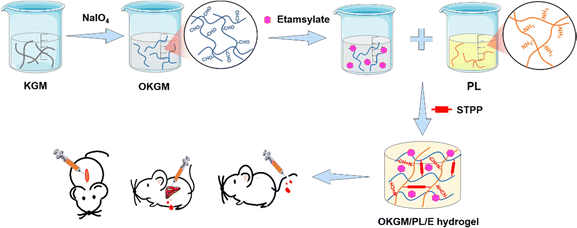
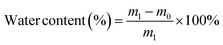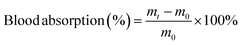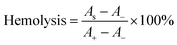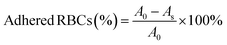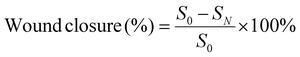Etamsylate loaded oxidized Konjac glucomannan-ε-polylysine injectable hydrogels for rapid hemostasis and wound healing
Shuo
Xu
a,
Jun
You
 b,
Shaorong
Yan
a,
Luting
Zhu
*c and
Xiaochen
Wu
b,
Shaorong
Yan
a,
Luting
Zhu
*c and
Xiaochen
Wu
 *a
*a
aDepartment of Pharmacy, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, China. E-mail: wxcguest@126.com
bMinistry-of-Education Key Laboratory for the Green Preparation and Application of Functional Materials, Hubei University, Youyi Road 368, Wuhan 430062, China
cSANKEN (The Institute of Scientific and Industrial Research), Osaka University, 8-1 Mihogaoka, Ibaraki, Osaka 5670047, Japan. E-mail: sharollzhu@eco.sanken.osaka-u.ac.jp
First published on 13th October 2023
Abstract
Uncontrollable bleeding is a crucial factor that can lead to fatality. Therefore, the development of hemostatic dressings that enable rapid hemostasis is of utmost importance. Hydrogels with injectability, self-healing ability, and adhesiveness hold significant potential as effective hemostatic dressings. Herein, a composite hydrogel was fabricated by the oxidized Konjac glucomannan and ε-polylysine. After the encapsulation of a hemostatic drug, etamsylate, an oxidized Konjac glucomannan/ε-polylysine/etamsylate (OKGM/PL/E) composite hydrogel that possesses favorable properties including injectability, self-healing ability, tissue adhesiveness, hemocompatibility and cytocompatibility was fabricated. The OKGM/PL/E hydrogel demonstrated the ability to effectively adhere red blood cells and seal wounds, enabling rapid control of hemorrhaging. In vivo wound healing experiments confirmed the hemostatic and wound healing efficacy of the OKGM/PL/E hydrogel, highlighting its potential as a valuable hemostatic dressing.
Introduction
Massive bleeding resulting from accidents and trauma can often lead to hemorrhagic shock and pre-hospital deaths, making it a critical health concern worldwide.1,2 It is crucial to address this issue promptly to prevent hemorrhage-related fatalities, particularly within the first few minutes after an accident. It is therefore essential to maintain immediate and effective hemorrhage control after the accidents to prevent hemorrhage-related deaths.3,4 To achieve this, traditional dressings with hemostatic ability, such as hemostatic gauze, cotton, and bandages, have been widely used. However, these products have limitations in controlling diffuse and incompressible bleeding, and can even cause secondary damage when removed from wounds.5 Hence, there is an urgent need to develop advanced hemostatic dressings that fulfill specific criteria. Firstly, they should be cost-effective, ensuring widespread accessibility for users across different backgrounds. Secondly, these dressings should possess biologically active properties, actively promoting blood clotting to effectively cease bleeding. Additionally, they must demonstrate biocompatibility, avoiding any adverse reactions or complications upon contact with the human body. Lastly, strong adhesive properties to tissue are essential for these dressings to maintain their position and effectiveness throughout use.Injectable, self-healing, and adhesive hydrogels have emerged as the ideal choice for hemostatic dressings due to their distinct properties. Extensive research has shown that these hydrogels can adapt to the shape and size of wounds, repair structural defects within the gel, and adhere to wound tissue even in the presence of exudate and blood.6–9 By effectively halting bleeding and providing continuous protection to the wound, these hydrogels offer significant advantages in managing hemostasis.5,10,11 Besides, the three-dimensional network structure of the hydrogels not only provides an optimal environment for tissue regeneration, but also functions as a physical barrier to restrain bacterial infections and create a moist atmosphere to accelerate wound healing.12 Natural polymer-based injectable hydrogels, in particular, are highly desirable options for hemostatic dressings. These hydrogels offer several advantages, including controllable physical and chemical properties, an excellent biosafety profile, and biodegradability. Among the natural polymers, Konjac glucomannan (KGM), a water-soluble polysaccharide extracted from the tubers of the Amorphophallus konjac plant, has shown great promise as a material for wound dressings due to its exceptional biocompatibility, ability to absorb water, capacity to form gels, ease of removal from the wound surface, stimulation of fibroblast proliferation, and capability to accelerate tissue regeneration.13–16 Moreover, KGM has been proved to be capable to promote the secretion of anti-inflammatory factors, such as IL-10, making it suitable as an immunomodulatory material for wound healing. In addition, hydrogels made from KGM can serve as carriers for various bioactive agents, including polysaccharides, proteins, drugs, growth factors, and nanoparticles. This allows for the incorporation of multiple functionalities, such as mimicking the extracellular matrix, targeted drug delivery, sustained drug release, antibacterial properties, antioxidant properties, and even photothermal therapy.17,18 For example, silver nanoparticles were incorporated into the composite hydrogels of KGM and chitosan to create a wound dressing with antimicrobial properties;19 matrine was incorporated into the composite hydrogels of KGM and fish gelatin to inhibit bacterial growth on the wound surface and maintain a suitable physiological environment for wound healing;20 polydopamine nanoparticles were loaded into composite hydrogels of KGM and xanthan gum for photothermally assisted antibacterial properties and to accelerate wound healing by downregulating the inflammatory response and upregulating vascular reconstruction.21 However, KGM hydrogels have been rarely utilized in the construction of injectable hydrogels for hemostatic dressings.
