Transdermal drug delivery via microneedles for musculoskeletal systems
Haibin
Zheng
ab,
Xuankun
Xie
ab,
Haocong
Ling
ab,
Xintong
You
b,
Siyu
Liang
b,
Rurong
Lin
b,
Renjie
Qiu
b and
Honghao
Hou
 *b
*b
aThe Second School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong 510280, P. R. China
bGuangdong Provincial Key Laboratory of Construction and Detection in Tissue Engineering, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong 510515, P. R. China. E-mail: ss.hhh89@hotmail.com
First published on 19th July 2023
Abstract
As the population is ageing and lifestyle is changing, the prevalence of musculoskeletal (MSK) disorders is gradually increasing with each passing year, posing a serious threat to the health and quality of the public, especially the elderly. However, currently prevalent treatments for MSK disorders, mainly administered orally and by injection, are not targeted to the specific lesion, resulting in low efficacy along with a series of local and systemic adverse effects. Microneedle (MN) patches loaded with micron-sized needle array, combining the advantages of oral administration and local injection, have become a potentially novel strategy for the administration and treatment of MSK diseases. In this review, we briefly introduce the basics of MNs and focus on the main characteristics of the MSK systems and various types of MN-based transdermal drug delivery (TDD) systems. We emphasize the progress and broad applications of MN-based transdermal drug delivery (TDD) for MSK systems, including osteoporosis, nutritional rickets and some other typical types of arthritis and muscular damage, and in closing summarize the future prospects and challenges of MNs application.
1. Introduction
The musculoskeletal (MSK) system consists of bones, cartilages, skeletal muscle, tendons, ligaments, and other connective tissues, which is the structural framework of the human body and plays an important role in movement.1 Therefore, it is subject to wear and tear throughout the whole life and vulnerable to trauma and degenerative diseases. Thus, the high prevalence of MSK disorders is conceivable, with about 1.7 billion people worldwide suffering from it. Also, the MSK diseases are the second leading cause of disability in the world, accounting for about 20% of all years of disability.2 Moreover, the morbidity will be gradually ascending with the prolongation of life expectancy and the trend of population aging. Diseases of the MSK system are mainly composed of skeletal diseases, joint diseases, and skeletal muscle diseases, most of which have effects that last a lifetime and seriously reduce the quality of people's lives. It has been reported that the disability and reduced abilities caused by MSK diseases are much more common than the damage caused by some chronic diseases, such as hypertension and hyperlipidemia.3 Meanwhile, MSK diseases are also prone to other complications. For example, in the physical state, the decline or loss of activity may lead to obesity, cardiovascular, and cerebrovascular diseases. Also, patients who have been bedridden for a long time may also be fatally threatened by collapsing pneumonia. On the other hand, in the mental state, due to long-term suffering from illness, patients have a higher risk of developing depression. Therefore, MSK diseases pose a huge challenge to global health, which requires great attention.Nowadays, although medical treatment has made great progress compared to the past, the drug treatment for some MSK diseases is still very limited. Traditional drug delivery methods, such as oral administration and injection, are sometimes not appropriate for treating the MSK diseases mainly due to the anatomical characteristics of the MSK system, which will be discussed in the following part. Briefly, it is difficult for circulating drugs to accumulate to an effective concentration on oral administration since the MSK system lacks blood flow,4 and it inevitably causes adverse effects to other tissues on account of the nonspecific distribution.5 As for conventional injection, it greatly creates fear in patients, causes damage to the injection site and risks infection, which is not conducive to the long-term management of chronic diseases in the MSK system. Therefore, there is an urgent need for a new drug delivery strategy to optimize the administration of MSK diseases at present. It is therefore expected to integrate the respective advantages of oral and injection administration, while avoiding their disadvantages. Transdermal drug delivery (TDD) seems to give a satisfactory answer to it. Attempting to realize the local or systemic treatment through skin, TDD can be divided into three generations according to their development (Table 1).6 Among them, microneedle patch (MNP), an array formed by hundreds of microscale needles attached to the patch, has its unique advantages that can better meet the needs of TDD.
| Generation | Principle | Representative | Advantage | Disadvantage |
|---|---|---|---|---|
| The first | Passive diffusion | Liquid spray, gel, cream | Convenient | Low efficiency |
| Lipophilic and small molecule only | ||||
| Enhancement technique | Vesicles and their analogues: liposomes, ethosomes, niosomes, phytosomes | Enhance penetration | Low reproducibility | |
| High biocompatibility | High cost | |||
| The second | Modifying the SC | Occlusive wet patches | Convenient | May be toxic and causes skin irritation, concentration-dependent |
| Chemical enhancers | Increase drug permeation | |||
| Surfactants | ||||
| Fatty acids, alcohols | ||||
| Providing the driving force | Iontophoresis | Increasing skin absorption; drug delivery scales with the electrical current, providing control over drug dosing | Skin irritation | |
| Skin damage | ||||
Small molecular drugs (<10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) 000 Da) |
||||
| Complex and expensive device | ||||
| Ultrasound | Safe, convenient | Affected by drug | ||
| Increases absorption | Dosage form | |||
| Small molecule only | ||||
| The third | Disrupt or remove the SC | Electroporation | Create microspores in the skin | High-cost; time-consuming; cell damage |
| Sonophoresis | Delivery of various drugs; minimal invasion | Time-consuming, sophisticated instrumentation, healthy skin condition needed | ||
| Thermal ablation | Increase permeability | Costly devices | ||
| Tape stripping/microdermabrasion | Simple and feasible; low price | Low reproducibility | ||
| Microneedles | Simple, efficient, sustainable, minimal invasion and high bioavailability; no extra energy device needed | Low drug-loading capacity | ||
Herein, we will first briefly demonstrate the anatomical foundation including both the skin and MSK system for better understanding how the microneedles (MNs) work in the MSK system. Also, we will explain the advantages and characteristics of MNs used in the MSK system compared with other methods of TDD such as sonophoresis and iontophoresis. Then, we will introduce some evaluations and clinical requirements of MNs used in the MSK system. Finally, we will discuss the application and current challenges as well as the future prospect of MNs in treating MSK diseases.
2. Anatomy foundation and characteristics of MNs for TDD in the MSK system
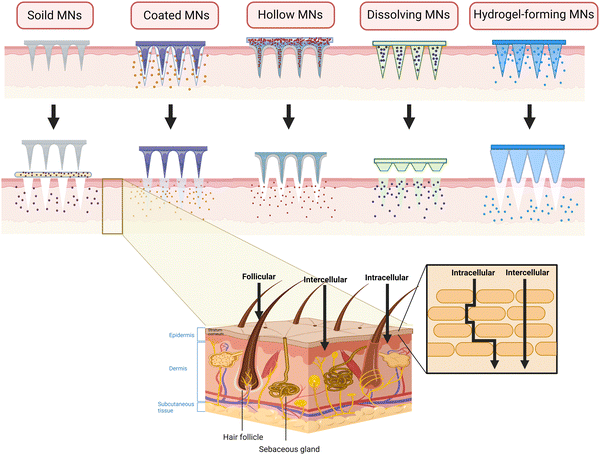
As the largest organ, the skin covers the entire surface of human body with an area of about 1.8 m2. It can be generally divided into three layers from the outside to the inside, which are epidermis, dermis, and subcutaneous tissue layer12 (Fig. 1). Devoid of blood vessels and nerves, the epidermis is free from pain while stimulated. Differently, consisting of the papillary layer and the reticular layer,13 the dermis is rich in blood vessels and free nerve endings. As for the transdermal absorption of drugs, it is now acknowledged that there are generally three pathways,14 including through the stratum corneum (SC), hair follicles, and sweat ducts. Among them, the most important one is the stratum corneum pathway because of its large surface area. However, as the outermost layer of the epidermis, SC is a hydrophobic barrier and only allows lipophilic small molecules (<500 Da) to pass through freely, limiting the effective absorption of transdermal administration on a large scale.15 The other two routes can only absorb negligibly small amount of drugs.16Located beneath the skin and subcutaneous tissue, bone is the supporting structure of the human body. The minerals of bone account for about 60–70%,5 which leads to poor permeability and low blood flow. It is difficult for circulating drugs to accumulate to an effective concentration in the target bone tissue,5 while nonspecific drug distribution inevitably causes adverse effects to other tissues. Joints, as the connection of the two or more bones, is surrounded by the joint capsule in which the articular cartilage is avascular.17 These anatomical properties may prevent the drug that absorbed into the bloodstream orally from reaching intra-articular lesions. As for intra-articular injection, drugs can easily leave the joint cavity through the blood and lymphatic vessels in the synovium within a short time. Thus, it requires an increased frequency of administration to maintain effective concentration in the joint cavity.18 Also, it greatly creates pain and fear in patients and causes a risk of infection. As the component of the peripheral fascial compartment, the skeletal muscle is characterized by low blood velocity and poor blood flow, resulting in efficacy similar to that of bones and joints when administered orally.4
Considering these, TDD seems to be a good option in the drug management of MSK diseases, which provides a satisfactory method for drug management, among which ultrasound and the use of electricity have been proven to be effective.19,20 However, in the MSK system, they may not be perfectly suitable. For instance, some characteristics of ultrasound may limit its application in the MSK system. Firstly, ultrasound has a good effect on the delivery of hydrophobic micromolecules instead of hydrophilic ones. Secondly, the penetration depth is mainly affected by ultrasonic frequency and intensity. The MSK system is located deeply and thus requires a high sound intensity if directly sending the drug to the lesion, which may do great damage to the tissues nearby. Also, achieving precise targeted therapy will be difficult because the microflow generated beside the highly reflective bones is unpredictable.21,22 Compared to ultrasound, electrical methods such as iontophoresis and electroporation, though extend the delivering drug to hydrophilic macromolecules, are still limited to charged molecules within a certain molecular weight and a small loading capacity. In addition, electrical methods can cause great disturbance to the cells and even lead to cell death. The thermal effect of the current can potentially harm the drug molecules. Moreover, it relies on professional personnel and expensive and sophisticated equipment, which is unfavorable for the long-term management of chronic diseases such as arthritis and osteoporosis.23,24 The characteristics of other types of TDD are listed in Table 1, omitting the examples here.
Fortunately, MNs are expected to overcome these limitations. Differently, MNs can directly reach the dermis, where there is abundant blood flow, and can better deliver various kinds of drugs from micromolecules to macromolecules and hydrophilic drugs to hydrophobic ones. They also allow the drugs to permeate to the target site such as bones,25 joint cavity26 and skeletal muscle27 nearby and reach the effective concentration. In addition, the operation of MNs in the drug delivery process, either with bare hands or with an applicator, is relatively simple, freeing from additional auxiliary equipment and professionals, which has great potential in the TDD of the MSK system. When applying, MNs can form microchannels to overcome the barrier of TDD, and the adverse reaction is mainly mild inflammation of the skin, which is often self-limited.28
In comparison to oral drug delivery, MNs can avoid the first-pass effect of the digestive enzymes in the gastrointestinal tract on the drug. Thus, the dosage of the drug can be reduced.29 In addition, it works locally to reduce the systemic adverse reactions of the drug when increasing the local drug concentration.30 In contrast with injection administration, MNs hardly touch the sensory nerve endings,31 making it painless and reducing the risk of infection, which can greatly improve patients’ compliance, thereby further contributing to the smooth and continuous running of healthcare. In short, MNs as a highly promising method for TDD combine the convenience of oral drug delivery with the high bioavailability of injection administration.32 However, due to the relatively deep anatomical position of the MSK system and slightly special construction of the MN system, there are few studies focusing on the application in this field. There is a very broad application prospect for MNs if designed properly.
Generally, MNs can be divided into five types: solid MNs, coated MNs, hollow MNs dissolving MNs, and hydrogel-forming MNs.33 To date, several articles have described each type of MNs in great detail. Herein, we only briefly introduce the characteristics related to TDD in the MSK system.
Made of materials with high hardness, solid MNs have excellent mechanical property and can easily penetrate the SC for pre-treatment by forming microchannels, through which the drugs can easily enter the epidermis and dermis.34 Also, if designed properly, such as the ST-needles, they are expected to reach the target site such as the surface of bones, joint cavity and muscles. However, since solid MNs are drug-free, it requires two-step administration, thus reducing patient compliance in the long-term management of chronic MSK diseases. Also, the microchannels tend to close as time goes by, leaving the drugs on the surface and epidermis, which causes irritation and errors in precise administration.35 The low-elastic tips are prone to be broken, resulting in local foreign body reaction and formation of granulation tissue. To simplify the administration process, drugs are loaded on the surface of the needle to fabricated coated MNs, which have the characteristics of high stability and fast release rate of loaded drugs. They are suitable for relieving the symptoms in acute inflammation such as muscle damage and gouty arthritis. However, their shortcomings are similar to those of solid ones. In addition, the total amount of drug loading is small36 and there is inevitable drug waste because of the mutual frictions with the skin during insertion. Coated MNs are quite suitable for the TDD of potent drugs such as hormones,37 among which PTH-coated MNs has been used to treat osteoporosis. For a better development, it is of great significance to solve the problems of drug loss and loading limitation of MNs. Characterized by a hollow structure in the central part, hollow MNs are similar to that of a syringe and can accommodate more drugs than the coated ones.38 There is no need for excessive processing on the drug formulation, avoiding the damage to the drug activity and allowing hollow MNs to load some macromolecular drugs such as proteins and biologics when treating MSK diseases such as arthritis and muscle fibrosis. However, the sharpness of MNs decreases and it is easily broken due to the hollow structure. The fabrication requirements are stricter and often require a micropump or other pressurized devices to facilitate drug entry into the tissue,39 inevitably increasing the cost.40 Furthermore, the pinhole is so tiny that it can be easily blocked by the relatively dense skin tissue, and the drug may be spilled, wasted, or delivered erroneously during the improper administration process.35 Also, the above three types are mostly disposable, which brings the issues of needle contamination and disposal.
