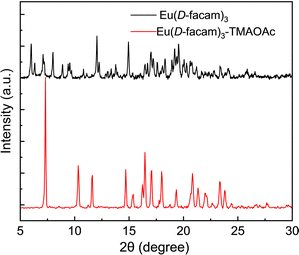
The hybrid material was crystallized to yield only tiny needle-like crystals. The powder X-ray diffraction (PXRD) of Eu(D-facam)3-TMAOAc was conducted for structure investigation (Fig. 8). Eu(D-facam)3 showed broad and weak signals which were attributed to its low crystallinity. In contrast, sharp and strong signals from Eu(D-facam)3-TMAOAc were observed. Although no single crystal for the structure determination was acquired, it verified the high crystallinity of Eu(D-facam)3-TMAOAc. From both ESI-mass spectrometry and PXRD measurements, Eu(D-facam)3-TMAOAc presumably acted as a chain structure, and the TMAOAc linkage resulted in a change in the coordination geometry of Eu(D-facam)3, as reflected from the PXRD signal shift. The preparation of a single crystal of Eu(D-facam)3-TMAOAc is still going on under various crystallization conditions, and the detailed crystalline structure of this new Eu(III) hybrid material will be presented in the near future.
Solubility of Eu(D-facam)3-TMAOAc
Most Eu(III)-based coordination polymer materials typically have a low solubility in organic solvents.46 However, Eu(D-facam)3-TMAOAc presented a decent solubility in most organic solvents, such as acetone, acetonitrile, chloroform, dimethyl sulfoxide and some alcohols.Interestingly, although acetone, one of the most common organic solvents, does not dissolve TMAOAc itself, the new Eu(III) hybrid material proposed in this study can be easily dissolved in acetone. This suggested that there was a stable interaction between TMAOAc and Eu(D-facam)3 in Eu(D-facam)3-TMAOAc. Therefore, acetone is the optimal solvent for the investigation of the luminescence performance of Eu(D-facam)3-TMAOAc in the solution state. Fig. 9 shows the emission spectra of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc in acetone. Eu(D-facam)3-TMAOAc exhibited a stronger emission intensity than that of Eu(D-facam)3. Correspondingly, the measured emission lifetime of Eu(D-facam)3-TMAOAc (363.0 μs) was longer than that of Eu(D-facam)3 (211.3 μs). Therefore, this new Eu(III) hybrid material, Eu(D-facam)3-TMAOAc, exhibited outstanding luminescence performance in both solid and solution states.
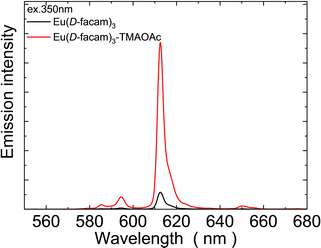 | ||
| Fig. 9 Emission spectra of Eu(D-facam)3 and Eu(D-facam)3-TMAOAc in acetone. The excitation wavelength was 350 nm. | ||
Conclusions
This study presented a new Eu(III) hybrid material, (Eu(D-facam)3-TMAOAc), prepared through a simple synthesis method, which is based on the deepening of luminescence improvement in our previous research. Differing from the earlier solution system, this study mainly targeted a solid system which is more suitable for practical applications. Compared to single complexes, Eu(D-facam)3-TMAOAc exhibited significant improvements in the luminescence, circular polarization, and thermal stability. This novel Eu(III) hybrid material well maintained its structure and luminescence properties even after 24 h of heat treatment at 200 °C. The enhancement was primarily attributed to its chain structure, in which TMAOAc acted as a bidentate-bridging molecule, linking with Eu(D-facam)3 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. In addition, outstanding luminescence performance of Eu(D-facam)3-TMAOAc was achieved in both solid and solution states. This novel Eu(III) hybrid material has considerable potential for applications, such as in CPL luminophores and bioassays.
1 ratio. In addition, outstanding luminescence performance of Eu(D-facam)3-TMAOAc was achieved in both solid and solution states. This novel Eu(III) hybrid material has considerable potential for applications, such as in CPL luminophores and bioassays.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by JSPS KAKENHI (grant numbers 17H06377, 20K05641 and 22H02154). Z. Li, one of the authors, received financial support from JST SPRING grant number JPMJFS2107 and an Initiative for Realizing Diversity in the Research Environment (Japan).Notes and references
- W. L. Scaff, D. L. Dyer and K. Mori, J. Bacteriol., 1969, 98, 246–248 CrossRef CAS PubMed.