Chemical cross-linking is a widely employed method for synthesizing injectable hydrogels. One common strategy involves the condensation of amine groups with aldehyde groups through a Schiff base reaction, resulting in the formation of imine bonds. The Schiff base reaction is easily attainable, reversible, and eliminates the need for external cross-linking agents, making it an appealing choice for the production of biocompatible and injectable hemostatic dressings.22,23 Aldehyde groups can be introduced into the structure of KGM by oxidizing vicinal hydroxyl groups with sodium periodate. The oxidized KGM (OKGM) can then form composite hydrogels via a Schiff base reaction between the aldehyde groups of OKGM and the amino groups of bioactive agents.24,25 In this study, an injectable and self-healing OKGM/PL hydrogel was fabricated by utilizing the Schiff base reaction between the aldehyde groups of OKGM and the amino-residues of ε-poly-L-lysine (PL). PL is a cationic homopolyamide that possesses remarkable properties such as high water solubility, high thermal stability, biodegradability, and excellent biocompatibility.26,27 PL possesses cationic properties in neutral aqueous solutions attributed to its amine groups, thereby exhibiting tissue adhesive properties through ionic attraction with the tissue. This characteristic allows for effective adherence between PL and the surrounding tissue.28 Moreover, PL has been extensively used to enhance cell adhesion,29 which is beneficial for effective bleeding control of wounds. To further enhance the hemostatic performance of the dressing, a hemostatic drug, etamsylate, was loaded into the OKGM/PL hydrogel (Fig. 1). Etamsylate possesses antihemorrhagic properties that can improve platelet adhesiveness and restore capillary resistance.30 The physical and chemical properties, biocompatibility, hemostatic performance, and wound healing efficiency of the as-formed OKGM/PL/E composite hydrogels were thoroughly investigated to assess their potential as hemostatic dressings.
Experimental
Materials
Konjac glucomannan and etamsylate were acquired from Shanghai Macklin Biochemical Co., Ltd. ε-Poly-L-lysine·HCl was purchased from Shanghai Aladdin Biochemical Co., Ltd. Sodium tripolyphosphate (STPP) and the dialysis bag (7 kDa cut-off) were purchased from Solarbio Science & Technology Co., Ltd. Other reagents were all from Sinopharm Chemical Reagent Co., Ltd.Characterization
Morphologies of the samples were analyzed by a JEOL 7401 emission scanning electron microscopy (Japan) at an acceleration voltage of 10 kV. FTIR measurements were performed on a VERTEX 70 Fourier transform infrared spectrometer. UV-vis spectra were obtained using a Molecular Devices SpectraMax i3 (Tacan Austria GmbH, Grodig, Austria). The adhesive strengths of the hydrogels were assessed using an electronic universal testing machine (UTM2502, Shenzhen Suns Technology STOCK Co., Ltd).Oxidation of KGM
1.32 g of NaIO4 was added to a 250 mL aqueous solution of 2% (w/v) KGM. The mixture was vigorously stirred at R.T. in a dark environment for 12 h. Then, 10 mL of ethylene glycol was added to consume the excess NaIO4, and the mixture was stirred for an additional 2 h. The reaction mixture was then dialyzed in H2O using a dialysis bag (7 kDa cut-off) for 3 days. After dialysis, the mixture was centrifuged at 6000 rpm for 10 min to collect the supernatant. Finally, the collected supernatant and freeze-dried to obtain the OKGM.Preparation of OKGM/PL/E hydrogels
Different amounts of etamsylate (10, 20, and 50 mg) was dissolved in 50 μL of PBS (pH 7.4, 0.01 M), respectively. The solutions were then mixed with 0.5 mL of OKGM solution (0.3 g mL−1, dissolved in PBS). Next, 0.2 mL of PL solution (1 g mL−1, dissolved in PBS) and 0.25 mL of STPP solution (0.28 g mL−1, dissolved in PBS) were added to the mixture. The resulting mixture was stirred evenly to obtain the OKGM/PL/E hydrogels. The OKGM/PL/E hydrogels were denoted OKGM/PL/Ex where x denotes the content of etamsylate (10, 20, or 50 mg mL−1 of the hydrogel).Physicochemical properties of OKGM/PL/E hydrogels
Biocompatibility of OKGM/PL/E hydrogels
Hemolysis of OKGM/PL/E hydrogels
A hemolysis assay was conducted to assess the hemocompatibility of OKGM/PL/E hydrogels. To carry out this assay, 0.1 mL of anticoagulant blood, 1 mL of the hydrogels, and 3.5 mL of normal saline were mixed and incubated at 37 °C for 1 hour. Next, the mixture was centrifuged at 3000 rpm for 5 minutes, and the ultraviolet absorption of the supernatant was measured at 545 nm (AS). A positive control (A+) was prepared by mixing 0.1 mL of anticoagulant blood with deionized water (3.5 mL), while a negative control (A−) was prepared by mixing 0.1 mL of anticoagulant blood with normal saline (3.5 mL).Hemostatic ability of OKGM/PL/E hydrogels
Male Kunming mice (aged 5–6 weeks and weighing 30–40 g) and male rats were obtained from Qingdao Darenfucheng Animal Technology Co., Ltd (Qingdao, China). All animal experiments were conducted in compliance with the guidelines of National Research Council's Guide for the Care and Use of Laboratory Animals and was approved of by the Qingdao University of Science and Technology Ethics Committee for Experimentation.In vivo wound healing efficacy of OKGM/PL/E hydrogels
A full-thickness skin defect model was employed to assess the effectiveness of wound healing. In this model, a 12 mm-long spindle-shaped full-thickness wound was created on the dorsal area of each mouse. The control group underwent no treatment, whereas the remaining groups received various treatments. Specifically, 0.3 mL of etamsylate, OKGM/PL hydrogels, OKGM/PL/E20 hydrogels, and OKGM/PL/E50 hydrogels were administered and applied to cover the wounds. The wounds were subsequently wrapped in gauze and secured with bandages. Dressings were changed every 2 days throughout the study. Photographs of the wounds were taken every two days using a camera. The wound area was measured and recorded at 3, 5, 7, and 9 days after the treatments, allowing the calculation of wound closure. S0 and SN represented the wound area of 0 and N days, respectively (N = 3, 5, 7 and 9).On the 5th day, the entire wound surface of skin tissue was collected and stained with Masson's trichrome.