Fortunately, dissolving MNs and hydrogel-forming MNs get rid of the hassle of needle disposal and the risk of cross infection41 because they are made of biodegradable polymers. Also, both of them have increased drug-loading capacity as the drug is encapsulated inside the MNs,42 enabling the MNs to release sufficient drugs to maintain effective concentration in the joint cavity. Better still, the drug release rate can be regulated by selecting different polymer types to meet the different drug release patterns in MSK disease.43 However, their mechanical property is relatively weak, so they sometimes fail to penetrate into the SC.44 Unlike the dissolving ones, hydrogel-forming MNs only swell without degrading when inserting the skin, presenting a crosslinking state as a whole and retaining the shape after water absorption and swelling.45 In addition, the characteristics of these five types of MNs have been summarized in Fig. 1 and Table 2.
| MN types | Solid MNs | Coated MNs | Hollow MNs | Dissolving MNs | Hydrogel-forming MNs |
|---|---|---|---|---|---|
| Disentangles | Poke and patch | Coat and poke | Poke and flow | Poke and dissolve | Poke and release |
| Preparation method | 3D printing, dry or wet etching, laser ablation, electroplating, micromolding etc. | Spray coating, dip coating, rolling coating based on solid MNs | Laser micromachining, deep reactive ion etching, lithographic molding, deep X-ray photolithography, wet chemical etching, and microfabrication | Solvent casting, droplet-born air blowing, laser machining, hot embossing, microinjection molding, ultrasonic welding, lithography, drawing lithography, 3D printing | Micromolding, lithography, etc. |
| Drug loading capacity | No drug loading | Small loading amount, usually less than 1 mg | Large loading amount, suitable for high molecular weight compounds | Flexible drug loading from small to large amount | Flexible drug loading from small to large amount |
| Mechanical property | Superior mechanical properties | High mechanical strength | Relatively low mechanical strength | Low mechanical strength | Low mechanical strength |
| Dimension for ideal targeted tissue | Reach the deep epidermis or the superficial dermis; sometimes can even enter the joint cavity, reach the bone surface, and muscles such as ST needles, if necessary | Can reach the deep epidermis or the superficial dermis | |||
| Advantages | Sharp tips to penetrate the skin effectively, with the possibility to combine with external forces (iontophoresis and sonophoresis) for better TDD | Have the advantages of solid MNs; convenient; improved stability of drugs; reduce exposure risks | Reduce the frequency of insertion with a higher drug loading capacity; realize TDD with a quantitative, precise dosage in a speed-controlled manner, have potential in the delivery of biomacromolecules | Excellent biocompatibility; easy to be fabricated and used, low production cost; no waste after use; good acceptance by patients | Relatively high drug-loading capacity, with the possibility to modify drug release rate; high biocompatibility; no residual in skin |
| Disadvantages | Need two steps to accomplishment, fragile and prone to breakage, low biocompatibility | Low capacity; insertion affected by the coating; drug retention caused by friction | Complex preparation process, high cost; fragile, prone to be broken and clogged; requires external force such as syringe plunger force, pressure difference, and spring elasticity | High requirements of the material; insufficient mechanical strength | Low mechanical strength; relatively higher risk of infection |
3 The evaluation and clinical requirements of MNs in the MSK system
3.1 Dimensional evaluation
The insertion depth and drug-loading amount are related to the dimensions of MNs, which are essential to the treatment of MSK diseases with MNs. Firstly, the longer the length, the better the TDD, especially in the MSK systems, as the target site tends to be internal to the body. Thus, the longer ones can reach deeper even to the target site for better drug diffusion to the lesion. For example, ST-needles, inspired by acupuncture, are long enough to reach the subchondral bone within the joints for TDD.49 Also, the longer ones can load more drugs due to the increased surface area or volume. However, overlong MNs may cause pain, and the efficiency of drug delivery can be lowered since a part of the needle body is exposed outside the skin during insertion. The overall volume or surface area of MNs determines the drug-loading amount, which is quite important to TDD in the MSK system. The MNs with large loading amount can meet the requirement for sustained drug release, effective drug concentration, and reduced frequency of TDD when treating chronic MSK disease such as arthritis. To maximize the loading amount, we can increase the density of the needles and enlarge the area of the patch. However, an enlarged patch can give rise to uneven force and incomplete insertion of needles,50 which can be avoided by using an applicator to reduce the variability of TDD and raise the efficiency of insertion,51,52 among which a film-trigger applicator (FTA) system has been developed to eliminate the complexity brought by the applicators.51 Unlike others, this applicator can deliver the drug into the skin layer through micropillars using the energy of the carboxymethyl cellulose (CMC) film fracture, which may optimize the process of TDD in MSK diseases if introduced properly. Meanwhile, MNP with high density sometimes cause the “bed of nails” effect mainly due to the dispersed and weakened force during insertion.53 A study with subjects found that firstly, high density of the microneedles could significantly lower transdermal efficiency, and secondly, the subjects could tolerate the MNs well when needle length exceeds 1000 μm. No evident skin irritation was observed despite the length being increased to 1500 μm.28 It can provide a guideline for designing an MNP with large loading capacity and the ability of sustained drug release when treating MSK disease.3.2 Mechanical property and behavior of insertion
Applied in the system, MNs are expected to possess excellent mechanical property to penetrate the SC to reach deep enough to the dermis and sometimes to the surface of bones, entering the joint cavity and muscle if needed, facilitating the drug to diffuse into the lesion,54 which puts forward higher requirements for the mechanical property. Insertion through the SC to the dermis can be examined by various experiments such compression test55 and nanoindentation.56 With the former test, the average fracture force57 and safety factor58 (defining as the fracture force/insertion force, usually the force is about 0.098 N per needle57,59) can be obtained. Also, the nanoindentation can obtain the hardness value and Young's modulus (usually more than 1 Gpa60) of each MN instead of the average value with the Oliver–Pharr method56 for more accurate results. Note that both the experiments are conducted on the machine instead of the real skin, unavoidably giving rise to errors. To overcome this, we can use skin simulants such as sealing film (Parafilm M®) or aluminum (Al) foil,61–63 and the skin of animals64 and subjects28 to characterize it. After insertion, the actual penetrating depth in the simulants can be speculated by the pierced layers while the insertion behavior in the skin can be studied by staining the skin with dye,64,65 cryosection,57 and even optical coherence tomography (OCT).55 When the needles are needed to reach the surface of the bones and enter the joint cavity and muscle, unlike the skin, there is no specific method or quantitative value to determine whether the MNs are qualified. In the study of ST-needle being directly inserted into the joint cavity, researchers cut the cartilage open to identify the actual position the needle had reached.49 Further study is expected to be conducted to fill the gaps for better promoting the development of MNs in the MSK system.3.3 Drug release property of MNs
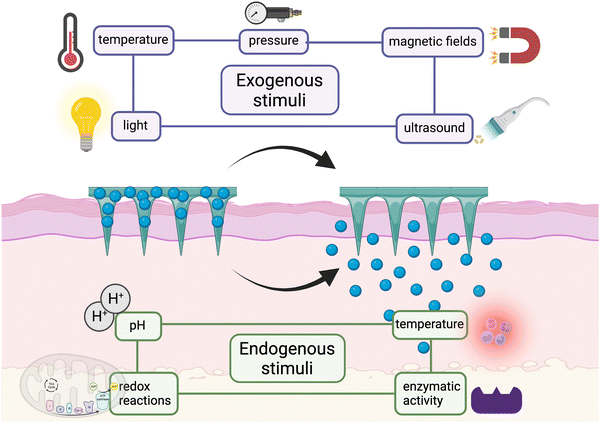
Commonly, the patterns of drug release can be classified as instant release, sustained release, and stimulus-responsive release, suitable for different circumstances. Instant drug delivery is mostly used for the treatment of acute states such as the attack of pain and acute inflammation by arthritis and muscle damage, which requires a massive release of the drug within a short period of time to relieve the symptoms. Differently, sustained drug release is suitable for the long-term management of chronic diseases such as chronic arthritis and osteoporosis for achieving a stable and effective drug concentration in the target site while reducing the adverse effects. As a kind of on-demand drug delivery, stimulus-responsive drug release is quite promising and can be used for some ever-changing or acute attack of MSK diseases such as arthritis and muscle damage. According to the source, stimulus can be divided into endogenous stimulus (pH, redox, and enzymes) and exogenous ones (temperature, pressure, and light) (Fig. 2). For example, during an acute phase of arthritis, the locally increased temperature and acid production can become the stimulus to design thermoresponsive66 and pH-responsive MNs,67 respectively. The strategies of detailed preparation have been mentioned in other articles68,69 so we don't discuss them here. Moreover, if different stimulating factors can be combined properly, undeniably the specificity of MNs can be improved greatly since single stimulant sometimes fails to mediate the TDD in the complex changes of signals and microenvironment.70 For instance, the MNs can run in the pattern of sustained release in the long term management of arthritis, but when acute attack occurs, it can quickly transform into an instant release one.To study the release behavior of MNs, some experiments need to be carried out. Usually, we can detect the releasing amount of the drug by putting the drug-loaded MNs in the medium fluid71 or using the Franz diffusion cells.72,73 The procedure of using the Franz cell have been shown in Fig. 3. Sometimes, if the result accuracy or the actual distribution of drugs in the lesion is required, we can determine it by labeling molecules with fluorescence.
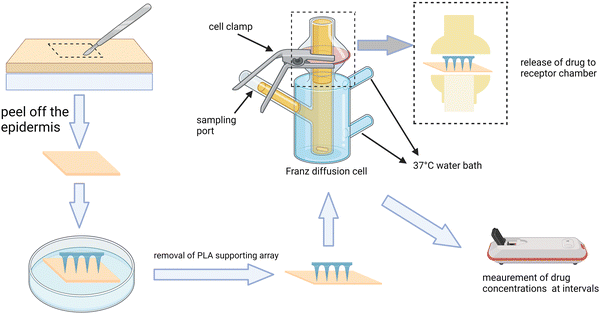 | ||
| Fig. 3 The procedure of using the Franz cell to determine the release property of MNs. This figure was adapted from the ref. 73. | ||
4. Application of MNs in the musculoskeletal system
Herein, the current treatments of some common MSK diseases will be described combined with MNs (Table 3). Furthermore, we will also draw the blueprint based on the advance treatments that can be hopefully integrated with MNs for the treatment of MSK diseases.| Disease | Administration route | Payload | Fabricating material | MNs' type | Advantage | Ref. |
|---|---|---|---|---|---|---|
| Poly(vinyl alcohol) (PVA), poly(vinyl pyrrolidone) (PVP), polyacrylamide (PAM). | ||||||
| Osteoporosis | Transdermal | Teriparatide | Polycarbonate, titanium metal | Coated MNs | Delivery of a consistent and therapeutically relevant PTH PK profile | 85 |
| Osteoporosis | Transdermal | Alendronate | HA | Dissolving MNs | Completely dissolves and resorbs in the skin; creates micron-scale pores into the skin for the transdermal delivery of macromolecular, highly polar, and hydrophilic drugs | 82 |
| Osteoporosis | Transdermal | Alendronate | Glycerin, BHT | Dissolving MNs | High absorbability of water-soluble drugs and high molecular drugs such as peptides and proteins. | 89 |
| Osteoporosis | Transdermal | Alendronate | HA | Dissolving MNs | Rapid release and absorption without skin irritation | 25 |
| Osteoporosis | Transdermal | PTH (1–34) | HA | Dissolving MNs | High storage stability; no skin irritation | 86 |
| Osteoporosis | Transdermal | Teriparatide | HA, TA, PU films | Dissolving MNs | Efficient transdermal delivery ability; minimizing TA activity reduction | 87 |
| Rheumatoid arthritis | Transdermal | Methotrexate | PVP K90 | Dissolving MNs | Better therapeutic performance compared with cream or conventional oral administration; stable drug loading and fast intradermal dissolution | 90 |
| Rheumatoid arthritis | Transdermal | Tetramethylpyrazine | PVP K90 | Dissolving MNs | Safety and biocompatibility to human fibroblast synovial cells; effective alleviation of inflammation and synovial tissue damage | 91 |
| Acute gout arthritis | Transdermal | Colchicine (Col) | PAM | Hydrogel-forming MNs | Mechanical and ultraswelling properties and sustain Col-releasing | 92 |
| Rheumatoid arthritis | Transdermal | MTX and polydopamine/PDA@MnO2 | PVP | Dissolving MNs | A new RA treatment by combing chemotherapy and antioxidative treatment | 93 |
| Rheumatoid arthritis | Transdermal | Melittin | MeHA | Dissolving MNs | Enhancing administration convenience and modulating release properties of the loaded drug | 94 |
| Rheumatoid arthritis | Transdermal | Tofacitinib | PVA | Dissolving MNs | Effective properties in restoring histological alterations (synovial hyperplasia and constriction, cartilage and articular erosions) | 95 |
| Psoriatic arthritis | Transdermal | Tacrolimus (TAC) and diclofenac (DIC) | HA, PVP K17, Dextran (w/v) | Dissolving MNs | Effectively alleviating skin lesions in psoriasis; reducing serum markers and arthritis symptoms caused by psoriatic arthritis | 26 |
| Collagen-Induced Arthritis | Transdermal | DEK-targeting aptamer (DTA) | HA | Dissolving MNs | Strong stability and anti-inflammatory efficacy; joints protection from cartilage/bone erosion in collagen-induced arthritis (CIA) mice | 96 |
| Juvenile idiopathic arthritis | Transdermal | Methotrexate | PVA, PVP | Hydrogel-forming MNs | Minimal toxicity and maximal biocompatibility of MTX | 97 |
| Rheumatoid Arthritis | Transdermal | Neurotoxin | PVP, CS and CMC | Dissolving MNs | Penetrate the skin in a biocompatible manner; strong therapeutic effects on rat RA by transdermal delivery | 98 |
| Rheumatoid Arthritis | Transdermal | Etanercept | HA | Dissolving MNs | Good biocompatibility; little influence on the bioactivity of EN; high anti-inflammatory efficacy | 99 |
| Arthritis | Transdermal | Paeoniflorin | silicon | Solid MNs | Enhance the penetration and storability of paeoniflorin | 100 |
| Arthritis | Transdermal | Meloxicam | PVA and PVP | Dissolving MNs | High penetration efficiency, advantageous stability and safety | 101 |
| Rheumatic arthritis | Transdermal | Powder capsaicin | HA, PVP | Dissolving MNs | Applicable for delivering drugs lack of appropriate solvents, with high molecular weight and low bioavailability | 102 |
| Rheumatoid arthritis/collagen-induced arthritis (CIA) | Transdermal | Triptolide | PVP K90 | Hydrogel-forming MNs | Effectively avoiding the toxic effects of triptolide and providing stable long-term release; significant therapeutic effects in the CIA model | 103 |
| Muscle injury | Transdermal | Aspirin or ibuprofen | PLGA | Dissolving MNs | Combined treatment using medication and electrostimulation; activated wirelessly for electrostimulation and sustainable delivery; fabricated with bioresorbable materials | 104 |
| Muscle injury | Transdermal | Carbonized wormwood and prostaglandin E2 | PVA | Dissolving MNs | Carbonized wormwood imparted the MNP with near infrared light heating characteristics that improved the efficiency of prostaglandin E2 delivery while also promoting circulation in the damaged muscle area | 27 |
| Achilles tendinopathy (AT) | Transdermal | Exosomes (EXO) derived from tendon stem cells (TSCs) | GelMA, PVA, HA | Hydrogel-forming MNs | The special array dedicated to joint parts keeps the MN tips stabilized after detachment; the NO in AT microenvironment enhances the treatment effect | 105 |
| OA | Transdermal | Hydrogel microspheres, PARKIN protein | Stainless steel | ST-needle | Using dynamic spiral Mosaic technology with thermosensitive adhesive triblock copolymer to transfer drugs and heat | 106 |
4.1 Osteoporosis
Osteoporosis is characterized by low bone mass and deterioration of the microstructure of bone tissue, followed by bone weakness and susceptibility to fracture74 as the rate of bone resorption is greater than that of bone formation.75 The current primary goal of treatment is to prevent or slow down the bone loss and avoid fracture by taking drugs such as bisphosphonates (BPs), parathyroid hormone (PTH), and estrogens orally or intravenously.76However, the side effects of such administration cannot be ignored. For example, oral BPs can cause nausea and gastrointestinal symptoms such as abdominal pain, gastritis, and erosive esophagitis,77,78 while intravenous BPs may lead to kidney damage, mandibular osteonecrosis, atrial fibrillation, and other serious side effects.79 Also, about 1/3 of patients may experience transient fever, muscle and joint pain, and other acute reactions for the first time.80 Therefore, it is urgent to seek out a new method to optimize the drug administration, and MNs seem to be an appropriate way to satisfy it.