- H. Tsukube and S. Shinoda, Chem. Rev., 2002, 102, 2389–2404 CrossRef CAS PubMed.
- J. Yuan and G. Wang, J. Fluoresc., 2005, 15, 559–568 CrossRef CAS PubMed.
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2009, 39, 189–227 RSC.
- Y. Hasegawa and Y. Kitagawa, J. Photochem. Photobiol., C, 2022, 51, 100485 CrossRef CAS.
- M. A. Martin, A. I. Olives, B. del Castillo and J. C. Menendez, Curr. Pharm. Anal., 2008, 4, 106–117 CrossRef CAS.
- M. D. McGehee, T. Bergstedt, C. Zhang, A. P. Saab, M. B. O’Regan, G. C. Bazan, V. I. Srdanov and A. J. Heeger, Adv. Mater., 1999, 11, 1349–1354 CrossRef CAS.
- R. C. Evans, P. Douglas and C. J. Winscom, Coord. Chem. Rev., 2006, 250, 2093–2126 CrossRef CAS.
- U. Giovanella, M. Pasini, C. Freund, C. Botta, W. Porzio and S. Destri, J. Phys. Chem. C, 2009, 113, 2290–2295 CrossRef CAS.
- K. Guido, Hybrid Materials: Synthesis, Characterization, and Applications, Wiley-VCH Verlag GmbH & Co. KGaA, Germany, 2007 Search PubMed.
- R. Carr, PhD thesis, Durham University, 2014.
- C. P. Montgomery, B. S. Murray, E. J. New, R. Pal and D. Parker, Acc. Chem. Res., 2009, 42, 925–937 CrossRef CAS PubMed.
- G. Muller, Dalton Trans., 2009, 9692–9707 RSC.
- S. M. Jeong, Y. Ohtsuka, N. Y. Ha, Y. Takanishi, K. Ishikawa, H. Takezoe, S. Nishimura and G. Suzaki, Appl. Phys. Lett., 2007, 90, 211106 CrossRef.
- D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331–1343 RSC.
- F. Zinna and L. Di Bari, Chirality, 2015, 27, 1–13 CrossRef CAS PubMed.
- Y. Kondo, S. Suzuki, M. Watanabe, A. Kaneta, P. Albertini and K. Nagamori, Front. Chem., 2020, 8, 527 CrossRef CAS PubMed.
- B. Yan, RSC Adv., 2012, 2, 9304–9324 RSC.
- M. Fernandes, S. S. Nobre, M. C. Gonçalves, A. Charas, J. Morgado, R. A. S. Ferreira, L. D. Carlos and V. de Zea Bermudez, J. Mater. Chem., 2009, 19, 733–742 RSC.
- Y. Hirai, T. Nakanishi, Y. Kitagawa, K. Fushimi, T. Seki, H. Ito and Y. Hasegawa, Angew. Chem., Int. Ed., 2016, 55, 12059–12062 CrossRef CAS PubMed.
- S. V. Eliseeva, D. N. Pleshkov, K. A. Lyssenko, L. S. Lepnev, J.-C. G. Bünzli and N. P. Kuzmina, Inorg. Chem., 2010, 49, 9300–9311 CrossRef CAS PubMed.
- H. Zhang, L. Zhou, J. Wei, Z. Li, P. Lin and S. Du, J. Mater. Chem., 2012, 22, 3457–3461 RSC.
- J.-C. Rybak, M. Hailmann, P. R. Matthes, A. Zurawski, J. Nitsch, A. Steffen, J. G. Heck, C. Feldmann, S. Götzendörfer, J. Meinhardt, G. Sextl, H. Kohlmann, S. J. Sedlmaier, W. Schnick and K. Müller-Buschbaum, J. Am. Chem. Soc., 2013, 135, 6896–6902 CrossRef CAS PubMed.