Statistical analysis
All experiments were replicated at least 3 times, and statistical analysis was conducted using one-way analysis of variance (ANOVA) to determine the significant differences between data. p < 0.05 was considered statistically significant.Results and discussion
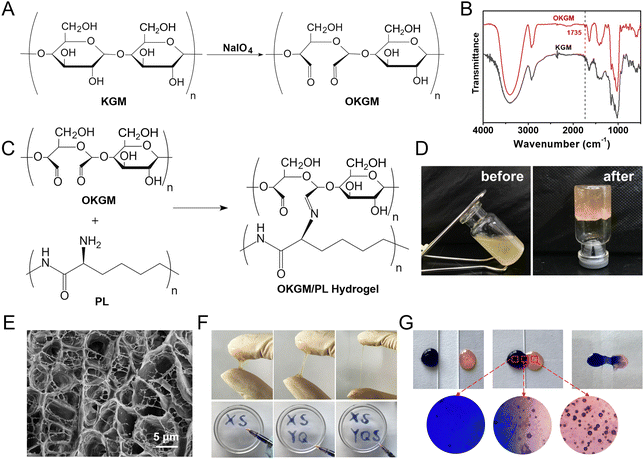
As depicted in Fig. 1 and 2A, aldehyde groups were incorporated into KGM following the protocol described in the literature.32 This involved the oxidation of vicinal hydroxyl groups with the use of NaIO4. FT-IR spectra were utilized to verify the presence of aldehyde in OKGM (Fig. 2B). The spectrum of OKGM exhibited a new peak at 1735 cm−1, indicating the characteristic vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the aldehyde. This confirmed the successful oxidation of KGM. Subsequently, OKGM acted as a supramolecular crosslinker and interacted with PL through the Schiff base reaction to form hydrogels (Fig. 2C). To enhance the stability of the hydrogel system, STPP was incorporated. STPP not only formed phosphate linkages between the intermolecular hydroxyl groups of OKGM,14 but also crosslinked ionically with the positively charged amine groups of PL via its negatively charged polyphosphate ions (Fig. 1).33,34 The crosslinking reaction occurred under mild conditions, resulting in the formation of a uniform, semi-transparent OKGM/PL hydrogel (Fig. 2D). The SEM image in Fig. 2E depicted the interconnected three-dimensional network structure of the OKGM/PL hydrogel. The presence of porous and irregular networks within the hydrogel may enhance its ability to absorb wound exudates and facilitate nutrient exchange at the wound site.
O group in the aldehyde. This confirmed the successful oxidation of KGM. Subsequently, OKGM acted as a supramolecular crosslinker and interacted with PL through the Schiff base reaction to form hydrogels (Fig. 2C). To enhance the stability of the hydrogel system, STPP was incorporated. STPP not only formed phosphate linkages between the intermolecular hydroxyl groups of OKGM,14 but also crosslinked ionically with the positively charged amine groups of PL via its negatively charged polyphosphate ions (Fig. 1).33,34 The crosslinking reaction occurred under mild conditions, resulting in the formation of a uniform, semi-transparent OKGM/PL hydrogel (Fig. 2D). The SEM image in Fig. 2E depicted the interconnected three-dimensional network structure of the OKGM/PL hydrogel. The presence of porous and irregular networks within the hydrogel may enhance its ability to absorb wound exudates and facilitate nutrient exchange at the wound site.
The OKGM/PL hydrogel displayed excellent adhesiveness and high stretchability, as it could be stretched to more than four times its initial length without cracking (Fig. 2F). Significantly, the OKGM/PL hydrogels demonstrated the ability to be extruded through a syringe needle and injected onto a glass dish to form specific shapes, as shown in Fig. 2F. These injected hydrogels adhered firmly to the surface of the dish, maintaining stable shapes without any displacement. This clearly indicates the injectability of the OKGM/PL hydrogels and highlights their adhesive properties. Consequently, the OKGM/PL hydrogel holds promise for filling irregular wounds and effectively sealing joint wounds. The self-healing properties of the OKGM/PL hydrogels were further investigated. As shown in the photo of Fig. 2G, two hydrogels with distinct colors were placed in contact with each other for a duration of 10 minutes, without any external intervention. Remarkably, it was observed that the two hydrogels seamlessly fused together, with no visible boundary at the contact interface. They merged completely, resulting in the formation of a homogeneous hydrogel, confirmed the self-healing capability of the hydrogels. The OKGM/PL hydrogel exhibited a multitude of favorable properties, including high stretchability, injectability, and self-healing, making it an ideal candidate for hemostatic dressings.