For this purpose, Katsumi et al.25 fabricated dissolving MNs with hyaluronic acid, which are only loaded with the alendronate, a kind of BPs, in the needle tip to treat osteoporosis. It shows that the bioavailability of the drug increases to 96% and in the rat model, it achieves the therapeutic efficacy without causing evident irritation in the treatment of osteoporosis by regulating bone resorption by osteoclasts. In contrast to the former one that is loaded with alendronate in the whole needle, the tip-loaded MNs can improve the bioavailability while reducing irritation to the skin.81,82
Unlike BPs, parathyroid hormone (PTH) is an anabolic hormone that can stimulate bone formation to exert anti-osteoporosis effects, mainly by coordinating the balance between bone resorption and formation. Low-dose PTH has synthetic metabolic function while the high-dose one exhibits the catabolic effect.83 Therefore, aiming to exert anti-osteoporosis effects, intermittently and tightly controlled dosage administration of PTH is required to achieve the anti-osteoporosis effect.84 In this case, the commonly used subcutaneous injection of human PTH (1–34) peptide such as FORTEO® has its shortcomings. For example, patient may fail to receive regular treatment since their compliance will be reduced due to the invasive operation, which will have a negative effect on the anabolic effect of PTH. Besides, it requires strict refrigerated storage conditions, which troubles the patients much.
To improve the treatment protocol, Daddona et al.85 developed an MNP system coated with PTH (1–34) (ZP-PTH) that can stay stable at room temperature for more than 2 years and causes little discomfort to the patient. Regrettably, its bioavailability is only 34% compared to the subcutaneous injection due to the short needle (190 μm), failing to reach the dermis effectively. Also, made of metal, the MNs have the risk of fracture during insertion, resulting in long-term residue in the body. In another research, Chihiro Naito et al.86 designed a dissolving MNs composed of hyaluronic acid (HA), a degradable material to avoid the residual needles. They also increase the length of the needle to 800 μm, enabling the MNs to reach the dermis layer directly, which can improve the bioavailability to 100 ± 4%, as expected. Undeniably, during the preparation, processes such as drying, may increase the drug loss and reduce the efficacy of the drugs. To maintain the stability, Jeeho Sim et al.87 added trehalose to the HA MNs loaded with teriparatide to form bonds with protein drugs, thus slowing down the protein dynamics. After adding 10% trehalose, the activity of the drugs increases to 75.4 ± 8.3% while the activity is only 59.0 ± 3.6% in the control group.88
Besides, estrogen has the effect of preventing osteoporosis by affecting the balance between bone resorption and formation.107 Due to the lack of estrogen, postmenopausal women are vulnerable to osteoporosis due to the decreased ovarian function and it is generally recommended for women with contraindications to BPs.108 In light of the adverse effects associated with high doses of estrogen intake, such as breast pain or postmenopausal vaginal bleeding, the clinical application is very strict in the selection of estrogen dosage, medication regimen, and indications.109 To avoid this, transdermal low-dose of estrogen with MNs can establish and maintain therapeutic levels in vivo as well as reduce the risk of gallbladder disease.110 For better dose control, the electronically-controlled MNs can be introduced to the transdermal management of estrogen, achieving automated on-demand drug release and regulating the drug release rate by applying different voltages.111
As mentioned above, though effective and with fewer adverse effects as expected from the MNs, the skin irritation mainly caused by the long retention time of the drug such as alendronate cannot be ignored sometimes.81 From this point, we can design an MNP combined with electroosmosis112 and gas-propelled technology to promote the drug to reach the blood flow, shortening the retention time in the upper dermis, which can reduce skin irritation to some extent. Reactive oxygen species (ROS), produced and released from the inflammatory such as macrophages and neutrophils, is the main cause of skin irritation.81 Therefore, adding some antioxidants such as butylhydroxytoluene (BHT)113 and selenium (Se)114 may be effective.
4.2 Nutritional rickets
Characterized by the decreased level of serum calcium and/or phosphate, rickets is associated with the defective mineralization of the growth plate in the childhood.115 There are many subtypes of rickets, among which nutritional rickets is the most common one and mainly results from the deficiency of vitamin D and/or calcium.116 Calcium salts, whose deficiency can be easily corrected with diet or calcium supplements, have the ability to mineralize osteoid into mature bone. VD3, also known as calcitriol, is the main active form that regulates bone mineralization by balancing calcium and phosphate levels. VD is synthesized mainly by endogenous pathways (as shown in the diagram), while a small amount of it is taken from food. VD deficiency is commonly seen due to (1) insufficient sunlight exposure, which leads to insufficient VD synthesis in the skin; (2) insufficient intake in diet; (3) VD malabsorption caused by hepatobiliary diseases and short bowel syndrome; (4) drug factors such as long-term use of anticonvulsants such as phenobarbital, which affects VD metabolism.At present, although it is relatively economical to prevent and treat rickets by appropriately increasing the time of sun exposure and by strengthening the diet,117 in some special factors, such as the dark-skinned who immigrate to high latitudes, special clothing for religious reasons, and excessive use of sunscreen, sunlight alone is possibly inadequate. In addition, the amount of VD contained in regular diet is very small, making it difficult to achieve effective supplementation, especially for patients with digestive system diseases. Thus, oral or intramuscular supplementation of VD3 is required occasionally. When taken orally, VD has poor solubility in the gastrointestinal tract since it is fat-soluble, along with low absorption rate, low bioavailability, and the first-pass effect in the liver, leading to an unsatisfactory effect.118 Intramuscular VD also has the corresponding disadvantages mentioned above, such as pain and infection. Therefore, there is a great need to seek new methods that overcome these shortcomings.
Considering the small recommended daily therapeutic dose of VD, MNs can be applied for transdermal administration, which can mimic natural light-based biosynthesis of VD when overcoming the above mentioned shortcomings of oral or injectable administration. In the delivery strategy of MNs-loaded VD, nanoparticles (NPs) are often used for VD encapsulation to reduce the inherent cytotoxicity and solve the problem of high sensitivity of VD to the external environment. Currently, some studies have adopted polylactic-co-glycolic acid (PLGA) enveloping VD to fabricate NPs, which are able to control the release rate and dose of VD flexibly by adjusting the molecular and composition proportion of NPs. Kim et al. used coated MNs loaded with PLGA-D3 nanoparticles and achieved an efficiency of 81.08% for TDD.119 In another research, Lalit K. Vora et al. loaded PLGA-D3 NPs into the tips via centrifugation and fabricated bilayer dissolving MNs, which exhibits a typical tri-phasic release profile in vitro for more than 5 days and effectively reduce the waste of drugs to a certain extent.120,121 Better still, both the MNs showed superior administration efficiency compared with the ointment patch without MNs.
However, when preparing NPs, the process such as heating and the use of organic solvents, will have a negative influence on the stability of VD. Made of cyclodextrin (CD) derivatives, the supramolecular dissolving MNs (CD DMN) have been developed to load hydrophobic drugs directly and free from the usage of NPs, which can be an alternative method to prepare VD-loaded MNs.122 Due to the various noncovalent bonds inside the MNs, it can provide sufficient mechanical property to penetrate the skin and enable the drug molecule to diffuse into deep layers of the skin rapidly. Though simplifying the process, the cytotoxicity of the VD in this case needs to be determined for safety.
4.3 Rheumatoid arthritis
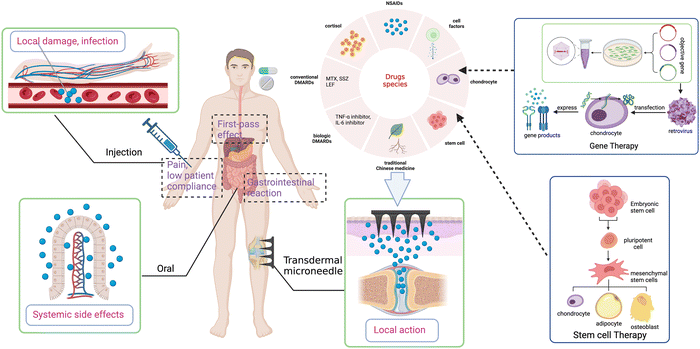
As a chronic systemic autoimmune disease, rheumatoid arthritis (RA) is characterized by synovial inflammation, leading to cartilage destruction and bone erosion eventually.123,124 The current drug treatments including taking nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), and disease-modifying anti-rheumatic drugs (DMARDs) have the effects of anti-inflammation, pain relief, and immunosuppression.125Oral NSAIDs is quite common in the treatment of RA but still has complex adverse effects such as gastrointestinal and cardiovascular hazards. Thus, long-term oral NSAIDs are not recommended for the treatment of RA.126,127 MNs seem to provide an alternative method to avoid the side effects and low bioavailability of NSAIDs in oral administration (Fig. 4). Sonja R et al.128 used poly(lactic acid) (PLA) as a carrier for ketoprofen, a kind of NSAIDs, to fabricate silicon MNs to increase the solubility, half-life, and bioavailability of the drug molecule, reaching a better topically therapeutic effect.
Though potent in the efficacy of anti-inflammation, taking GCs orally has more serious and extensive side effects than NSAIDs, such as osteoporosis, femoral head necrosis, endocrine imbalance, and immunosuppression. Therefore, it is not adopted as a long-term treatment of RA, but is only used in low doses during acute episodes. Topical administration such as intra-articular injection can send the GC directly to the target site, reducing the systemic side effects, but the pain, secondary damage, and rapid clearance cannot be ignored.129 At this point, MNs can be an ideal choice, but unfortunately, there are very few studies that develop MNs to load GC for the treatment of RA, which is worth further study.
Differently, as the second-line treatment, DMARDs can delay the progression of RA but act slowly and have no effect against acute inflammation. Some DMARDs such as minocycline are administered intravenously, which is an invasive operation, damaging the blood vessels and skin in a long-term management. It also reduces the compliance of the patients and sometimes results in infection. Others such as MTX and leflunomide are generally oral administration and have the gastrointestinal symptoms and extensive systemic side effects. Fortunately, it proves that MNs can be an excellent alternative strategy for the prolonged administration of DMARDs without much side effects mentioned above.130 Vemulapalli et al. fabricate MTX-loaded MNs combining iontophoretic (ITP), which can promote the permeation of the drugs via local current on the skin surface, enabling the drugs to reach Cmax easily and controlling the dosage better.131 In another study, hydrogel-forming MNs have been designed for MTX delivery, which can delay the peak time while the total amount of drugs is 1.2 times higher than taking orally within 48 hours.97 If ITP is combined, about 25-fold of the drug amount is observed, which is quite promising.
In addition, as a kind of acupuncture techniques of TCM, the bee sting therapy is often applied to arthritis treatment. Based on MNs system, a bee venom gel (BVG) has been designed to mimic bee sting therapy and exert anti-inflammation effect,132 which is consistent with the previous clinical trials.133 In another research, researchers prepared HA MNs loaded with melittin, releasing the drug in a sustained way to maintain a stable drug concentration topically.94 Also, it functions by suppressing the levels of pro-inflammatory cytokines such as IL-17 and TNF-α as well as upregulating the percentage of CD4 T cells. Though effective, fabricated on a plane, MNs sometimes cannot be fully inserted into the skin and release enough drugs due to the individual differences and curved surface of the joints.134 To address this, MNs with specific curve surface have been fabricated via 3D printing, achieving the goal of personalized medicine. Unfortunately, mainly because the thickness of the skin samples has not been assessed yet, its penetrating rate is only 64%, lower than that of the average rate of 90%.134 For a better effect, the condition and thickness of patients’ skin should also be fully taken into account in further studies.