- N. N. Golovnev, M. S. Molokeev, S. N. Vereshchagin and V. V. Atuchin, J. Coord. Chem., 2015, 68, 1865–1877 CrossRef CAS.
- A. A. Ansari, A. K. Parchur, M. K. Nazeeruddin and M. M. Tavakoli, Coord. Chem. Rev., 2021, 444, 214040 CrossRef CAS.
- X. Li, Y. Xie, B. Song, H.-L. Zhang, H. Chen, H. Cai, W. Liu and Y. Tang, Angew. Chem., Int. Ed., 2017, 56, 2689–2693 CrossRef CAS PubMed.
- M. L. Aulsebrook, B. Graham, M. R. Grace and K. L. Tuck, Coord. Chem. Rev., 2018, 375, 191–220 CrossRef CAS.
- Z. Li, H. Minami, K. Nakamura and N. Kobayashi, ChemPhysChem, 2021, 22, 2511–2516 CrossRef CAS PubMed.
- Z. Li, K. Nakamura and N. Kobayashi, J. Imaging Soc. Jpn., 2021, 61, 194–199 Search PubMed.
- H. Tsumatori, T. Nakashima and T. Kawai, Org. Lett., 2010, 12, 2362–2365 CrossRef CAS PubMed.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- W. C. Nieuwpoort and G. Blasse, Solid State Commun., 1966, 4, 227–229 CrossRef CAS.
- V. V. Atuchin, A. S. Aleksandrovsky, O. D. Chimitova, T. A. Gavrilova, A. S. Krylov, M. S. Molokeev, A. S. Oreshonkov, B. G. Bazarov and J. G. Bazarova, J. Phys. Chem. C, 2014, 118, 15404–15411 CrossRef CAS.
- V. V. Atuchin, A. K. Subanakov, A. S. Aleksandrovsky, B. G. Bazarov, J. G. Bazarova, T. A. Gavrilova, A. S. Krylov, M. S. Molokeev, A. S. Oreshonkov and S. Y. Stefanovich, Mater. Des., 2018, 40, 488–494 CrossRef.
- M. H. V. Werts, R. T. F. Jukes and J. W. Verhoeven, Phys. Chem. Chem. Phys., 2002, 4, 1542–1548 RSC.
- C. Görller-Walrand, L. Fluyt, A. Ceulemans and W. T. Carnall, J. Chem. Phys., 1991, 95, 3099–3106 CrossRef.
- J. P. Riehl and G. Muller, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneidner, Jr., J.-C. G. Bünzli and V. K. Pecharsky, Elsevier, Amsterdam, 2004, 220, 289–357 Search PubMed.
- Y. Hasegawa, M. Yamamuro, Y. Wada, N. Kanehisa, Y. Kai and S. Yanagida, J. Phys. Chem. A, 2003, 107, 1697–1702 CrossRef CAS.
- J. H. S. K. Monteiro, A. de Bettencourt-Dias and F. A. Sigoli, Inorg. Chem., 2017, 56, 709–712 CrossRef CAS PubMed.
- L. Thompson, J. Legendziewicz, J. Cybinska, L. Pan and W. Brennessel, J. Alloys Compd., 2002, 341, 312–322 CrossRef CAS.
- V. L. Garza and N. Purdie, J. Phys. Chem., 1970, 74, 275–280 CrossRef CAS.
- K. Miyata, T. Ohba, A. Kobayashi, M. Kato, T. Nakanishi, K. Fushimi and Y. Hasegawa, ChemPlusChem, 2012, 77, 277–280 CrossRef CAS.
- H. Minami, M. Miyazato, Z. Li, K. Nakamura and N. Kobayashi, Chem. Commun., 2020, 56, 13532–13535 RSC.
- C. C. R. Sutton, G. da Silva and G. V. Franks, Chem. – Eur. J., 2015, 21, 6801–6805 CrossRef CAS PubMed.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley & Sons, Inc., Hoboken, 1986 Search PubMed.
- G. Calvez, F. Le Natur, C. Daiguebonne, K. Bernot, Y. Suffren and O. Guillou, Coord. Chem. Rev., 2017, 340, 134–153 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc04670a |
| This journal is © The Royal Society of Chemistry 2023 |