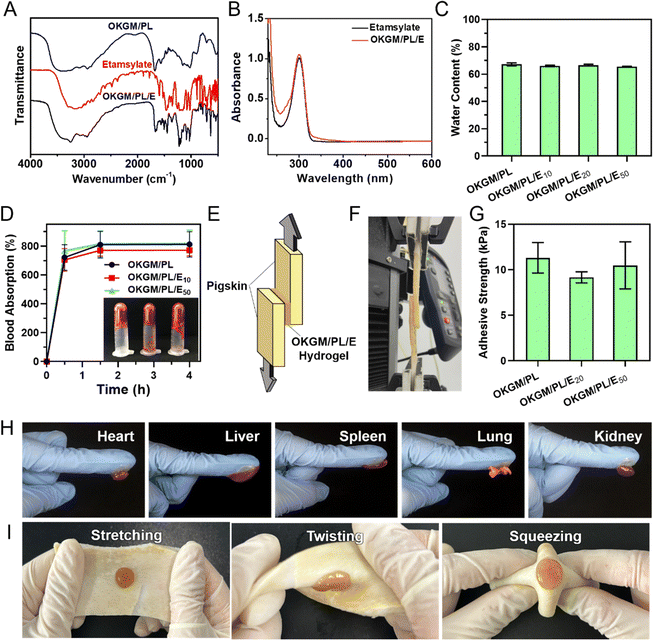
To enhance the functionality of the hydrogel, a hemostatic agent, etamsylate,35 was incorporated into the matrix of the OKGM/PL hydrogel to form OKGM/PL/E composite hydrogels. As illustrated in Fig. 3A, the FT-IR spectrum of OKGM/PL revealed an important difference compared to the spectrum of OKGM. Specifically, the characteristic peak associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of the aldehyde groups at 1735 cm−1 disappeared. This disappearance can be attributed to the reaction between OKGM and PL, resulting in the formation of OKGM/PL. After the loading of etamsylate, the OKGM/PL/E hydrogels exhibited characteristic peaks of etamsylate, indicating its successful loading. The UV-vis spectrum presented similar results: the OKGM/PL/E hydrogels exhibited the characteristic absorption peak of etamsylate at 300 nm (Fig. 3B), confirming the loading of etamsylate.
O vibration of the aldehyde groups at 1735 cm−1 disappeared. This disappearance can be attributed to the reaction between OKGM and PL, resulting in the formation of OKGM/PL. After the loading of etamsylate, the OKGM/PL/E hydrogels exhibited characteristic peaks of etamsylate, indicating its successful loading. The UV-vis spectrum presented similar results: the OKGM/PL/E hydrogels exhibited the characteristic absorption peak of etamsylate at 300 nm (Fig. 3B), confirming the loading of etamsylate.
A desirable hydrogel dressing should have a high-water content to effectively absorb the wound fluid and the exudate, maintain an appropriate moisture level in the wound, facilitate softening of necrotic tissue, and aid in debridement. The OKGM/PL/E hydrogels demonstrated high water content, and the incorporation of etamsylate did not negatively affect the water content (Fig. 3C). For instance, the water content of OKGM/PL, OKGM/PL/E10, OKGM/PL/E20 and OKGM/PL/E50 hydrogels was 67.2%, 66.0%, 66.6% and 65.6%, respectively. The blood absorption capacity of the OKGM/PL/E hydrogels was depicted in Fig. 3D. The freeze-dried OKGM/PL/E hydrogels possessed a remarkable ability to quickly absorb a substantial amount of blood. Notably, the blood absorption content and the time taken to reach equilibrium remained relatively consistent across different loading amounts of etamsylate in the composite hydrogels. For instance, the OKGM/PL hydrogel exhibited a blood absorption capacity of 811%, while the OKGM/PL/E50 hydrogel demonstrated a blood absorption capacity of 818%. The blood absorption capabilities of these hydrogels make them well-suited for use as hemostatic dressings.
To effectively stop bleeding, absorb blood, and prevent potential bacterial colonization, hemostatic hydrogel dressings should possess strong tissue adhesiveness. This enables them to adhere effectively to the wound site. A lap shear test was applied to assess the adhesive properties of the OKGM/PL/E hydrogels (Fig. 3E–G). As depicted schematically and exemplified in the photograph, the OKGM/PL/E hydrogel was sandwiched between two pieces of freshly wetted porcine skin, which mimics the biological properties of the human skin. The adhesive strength of the OKGM/PL/E hydrogels, as demonstrated in Fig. 3G, showed an adhesion strength of 9.1–11.3 kPa with no significant difference between groups. This indicated that the varying amounts of loaded etamsylate had no adverse effect on the adhesive strength values of the hydrogel. The OKGM/PL/E hydrogel demonstrated excellent adhesive behavior to various visceral tissues of mice (Fig. 3H). The organs (e.g., heart, liver, spleen, lungs, and kidneys) can firmly adhere to the OKGM/PL/E hydrogels without any external assistance. Additionally, the hydrogel displayed a strong adherence to stretched, twisted, and squeezed porcine skin, as shown in Fig. 3I. These consistent results highlighted the remarkable adhesion ability of the OKGM/PL/E hydrogels under wet and dynamic conditions,36 indicating their potential for tightly adhering to tissues and delivering hemostatic effects.