4.4 Osteoarthritis
As a chronic inflammatory disease, OA is manifested as pain, loss of function, stiffness, and swelling in multiple joints of the body.135 The pathogenesis of OA is complex and has not yet been completely elucidated, mainly involving metabolic and physical injury and inflammatory factors.136 Therefore, the treatment in clinical practice is symptomatic, aiming to control the inflammation, relieve pain, and improve the quality of life. Similar to the RA, taking NSAIDs and GCs is a conventional treatment. Among them, celecoxib (CXB), as a novel NSAID, can selectively inhibit cyclooxygenase (COX)-2 and has better anti-inflammatory and analgesic effects and fewer adverse effects compared to others in the treatment of OA.137 But its poor water solubility and low bioavailability have limited the clinical application on oral intake.138 Besides, the intra-articular injection of some nutritional supplements to protect the cartilage from damage and taking some disease-modifying OA drugs (DMOADs) to control the process of OA are applied to the treatment of OA.139 Also, traditional Chinese medicine (TCM) has also been explored for the better treatment of OA. However, conventional administrations of such drugs have the similar side effects mentioned above, and MNs seem to offer a perfect solution.For a better therapeutic effect, Qiuyue Wang et al.140 developed dissolving MNs loaded with CXB-nanocrystals (CXB-NCs), which can significantly improve the water solubility and loading capacity of CXB in MNs while avoiding the first-pass effect. It can effectively modify the inflammatory cell infiltration, joint destruction, and swelling within the joints in the rat model with sustained drug release. Made into microemulsion, α-linolenic acid can be also used to improve the water solubility of CXB.141,142 Notably, α-linolenic acid has also been shown to have a pharmacological effect of inhibiting the production of COX (especially COX-2) at the site of inflammation.143 Therefore, when loaded in MNs, CXB and α-linolenic acid can exert synergistic anti-inflammatory benefits, allowing MNs to carry a relatively low dose of CXB to alleviate the inflammation of joints with less side effects compared with the conventional administrations.141 As the main active ingredient of tripterygium in TCM, triptolide (TP) has immunosuppressive and anti-inflammatory effects by reducing inflammatory factors and downregulating the expression of matrix metalloprotein,144 which is appropriate for the treatment of OA. Unfortunately, its poor water solubility and severe toxicity to several systems of the body have limited its clinical application.145,146 To overcome this, Ping zhou et al.144 prepared dissolved MNs loaded with triptolide liposome (TP-Lipo) via ethanol injection method. It can markedly alleviate the symptoms, cartilage damage, and swelling of the joints as well as reduce the levels of inflammatory factors such as TNF-α, IL-1β, and IL-6. Better still, after applying the MNs, blood biochemical analysis shows lowered systemic toxicity. Besides the OA, triptolide has also been encapsulated into the liposome to fabricate hydrogel MNs patch, which functions in the collagen-induced arthritis (CIA).103 Also, for MNs delivery of TP, choosing an appropriate encapsulation method to increase its water solubility and reduce the toxicity is of significance to clinical application.
Currently, several anti-arthritis drugs suffer from poor water solubility and/or route of administration-dependent adverse effects to varying degrees. Also, MNs are combined with techniques such as solubilizers,147 salinization,148 solid dispersion,149 nanoscale bar150 as well as the nanocrystals and microemulsions mentioned above to increase the solubility of the drug molecules for better TDD. In addition, the rapid clearance of the joint cavity will greatly reduce the efficacy of TDD.151 In this situation, some nanomaterials such as liposomes, micelles, and nanoparticles have been combined with MNs to solve the problems, improving the bioavailability and half-life of the drugs as well.97,140,150,152 However, the toxicity of nanomaterials is not negligible, though sometimes their surface has been modified with certain polymers or compounds to minimize the toxicity.153 Further elucidating and eliminating the toxicological effects of nanomaterials to obtain the optimal therapeutic effects is of great value for the TDD administration of various types of arthritis with MNs.
Acupuncture, as a mature traditional Chinese medicine therapy, occupies a certain position in the treatment of osteoarthritis. Inspired by acupuncture, Feng Lin et al. modified the Chinese acupuncture needle (CA needle) to design ST needle loading Lipo as the carrier of baicalin with a threaded tip structure for transporting the hydrogel to the subchondral bone.49 After applying the ST needle, the levels of inflammatory cytokines 15-LOX-1 and TGF-β1, which function in the development of OA, were significantly decreased after treatment with ST needle. Also, it promotes the bone marrow mesenchymal stem cells’ migration to the surface of the cartilage through the micron-sized pores left after the ST needle to participate in repair. Furthermore, loss of autophagy resulting in increased apoptosis in mitochondria in aging cells is another important mechanism in the development of OA,154,155 which means if mitochondrial autophagy can be restarted and mitochondrial apoptosis can be inhibited, the repair of chondrocytes could be dramatically improved. Studies have shown that PARKIN protein plays an important role in the regulation of mitochondrial autophagy, and thermal energy can rapidly inhibit chondrocyte apoptosis caused by mitochondria. Lin et al. designed heat transfer microneedles with spiral Mosaic micro/nanohydrogel microspheres (ST needles).106 Hydrogel microspheres containing the biological factor PARKIN protein are loaded into the threaded groove of the ST needle using a thermosensitive adhesive triblock copolymer so that they can pass through the barrier of cartilage on the ST needle with the double protection provided by the threaded groove and the viscous polymer. In response to thermal stimulation with molecular chain motion, the hydrogel microspheres will detach from the needle body. The results showed that the hydrogel microspheres could accumulate stably in the cartilage for a long time without being cleared and release PARKIN protein to induce mitochondrial autophagy. At the same time, by heating the needle with specific instrument, the good thermal conductivity of ST needle enables the transference of heat to deep lesion site and inhibit mitochondrial apoptosis rapidly. In this way, the targeted regulation of mitochondria to treat OA can be achieved.
In addition, there are numerous cutting-edge treatments such as platelet-rich plasma (PRP) injection and gene and stem cell therapy for arthritis that deserve attention. Obtained from autologous whole blood after centrifugation, PRP contains high concentrations of platelets, white blood cells, and fibrin.156 After transfusion to the lesion site, various growth factors released from activated platelet can stimulate and accelerate tissue repair and regeneration.157,158 PRP is the best source of natural growth factors, without the risk of disease transmission and rejection.159 This technique has been clinically verified and is approaching maturity. So far, PRP treatment has been performed by traditional injection. If we introduce microneedles to deliver PRP, it is expected to improve the accuracy and continuity of injection and shows promising potential to bring better therapeutic effect and acceptance of patients. In gene therapy, after transduction by a retroviral, chondrocytes that can express the genes encoding chondrogenic growth factors and anti-inflammatory cytokines are injected intraarticularly or intravenously into the targeted joint, leading to sustained intra-articular synthesis of the gene products for therapeutic effects,160 which has been proved in animal models of OA and RA.161 It is revealed that mesenchymal stem cells (MSCs) can control inflammation and immune response, repair cartilage, and improve clinical symptoms in arthritis,162 which is available in the therapy of arthritis. However, gene or stem therapy is mainly delivered by injection, with the uncertainty of cell viability and migration to target tissues due to the limited migratory capacity of MSCs, which directly affects clinical translation.163 Recently, the GelMA-MNs system has been applied to culture and deliver MSCs, improve the viability of MSCs, and achieve more precise delivery.163,164 Unfortunately, in the field of arthritis, gene or stem therapy combined with MNs remains a blank and has great potential.
4.5 Skeletal muscle damage
Skeletal muscle is mainly composed of muscle fibers, extracellular matrix (ECM), and other supporting tissues, among which ECM constitutes about 10% of the skeletal muscle framework structure, playing an important role in force transmission and repair of muscle fibers after injury.165 Commonly seen daily, skeletal muscle damage varies from minor injuries (strains and contusions) to severe ones such as volumetric muscle loss (VML), resulting from high-energy traffic injuries, blast injuries, surgery, and exercises.166 Generally, skeletal muscle has a certain capacity for self-repair, while beyond the threshold, complete regeneration seems to be impossible, which will result in a loss of contractile tissue and excessive fibrosis of ECM, leading to muscle fibrosis.167 Though nonfatal, muscle fibrosis will damage the structure and function of skeletal muscle and impair muscle regeneration while increasing susceptibility to re-injury,168 thus reducing the patients’ quality of life in the long term.Early inflammatory response is mainly about recruiting inflammatory cells, producing inflammatory cytokines and clearing damaged tissue in preparation for the subsequent repair process, which is critical for muscle repair.169 Actual repair begins with two simultaneous processes, the regeneration of damaged muscle fibers (and nerves) and the formation of connective tissue scarring, which are in synergy and competition at the same time. Once the balance is disturbed, excessive fibrosis will occur, mainly resulting from the persistent inflammation170,171 and high level of ROS. The persistent inflammation will increase the secretion of profibrotic cytokines such as transforming growth factor-β1 (TGF-β1), CTGF, and muscle inhibitors.165 Among them, TGF-β1 plays a central role in the fibrosis process,172 which is consistent with the result that in rats with glycerol-induced skeletal muscle injury,173 the treatment with TGF-β1 can bring about extensive fibrosis due to the inhibition of the muscle regeneration, especially in the early stage. Similar to the early inflammatory response to injury, a moderate level of ROS can promote the repair of skeletal muscle while excessive ROS, caused by the mitochondrial dysfunction in the myocyte, will impair the number and activity of satellite cells (SCs), which initiate muscle regeneration, thus contributing to a blockage of muscle regeneration.174 Although the inflammatory response and ROS are two different major factors in fibrogenesis, they do promote and influence each other.175
Currently, the treatments for muscle damage are mainly related to RICE principles, drugs, and surgery. The conventional drug treatments are nonspecific and often inhibit inflammatory pathways, including NSAIDs, COX-2 inhibitors, and corticosteroids, which may lead to poor contractility and progressive loss of muscle strength when taken for a long term,176 making serious muscle injury difficult to deal. Here, based on the key aspects of the pathophysiological process after injury, we will focus on how to promote repair and inhibit the fibrosis of muscle with MNs to paint a blueprint for MNs in the field of skeletal muscle injury (Fig. 5).
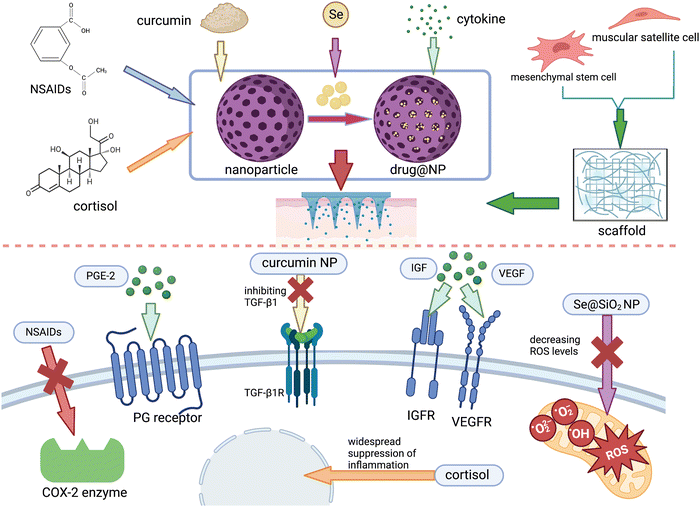 | ||
| Fig. 5 A summary of advanced treatments combined with MNs in muscle damage from ref. 27, 167, 182 and 186 and created by authors. | ||
In terms of promoting repair, Chuxi Zhang et al.27 developed a MNP photothermal system containing carbonized wormwood and prostaglandin E2 (PGE-2), in which PGE-2 can promote the proliferation of muscle stem cells via signal transduction at EP4 receptors, while carbonized wormwood can stimulate microvascular dilation and improve local blood circulation due to its property to impart near-infrared photothermal. This system can avoid the problems of local overheating and smoke contamination that occur during traditional moxibustion and is proved to be effective in the rat model. In addition, growth factors secreted after the onset of injury are important for endogenous repair, the absence of which will contribute to poor muscle regeneration and scar formation.177 To promote regeneration, appropriate amounts of growth factors such as VEGF, IGF, and SDF-1a are supplemented either intramuscularly or intravenously178 and have several side effects. For example, when delivered intravenously, the concentration at the target site will be very low since the half-life is very short and easily removed in the blood circulation. Worse, it may also trigger cardiovascular complications and tumors. In the case of intramuscular injection, although with less frequent systemic complications, the injection itself is an invasive operation that may cause additional damage. To avoid these, MNs loaded with growth factors can be designed to achieve minimally invasive drug delivery, and the stability of the drug molecule may be improved while achieving a controlled release pattern when encapsulating with the appropriate nanomaterials.
For muscle fibrosis, the state of persistent inflammation and excessive oxidative stress are important promoting factors,179,180 which we can inhibit the process of fibrosis. Ya Huang et al.104 developed an anti-inflammatory electronic MNs device consisting mainly of a drug-loaded MNs and a radio frequency-based wireless power transmission system. It reduces inflammation and relieves pain by releasing anti-inflammatory drugs aspirin and ibuprofen, as well as regulate the behavior of skeletal muscle cells and tissue regeneration through the periodic electrostimulation generated by the electrical device. However, this system requires an extra RF-powered device, making it complex and unusable when the device is damaged or not available. Considering this, self-power electrical stimulation system can be combined with MNs, which can convert the mechanical energy of finger sliding into electrical energy via the sliding independent friction nanogenerator, free from the external power source. This system has been applied to the research in the field of vascular regeneration and wound healing,181 from which the treatment of skeletal muscle injury can learn. However, it has been verified that taking NSAIDs for a long term can negatively affect skeletal muscle regeneration and contractile function, probably due to their extensive effects on the immune system, affecting the central role of inflammation in the healing process. Accordingly, studies have now targeted inhibition on the key targets of fibrosis such as TGF-β1, CTGF, and muscle inhibitors. Chitosan (Cs) and curcumin (Cn) can inhibit TGF-β1 and thus reduce inflammation and muscle fibrosis,182 but their large molecular weight and poor water solubility make it difficult to achieve effective blood concentrations at the injury site via conventional administrations.183 To conquer this, Mohamed A. A. Mahdy et al.167 prepared Cs and Cn as nanoparticles for intraperitoneal injection in a rat model of glycerol-induced skeletal muscle injury and found that they were able to significantly reduce fibrosis, attenuate inflammation during the regeneration phase, and promote muscle regeneration. In a way, MNs system can substitute for intraperitoneal or intravenous administration to reduce the dose and systemic side effects.
Besides persistent inflammation, oxidative stress is another key factor in fibrosis; thus, ways to avoid excessive oxidation is of great significance in anti-fibrosis. Based on this, we can prepare drugs with antioxidant properties such as selenium,184 melatonin,185 angiotensin II antagonist,178 and acetylcysteine178 by wrapping them with nanomaterials and using MNs as a carrier to improve the oxidative environment at the site of injury. Yu–Xia Yang et al.186 prepared porous Se@SiO2 NP by encapsulating Se element with SiO2, allowing the slow release of Se from the porous material to attenuate its toxicity. Also, it has been confirmed that the mitochondrial function and high level of oxidative environment were improved in the rat models via intraperitoneal injection. They found that after treatment by intraperitoneal injection, the mitochondrial function and high level of oxidative environment were improved in the rat models, promoting the proliferation and functional differentiation of SCs and attenuating fibrosis. We can accordingly load Se@SiO2 NP on MNs to improve the targeted delivery level of this drug, thus reducing the dose of Se elements and minimizing its toxicity to the organism. In addition, melatonin has also been studied in aged mice and found to reduce mitochondrial damage, decrease intracellular oxidative stress and autophagic state, increase the number of SCs, and inhibit skeletal muscle atrophy. Thus, melatonin could be considered for use in the treatment of skeletal muscle injury in a high level of oxidative stress sate.187,188 However, it is known that melatonin is secreted in vivo by the pineal gland and has a circadian rhythm-regulating effect, which may lead to sleep disorders if excessive exogenous melatonin is ingested; moreover, there may be a risk of hypogonadism or infertility. Therefore, the topical delivery of melatonin by MNs could be included in future studies for the treatment of skeletal muscle injury.
In severe injuries such as VML, when the extent of damaged is beyond the ability of complete muscle repair, cell-based or tissue-engineered therapies may be potential ways to minimize functional impairment when fibrosis exists. Since the ultimate goal of injury repair is the formation of contractible muscle fibers, this therapy is promising in the study of skeletal muscle repair.178 Presently, cell-based therapy mainly consists of delivering myogenic or nonmyogenic cells to the injury site.176 Myogenic cells include SCs, pericytes, mesenchymal stem cells, and some other cells that can proliferate and differentiate to produce muscle fibers, and experiments have been conducted to transplant SCs into dystrophin-deficient mdx mice, and it was found that the transplantation improved the muscle contractile function of the mice.189 In nonmyogenic cell therapy, cells that can secrete cytokines required for muscle regeneration are majorly selected, such as MSCs, to achieve the stimulation of endogenous muscle regeneration signaling pathways.190 Muscle-induced decellularized scaffolds are also widely used for VML repair these days as the microenvironment of this scaffold can better mimic human tissue.166 Compared with conventional ECM, the decellularized scaffold can obtain more blood vessels due to the rich matrix, thus better promoting the proliferation of myofibers. Hydrogels containing muscle-derived decellularized scaffolds have been injected into rat models and have shown excellent myo-proliferative capacity. Although there are studies related to cell delivery by MNs, few of them have been associated with skeletal muscle injury. MNs are quite promising in this field. After all, the form of injection needles is still universally adopted to deliver cells or scaffolds, and secondary damage from needles along with the shear force generated by needles to inject viscous cell suspensions are problems that need to be solved.176 At the same time, the host reactivity and rejection invoked by the decellularized scaffolds resulting from the residual cell debris and potential immunogenic substances in the scaffold also need further exploration.191
5. Clinical translation of MNs in the MSK system
Achieving clinical translation of the research is the ultimate goal of the studies on MN for TDD. Also, 141 clinical trials have been found on MNs so far (clinicaltrials.gov), only five of which are related to the transdermal treatment of the MSK system (as shown in Table 4). It is almost a blank field thus worthy of attention. By summarizing the current clinical trials of MNs in other fields, some common features of MNs that are able to be clinically translated can be derived, which can better provide the theoretical and practical basis for future preclinical studies of microneedles in the musculoskeletal system.| Interventions | Type of application/dose | Trade name | No. enrolled/age | Phase | Status | NCT number | Sponsor/collaborator | Summary |
|---|---|---|---|---|---|---|---|---|
| Abaloparatide | Transdermal microneedle patch/50–150 μg daily for 6 months | BA058 | 250/up to 85 or postmenopausal women with osteoporosis | II | Completed | NCT01674621 | Radius Health, Inc. and Nordic Bioscience A/S | BMD and serum markers of bone metabolism greatly improved in Abaloparatide transdermal treatment for 6 months. |
| Zosano Pharma Parathyroid Hormone (ZP-PTH) | Transdermal Microneedle Patch/40 mg daily for 30 minutes once for 14 days | — | 24/55–85 or postmenopausal women with osteoporosis | I | Completed | NCT02478879 | Zosano Pharma Corporation | Subjects’ preference for ZP-PTH and FORTEO(R) Pen overall in the safety evaluation. No results are posted yet. |
| Abaloparatide-sMTS | Solid microneedle transdermal patch/300 μg daily for 5 minutes once for 29 days | BA058 | 22/50–85 or postmenopausal women with osteoporosis | I | Completed | NCT04366726 | Radius Health, Inc. | The same pharmacokinetic characteristics as abaloparatide-SC in 29 days. High subject-rated satisfaction, convenience, and acceptability |
| Abaloparatide-sMTS | Solid microneedle transdermal patch/300 μg daily for 5 minutes once for 29 days | BA058 | 511/50–85 or postmenopausal women with osteoporosis | III | Completed | NCT04064411 | Radius Health, Inc. | Similar BMD changes in lumbar spine, total hip, and femoral neck were observed in patients applying sMTS as in those with SC administered daily (80 g). |
| Abaloparatide-sMTS (Patheon)/abaloparatide-sMTS (kindeva) | Solid microneedle transdermal patch/300 μg/4 or 7 minutes | BA058 | 36/40–65 years or postmenopausal women with osteoporosis | I | Completed | NCT04936984 | Radius health, Inc. and Medpace, Inc. | Evaluation of the bioequivalence of two groups of doses 300 μg (Patheon sterile and Kindeva ultra-low bioburden abaloparatide-sMTS) applied to the thigh for 5 minutes. No results are posted yet. |
Firstly, MNs must be proven safe, nontoxic, and minimally invasive in clinical trials, which are the most essential characteristics they should possess. At the cellular level, cytotoxicity tests are commonly carried out to verify the biocompatibility of MNs in vitro, mainly including LIVE/DEAD staining and cell counting-8 kit (CCK-8) assay.63,192 To identify whether the MNs induce evident inflammation or damage to the skin, the Draize grading scale193 and the transepidermal water loss (TEWL)194 have been performed at times. The former is used to rate the degree of skin irritation such as erythema, edema, and scabbing, and the latter is used to reflect the degree of structural and functional damage of the skin. It is worth mentioning that the skin damage and irritation caused by the MNs is only mild and temporary, a vast majority of which can subside within a few hours among the subjects.28
Besides, the degree of pain is another important factor to the clinical translation of MNs in the MSK system. Some widely accepted scales such as the visual analog scale (VAS) are available for assessing the pain level of MNs.195 Notably, it is considered that length contributes a greater value to pain level compared to needle density and number.196 Though common MNs cause negligible pain in some clinical trials,195 MNs used in the MSK system are expected to have longer needle bodies, allowing closer access to the lesion site. Thus, further investigation of the relationship between pain and MNs parameters is necessary for the clinical translation of MNs in the MSK system.
Next, increased stability and high loading capacity of the drugs are also essential properties before MNs can be put into clinical use of MSK disorders. At the stability aspect, it is expected that the drug loaded on MNs remains stable for a long time at ambient conditions and can tolerate long transport distances to some remote areas, reducing the additional expense of storage conditions and drug loss. In terms of drug loading capacity in the treatment of arthritis with acute exacerbations, for example, the increased drug-loading capacity enables to reduce the frequency and cost of TDD, reaching the effective drug concentration at the lesion site earlier. However, as a matter of fact, it is generally limited by their size.197 To make a change, Suping Jiang et al. developed a hydrogel MNP for the treatment of gouty arthritis that possesses both good loading capacity and delivery effect, carrying 1 mg colchicine (to meet the clinical dose requirement) and achieving a release rate of 80% within 48 hours.92
Lastly, reducing the production costs is also integral to the clinical translation of MNs as high manufacturing costs and expenses inevitably limit their feasibility for scale-up and market introduction.198 Selecting low-cost material alternatives and simplifying the fabricating process can definitely lower the cost.199 In addition, improving the fabricating technology to enable the mass production of MNs and promoting efficient production models that can then be transformed into clinical applications200 are also ideal steps.
6. Conclusion and future perspectives
MNs offer a painless and minimally invasive way for drug delivery to the MSK system, combining the advantages as well as circumventing the disadvantages of traditional oral and injectable ways. Nevertheless, relatively few studies have been conducted on the application of MNs in the MSK system due to the specificity of the MSK system. Many issues need to be addressed to facilitate its translation from basic research to the clinic and further practically bring benefits to patients.At the level of MNs, given their micrometer sizes, how to further improve their loading capacity and the efficacy of the drugs is a topic worthy of in-depth discussion, which directly affects the curative effects. Drug loading has the possibility to weaken the mechanical strength of MNs and thus affect their insertion behavior. Therefore, it is of great necessity to study increasing drug loading while maintaining the effective transdermal delivery of MNs at the same time. Although the current studies confirm that the side effects of MNs are minimal, we must take into account the following questions: can safety and satisfaction be guaranteed if MNs are applied to a broader patient population, leaving aside the controlled conditions during the experiments? If MNs are applied frequently for drug delivery over a long period of time, are there toxic reactions and immune responses that can build up? In addition, the industrial production of MNs through process improvement to reduce costs and bring true benefits to the general public is crucial to the success of clinical translation and will promote innovation in drug delivery methods across the board. There is no pharmacopeial standard related to MNs that has been put forward so far; thus, corresponding application regulations shall be prepared and improved as soon as possible to standardize the development and use of MNs.
At the level of MSK diseases, as the related position is located so deep that the MNs sometimes cannot reach directly so the drugs can only enter the lesion site from the dermis through penetration. Therefore, it is particularly important to promote the permeation to the target site with the help of some advanced techniques. Firstly, the photothermal and photodynamic therapy can be applied with MNs. Preparing MNs made of photosensitive materials can enhance the release and permeation of the drugs by means of infrared light irradiation.201 Besides, combining the MNs with other TDD methods such as ultrasound202 and iontophoresis203 can also be a good choice. Moreover, together with gas (including O2 and CO2) propulsion devices, diffusion can be promoted through thrust and convection204,205 In addition, in the MSK system, targeted therapy also deserves further study. The carriers of neutrophils,206 cell penetrating peptides (CPPs),207 as well as the targeting of macrophages208 provide new strategies for this. It is expected that activated neutrophils and derived vesicles as carriers can directly target the inflammation site and alleviate the early inflammatory state. Playing an important role in the arthritis and inflammation of later stage, macrophages can be targeted for the treatment of arthritis by combining the MNs with nanotechnology.209 As the promoters of intracellular delivery, CPPs can deliver drugs directly into the cell, which is expected to target the main pathways of inflammation, thus accurately alleviating fibrosis after muscle injury.
In addition, the current drug treatments for MSK diseases are limited and mostly nonspecific, which may require the further elucidation of the pathophysiological process of the diseases and development of targeted drugs as well as the introduction of new strategies such as stem cells and gene therapy to make MNs treatments more effective. With the development of precision medicine, smart MNs that integrate analysis, diagnosis, and drug delivery are expected to be developed, which has the potential to greatly broaden the prospects of MNs applications in the MSK system by interweaving with other high-tech fields. For example, by capturing changes in various endogenous and exogenous indicators during the development of diseases in the MSK system and combining them with signal monitoring and robotic drug management systems, early autonomous drug delivery is expected to be realized along with disease diagnosis. Achieving the clinical translation of MNs in drug delivery to the MSK system is the ultimate goal of conducting related research, which does not rely solely on the efforts of basic researchers but also requires a full understanding of the technology and multi-party collaboration among clinicians, especially orthopedic surgeons, who participate together in the design and development of MNs products based on clinical practice.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52003113), and Guangdong Basic and Applied Basic Research Foundation (2021A1515010745, 2020A1515110356).References
- A. Salhotra, H. N. Shah, B. Levi and M. T. Longaker, Nat. Rev. Mol. Cell Biol., 2020, 21, 696–711 CrossRef CAS PubMed.
- R. Lozano, M. Naghavi, K. Foreman, S. Lim, K. Shibuya, V. Aboyans, J. Abraham, T. Adair, R. Aggarwal, S. Y. Ahn, M. Alvarado, H. R. Anderson, L. M. Anderson, K. G. Andrews, C. Atkinson, L. M. Baddour, S. Barker-Collo, D. H. Bartels, M. L. Bell, E. J. Benjamin, D. Bennett, K. Bhalla, B. Bikbov, A. Bin Abdulhak, G. Birbeck, F. Blyth, I. Bolliger, S. Boufous, C. Bucello, M. Burch, P. Burney, J. Carapetis, H. Chen, D. Chou, S. S. Chugh, L. E. Coffeng, S. D. Colan, S. Colquhoun, K. E. Colson, J. Condon, M. D. Connor, L. T. Cooper, M. Corriere, M. Cortinovis, K. C. de Vaccaro, W. Couser, B. C. Cowie, M. H. Criqui, M. Cross, K. C. Dabhadkar, N. Dahodwala, D. De Leo, L. Degenhardt, A. Delossantos, J. Denenberg, D. C. Des Jarlais, S. D. Dharmaratne, E. R. Dorsey, T. Driscoll, H. Duber, B. Ebel, P. J. Erwin, P. Espindola, M. Ezzati, V. Feigin, A. D. Flaxman, M. H. Forouzanfar, F. G. Fowkes, R. Franklin, M. Fransen, M. K. Freeman, S. E. Gabriel, E. Gakidou, F. Gaspari, R. F. Gillum, D. Gonzalez-Medina, Y. A. Halasa, D. Haring, J. E. Harrison, R. Havmoeller, R. J. Hay, B. Hoen, P. J. Hotez, D. Hoy, K. H. Jacobsen, S. L. James, R. Jasrasaria, S. Jayaraman, N. Johns, G. Karthikeyan, N. Kassebaum, A. Keren, J. P. Khoo, L. M. Knowlton, O. Kobusingye, A. Koranteng, R. Krishnamurthi, M. Lipnick, S. E. Lipshultz, S. L. Ohno, J. Mabweijano, M. F. MacIntyre, L. Mallinger, L. March, G. B. Marks, R. Marks, A. Matsumori, R. Matzopoulos, B. M. Mayosi, J. H. McAnulty, M. M. McDermott, J. McGrath, G. A. Mensah, T. R. Merriman, C. Michaud, M. Miller, T. R. Miller, C. Mock, A. O. Mocumbi, A. A. Mokdad, A. Moran, K. Mulholland, M. N. Nair, L. Naldi, K. M. Narayan, K. Nasseri, P. Norman, M. O'Donnell, S. B. Omer, K. Ortblad, R. Osborne, D. Ozgediz, B. Pahari, J. D. Pandian, A. P. Rivero, R. P. Padilla, F. Perez-Ruiz, N. Perico, D. Phillips, K. Pierce, C. A. Pope, 3rd, E. Porrini, F. Pourmalek, M. Raju, D. Ranganathan, J. T. Rehm, D. B. Rein, G. Remuzzi, F. P. Rivara, T. Roberts, F. R. De León, L. C. Rosenfeld, L. Rushton, R. L. Sacco, J. A. Salomon, U. Sampson, E. Sanman, D. C. Schwebel, M. Segui-Gomez, D. S. Shepard, D. Singh, J. Singleton, K. Sliwa, E. Smith, A. Steer, J. A. Taylor, B. Thomas, I. M. Tleyjeh, J. A. Towbin, T. Truelsen, E. A. Undurraga, N. Venketasubramanian, L. Vijayakumar, T. Vos, G. R. Wagner, M. Wang, W. Wang, K. Watt, M. A. Weinstock, R. Weintraub, J. D. Wilkinson, A. D. Woolf, S. Wulf, P. H. Yeh, P. Yip, A. Zabetian, Z. J. Zheng, A. D. Lopez, C. J. Murray, M. A. AlMazroa and Z. A. Memish, Lancet, 2012, 380, 2095–2128 CrossRef.