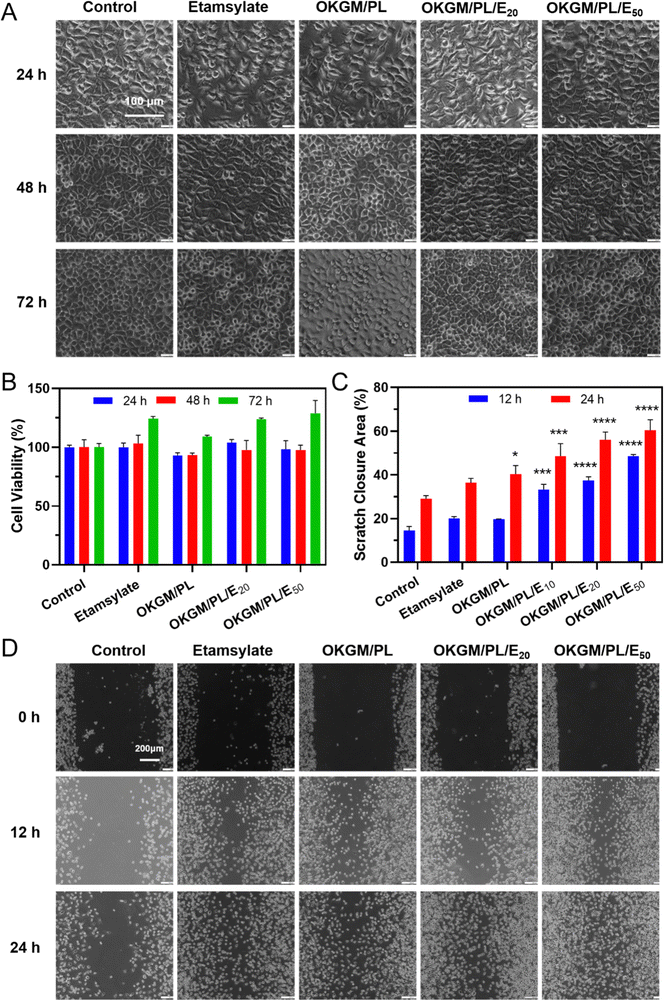
In order to be used as wound dressings, the hydrogel must exhibit excellent biocompatibility. The cytotoxicity of the hydrogels was evaluated by an MTT assay against L929 cells (Fig. 4A and B). During 3 days of incubation, the viability of cells treated with OKGM/PL hydrogels was comparable to the control group, indicating the good biocompatibility of the OKGM/PL hydrogels. The presence of etamsylate did not negatively affect the cytocompatibility of the OKGM/PL hydrogels. For example, after 3 days of incubation, the viability of L929s treated with OKGM/PL/E20 hydrogels was 123.8%, and the viability of L929s treated with OKGM/PL/E50 hydrogels was 128.6%, exhibiting the satisfactory cytocompatibility of the OKGM/PL/E hydrogels.
Furthermore, a scratch assay was conducted to evaluate the effect of the OKGM/PL/E hydrogels on the migration of L929s (Fig. 4C and D). Both the OKGM/PL and OKGM/PL/E hydrogels exhibited a tendency to promote cell migration. For example, the OKGM/PL/E50 hydrogel significantly enhanced the migration of L929s within 24 hours, with approximately 60.4% of the wound area healed due to cell migration, compared with 29.1% of the control group. The effect of OKGM/PL/E hydrogels was statistically different from that of the control group, highlighting the great potential of the OKGM/PL/E hydrogels in promoting wound healing. KGM stands out as an excellent matrix material due to its favorable biocompatibility and biodegradability.37 Similarly, PL also possesses remarkable biocompatibility and has been extensively employed to enhance cell adhesion.29,38 Consequently, the incorporation of PL in the OKGM/PL/E hydrogel further enhances its cell affinity. This synergistic effect likely contributes to the hydrogel's ability to promote L929 cells migration.
When used as dressings, it is crucial for the OKGM/PL/E hydrogels to not cause irritation to biological membranes. In the case of direct contact with CAM for 5 minutes, the hydrogels showed no signs of injuring or causing hemorrhage to the veins, arteries, or capillaries of the CAM, as depicted in Fig. 5A. To assess the level of CAM irritation, the absorption of trypan blue at the hydrogel treatment site was measured. Fig. 5B clearly indicated that the OKGM/PL/E hydrogels caused minimal irritation to the CAM, with significantly different results from the positive control, confirming the excellent biocompatibility of the hydrogels. Furthermore, for effective hemostatic dressings, it is important for them to have a minimal impact on hemolysis when in contact with bleeding wounds. After incubation with blood samples for an hour, no observable toxicity against erythrocytes was noticed, as shown in Fig. 5C. None of the OKGM/PL/E hydrogels, regardless of drug loading amount, resulted in any hemolysis. The hemolysis rate of the hydrogels was below 3%, indicating exceptional hemocompatibility and suggesting their safety as hemostatic materials.