- Z. A. Cupler, M. Alrwaily, E. Polakowski, K. S. Mathers and M. J. Schneider, Chiropr. Man. Therap., 2020, 28, 52 CrossRef.
- C. Shi, T. Wu, Y. He, Y. Zhang and D. Fu, Pharmacol. Ther., 2020, 207, 107473 CrossRef CAS PubMed.
- E. J. Carbone, K. Rajpura, B. N. Allen, E. Cheng, B. D. Ulery and K. W. Lo, Nanomedicine, 2017, 13, 37–47 CrossRef CAS PubMed.
- S. A.-J. K. Ahmed, S. Mahmood, A. S. Hamed, V. J. Reddy, H. A. Rebhi, M. Azmana and S. Raman, Int. J. Pharm., 2020, 587, 119673 CrossRef.
- F. Qu, R. Geng, Y. Liu and J. Zhu, Theranostics, 2022, 12, 3372–3406 CrossRef CAS PubMed.
- Y. Zhang, J. Yu, A. R. Kahkoska, J. Wang, J. B. Buse and Z. Gu, Adv. Drug Delivery Rev., 2019, 139, 51–70 CrossRef CAS PubMed.
- S. Kumari, A. Goyal, E. Sönmez Gürer, E. Algın Yapar, M. Garg, M. Sood and R. K. Sindhu, Pharmaceutics, 2022, 14, 1091 CrossRef CAS.
- A. Kováčik, M. Kopečná and K. Vávrová, Expert Opin. Drug Delivery, 2020, 17, 145–155 CrossRef.
- B. C.-Q. Seah and B. M. Teo, Int. J. Nanomed., 2018, 13, 7749 CrossRef CAS.
- M. S. Lhernould, M. Deleers and A. Delchambre, Int. J. Pharm., 2015, 480, 152–157 CrossRef CAS PubMed.
- L. M. Mikesh, L. R. Aramadhaka, C. Moskaluk, P. Zigrino, C. Mauch and J. W. Fox, J. Proteomics, 2013, 84, 190–200 CrossRef CAS PubMed.
- B. W. Barry, Eur. J. Pharm. Sci., 2001, 14, 101–114 CrossRef CAS PubMed.
- S. Chandrasekhar, L. K. Iyer, J. P. Panchal, E. M. Topp, J. B. Cannon and V. V. Ranade, Expert Opin. Drug Delivery, 2013, 10, 1155–1170 CrossRef CAS.
- E. Larrañeta, M. T. McCrudden, A. J. Courtenay and R. F. Donnelly, Pharm. Res., 2016, 33, 1055–1073 CrossRef.
- C. Loebel and J. A. Burdick, Cell Stem Cell, 2018, 22, 325–339 CrossRef CAS PubMed.
- T. K. Mwangi, I. M. Berke, E. H. Nieves, R. D. Bell, S. B. Adams and L. A. Setton, J. Controlled Release, 2018, 283, 76–83 CrossRef CAS.
- V. S. Bachu, J. Kedda, I. Suk, J. J. Green and B. Tyler, Ann. Biomed. Eng., 2021, 49, 1975–1991 CrossRef PubMed.
- S. Shin Low, C. Nong Lim, M. Yew, W. Siong Chai, L. E. Low, S. Manickam, B. Ti Tey and P. L. Show, Ultrason. Sonochem., 2021, 80, 105805 CrossRef.
- T. Han and D. B. Das, Eur. J. Pharm. Biopharm., 2015, 89, 312–328 CrossRef CAS PubMed.
- J. Sitta and C. M. Howard, Int. J. Mol. Sci., 2021, 22, 11491 CrossRef CAS PubMed.
- A. K. Banga, S. Bose and T. K. Ghosh, Int. J. Pharm., 1999, 179, 1–19 CrossRef CAS.
- D. Omata, L. Munakata, K. Maruyama and R. Suzuki, Biol. Pharm. Bull., 2021, 44, 1391–1398 CrossRef CAS PubMed.
- H. Katsumi, Y. Tanaka, K. Hitomi, S. Liu, Y.-S. Quan, F. Kamiyama, T. Sakane and A. Yamamoto, Pharmaceutics, 2017, 9, 29 CrossRef.
- K. Yu, X. Yu, S. Cao, Y. Wang, Y. Zhai, F. Yang, X. Yang, Y. Lu, C. Wu and Y. Xu, Acta Pharm. Sin. B, 2021, 11, 505–519 CrossRef CAS.
- C. Zhang, S. Jia, J. Huang, H. Peng, J. Zhang, L. Liu, W. Zhang, H. Xin and X. Wang, J. Mater. Chem. B, 2021, 9, 8014–8020 RSC.
- W. Li, S. Li, X. Fan and M. R. Prausnitz, J. Controlled Release, 2021, 339, 350–360 CrossRef CAS.
- M. Chen, G. Quan, Y. Sun, D. Yang, X. Pan and C. Wu, J. Controlled Release, 2020, 325, 163–175 CrossRef CAS.
- S. Mahmood, M. Taher and U. K. Mandal, Int. J. Nanomed., 2014, 9, 4331–4346 Search PubMed.
- G. Ma and C. Wu, J. Controlled Release, 2017, 251, 11–23 CrossRef CAS PubMed.
- N. S. Rejinold, J. H. Shin, H. Y. Seok and Y. C. Kim, Expert Opin. Drug Delivery, 2016, 13, 109–131 CrossRef CAS.
- A. Tucak, M. Sirbubalo, L. Hindija, O. Rahić, J. Hadžiabdić, K. Muhamedagić, A. Čekić and E. Vranić, Micromachines, 2020, 11, 961 CrossRef PubMed.
- R. Li, X. Liu, X. Yuan, S. Wu, L. Li, X. Jiang, B. Li, X. Jiang and M. Gou, Int. J. Bioprint, 2022, 8, 553 CAS.
- Z. Hu, C. S. Meduri, R. S. J. Ingrole, H. S. Gill and G. Kumar, Appl. Phys. Lett., 2020, 116, 203703 CrossRef CAS.
- Y. Chen, B. Z. Chen, Q. L. Wang, X. Jin and X. D. Guo, J. Controlled Release, 2017, 265, 14–21 CrossRef CAS PubMed.
- K. van der Maaden, E. Sekerdag, P. Schipper, G. Kersten, W. Jiskoot and J. Bouwstra, Langmuir, 2015, 31, 8654–8660 CrossRef CAS PubMed.
- Q. K. Anjani, A. D. Permana, Á. Cárcamo-Martínez, J. Domínguez-Robles, I. A. Tekko, E. Larrañeta, L. K. Vora, D. Ramadon and R. F. Donnelly, Eur. J. Pharm. Biopharm., 2021, 158, 294–312 CrossRef CAS.
- C. J. W. Bolton, O. Howells, G. J. Blayney, P. F. Eng, J. C. Birchall, B. Gualeni, K. Roberts, H. Ashraf and O. J. Guy, Lab Chip, 2020, 20, 2788–2795 RSC.
- Á. Cárcamo-Martínez, B. Mallon, J. Domínguez-Robles, L. K. Vora, Q. K. Anjani and R. F. Donnelly, Int. J. Pharm., 2021, 599, 120455 CrossRef.
- A. Z. Alkilani, M. T. McCrudden and R. F. Donnelly, Pharmaceutics, 2015, 7, 438–470 CrossRef CAS PubMed.
- E. Caffarel-Salvador, S. Kim, V. Soares, R. Y. Tian, S. R. Stern, D. Minahan, R. Yona, X. Lu, F. R. Zakaria, J. Collins, J. Wainer, J. Wong, R. McManus, S. Tamang, S. McDonnell, K. Ishida, A. Hayward, X. Liu, F. Hubálek, J. Fels, A. Vegge, M. R. Frederiksen, U. Rahbek, T. Yoshitake, J. Fujimoto, N. Roxhed, R. Langer and G. Traverso, Sci. Adv., 2021, 7, e2620 CrossRef.
- K. J. Lee, S. S. Jeong, D. H. Roh, D. Y. Kim, H. K. Choi and E. H. Lee, Int. J. Pharm., 2020, 573, 118778 CrossRef CAS.
- R. Ali, P. Mehta, M. S. Arshad, I. Kucuk, M. W. Chang and Z. Ahmad, AAPS PharmSciTech, 2019, 21, 12 CrossRef CAS PubMed.
- R. F. Donnelly, T. R. Singh, A. Z. Alkilani, M. T. McCrudden, S. O'Neill, C. O'Mahony, K. Armstrong, N. McLoone, P. Kole and A. D. Woolfson, Int. J. Pharm., 2013, 451, 76–91 CrossRef CAS.
- P. Gadziński, A. Froelich, M. Wojtyłko, A. Białek, J. Krysztofiak and T. Osmałek, Beilstein J. Nanotechnol., 2022, 13, 1167–1184 CrossRef PubMed.
- X. Li, X. Xie, Y. Wu, Z. Zhang and J. Liao, Drug Delivery Transl. Res., 2023, 1–18 Search PubMed.
- D. Kulkarni, F. Damiri, S. Rojekar, M. Zehravi, S. Ramproshad, D. Dhoke, S. Musale, A. A. Mulani, P. Modak, R. Paradhi, J. Vitore, M. H. Rahman, M. Berrada, P. S. Giram and S. Cavalu, Pharmaceutics, 2022, 14, 1097 CrossRef CAS.
- F. Lin, Z. Wang, L. Xiang, L. Wu, Y. Liu, X. Xi, L. Deng and W. Cui, Adv. Sci., 2022, 9, 2200079 CrossRef CAS.
- H. Kathuria, H. Li, J. Pan, S. H. Lim, J. S. Kochhar, C. Wu and L. Kang, Pharm. Res., 2016, 33, 2653–2667 CrossRef CAS PubMed.
- Y. Kim, H. S. Min, J. Shin, J. Nam, G. Kang, J. Sim, H. Yang and H. Jung, Biomater. Res., 2022, 26, 1–14 CrossRef PubMed.
- J. W. Coffey, N. M. D. van der Burg, T. Rananakomol, H.-I. Ng, G. J. P. Fernando and M. A. F. Kendall, Adv. Nanobiomed. Res., 2022, 2100151 CrossRef CAS.
- P. Makvandi, M. Kirkby, A. R. J. Hutton, M. Shabani, C. K. Y. Yiu, Z. Baghbantaraghdari, R. Jamaledin, M. Carlotti, B. Mazzolai, V. Mattoli and R. F. Donnelly, Nanomicro Lett., 2021, 13, 93 CAS.
- J. Li, M. Zeng, H. Shan and C. Tong, Curr. Med. Chem., 2017, 24, 2413–2422 CAS.
- O. Howells, G. J. Blayney, B. Gualeni, J. C. Birchall, P. F. Eng, H. Ashraf, S. Sharma and O. J. Guy, Eur. J. Pharm. Biopharm., 2022, 171, 19–28 CrossRef CAS PubMed.
- M. S. Azizi, A. K. Nguyen, L. Vanderwal, S. Stafslien and R. J. Narayan, Pharmaceutics, 2022, 14, 2413 CrossRef PubMed.
- A. S. Al-Badry, M. H. Al-Mayahy and D. J. Scurr, J. Pharm. Sci., 2022, 112, 1011–1019 CrossRef PubMed.
- C. O'Mahony, Biomed. Microdevices, 2014, 16, 333–343 CrossRef.
- W. Yu, G. Jiang, Y. Zhang, D. Liu, B. Xu and J. Zhou, Mater. Sci. Eng., C, 2017, 80, 187–196 CrossRef CAS.
- J. H. Park, M. G. Allen and M. R. Prausnitz, J. Controlled Release, 2005, 104, 51–66 CrossRef CAS PubMed.
- T. Wu, X. You and Z. Chen, Sensors, 2022, 22, 4253 CrossRef PubMed.
- J. G. Turner, L. R. White, P. Estrela and H. S. Leese, Macromol. Biosci., 2021, 21, 2000307 CrossRef CAS PubMed.
- Y. Yang, H. Chu, Y. Zhang, L. Xu, R. Luo, H. Zheng, T. Yin and Z. Li, Nano Res., 2022, 15, 8336–8344 CrossRef CAS.
- M. Kirkby, A. B. Sabri, D. Scurr and G. Moss, Pharm. Res., 2022, 39, 1945–1958 CrossRef CAS PubMed.
- J. Chen, X. Liu, S. Liu, Z. He, S. Yu, Z. Ruan and N. Jin, Drug Dev. Ind. Pharm., 2021, 47, 1578–1586 CrossRef CAS PubMed.
- V. Ben-Yashar and Y. Barenholz, Biochim. Biophys. Acta, 1989, 985, 271–278 CrossRef CAS PubMed.
- X. M. Liu, L. D. Quan, J. Tian, Y. Alnouti, K. Fu, G. M. Thiele and D. Wang, Pharm. Res., 2008, 25, 2910–2919 CrossRef CAS PubMed.