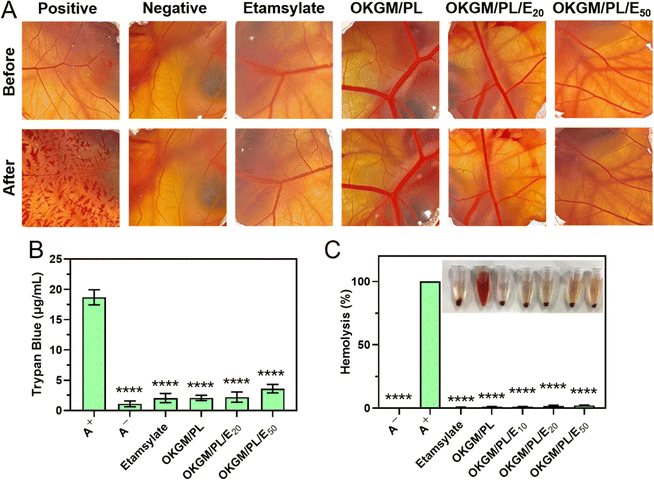 | ||
| Fig. 5 (A) Photographs of CAM after different treatments. (B) Trypan blue staining results of CAM. (C) Hemolysis of the OKGM/PL/E hydrogels. ****p < 0.0001 compared to the positive control group. | ||
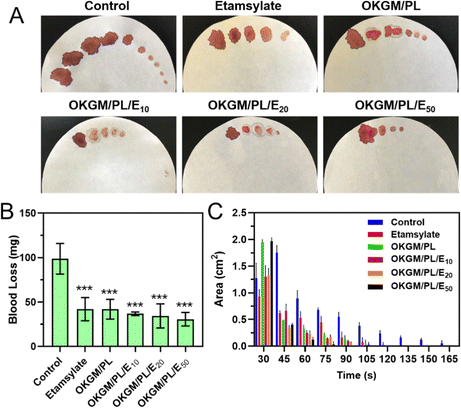
The potential application of OKGM/PL/E hydrogels as hemostatic dressings is promising due to their injectability, adhesiveness, and biocompatibility. To evaluate their hemostatic potential, tail amputation was performed as a typical bleeding model (Fig. 6). The hydrogels were immediately applied to the cut tail to assess the hemostatic efficacies. The untreated tail generated a large area of bloodstain on the filter paper, with a total blood loss of 98.7 mg after 165 seconds. Both the individual etamsylate and the OKGM/PL hydrogel can efficiently reduce the blood loss to some extent, which was significantly different from the non-treated control group. After applying the OKGM/PL/E20 and OKGM/PL/E50 hydrogel, the total blood loss was dramatically reduced, for example, only 30.7 mg of blood was collected after treatment of OKGM/PL/E50, which was ∼3 times lower than the control group (Fig. 6B). Moreover, there was almost no subsequent bleeding from the wound to the filter paper after 75 s treatment of the OKGM/PL/E50, as well as 90 s treatment of the OKGM/PL/E20 (Fig. 6A and C). That is, the bleeding was rapidly controlled within 75 s of treatment with the OKGM/PL/E50 dressing, which was ∼2.2 times faster than the control group, suggesting that the hydrogels can act as a barrier to seal the wound and stop bleeding rapidly and constantly.
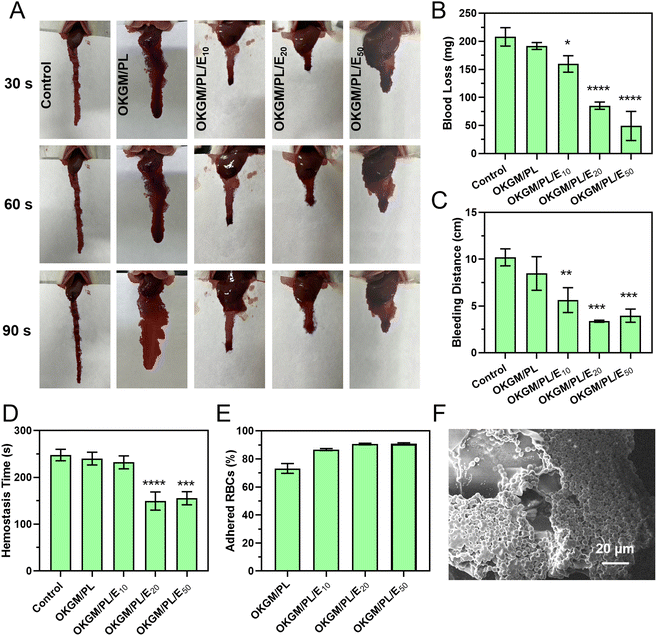
Similar hemostatic results were observed in the liver incision model of rats (Fig. 7A–D). The application of OKGM/PL/E hydrogels quickly inhibited liver bleeding. For example, the average value of the total blood loss, bleeding distance, and hemostasis time of the OKGM/PL/E50 treated group was 49.2 mg, 4.0 cm and 155.3 s, respectively, significantly lower than the non-treated control group, which was 207.9 mg, 10.2 cm and 247.7 s, respectively. Furthermore, the OKGM/PL/E hydrogels demonstrated excellent adhesiveness to bleeding tissues, remaining at the bleeding site without being swept away for more than 5 minutes. This highlighted their ability to effectively adhere to the bleeding tissues. The OKGM/PL/E50 hydrogel combined the hemostatic ability of etamsylate with the tissue adhesiveness of the OKGM/PL hydrogel. As a result, it exhibited positive hemostatic properties, quickly and thoroughly inhibiting blood loss.
The hemostatic properties of the OKGM/PL/E hydrogels may be partially attributed to their ability to promote surface adhesion of red blood cells. The OKGM/PL/E hydrogels exhibited a strong affinity for red blood cells. Approximately 73.2% of the red blood cells were adhered to the OKGM/PL hydrogel, while 90.9% of the red blood cells were adhered to the OKGM/PL50 hydrogel after incubation for 30 min (Fig. 7E). As depicted in Fig. 7F, the OKGM/PL/E50 hydrogels displayed significant adherence of red blood cells to their surfaces. The interconnected porous structure and blood absorption ability of the OKGM/PL/E50 hydrogel enabled it to rapidly absorb plasma and efficiently concentrate blood, thereby trapping aggregated hemocytes.39 By promoting the adhesion of red blood cells, the OKGM/PL/E hydrogels enhanced coagulation and facilitated a rapid hemostatic effect.
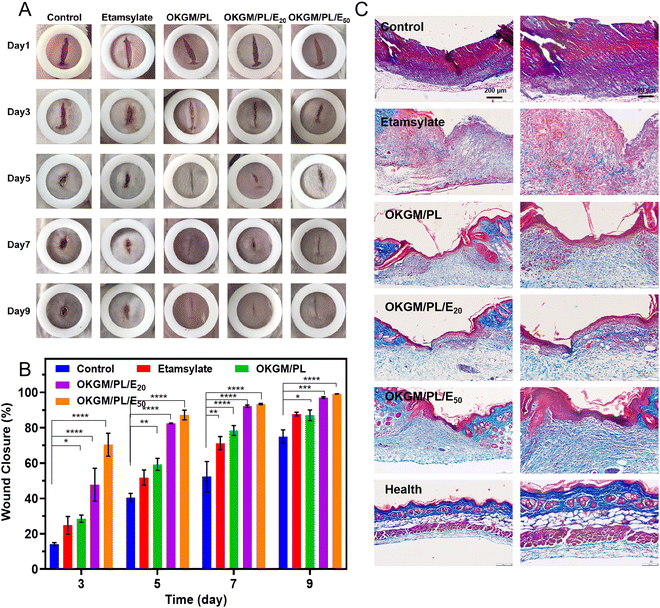
The in vivo hemostatic performance as well as the wound healing efficiency of the OKGM/PL/E hydrogels was further assessed on a full-thickness skin defect model (Fig. 8). The wound closure was monitored every two days from the day of wound creation. Generally, in comparison with the non-treated control group and that treated by etamsylate or OKGM/PL, the wounds managed by the OKGM/PL/E hydrogels exhibited better healing effects. For instance, quantitative statistical analysis showed that on day 5, the wound closure was 87.2% for the OKGM/PL/E50 hydrogel treated group, while the value was 40.4%, 51.8%, 59.3%, and 82.5% for the blank control, the etamsylate treated group, the OKGM/PL hydrogel treated group, and the OKGM/PL/E20 hydrogel treated group, respectively, demonstrating the high efficiency of the OKGM/PL/E50 hydrogel in promoting wound healing (Fig. 8A and B). The OKGM/PL/E hydrogel can firmly adhere to the tissue, absorb wound exudates, prevent wound dehydration while inhibit bleeding to facilitate healing. Masson's trichrome staining of the wound site showed that after the treatment of the OKGM/PL/E50 hydrogel, the healed skin was comparable with the normal skin, confirming the overall healing efficacy of the OKGM/PL/E hydrogels (Fig. 8C).
Conclusions
In summary, an OKGM/PL/E hydrogel was fabricated with favorable properties. This hydrogel was crosslinked through a Schiff base reaction between the aldehyde group of OKGM and the amino group of PL, followed by the loading of a hemostatic drug etamsylate. The OKGM/PL/E hydrogel possessed several remarkable properties, including injectability, self-healing ability, tissue adhesiveness, blood adsorption ability, and biocompatibility, thus could effectively seal different shapes of wounds and realize excellent in vivo hemostatic performance. Moreover, the OKGM/PL/E hydrogel could efficiently accelerate wound healing, making it an ideal material for the development of hydrogel dressings with rapid hemostatic properties.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Talent Foundation funded by Province and Ministry Co-construction Collaborative Innovation Center of Eco-chemical Engineering (STHGYX2206).References
- A. Kumar, D. K. Sah, K. Khanna, Y. Rai, A. K. Yadav, M. S. Ansari and A. N. Bhatt, Carbohydr. Polym., 2023, 299, 120186 CrossRef CAS PubMed.
- C. Cao, N. Yang, Y. Zhao, D. Yang, Y. Hu, D. Yang, X. Song, W. Wang and X. Dong, Nano Today, 2021, 39, 101165 CrossRef CAS.
- S. Indrakumar, S. Ghosh, T. K. Dash, V. Mishra, B. Tandon and K. Chatterjee, Carbohydr. Polym., 2023, 304, 120479 CrossRef CAS PubMed.
- Y. Shi, W. Yu, X. Liang, J. Cheng, Y. Cao, M. Liu, Y. Fang, Z. Yang, H. Liu, H. Wei and G. Zhao, Carbohydr. Polym., 2023, 307, 120590 CrossRef CAS PubMed.
- Z. Chen, J. Zhao, H. Wu, H. Wang, X. Lu, M.-A. Shahbazi and S. Wang, Carbohydr. Polym., 2023, 303, 120434 CrossRef CAS PubMed.
- J. He, Z. Zhang, Y. Yang, F. Ren, J. Li, S. Zhu, F. Ma, R. Wu, Y. Lv, G. He, B. Guo and D. Chu, Nano-Micro Lett., 2021, 13, 80 CrossRef CAS.
- Y. Liang, H. Xu, Z. Li, A. Zhangji and B. Guo, Nano-Micro Lett., 2022, 14, 185 CrossRef CAS PubMed.
- B. Guo, R. Dong, Y. Liang and M. Li, Nat. Rev. Chem., 2021, 5, 773–791 CrossRef CAS PubMed.
- Y. Liang, M. Li, Y. Yang, L. Qiao, H. Xu and B. Guo, ACS Nano, 2022, 16, 3194–3207 CrossRef CAS PubMed.
- Y. Lv, F. Cai, Y. He, L. Li, Y. Huang, J. Yang, Y. Zheng and X. Shi, Acta Biomater., 2023, 159, 95–110 CrossRef CAS PubMed.
- Y. Wang, M. Yang and Z. Zhao, Carbohydr. Polym., 2023, 310, 120723 CrossRef CAS PubMed.
- H. Cao, D. Xiang, X. Zhou, P. Yue, Y. Zou, Z. Zhong, Y. Ma, L. Wang, S. Wu and Q. Ye, Carbohydr. Polym., 2023, 307, 120609 CrossRef CAS PubMed.
- H. Chen, J. Cheng, L. Ran, K. Yu, B. Lu, G. Lan, F. Dai and F. Lu, Carbohydr. Polym., 2018, 201, 522–531 CrossRef CAS PubMed.
- P. K. Veerasubramanian, P. Thangavel, R. Kannan, S. Chakraborty, B. Ramachandran, L. Suguna and V. Muthuvijayan, Colloids Surf., B, 2018, 165, 92–102 CrossRef CAS PubMed.
- Y. Xie, Z.-x Yi, J.-x Wang, T.-g Hou and Q. Jiang, Int. J. Biol. Macromol., 2018, 112, 1225–1233 CrossRef CAS PubMed.
- L. Zhu, J. Chen, X. Mao and S. Tang, Mater. Sci. Eng., C, 2021, 129, 112374 CrossRef CAS PubMed.
- N. Zhou, S. Zheng, W. Xie, G. Cao, L. Wang and J. Pang, J. Appl. Polym. Sci., 2022, 139, 51780 CrossRef CAS.
- R. D. Devaraj, C. K. Reddy and B. Xu, Int. J. Biol. Macromol., 2019, 126, 273–281 CrossRef CAS PubMed.
- Y. Jiang, J. Huang, X. Wu, Y. Ren, Z. Li and J. Ren, Int. J. Biol. Macromol., 2020, 149, 148–157 CrossRef CAS PubMed.
- L. Zhou, T. Xu, J. Yan, X. Li, Y. Xie and H. Chen, Food Hydrocolloids, 2020, 104, 105702 CrossRef CAS.
- Q. Zeng, Y. Qian, Y. Huang, F. Ding, X. Qi and J. Shen, Bioact. Mater., 2021, 6, 2647–2657 CrossRef CAS PubMed.
- C. Mo, L. Xiang and Y. Chen, Macromol. Rapid Commun., 2021, 42, 2100025 CrossRef CAS PubMed.
- J. Yang and S. Wang, Gels, 2023, 9, 138 CrossRef CAS PubMed.
- D. Yang, Y. Yuan, L. Wang, X. Wang, R. Mu, J. Pang, J. Xiao and Y. Zheng, Int. J. Mol. Sci., 2017, 18, 2250 CrossRef PubMed.
- F. Zhu, Food Chem., 2018, 256, 419–426 CrossRef CAS PubMed.
- Y. Wang, H. Cao and X. Wang, Mater. Chem. Phys., 2020, 248, 122902 CrossRef CAS.
- H. Liu, Z. Li, Y. Zhao, Y. Feng, A. V. Zvyagin, J. Wang, X. Yang, B. Yang and Q. Lin, ACS Appl. Mater. Interfaces, 2021, 13, 26770–26781 CrossRef CAS PubMed.
- W. Nie, X. Yuan, J. Zhao, Y. Zhou and H. Bao, Carbohydr. Polym., 2013, 96, 342–348 CrossRef CAS PubMed.
- Y. Sun, X. Chi, H. Meng, M. Ma, J. Wang, Z. Feng, Q. Quan, G. Liu, Y. Wang, Y. Xie, Y. Zheng and J. Peng, Bioact. Mater., 2021, 6, 3987–3998 CrossRef CAS PubMed.
- P. Suchý, A. Paprskářová, M. Chalupová, L. Marholdová, K. Nešporová, J. Klusáková, G. Kuzmínová, M. Hendrych and V. Velebný, Materials, 2020, 13, 1627 CrossRef PubMed.
- W. Feng and Z. Wang, Carbohydr. Polym., 2022, 294, 119824 CrossRef CAS PubMed.
- J. Luan, K. Wu, C. Li, J. Liu, X. Ni, M. Xiao, Y. Xu, Y. Kuang and F. Jiang, Carbohydr. Polym., 2017, 171, 9–17 CrossRef CAS PubMed.
- C. Wu, Y. Li, Y. Du, L. Wang, C. Tong, Y. Hu, J. Pang and Z. Yan, Food Hydrocolloids, 2019, 89, 682–690 CrossRef CAS.
- G. Patil, A. Torris, P. R. Suresha, S. Jadhav, M. V. Badiger and V. Ghormade, Colloids Surf., B, 2021, 198, 111454 CrossRef CAS PubMed.
- M. Y. Cobo-Nuñez, M. El Assar, P. Cuevas, A. Sánchez-Ferrer, J. Martínez-González, L. Rodríguez-Mañas and J. Angulo, Eur. J. Pharmacol., 2018, 827, 167–172 CrossRef PubMed.
- W. Xue, R. Yang, S. Liu, Y. Pu, P. Wang, W. Zhang, X. Tan and B. Chi, Biomater. Sci., 2022, 10, 2417–2427 RSC.
- H. R. Nie, X. X. Shen, Z. H. Zhou, Q. S. Jiang, Y. W. Chen, A. Xie, Y. Wang and C. C. Han, Carbohydr. Polym., 2011, 85, 681–686 CrossRef CAS.
- S. Some, S.-M. Ho, P. Dua, E. Hwang, Y. H. Shin, H. Yoo, J.-S. Kang, D.-K. Lee and H. Lee, ACS Nano, 2012, 6, 7151–7161 CrossRef CAS PubMed.
- R. Cui, F. Chen, Y. Zhao, W. Huang and C. Liu, J. Mater. Chem. B, 2020, 8, 8282–8293 RSC.
| This journal is © The Royal Society of Chemistry 2023 |