- C. Li, H. Li, Q. Wang, M. Zhou, M. Li, T. Gong, Z. Zhang and X. Sun, J. Controlled Release, 2017, 246, 133–141 CrossRef CAS PubMed.
- C. M. Wells, M. Harris, L. Choi, V. P. Murali, F. D. Guerra and J. A. Jennings, J. Funct. Biomater., 2019, 10, 34 CrossRef CAS PubMed.
- B. Yang, Y. Dong, Y. Shen, A. Hou, G. Quan, X. Pan and C. Wu, Bioact Mater., 2021, 6, 2400–2411 CrossRef CAS PubMed.
- M. Yuan, K. Liu, T. Jiang, S. Li, J. Chen, Z. Wu, W. Li, R. Tan, W. Wei, X. Yang, H. Dai and Z. Chen, J. Nanobiotechnol., 2022, 20, 147 CrossRef CAS PubMed.
- T. J. Franz, J. Invest. Dermatol., 1975, 64, 190–195 CrossRef CAS PubMed.
- M.-C. Chen, S.-F. Huang, K.-Y. Lai and M.-H. Ling, Biomaterials, 2013, 34, 3077–3086 CrossRef CAS PubMed.
- W. A. Peck, Am. J. Med., 1993, 94, 646–650 CrossRef PubMed.
- A. Feldstein, P. J. Elmer, E. Orwoll, M. Herson and T. Hillier, Arch. Intern. Med., 2003, 163, 2165–2172 CrossRef PubMed.
- T. Luhmann, O. Germershaus, J. Groll and L. Meinel, J. Controlled Release, 2012, 161, 198–213 CrossRef CAS PubMed.
- S. T. Harris, N. B. Watts, H. K. Genant, C. D. McKeever, T. Hangartner, M. Keller, C. H. Chesnut Iii, J. Brown, E. F. Eriksen and M. S. Hoseyni, JAMA, 1999, 282, 1344–1352 CrossRef CAS PubMed.
- P. C. De Groen, D. F. Lubbe, L. J. Hirsch, A. Daifotis, W. Stephenson, D. Freedholm, S. Pryor-Tillotson, M. J. Seleznick, H. Pinkas and K. K. Wang, N. Engl. J. Med., 1996, 335, 1016–1021 CrossRef CAS PubMed.
- D. M. Black, I. R. Reid, J. A. Cauley, F. Cosman, P. C. Leung, P. Lakatos, K. Lippuner, S. R. Cummings, T. F. Hue and A. Mukhopadhyay, J. Bone Miner. Res., 2015, 30, 934–944 CrossRef CAS PubMed.
- R. E. Coleman, Br. J. Cancer, 2008, 98, 1736–1740 CrossRef CAS PubMed.
- H. Katsumi, M. Nakatani, J.-I. Sano, M. Abe, K. Kusamori, M. Kurihara, R. Shiota, M. Takashima, T. Fujita and T. Sakane, Int. J. Pharm., 2010, 400, 124–130 CrossRef CAS PubMed.
- H. Katsumi, S. Liu, Y. Tanaka, K. Hitomi, R. Hayashi, Y. Hirai, K. Kusamori, Y.-S. Quan, F. Kamiyama and T. Sakane, J. Pharm. Sci., 2012, 101, 3230–3238 CrossRef CAS PubMed.
- S. Lotinun, J. D. Sibonga and R. T. Turner, Endocrine, 2002, 17, 29–36 CrossRef CAS PubMed.
- M. E. Kraenzlin and C. Meier, Nat. Rev. Endocrinol., 2011, 7, 647–656 CrossRef CAS PubMed.
- P. E. Daddona, J. A. Matriano, J. Mandema and Y.-F. Maa, Pharm. Res., 2011, 28, 159–165 CrossRef CAS PubMed.
- C. Naito, H. Katsumi, T. Suzuki, Y.-S. Quan, F. Kamiyama, T. Sakane and A. Yamamoto, Pharmaceutics, 2018, 10, 215 CrossRef CAS PubMed.
- J. Sim, G. Kang, H. Yang, M. Jang, Y. Kim, H. Ahn, M. Kim and H. Jung, Polymers, 2022, 14, 4027 CrossRef CAS PubMed.
- E. Jacoby, C. Jarrahian, H. F. Hull and D. Zehrung, Vaccine, 2015, 33, 4699–4704 CrossRef CAS PubMed.
- H. Katsumi, Yakugaku Zasshi, 2014, 134, 427–431 CrossRef CAS PubMed.
- W. Zhao, L. Zheng, J. Yang, Z. Ma, X. Tao and Q. Wang, Drug Delivery, 2023, 30, 121–132 CrossRef CAS PubMed.
- W. Zhao, L. Zheng, J. Yang, Y. Li, Y. Zhang, T. Ma and Q. Wang, Eur. J. Pharm. Sci., 2023, 106409 CrossRef CAS PubMed.
- S. Jiang, W. Wang, J. Ke, S. Huang, J. Wang, C. Luo, X. Li, K. Zhang, H. Liu and W. Zheng, Biomater. Sci., 2023, 11, 1714–1724 RSC.
- C. Wu, J. Cheng, W. Li, L. Yang, H. Dong and X. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 55559–55568 CrossRef CAS PubMed.
- G. Du, P. He, J. Zhao, C. He, M. Jiang, Z. Zhang, Z. Zhang and X. Sun, J. Controlled Release, 2021, 336, 537–548 CrossRef CAS PubMed.
- Y. Li, Y. Sun, S. Wei, L. Zhang and S. Zong, Drug Dev. Ind. Pharm., 2021, 47, 878–886 CrossRef CAS PubMed.
- J. Cao, J. Su, M. An, Y. Yang, Y. Zhang, J. Zuo, N. Zhang and Y. Zhao, Mol. Pharm., 2021, 18, 305–316 CrossRef CAS PubMed.
- I. A. Tekko, G. Chen, J. Domínguez-Robles, R. R. S. Thakur, I. M. N. Hamdan, L. Vora, E. Larrañeta, J. C. McElnay, H. O. McCarthy, M. Rooney and R. F. Donnelly, Int. J. Pharm., 2020, 586, 119580 CrossRef CAS PubMed.
- W. Yao, C. Tao, J. Zou, H. Zheng, J. Zhu, Z. Zhu, J. Zhu, L. Liu, F. Li and X. Song, Int. J. Pharm., 2019, 563, 91–100 CrossRef CAS PubMed.
- J. Cao, N. Zhang, Z. Wang, J. Su, J. Yang, J. Han and Y. Zhao, Pharmaceutics, 2019, 11, 235 CrossRef CAS PubMed.
- Y. Cui, Y. Mo, Q. Zhang, W. Tian, Y. Xue, J. Bai and S. Du, Molecules, 2018, 23, 3371 CrossRef PubMed.
- S. Amodwala, P. Kumar and H. P. Thakkar, Eur. J. Pharm. Sci., 2017, 104, 114–123 CrossRef CAS PubMed.
- M. Dangol, H. Yang, C. G. Li, S. F. Lahiji, S. Kim, Y. Ma and H. Jung, J. Controlled Release, 2016, 223, 118–125 CrossRef CAS PubMed.
- G. Chen, B. Hao, D. Ju, M. Liu, H. Zhao, Z. Du and J. Xia, Acta Pharm. Sin. B, 2015, 5, 569–576 CrossRef PubMed.
- Y. Huang, H. Li, T. Hu, J. Li, C. K. Yiu, J. Zhou, J. Li, X. Huang, K. Yao, X. Qiu, Y. Zhou, D. Li, B. Zhang, R. Shi, Y. Liu, T. H. Wong, M. Wu, H. Jia, Z. Gao, Z. Zhang, J. He, M. Zheng, E. Song, L. Wang, C. Xu and X. Yu, Nano Lett., 2022, 22, 5944–5953 CrossRef CAS PubMed.
- A. Liu, Q. Wang, Z. Zhao, R. Wu, M. Wang, J. Li, K. Sun, Z. Sun, Z. Lv and J. Xu, ACS Nano, 2021, 15, 13339–13350 CrossRef CAS PubMed.
- F. Lin, Y. Zhuang, L. Xiang, T. Ye, Z. Wang, L. Wu, Y. Liu, L. Deng and W. Cui, Adv. Funct. Mater., 2023, 2212730 CrossRef CAS.
- C.-H. Cheng, L.-R. Chen and K.-H. Chen, Int. J. Mol. Sci., 2022, 23, 1376 CrossRef CAS PubMed.
- M. Gambacciani and M. Levancini, Przegl. Menopauzalny, 2014, 13, 213–220 Search PubMed.
- A. M. S. North, Menopause, 2017, 24, 728–753 CrossRef PubMed.
- N. Carroll, J. Womens Health, 2010, 19, 47–55 CrossRef PubMed.
- Y. Yang, B. Z. Chen, X. P. Zhang, H. Zheng, Z. Li, C. Y. Zhang and X. D. Guo, ACS Appl. Mater. Interfaces, 2022, 14, 31645–31654 CrossRef CAS PubMed.
- D. Terutsuki, R. Segawa, S. Kusama, H. Abe and M. Nishizawa, J. Controlled Release, 2023, 354, 694–700 CrossRef CAS PubMed.
- K. Kusamori, H. Katsumi, M. Abe, A. Ueda, R. Sakai, R. Hayashi, Y. Hirai, Y. S. Quan, F. Kamiyama and T. Sakane, J. Bone Miner. Res., 2010, 25, 2582–2591 CrossRef PubMed.
- S. M. Heffernan, K. Horner, G. De Vito and G. E. Conway, Nutrients, 2019, 11, 696 CrossRef CAS PubMed.
- S. Uday and W. Högler, Indian J. Med. Res., 2020, 152, 356 CrossRef CAS PubMed.
- S. Uday and W. Högler, J. Steroid Biochem. Mol. Biol., 2019, 188, 141–146 CrossRef CAS PubMed.
- R. Chanchlani, P. Nemer, R. Sinha, L. Nemer, V. Krishnappa, E. Sochett, F. Safadi and R. Raina, Kidney Int. Rep., 2020, 5, 980–990 CrossRef PubMed.
- L. Junqueira, H. Polonini, C. Ramos, A. O. Ferreira, N. Raposo and M. Brandão, Curr. Drug Delivery, 2022, 19, 614–624 CrossRef CAS PubMed.
- H.-G. Kim, D. L. Gater and Y.-C. Kim, Drug Delivery Transl. Res., 2018, 8, 281–290 CrossRef CAS PubMed.
- R. R. S. Thakur, I. A. Tekko, F. Al-Shammari, A. A. Ali, H. McCarthy and R. F. Donnelly, Drug Delivery Transl. Res., 2016, 6, 800–815 CrossRef CAS PubMed.
- L. K. Vora, R. F. Donnelly, E. Larrañeta, P. González-Vázquez, R. R. S. Thakur and P. R. Vavia, J. Controlled Release, 2017, 265, 93–101 CrossRef CAS PubMed.
- H. Wang, Y. Fu, J. Mao, H. Jiang, S. Du, P. Liu, J. Tao, L. Zhang and J. Zhu, Adv. Mater., 2022, 34, e2207832 CrossRef PubMed.
- M. Auréal, I. Machuca-Gayet and F. Coury, Biomolecules, 2020, 11, 48 CrossRef PubMed.
- E. A. Littlejohn and S. U. Monrad, Prim. Care, 2018, 45, 237–255 CrossRef PubMed.
- Y.-J. Lin, M. Anzaghe and S. Schülke, Cells, 2020, 9, 880 CrossRef CAS PubMed.
- C. A. Heyneman, C. Lawless-Liday and G. C. Wall, Drugs, 2000, 60, 555–574 CrossRef CAS PubMed.
- F. Salvo, A. Fourrier Réglat, F. Bazin, P. Robinson, N. Riera Guardia, M. Haag, A. P. Caputi, N. Moore, M. C. Sturkenboom and A. Pariente, Clin. Pharmacol. Ther., 2011, 89, 855–866 CrossRef CAS PubMed.
- S. R. Vučen, G. Vuleta, A. M. Crean, A. C. Moore, N. Ignjatović and D. Uskoković, J. Pharm. Pharmacol., 2013, 65, 1451–1462 CrossRef PubMed.
- B. Combe, R. Landewe, C. I. Daien, C. Hua, D. Aletaha, J. M. Álvaro-Gracia, M. Bakkers, N. Brodin, G. R. Burmester and C. Codreanu, Ann. Rheum. Dis., 2017, 76, 948–959 CrossRef PubMed.
- M. Qindeel, D. Khan, N. Ahmed, S. Khan and U. R. Asim, ACS Nano, 2020, 14, 4662–4681 CrossRef CAS PubMed.
- V. Vemulapalli, Y. Yang, P. M. Friden and A. K. Banga, J. Pharm. Pharmacol., 2008, 60, 27–33 CrossRef CAS PubMed.
- M. Zhao, J. Bai, Y. Lu, S. Du, K. Shang, P. Li, L. Yang, B. Dong and N. Tan, J. Tradit. Chin. Med., 2016, 3, 256–262 Search PubMed.
- J.-D. Lee, H.-J. Park, Y. Chae and S. Lim, J. Evidence-Based Complementary Altern. Med., 2005, 2, 79–84 CrossRef PubMed.
- S. F. Lahiji, M. Dangol and H. Jung, Sci. Rep., 2015, 5, 7914 CrossRef CAS PubMed.
- J. Wang, J. Zeng, Z. Liu, Q. Zhou, X. Wang, F. Zhao, Y. Zhang, J. Wang, M. Liu and R. Du, Pharmaceutics, 2022, 14, 1736 CrossRef CAS PubMed.
- D. J. Hunter and S. Bierma-Zeinstra, Lancet, 2019, 393, 1745–1759 CrossRef CAS PubMed.
- M. Krasselt and C. Baerwald, Expert Opin. Pharmacother., 2019, 20, 1689–1702 CrossRef CAS PubMed.
- S. B. Murdande, D. A. Shah and R. H. Dave, J. Pharm. Sci., 2015, 104, 2094–2102 CrossRef CAS PubMed.
- H. A. Wieland, M. Michaelis, B. J. Kirschbaum and K. A. Rudolphi, Nat. Rev. Drug Discovery, 2005, 4, 331–344 CrossRef CAS PubMed.
- Q. Wang, X. Yang, X. Gu, F. Wei, W. Cao, L. Zheng, Y. Li, T. Ma, C. Wu and Q. Wang, Int. J. Pharm., 2022, 625, 122108 CrossRef CAS PubMed.
- J. Li, X. Tian, K. Wang, Y. Jia and F. Ma, J. Drug Targeting, 2022, 1–11 CrossRef PubMed.
- Q. Li, X. Liu, X. Wang, S. Qiu, K. Byambasuren, L. Dang and Z. Wang, J. Agric. Food Chem., 2019, 67, 11518–11526 CrossRef CAS PubMed.
- R. Anand and G. Kaithwas, Inflammation, 2014, 37, 1297–1306 CrossRef CAS PubMed.
- P. Zhou, C. Chen, X. Yue, J. Zhang, C. Huang, S. Zhao, A. Wu, X. Li, Y. Qu and C. Zhang, Int. J. Pharm., 2021, 609, 121211 CrossRef CAS PubMed.
- L. Liu, Z. Jiang, J. Liu, X. Huang, T. Wang, J. Liu, Y. Zhang, Z. Zhou, J. Guo and L. Yang, Toxicology, 2010, 271, 57–63 CrossRef CAS PubMed.
- Z.-L. Zhou, Y.-X. Yang, J. Ding, Y.-C. Li and Z.-H. Miao, Nat. Prod. Rep., 2012, 29, 457–475 RSC.
- Y. Zhao, P. Wan, J. Wang, P. Li, Q. Hu and R. Zhao, Carbohydr. Polym., 2020, 229, 115473 CrossRef CAS PubMed.
- D. Gupta, D. Bhatia, V. Dave, V. Sutariya and S. Varghese Gupta, Molecules, 2018, 23, 1719 CrossRef PubMed.
- A. R. Tekade and J. N. Yadav, Adv. Pharm. Bull., 2020, 10, 359 CrossRef CAS PubMed.
- K. S. Kim, K. Suzuki, H. Cho, Y. S. Youn and Y. H. Bae, ACS Nano, 2018, 12, 8893–8900 CrossRef CAS PubMed.
- P. Maudens, O. Jordan and E. Allémann, Drug Discovery Today, 2018, 23, 1761–1775 CrossRef CAS PubMed.
- D. Zhou, F. Zhou, S. Sheng, Y. Wei, X. Chen and J. Su, Drug Discovery Today, 2023, 28, 103482 CrossRef CAS PubMed.
- S. M. Hosseinikhah, M. Barani, A. Rahdar, H. Madry, R. Arshad, V. Mohammadzadeh and M. Cucchiarini, Int. J. Mol. Sci., 2021, 22, 3092 CrossRef CAS PubMed.
- M. Abate, A. Festa, M. Falco, A. Lombardi, A. Luce, A. Grimaldi, S. Zappavigna, P. Sperlongano, C. Irace and M. Caraglia, Semin. Cell Dev. Biol., 2020, 139–153 CrossRef CAS PubMed.
- N. Hafsia, M. Forien, F. Renaudin, D. Delacour, P. Reboul, P. Van Lent, M. Cohen-Solal, F. Lioté, F. Poirier and H. K. Ea, Int. J. Mol. Sci., 2020, 21, 1486 CrossRef CAS PubMed.
- K. Riewruja, S. Phakham, P. Sompolpong, R. Reantragoon, A. Tanavalee, S. Ngarmukos, W. Udomsinprasert, T. Suantawee, S. Dechsupa and S. Honsawek, Int. J. Mol. Sci., 2022, 23, 890 CrossRef CAS PubMed.
- A. Di Martino, A. Boffa, L. Andriolo, I. Romandini, S. A. Altamura, A. Cenacchi, V. Roverini, S. Zaffagnini and G. Filardo, Am. J. Sports Med., 2022, 50, 609–617 CrossRef PubMed.
- B. O. Connell, N. M. Wragg and S. L. Wilson, Cell Tissue Res., 2019, 376, 143–152 CrossRef PubMed.
- S. Wang, X. Liu and Y. Wang, Front. Bioeng. Biotechnol., 2022, 10, 808248 CrossRef PubMed.
- C. H. Evans, S. C. Ghivizzani and P. D. Robbins, Transl. Res., 2013, 161, 205–216 CrossRef CAS PubMed.
- S. C. Ghivizzani, E. Gouze, J. N. Gouze, J. D. Kay, M. L. Bush, R. S. Watson, P. P. Levings, D. M. Nickerson, P. T. Colahan, P. D. Robbins and C. H. Evans, Curr. Gene Ther., 2008, 8, 273–286 CrossRef CAS PubMed.
- J. J. Hwang, Y. A. Rim, Y. Nam and J. H. Ju, Front. Immunol., 2021, 12, 631291 CrossRef CAS PubMed.
- K. Lee, Y. Xue, J. Lee, H. J. Kim, Y. Liu, P. Tebon, E. Sarikhani, W. Sun, S. Zhang, R. Haghniaz, B. Çelebi-Saltik, X. Zhou, S. Ostrovidov, S. Ahadian, N. Ashammakhi, M. R. Dokmeci and A. Khademhosseini, Adv. Funct. Mater., 2020, 30, 2000086 CrossRef CAS PubMed.
- M. Han, H. Yang, X. Lu, Y. Li, Z. Liu, F. Li, Z. Shang, X. Wang, X. Li, J. Li, H. Liu and T. Xin, Nano Lett., 2022, 22, 6391–6401 CrossRef CAS PubMed.
- M. A. A. Mahdy, Cell Tissue Res., 2019, 375, 575–588 CrossRef PubMed.
- J. Liu, D. Saul, K. O. Böker, J. Ernst, W. Lehman and A. F. Schilling, BioMed Res. Int., 2018, 2018, 1984879 Search PubMed.
- M. A. A. Mahdy, M. A. Akl and F. A. Madkour, BMC Musculoskeletal Disord., 2022, 23, 670 CrossRef CAS PubMed.
- P. Prazeres, A. O. M. Turquetti, P. O. Azevedo, R. S. N. Barreto, M. A. Miglino, A. Mintz, O. Delbono and A. Birbrair, Int. J. Biochem. Cell Biol., 2018, 99, 109–113 CrossRef CAS PubMed.
- S. M. Greising, B. T. Corona and J. A. Call, Int. J. Sports Med., 2020, 41, 495–504 CrossRef PubMed.
- B. T. Corona, J. C. Rivera, K. A. Dalske, J. C. Wenke and S. M. Greising, Tissue Eng., Part A, 2020, 26, 636–646 CrossRef CAS PubMed.
- J. Zhang, S. Shen, R. Lin, J. Huang, C. Pu, P. Chen, Q. Duan, X. You, C. Xu and B. Yan, Adv. Mater., 2022, 35, 2209497 CrossRef PubMed.
- K. Delaney, P. Kasprzycka, M. A. Ciemerych and M. Zimowska, Cell Biol. Int., 2017, 41, 706–715 CrossRef CAS PubMed.
- M. A. A. Mahdy, K. Warita and Y. Z. Hosaka, Anim. Sci. J., 2017, 88, 1811–1819 CrossRef CAS PubMed.
- E. Le Moal, V. Pialoux, G. Juban, C. Groussard, H. Zouhal, B. Chazaud and R. Mounier, Antioxid. Redox Signaling, 2017, 27, 276–310 CrossRef CAS PubMed.
- K. Richter, A. Konzack, T. Pihlajaniemi, R. Heljasvaara and T. Kietzmann, Redox Biol., 2015, 6, 344–352 CrossRef CAS PubMed.
- T. H. Qazi, G. N. Duda, M. J. Ort, C. Perka, S. Geissler and T. Winkler, J. Cachexia Sarcopenia Muscle, 2019, 10, 501–516 CrossRef PubMed.
- I. M. Aragón, B. H. Imbroda and M. F. Lara, Int. J. Med. Sci., 2018, 15, 195–204 CrossRef PubMed.
- S. Bersini, M. Gilardi, M. Mora, S. Krol, C. Arrigoni, C. Candrian, S. Zanotti and M. Moretti, Adv. Drug Delivery Rev., 2018, 129, 64–77 CrossRef CAS PubMed.
- Y. He, Q. Li, P. Chen, Q. Duan, J. Zhan, X. Cai, L. Wang, H. Hou and X. Qiu, Nat. Commun., 2022, 13, 7666 CrossRef CAS PubMed.
- K. Mukund and S. Subramaniam, Wiley Interdiscip. Rev.: Syst. Biol. Med., 2020, 12, e1462 Search PubMed.
- Y. Yang, R. Luo, S. Chao, J. Xue, D. Jiang, Y. H. Feng, X. D. Guo, D. Luo, J. Zhang, Z. Li and Z. L. Wang, Nat. Commun., 2022, 13, 6908 CrossRef CAS PubMed.
- M. Ashrafizadeh, A. Zarrabi, K. Hushmandi, V. Zarrin, E. R. Moghadam, F. Hashemi, P. Makvandi, S. Samarghandian, H. Khan, F. Hashemi, M. Najafi and H. Mirzaei, Front. Pharmacol., 2020, 11, 585413 CrossRef CAS PubMed.
- X. Cai, Y. He, C. Liu, J. Zhan, Q. Li, S. Zhong, H. Hou, W. Wang and X. Qiu, Biomater. Sci., 2023, 11, 3227–3240 RSC.
- M. P. Rayman, Lancet, 2000, 356, 233–241 CrossRef CAS PubMed.
- D. X. Tan, L. C. Manchester, M. P. Terron, L. J. Flores and R. J. Reiter, J. Pineal. Res., 2007, 42, 28–42 CrossRef CAS PubMed.
- Y. X. Yang, M. S. Liu, X. J. Liu, Y. C. Zhang, Y. Y. Hu, R. S. Gao, E. K. Pang, L. Hou, J. C. Wang and W. Y. Fei, Nanomedicine, 2022, 17, 1547–1565 CrossRef CAS PubMed.
- J. A. Boga, B. Caballero, Y. Potes, Z. Perez-Martinez, R. J. Reiter, I. Vega-Naredo and A. Coto-Montes, J. Pineal. Res., 2019, 66, e12534 CrossRef PubMed.
- R. K. A. Sayed, M. Fernández-Ortiz, M. E. Diaz-Casado, I. Rusanova, I. Rahim, G. Escames, L. C. López, D. M. Mokhtar and D. Acuña-Castroviejo, J. Gerontol., Ser. A, 2018, 73, 1330–1338 CrossRef CAS PubMed.
- M. Cerletti, S. Jurga, C. A. Witczak, M. F. Hirshman, J. L. Shadrach, L. J. Goodyear and A. J. Wagers, Cell, 2008, 134, 37–47 CrossRef CAS PubMed.
- J. H. Oh, S. W. Chung, S. H. Kim, J. Y. Chung and J. Y. Kim, J Shoulder Elbow Surg, 2014, 23, 445–455 CrossRef PubMed.
- H. Wu, R. Zhang, B. Hu, Y. He, Y. Zhang, L. Cai, L. Wang, G. Wang, H. Hou and X. Qiu, Chin. Chem. Lett., 2021, 32, 3940–3947 CrossRef CAS.
- Y. Shan, B. Tan, M. Zhang, X. Xie and J. Liao, J. Nanobiotechnol., 2022, 20, 238 CrossRef CAS PubMed.
- C. Charmeau-Genevois, S. Sarang, M. Perea, C. Eadsforth, T. Austin and P. Thomas, Regul. Toxicol. Pharmacol., 2021, 123, 104922 CrossRef CAS PubMed.
- Q. Zhang, M. Murawsky, T. LaCount, G. B. Kasting and S. K. Li, Toxicol. In Vitro, 2018, 51, 129–135 CrossRef CAS PubMed.
- H. X. Nguyen and C. N. Nguyen, Pharmaceutics, 2023, 15, 277 CrossRef CAS PubMed.
- T. T. Nguyen and J. H. Park, Expert Opin. Drug Delivery, 2018, 15, 235–245 CrossRef PubMed.
- K. Tran, T. D. Gavitt, N. J. Farrell, E. J. Curry, A. B. Mara, A. Patel, L. Brown, S. Kilpatrick, R. Piotrowska and N. Mishra, Nat. Biomed. Eng., 2021, 5, 998–1007 CrossRef CAS PubMed.
- M. Olowe, S. K. Parupelli and S. Desai, Pharmaceutics, 2022, 14, 2693 CrossRef CAS PubMed.
- K. J. Cha, T. Kim, S. J. Park and D. S. Kim, J. Micromech. Microeng., 2014, 24, 115015 CrossRef.
- B. Z. Chen, M. C. He, X. P. Zhang, W. M. Fei, Y. Cui and X. D. Guo, Drug Delivery Transl. Res., 2022, 12, 2730–2739 CrossRef CAS PubMed.
- D. Zhi, T. Yang, J. O'Hagan, S. Zhang and R. F. Donnelly, J. Controlled Release, 2020, 325, 52–71 CrossRef CAS PubMed.
- T. Han and D. B. Das, J. Pharm. Sci., 2013, 102, 3614–3622 CrossRef CAS PubMed.
- V. Sachdeva, Y. Zhou and A. K. Banga, Curr. Pharm. Biotechnol., 2013, 14, 180–193 CAS.
- P. Liu, Y. Fu, F. Wei, T. Ma, J. Ren, Z. Xie, S. Wang, J. Zhu, L. Zhang, J. Tao and J. Zhu, Adv. Sci., 2022, 9, e2202591 CrossRef PubMed.
- M. Zhang, B. Yang, X. Luan, L. Jiang, C. Lu, C. Wu, X. Pan and T. Peng, Pharmaceutics, 2023, 15, 1059 CrossRef CAS PubMed.
- D. Chu, X. Dong, X. Shi, C. Zhang and Z. Wang, Adv. Mater., 2018, 30, e1706245 CrossRef PubMed.
- M. Zorko, S. Jones and Ü. Langel, Adv. Drug Delivery Rev., 2022, 180, 114044 CrossRef CAS PubMed.
- M. Mukhtar, H. Ali, N. Ahmed, R. Munir, S. Talib, A. S. Khan and R. Ambrus, Expert Opin. Drug Delivery, 2020, 17, 1239–1257 CrossRef CAS PubMed.
- E. Nogueira, A. C. Gomes, A. Preto and A. Cavaco-Paulo, Nanomedicine, 2016, 12, 1113–1126 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |