Nanoscale MOFs in nanomedicine applications: from drug delivery to therapeutic agents
Zeyi
Sun†
ad,
Tieyan
Li†
b,
Tianxiao
Mei
a,
Yang
Liu
c,
Kerui
Wu
a,
Wenjun
Le
*a and
Yihui
Hu
 *a
*a
aInstitute for Regenerative Medicine, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai 200092, China. E-mail: wenjunle@tongji.edu.cn; yihuihu2020@163.com
bDepartment of Cardiovascular Surgery, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai 200092, China
cShanghai Heart Failure Research Center, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai 200092, China
dShanghai East Hospital, Jinzhou Medical University, Jinzhou 121001, China
First published on 3rd March 2023
Abstract
Metal–organic frameworks (MOFs) hold great promise for widespread applications in biomedicine and nanomedicine. MOFs are one of the most fascinating nanocarriers for drug delivery, benefiting from their high porosity and facile modification. Furthermore, the tailored components of MOFs can be therapeutic agents for various treatments, including drugs as organic ligands of MOFs, active metal as central metal ions of MOFs, and their combinations as carrier-free MOF-based nanodrug. In this review, the advances in delivery systems and applications as therapeutic agents for nanoscale MOF-based materials are summarized. The challenges of MOFs in clinical translation and the future directions in the field of MOFs therapy are also discussed. We hope that more researchers will focus their attention on advancing and translating MOF-based nanodrugs into pre-clinical and clinical applications.
1. Introduction
The recent decades have witnessed rapid developments and multitudinous achievements in nanomedicine. Intensive efforts have been devoted to exploiting biomaterials for preclinical or clinical applications,1,2 such as diagnosis, therapy and beyond.3–5 To date, a variety of nanomaterials have been developed as diagnostic agents, therapeutic agents, and even theranostic agents and have made enormous progress in the field of biomedicine due to their tunable size, unique surface characterizations, and high cargo loadings. Notably, nanomaterial-based agents have some intrinsic advantages over their counterparts, such as controllable release, enhanced accumulation, and augmented blood circulation, allowing for boosting the therapeutic efficacy and alleviating adverse reactions.6 Nowadays, the vast majority of nanomaterials can be categorized into either inorganic nanomaterials, including metal-based nanomaterials, metal oxide-based nanomaterials, and carbon-based nanomaterials, or organic nanomaterials including polymer nanoparticles, liposomes, micelles, and dendrimers.7,8 However, these nanomaterials suffer from some inherent disadvantages, such as inorganic materials that are difficult to degrade and organic materials with undesirable cargo loadings. To tackle these challenges of purely inorganic or organic materials in biomedical applications, hybrid nanomaterials have been constructed.Nanoscale metal–organic frameworks (NMOFs) are emerging hybrid materials assembled from metal ions and organic ligands via coordination bonds. Importantly, they combined the benefits of inorganic and organic materials, making them more applicable in biomedicine. NMOFs possess several advantages in the field of nanomedicine: (1) they are readily biodegradable due to the relatively weak metal–ligand coordination bonds; (2) they are conducive to favourable loading efficiency of cargoes because of the high porosity and large pores; (3) they are easy to adjust, allowing for encapsulating or modifying diverse cargoes. Therefore, NMOFs have been widely explored for medical applications.
In this review, we focus on summarizing the nanomedical applications of NMOFs in the last few years, ranging from drug delivery to therapeutic agents (Fig. 1). Compared with the published overview articles, we categorize the delivered drugs according to their properties, highlight the advantages of MOFs in the delivery of various drug components, and pay more attention to the structural therapeutic components of MOFs in terms of treatment. By incorporating MOFs, our article covers a wider range of diseases, not limited to cancer. Moreover, we discuss the challenges and further development of NMOFs, in order to provide new insights and comprehensive understanding of NMOFs in nanomedical applications.
 | ||
| Fig. 1 Schematic diagram of the transportation and therapeutic effects of nanoscale MOFs-based materials. | ||
2. MOFs as delivery systems
MOFs, as an inorganic–organic hybrid nanocarrier, hold its intrinsic advantages over inorganic and organic carriers, owing to its high loading capacity and great biodegradability. Therefore, MOFs could be regarded as a promising candidate for further development of carriers to deliver therapeutic agents, ranging from small molecule drugs to biomacromolecules, such as chemical drugs, proteins, nucleic acids, saccharides, antigens, adjuvants, and gasotransmitters.2.1. Small molecule chemical drugs delivery
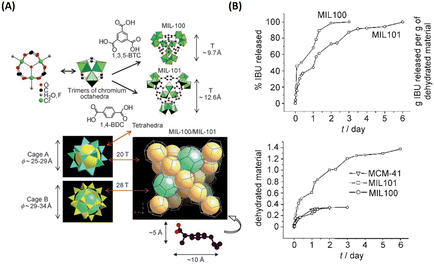 | ||
| Fig. 2 MIL family materials as drug delivery systems that slowly released ibuprofen. (A) 3D representation of MIL-101 (Cr) and MIL-100 (Cr). (B) Ibuprofen delivery from MIL-101 (Cr) and MIL-100 (Cr).9 Copyright 2006, Angewandte Chemie International Edition. | ||
Since then, many MOFs have been used as nanocarriers or intelligent carriers for the sustained and controlled release of drugs, as shown in Table 1. Up until now, MOFs have gained great success in the delivery of active pharmaceutical ingredients (API) and prodrugs, including MIL, zeolitic imidazolate framework (ZIF), porous coordination network (PCN), university of oslo (UiO), isoreticular metal-organic framework (IRMOF), biomolecule-based MOFs (bio-MOFs) and cyclodextrin (CD)-based MOFs. These MOFs are superior to other delivery materials in protecting drugs from degradation, precise delivery, and controllable release.
| MOF-based nanocarriers | Drug | Loading capacity [wt%] | Release behaviour | Ref. |
|---|---|---|---|---|
| MIL-100 (Cr) | Ibuprofen | 25.8 | Sustained release 3 days | 9 |
| MIL-101 (Cr) | Ibuprofen | 58 | Sustained release 6 days | |
| MIL-53(Cr) | Ibuprofen | 19.0 | Sustained release 21 days | 10 |
| MIL-53(Fe) | Ibuprofen | 17.5 | Sustained release 21 days | |
| MIL-101 (Fe) | Cisplatin prodrug | 12.8 | Sustained release 3 days | 42 |
| Cidofovir | 42 | — | 14 | |
| Azidothymidine triphosphate | 42 | — | ||
| Azidothymidine triphosphate | 21 | Sustained release 3 days | 12 | |
| Cidofovir | 29 | Sustained release 5 days | ||
| Doxorubicin | 9 | Sustained release 14 days | ||
| Flurbiprofen | 37 | Sustained release 72 hours | 18 | |
| Indocyanine green | 16.9 | Sustained release | 20 | |
| MIL-101 (Al) | Indocyanine green | 18.2 | Sustained release | |
| MIL-88 (Fe) | Indocyanine green | 10.6 | Sustained release | |
| MIL-88A | Busulfan | 8 | Sustained release | 13 |
| MIL-89 | Busulfan | 10 | Sustained release | |
| MIL-100 | Busulfan | 26 | Sustained release 24 hours | |
| MIL-53 | Busulfan | 14 | Sustained release 24 hours | 13 |
| Flurbiprofen | 20 | Sustained release 72 hours | 18 | |
| Vancomycin | 11.5 | Sustained release | 21 | |
| MIL-100 | TPT | 11.6 | Photon-induced release | 36 |
| [Ru(pcymene)Cl2(pta)] | — | Sustained release | 15 | |
| Caffeine | 42 | Sustained release 48 hours | 16 | |
| Phosphated gemcitabin | 30 | Sustained release 24 hours | 17 | |
| Flurbiprofen | 46 | Sustained release 72 hours | 18 | |
| Gemcitabine monophosphate | 30 | pH-Responsive release | 19 | |
| Ca-MOF | Flurbiprofen | 10 | Sustained release 72 hours | 18 |
| MOF-5 | 5-FU | 84.1 | pH-Responsive release | 43 |
| Fe3O4 nanorods@HKUST-1 | NIM | 16.7 | Sustained release 11 days | 29 |
| HKUST-1 | 5-FU | 40.2 | Sustained release 96 hours | 44 |
| ZIF-8 | 5-FU | 39.8 | pH-Responsive release | 32,45 |
| Doxorubicin | 4.67 | Sustained release 30 days | 46 | |
| 20 | pH-Responsive release | 47 | ||
| Curcumin | 12.7 | pH-Responsive release | 48 | |
| Curcumin | 3.4 | pH-Responsive release | 49 | |
| Verapamil hydrochloride | 32.0 | pH-Responsive release | 50 | |
| Doxorubicin | 8.9 | |||
| 3-Methyladenine | 19.8 | pH-Responsive release | 51 | |
| Chloroquine diphosphate | 18.0 | pH-Responsive release | 52 | |
| Cytarabine-IR820 | 39.8 | pH-Responsive release | 40 | |
| Tetracycline | 59.7 | pH-Responsive release | 53 | |
| ZIF-90 | Doxorubicin | 13.5 | Sustained release 18 hours | 54 |
| 5-FU | 36.4 | Sustained release 25 hours | ||
| Zn-cpon-1 | 5-FU | 44.7 | pH and temperature dual-triggered release | 55 |
| 6-Mercaptopurine | 4.8 | |||
| Zn8(O)2(CDDB)6(DMF)4(H2O) | 5-FU | 53.3 | Sustained release 72 hours | 56 |
| Cu-BTC MOF | 5-FU | 8.2 | Sustained release 2 days | 57 |
| Cu2(COO)4(H2O)2 | Ibuprofen | 45 | Sustained release 96 hours | 58 |
| Guaiacol | 62 | Sustained release 48 hours | ||
| Anethole | 60 | Sustained release 3.5 hours | ||
| mesoMOFs | Doxorubicin | 55 | Sustained release 72 hours | 59 |
| MOF-74 (Fe) | Ibuprofen | 15.9 | Anion-exchange controlled release | 60 |
| Gd-pDBI | Doxorubicin | 12 | pH-Response release | 61 |
| UiO-66 | Cisplatin prodrug | 12.3 | Sustained release 8 hours | 23 |
| Alendronate | 51.4 | pH-Response release | 24 | |
| 5-FU | — | Zn2+ or thermal-triggered release | 25 | |
| Taxol | 14 | Sustained release 300 hours | 26 | |
| Cisplatin | 4.8 | |||
| Dichloroacetic acid | 13.7 | pH-Response release | 27 | |
| Dichloroacetate | 20.7 | Sustained release | 28 | |
| UiO-67 | Taxol | 10 | Sustained release 300 hours | 26 |
| Cisplatin | 1 | |||
| ZJU-800 | Diclofenac sodium | 58.8 | Pressure-controlled release | 62 |
| ZJU-64 | MTX | 13.45 | Sustained release 72 hours | 63 |
| ZJU-101 | Diclofenac sodium | 54.6 | pH-Responsive release | 64 |
| NU-901 | α-Cyano-4-hydroxycinnamic acid | 79.0 | Sustained release 30 days | 65 |
| NU-1000 | α-Cyano-4 hydroxycinnamic acid | 81.0 | ||
| Bio-MOF-1 | Procainamide | 22 | Cation-triggered release | 11 |
| Bio-MOF-Zn | Diclofenac sodium | 172 | Sustained release 48 hours | 66 |
| ZFH-DGR | Dox | 5.4 ± 0.1 | pH-Responsive release | 41 |
| FITC-OVA | 6.9 ± 0.1 | |||
| UMCM-1 | Doxorubicin | — | pH- or competitive binding agent-triggered release | 67 |
| IRMOF-74 | Gemcitabine | 113 | Sustained release | 68 |
| IRMOF-3 | 5-FU | 20.4 | Sustained release 96 hours | 69 |
| Celecoxib | 26.4 | pH-Responsive release | 70 | |
| Doxorubicin | 46.8 | |||
| MOF-In1 | 5-FU | 34.3 | Zn2+-triggered release | 71 |
| γ-CD-MOF | Captopril | 19.3 | Sustained release | 72 |
| Ibuprofen | 26 | Sustained release | 73 | |
| Ibuprofen | 12 | Sustained release 48 hours | 74 | |
| Lansoprazole | 9.4 | |||
| α-CD-MOF | 5-FU | 25.7 | Sustained release 36 hours | 75 |
Among tens of thousands of known MOFs, UiO-66 constructed from zirconium(IV) oxoclusters and terephthalate anions, possessed high stability and porosity as one of the most promising nanocarriers for drug delivery. Furthermore, it is more feasible to modify functional groups on UiO-66, so that both hydrophilic and hydrophobic drugs can be encapsulated through reasonable functionalization. For example, Serre and coworkers explored the relationships between drug encapsulation performances and the functionalized organic linker of UiO-66.22 It was first demonstrated that polar or apolar functional groups on UiO-66 exhibited different drug payloads, in which it appeared that the large octanol-water partition coefficient and low hydrogen bond functional groups were conducive to loading amphiphilic cosmetic caffeine, whereas the loading of the hydrophobic drug ibuprofen was enhanced with large solvent surface and free volume functional groups. It highlighted that the competition between the adsorption of the solvent and the drug during the encapsulation process played a crucial role.
In 2014, Lin and his group first used UiO with hexagonal-plate morphologies for the co-delivery of cisplatin prodrug and small interfering RNAs (siRNAs) via encapsulation and surface coordination to enhance therapeutic efficacy in drug-resistant ovarian cancer cells.23 As shown in Fig. 3, UiO with high porosity allows the cisplatin prodrugs to encapsulate into the channels and metal sites on the surfaces to enable the phosphate group to coordinate binding with siRNA. UiO could effectively protect siRNA from degradation by nuclease, exhibited multiple therapeutics for drug-resistant cancer therapy, and represented a unique nanocarrier platform for co-delivery. Afterwards, UiO-based platforms (i.e., UiO-66, UiO-67) were also been utilized to deliver various chemical drugs, such as 5-FU, taxol, cisplatin, and dichloroacetate, to defeat tumors.24–28 On the one hand, UIO kept the structure and efficacy of anticancer drugs intact; on the other hand, the high porosity of UIO boosted drug loading capacity and achieved better anticancer effects.
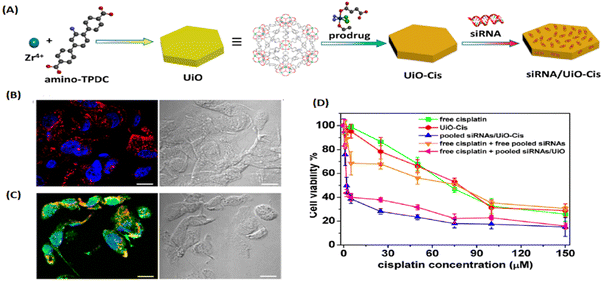 | ||
| Fig. 3 UIO as a unique co-delivery platform. (A) Schematic presentation of siRNA/UiO-Cis synthesis and drug loading; (B) CLSM images showing cell apoptosis and siRNA (TAMRA-labelled, red) internalization in SKOV-3 cells after incubation with siRNA/UiO; and (C) siRNA/UiO-Cis for 24 h; (D) SKOV-3 cells were incubated with free cisplatin, UiO-Cis, pooled siRNAs/UiO-Cis, free cisplatin plus free pooled siRNAs, and free cisplatin plus pooled siRNAs/UiO at different concentrations for 72 h, and then the cytotoxicity was determined by MTT assay.23 Copyright 2014, Journal of the American Chemical Society. | ||
Zhang et al. firstly fabricated MOF-based nanocomposites (named Fe3O4 nanorods@HKUST-1) for magnetic targeted drug delivery.29 As shown in Fig. 4, the magnetic MOF nanocomposites were obtained by incorporating Fe3O4 nanorods into 3D HKUST-1, conferring them excellent candidates for magnetic targeted drug delivery and controlled release. Then, Nimesulide (NIM), an anti-cancer drug for pancreatic cancer treatment, was laden into Fe3O4 nanorods@HKUST-1. There were up to 0.2 g NIM adsorbed per gram Fe3O4 nanorods@HKUST-1 composite. Interestingly, it took as long as 11 days for NIM release in physiological saline at 37 °C. Importantly, a variety of conjugation methods have been developed to attach functional moieties to MOFs.30
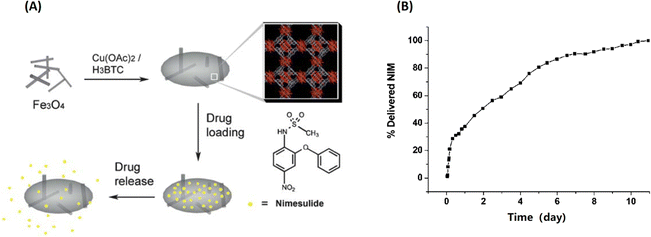 | ||
| Fig. 4 Fe3O4@MOF for magnetic targeted drug delivery and controlled release. (A) The synthesis and action of Fe3O4/Cu3(BTC)2 nanocomposites for potential targeted drug release; (B) the NIM release process in physiological saline at 37 °C.29 Copyright 2011, Journal of Materials Chemistry. | ||
Therefore, covalent attachment targeting cargoes onto MOFs is another important approach for targeted delivery. For example, Dong and coworkers designed and developed a folic acid (FA)-targeting bearing UiO-based drug delivery platform (Fig. 5(A)). The doxorubicin (DOX)-loaded Mi-UiO-68 was covalently modified with FA targeting agent (DOX@UiO-68-FA) via thiol-maleimide Michael-type addition for highly effective hepatoma therapy. Compared with free DOX and DOX@Mi-UiO-68, DOX@Mi-UiO-68-FA possessed the best therapeutic effect.31 As shown in Fig. 5(B), Forgan et al. reported three surface modification protocols, including coordination modulation, postsynthetic exchange and covalent click modulation, to attach biomolecules (i.e., FA, biotin, PEG) on the surface of UiO-66 for dichloroacetic acid (DCA) targeting delivery. They not only demonstrated that biomolecule functionalization could improve the properties of UiO-66, but also confirmed that the therapeutic efficiency was drastically enhanced with a 300-fold increase in the selective cytotoxicity of DCA@UiO-66-FA toward the overexpression of FR of cancer cells.28
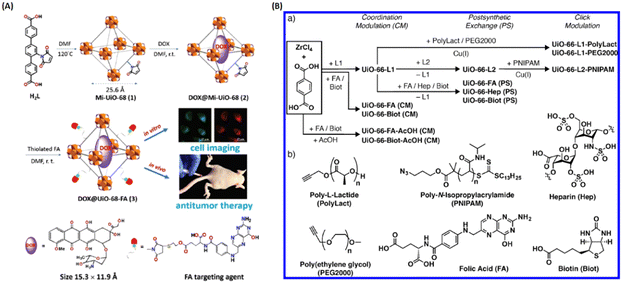 | ||
| Fig. 5 Covalent attachment of targeting agents to MOFs for targeted delivery. (A) Design and fabrication of FA targeting agent-decorated drug delivery system and its application in cell imaging and in vivo antitumor therapy.31 Copyright 2016, Chemical Communications (Camb). (B) Synthetic scheme for the three surface-modified protocol of UiO-66 and the attachment of chemical structures.28 Copyright 2018, ACS Applied Materials & Interfaces. | ||
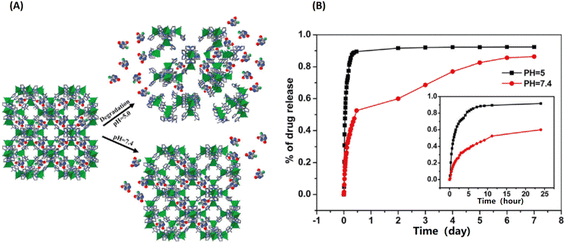 | ||
| Fig. 6 ZIF-8 as a pH-sensitive delivery vehicle for the controlled release of 5-fluorouracil (5-FU). (A) The scheme of pH-response of the encapsulated 5-Fu release from ZIF-8; (B) the NIM release process in physiological saline at 37 °C.32 Copyright 2014, Dalton Transactions. | ||
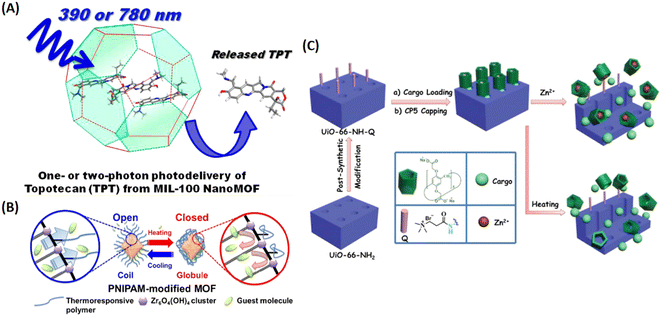 | ||
| Fig. 7 MIL-100 as a light triggered delivery system for transporting topotecan (TPT). (A) Schematic representation of one- or two-photon photodelivery of TPT from MIL-100.36 Copyright 2014, Journal of Medicinal Chemistry. (B) Schematic representation of the thermoresponsive controlled release from UiO-66-PNIPAM.37 Copyright 2015, Chemical Communications (Camb). (C) Schematic representation of the stimuli-responsive mechanized UiO-66-NH2 MOFs equipped with positive charged quaternary ammonium salt (Q) encircled by pillarene[2]pseudorotaxanes. The mechanized nanoUiO-66-NH2 MOFs can be operated either by thermal heating or by Zn2+ competitive binding in regulation of the release of cargo molecules.25 Copyright 2015, Small. | ||
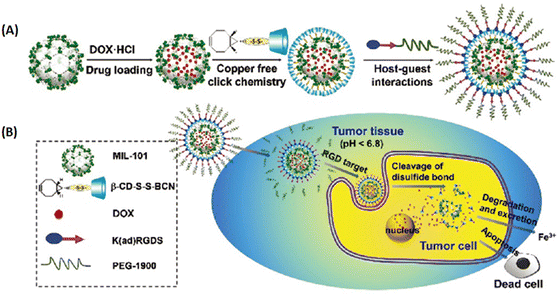 | ||
| Fig. 8 MIL-101 as a multifunctional drug delivery system for targeting and pH/GSH dual-responsive release. (A) Schematic illustration of drug loading and post-synthetic modification procedure, and (B) the tumor-targeting drug delivery and cancer therapy procedure of the multifunctional MOF based DDS.39 Copyright 2015, Nanoscale. | ||
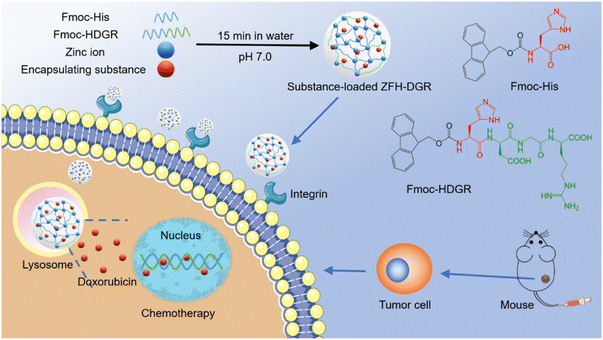 | ||
| Fig. 9 Bio-MOFs construction from amino acids/peptides and bimetallic ions as green carriers for targeting delivery. One-pot construction of targeted bio-MOF/drugs and their targeted therapy.41 Copyright 2021, ACS Applied Materials & Interfaces. | ||
2.2. Protein delivery
It is well known that proteins are prone to deactivation by degradation, and most native proteins are membrane impermeable. So, it is crucial to develop carrier systems for the efficient delivery of functional proteins. Nowadays, there are three main approaches to fabricate MOF-based nanocarriers for protein protection/delivery, including encapsulation, covalent conjugation and protein/peptide as organic linkers, as shown in Table 2.| MOFs | Proteins | Methods | Ref. |
|---|---|---|---|
| [Cu(OOC–C6H4-C6H4–COO).1/2 C6H12N2]n MOF | MP-11 | Immersed method | 76 |
| Tb-mesoMOF | MP-11 | Immersed method | 77,95 |
| Tb-mesoMOF | Myoglobin | Immersed method | 96 |
| Tb-mesoMOF | Cyt c | Immersed method | 97 |
| PCN-333 | HRP, Cyt c, MP-11 | Immersed method | 88 |
| ZIF-8 | Cyt c, HRP, lipose | One-pot coprecipitation method | 78 |
| ZIF-10 | |||
| Cyt c | |||
| ZIF-90 | Catalase | One-pot coprecipitation method | 80 |
| ZIF | Catalase | One-pot coprecipitation method | 82 |
| ZIF-8 | GOx | One-pot coprecipitation method | 86 |
| ZIF-8 | GOx&HRP | One-pot coprecipitation method | 81 |
| ZIF-8 | BSA, β-Gal, HAS, caspase3, EGFP, RFP, | One-pot coprecipitation method | 84 |
| ZIF-8 | BSA, Cyt c, geloion | One-pot coprecipitation method | 85 |
| ZIF-8 | GOx&insulin | One-pot coprecipitation method | 87 |
| ZIF-8, HKUST-1, MIL-88A, Eu/Tb-BDC | BSA/HAS/OVA/lysozyme, HRP/ribonucleaseA/haemoglobin/trypsin/lipase/insulin/glucose-dehydrogenase | Biomimetic mineralization | 79 |
| PCN-224 | GOx&catalase | Immersed method | 83 |
| PCN-333 | SOD&catalase | Immersed method | 98 |
| [(Et2NH2)(In(pda)2)]n, [Zn(bpydc)(H2O). (H2O)]n, IRMOF-3 | EPGF, CAL-B | Bioconjugation | 89 |
| MIL-88B-NH2 | Trypsin | Bioconjugation | 90 |
| CYCU-4 | Trypsin-FITC | Vortex-assisted host–guest interaction | 91 |
| Zn-ferritin MOF | Ferritin | As modular component | 93 |
| Metal-ferritin MOFs | Ferritin | As modular component | 94 |
Taking advantage of the simple and friendly synthesis of ZIF, the Liu group developed a facile and general method for the one-pot synthesis of protein-embedded ZIF-8/10 by coprecipitation method.78 As shown in Fig. 10, it demonstrated that the peroxidase activity of cytochrome c (Cyt c)-embedded in ZIF-8 increased 10-fold compared to free Cyt c in solution. Following up on this work, it was a rapid development of ZIF-encapsulated enzymes and functional proteins for pH-responsive delivery or co-delivery in imaging and therapy applications.79–87 Afterwards, Zhou and coworkers rationally designed ultra-large mesoporous cages of PCN-332/333 and developed single-enzyme encapsulation (SEE).88 The size of the cage could be larger than the enzyme, but cannot accommodate two enzymes due to size limitation, for the encapsulation of a single-enzyme molecule in one cage. Thus, the SEE could effectively prevent enzyme aggregation and denaturation.
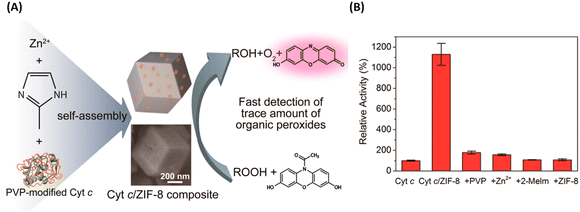 | ||
| Fig. 10 ZIF-8 for enzymes and functional proteins delivery. (A) Preparation of the Cyt c-embedded ZIF-8 and its biocatalysis. (B) The relative peroxidase activity of Cyt c, Cyt c/ZIF-8 composite, PVP/Cyt c mixture, Cyt c/zinc ion mixture, Cyt c/2-methylimidazole mixture, and Cyt c/ZIF-8 mixture.78 Copyright 2014, Nano Letters. | ||
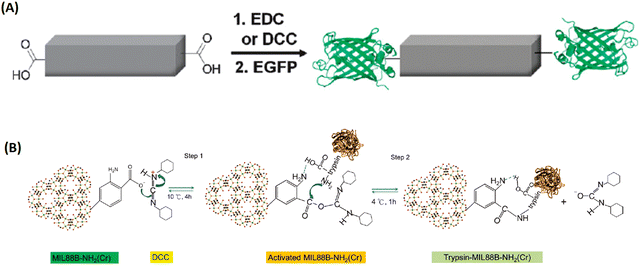 | ||
| Fig. 11 The bioconjugation of proteins onto the surface of MOFs via functional groups of the organic linkers. (A) The bioconjugation of the 1D-MOFs, [(Et2NH2)(In(pda)2)]n with EGFP.89 Copyright 2011, Chemical Communications (Camb). (B) Trypsin immobilization onto DCC-activated MIL-88B-NH2.90 Copyright 2012, ChemPlusChem. | ||
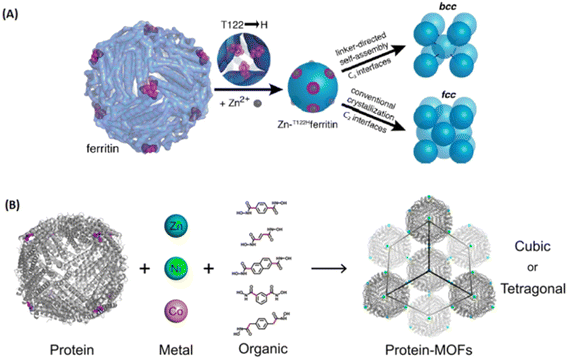 | ||
| Fig. 12 Metal-peptide/protein frameworks as robust vehicles to deliver peptides or proteins. (A) Scheme for metal/linker-directed self-assembly of ferritin into 3D crystals.93 Copyright 2015, Journal of The American Chemical Society. (B) The construction of a library of 3D ferritin-MOFs with different modular components.94 Copyright 2017, Journal of The American Chemical Society. | ||
2.3. Nucleic acid delivery
It is crucial to develop non-viral vectors for nucleic acid delivery, which can promote applications in the field of gene therapy and gene editing. MOFs, as novel carriers, play vital roles in nucleotide delivery. Nucleic acid with some intrinsic advantages, such as excellent biocompatibility, high electronegativity, multiple functional groups, etc., which enabled MOFs to load nucleic acid with various methods, ranging from adsorption, encapsulation and covalent attachment to as building blocks.99Nucleic acids with rich structure and multiple functional groups could sever as organic linkers to coordinate with metal ions, forming supramolecular networks. Kimizuka et al. reported on the supramolecular networks self-assembled from nucleotide (5′-AMP, 5′-GMP, 5′-UMP, 5′-CMP) and lanthanide ions, which can even encapsulate fluorescent dyes, proteins, drugs or nanoparticles for biomedical applications.100
Later, Zhou and colleagues synthesized two nucleobase-incorporated MOFs (PCN-530 and TMOP-1) by introducing adenine as co-ligand.101 Gref and coworkers explored the impact of phosphate groups on nucleoside analogue drugs (i.e., azidothymidine and its phosphorylated derivatives) of loading capacity, and demonstrated that the interaction between drugs’ phosphate groups and the Lewis iron(III) acid site from MIL-100(Fe) increased encapsulation efficiency.102 Inspired by this, Liu et al. synthesized Se/Ru@MIL-101 composite to load siRNAs via surface coordination between the unsaturated Fe(III) sites of MIL-101 and phosphate residues on the backbone of siRNA, for delivery of siRNAs to reverse multidrug resistance therapy.103
The research team of Mirkin constructed UiO-66-nucleic acid conjugates through click reaction, which exhibited increased stability and enhanced cellular uptake.104 UiO-66-N3 were synthesized by 2-azidoterephthalic acid and ZrOCl2.8H2O via solvothermal reaction, and then dibenzylcyclooctyne-functionalized DNA reacted with the –N3 group of UiO-66 to obtain DNA-UiO-66. Wang et al. developed a versatile nanosystem (Ce6-DNAzyme@ZIF-8) by encapsulating a therapeutic DNAzyme agent into ZIF-8 for self-sufficient gene therapy and photodynamic therapy (PDT).105 The as-prepared Ce6-DNAzyme@ZIF-8 exhibited efficient intracellular delivery and pH-responsive release of Ce6-DNAzyme, and Zn2+ ions as co-factor concomitantly supplied to boost gene therapy, along with Ce6-based PDT. Zhou et al. tuned the pore size of IRMOF-74 with different organic linkers to precisely include different lengths of ssDNA.106 IRMOF-74 can protect ssDNA excellently, because the entire nucleic acid completely confining inside the pores, and exhibited high transfection efficiency in immune cells.
2.4. Antigens/adjuvant delivery
Tumor vaccines, consisting of antigen and adjuvant, have been considered as a promising therapy. Therefore, nanocarrier delivery systems afford benefits for promoting the development of vaccines. As a well-known class of porous hybrids, MOFs have been used as carriers to stimulate the development of vaccines for tumor therapy.As shown in Fig. 13, Qu et al. developed a ZIF-8-based vaccine by in situ encapsulating ovalbumin (OVA) antigens and absorbing unmethylated cytosine-phosphate-guanine oligodexynucleotides (CpG ODNs) adjuvants, donated as OVA@ZIF-8-CpG.107 The obtained vaccines have good biocompatibility and are pH-responsive to the delivery of OVA and CpG ODNs into the same antigen presenting cells (APCs) efficiently, which induced a strong systemic immune and potent immune memory response. Zhang's research group also fabricated OVA and CpG codelivery based on pH-responsive MOFs nanocarrier for enhanced cancer immunotherapy.108 Similar with proteins and nucleic acids delivery, MOFs could be applyed to the delivery of antigens/adjuvants for enhanced immune and synergistic therapy.109–112
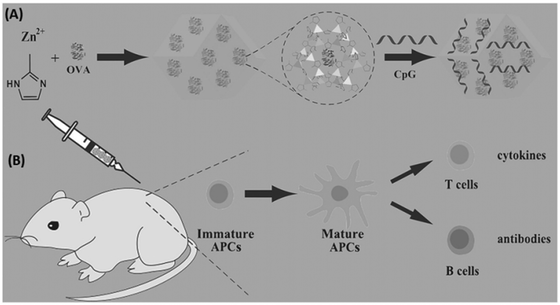 | ||
| Fig. 13 MOFs used for co-delivery of antigens and adjuvants to construct tumor vaccines. (A) The preparation of the OVA@ZIF-8-CpG vaccine. (B) The use of the OVA@ZIF-8-CpG vaccine to induce a humoral and cellular immunity response.107 Copyright 2016, Advanced Functional Materials. | ||
2.5. Gasotransmitter delivery
Recently, gasotransmitter-based therapy has attracted tremendous attention, such as nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), and hydrogen (H2). It is vital to develop gas releasing systems because of their short half-life. MOFs, a “star” hybrid, hold great promise for gas storage and delivery in biological applications.Morris et al. found that NO and H2 have relatively weak interaction with metals in HKUST-1, which is an advantage for gas delivery.113 In particular, the adsorption capacity of NO on HKUST-1 is significantly high, and is enough to be biologically active and inhibit platelet aggregation. Based on gas–metal interaction, they further developed CPO-27, MIL-88(Fe), Ca-MOF, etc. to store and deliver NO or H2S for potential biomedical applications.114–117 Metzler-Nolte and coworkers prepared high-quality MIL-88B-Fe and NH2-MIL-88B-Fe with a large number of unsaturated metal sites for loading and delivery CO.118 CO was captured by the iron unsaturated coordination site of MIL-88, and released during the decomposition.
Gas-releasing molecules were recently developed for controllable release, such as NO donors and CO donors. Diazeniumdiolate is attractive for NO release and can be facilitated to modify nanomaterials through the reaction of the amine with NO/NO2− to form N-nitrosamine. Based on this, NO donor-modified MOFs were developed by covalent modification for controlled release of NO.119–121 A few research groups constructed stimulus-responsive CO-releasing MOFs by using the open 2,2′-bipyridine (bpy) centers in MOFs (UiO-67-bpy, UiO-66-BDP, Ti-MOFs) to coordinate with CO donors (MnBr(CO)5), leading to the formation of MnBr(bpydc)(CO)3@MOFs.122–124 They achieved efficient and controllable CO release by light-/H2O2-triggered therapy. Additionally, Furukawa and coworkers used 2-nitroimidazole and 5-methyl-4-nitroimidazole as organic ligands to construct NO-based ZIFs, and achieved precisely controlled NO release via two-photo laser activation.125
2.6. Saccharide delivery
Heparin, a linear glycosaminoglycan, has been extensively used as an anticoagulant. Pidko and coworkers synthesized a de novo heparin-MIL-101(Fe) composite with excellent biocompatibility and pronounced anticoagulant activity. Furthermore, they used the as-prepared heparin-MIL-101(Fe) as a drug-releasing depot to coat on the structure of streptokinase (SK)-entrapped alumina, named as Hep-MIL-101(Fe) + SK@alumina, which was applied on polytetrafluoroethylene vein implants.1263. MOFs as therapeutic agents
Some therapeutic drugs with several functional groups make them possible to directly construct MOFs as bridging ligands, or biologically active cations as central metal ions or even their combinations could build therapeutic MOFs for biomedical applications.3.1. Therapeutic ligands
Therapeutic ligands, as linkers to construct nanoscale coordination polymers (NCPs) or MOFs, afford high atomic economy to generate high drug capacity and avoid the toxicity of exotic organic ligands. Therefore, it is a judicious approach to develop therapeutic MOFs for broadening applications.As early as 2005, Li et al. synthesized a 2D coordination polymer, which was self-assembled from enoxacin and Mn2+ ions through covalent coordination and hydrogen bonds under hydrothermal conditions.127 However, a majority of drugs or prodrugs lacked molecular rigidity and were unstable in high temperatures/pressures conditions. Thus, some facile, simple and general methods were developed to construct drug/prodrug-based amorphous NCPs for therapy. In 2008, Lin and coworkers first reported a NCPs forming from Tb3+ and platinum prodrug by poor solvent precipitation method for drug delivery.128 As shown in Fig. 14, NCP-1 was built from cisplatin-based prodrug with two carboxyl groups and lanthanide metal ions (Tb3+), then NCP-1′ obtained by coating a silica shell on NCP-1 to improve the water stability (NCP-1′-a with a silica shell thickness of ∼2 nm, NCP-1′-b with ∼7 nm silica shell). Furthermore, a targeted peptide c(RGDfK) was modified on silica shell via covalent conjugation. They demonstrated that the thickness of the silica shell could improve the stability, and also efficiently control the release of Pt species for targeted drug delivery.
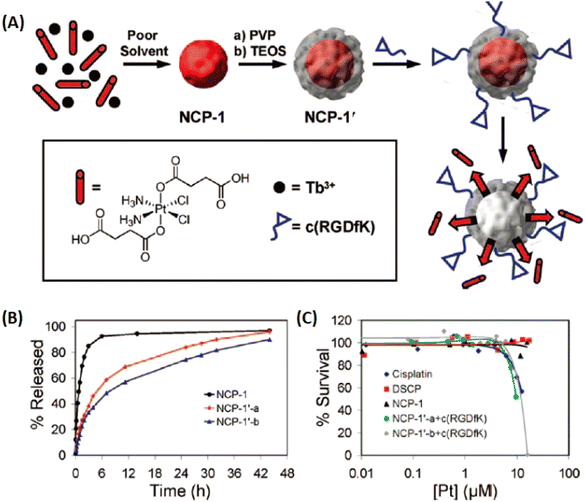 | ||
| Fig. 14 Cisplatin-based prodrug as organic linkers to construct NCPs for the controllable release of the Pt species. (A) The preparation of NCPs; (B) the Pt release from NCPs against time; (C) cytotoxicity assay for HT-29 cells against Pt concentration of NCPs.128 Copyright 2008, Journal of the American Chemical Society. | ||
In their follow up work, Lin and his team refined the synthetic strategy and formulated a series of NCPs for anticancer therapy. As shown in Fig. 15, drugs with double carboxyl (methotrexate, folic acid) or bisphosphonates (pamidronate, zoledronate) as building blocks coordinate with metal connecting (Zn2+, Zr4+, Gd3+, Ca2+) to form different morphology NCPs by hydrothermal reaction. Encapsulation of the NCPs within a functionalized lipid bilayer could achieve targeted delivery and controlled release to cancer cells.129–131 Subsequently, they further developed reverse microemulsion and pegylated method to obtain multifunctional NCPs for in vivo therapy. They constructed lipid-coated NCPs via reverse microemulsion, and then modified PEG-functionalized lipid or even siRNA-functionalized lipid (Fig. 16). The as-prepared NCPs exhibited excellent blood circulation, superior potency and efficacy for chemotherapy, gene silencing, immunotherapy or their combined therapy.132–140 Importantly, they have made significant progress and promoted clinical translation of some NCPs.
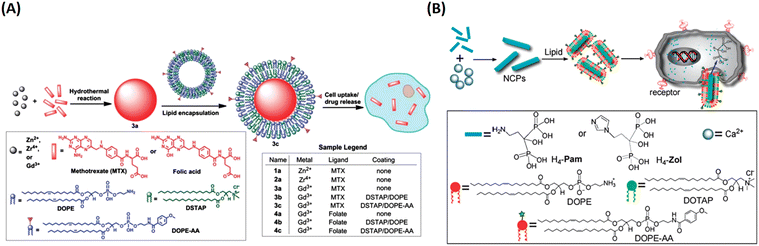 | ||
| Fig. 15 The synthesis of NCPs via hydrothermal reaction and functionalization with a lipid bilayer for targeted delivery and controlled release. (A) MTX-based NCPs for targeted delivery to cancer cells;129 Copyright 2012, Chemical science. (B) H4-Pam/H4-Zol-based NCPs and their targeted delivery to cancer cells.130 Copyright 2012, Chemical Communications (Camb). | ||
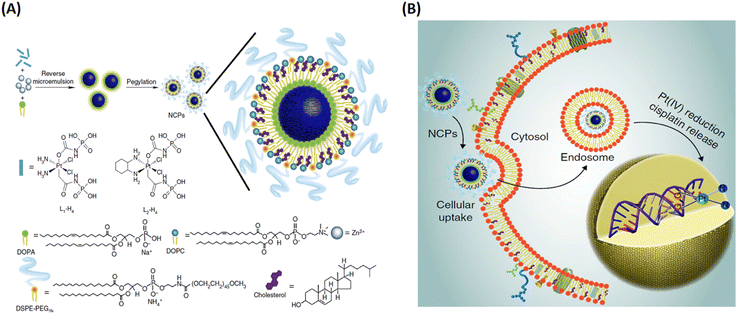 | ||
| Fig. 16 The construction of lipid-coated NCPs via reverse microemulsion for delivery. (A) General procedure for self-assembly of NCPs with lipid and PEG coatings; (B) schematic showing endocytosis of NCPs and subsequent reduction of prodrug ligands by intracellular reducing agents, such as glutathione, to release cytotoxic cisplatin and oxaliplatin.132 Copyright 2014, Nature Communications. | ||
Inspired by these, Liu's group fabricated nanoscale metal–organic particles (NMOPs) composed of Mn2+ ions and a NIR dye, IR825 photothermal agents via coordinated self-assembly, obtaining Mn-IR825 NMOPS as the core. Then, a shell of polydopamine (PDA) was also coated along with PEG functionalization, achieving Mn-IR825@PDA-PEG NMOPs.141 Among them, IR825 exhibited strong photothermal conversion and high photostability for efficient photothermal therapy (PTT), and Mn2+ ions were endowed with T1-weighted MR imaging. Thus, Mn-IR825@PDA-PEG NMOPs can be applied for MRI-guided PTT. Furthermore, the Mn-IR825 core was mixed with Hf4+ ions via post-synthesis cation exchange, and then they obtained co-doped Mn/Hf-IR825@PDA-PEG NMOPs. Due to Hf4+ with its excellent computed tomography (CT) enhancement ability and radio-sensitization (RT) capability, Mn/Hf-IR825@PDA-PEG NMOPs can be utilized for MR&CT&PA multimode imaging and PTT&RT synergistic treatment of tumors with high therapeutic efficacy.137
Recently, we designed and constructed novel NCPs, integrating two prodrugs (Pt(IV) prodrug and NO donor) as organic ligands and Fe3+ as coordinative centers.142 As shown in Fig. 17, this unique NCPs contained two prodrugs of bio-orthogonal chemistry, the masked trigger of the Pt(IV) prodrug and the caged active group of the NO donor. In a tumor microenvironment, the Pt(IV) prodrug can be reduced to catalytically active Pt(II) cisplatin, which triggers depropargylation of the NO donor to release high levels of NO. Thus, the DNA crosslinking effect of Pt(II) cisplatin and the anticancer activity of NO achieved synergistic therapy for triple-negative breast cancer.
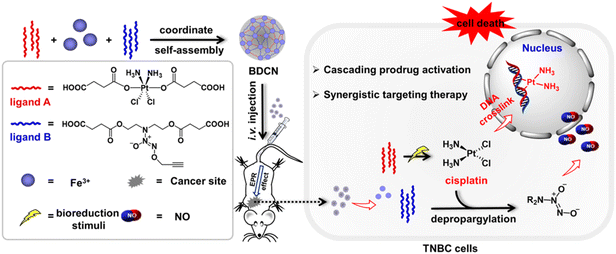 | ||
| Fig. 17 The construction of integrated bio-orthogonal NCPs and their therapeutic mechanism of tumor-specific initiating cascade reactions.142 Copyright 2022, JACS Au. | ||
Compared to the above-mentioned small molecular therapeutic agents as building blocks, porphyrins and porphyrin derivatives are macromolecular heterocyclic compounds with molecular rigidity, which have been widely used as photosensitizers (PSs) for photodynamic therapy (PDT). Therefore, the development of porphyrinic MOFs for PDT has attracted tremendous attention. For instance, Lin and coworkers were the first to construct MOFs with porphyrin derivative-based linkers for highly effective PDT.143 As shown in Fig. 18, the porphyrinic MOFs (DBP-UiO) were built from a porphyrin derivative, 5,15-di(p-benzoato)porphyrin (H2DBP) linkers and Hf12(μ3-O)8(μ3-OH)8(μ2-OH)6 by a solvothermal reaction. The as-prepared DBP-UiO MOFs were highly stable in physiological media, and efficiently generated singlet oxygen (1O2) upon 640 nm irradiation. They demonstrated high anticancer efficacy of DBP-UiO-enabled PDT in vitro cytotoxicity and human head and neck cancer models in vivo. Furthermore, they optimized the design of the chlorin-based MOFs by reducing the ligands of 5,15-di(p-benzoato)chlorin (H2DBC) in DBC-UiO.144 Compared with DBP-UiO, DBC-UiO was redshifted by 13 nm to provide improved tissue penetration, and the extinction coefficient at the lowest energy Q-band was increased 11-fold to generate more efficient 1O2 for PDT. The combination of Hf-porphyrin or Hf-porphyrinic derivate MOF-mediated PDT with immunotherapy has been explored.145,146
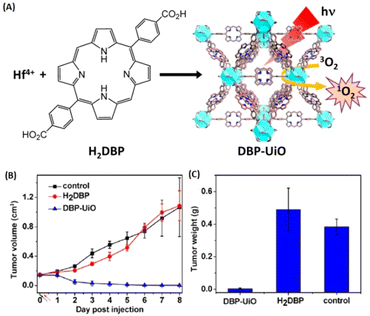 | ||
| Fig. 18 MOFs constructing with porphyrin derivative-based linkers for highly efficient PDT. (A) Synthesis of Hf-DBP NMOF and the schematic description of the 1O2 generation process. (B) Tumor growth inhibition curve after PDT treatment. Black and red arrows refer to injection and irradiation time points, respectively. (C) Tumor weight after PDT treatment.143 Copyright 2014, Journal of the American Chemical Society. | ||
Subsequently, several other research groups have developed more porphyrinic MOFs (PCN-224, PCN-222, MOF-545 etc.) for PDT or PDT-based combination therapy.147–162 For example, Zhou and colleagues reported a size-controlled synthesis of Zr-TCPP MOF (named PCN-224) ranging from 30 nm to 190 nm and functionalized with folic acid (FA) onto Zr6 cluster in PCN-224 for targeted PDT.148 They demonstrated that PCN-224 of 90 nm has preferential cellular uptake and remarkable PDT efficacy over other sizes. By using PCN-224, Zhang and coworkers constructed a cancer cell membrane-camouflaged cascade bioreactor by loading glucose oxidase (GOx) and catalase on the surface of PNC-224 for targeted starvation therapy-PDT synergistic antitumor therapy.149
Noteworthily, the controlled generation of ROS in PDT has attracted considerable attention. Zhou and coworkers developed photochromic porphyrin-based MOFs for reversible control of 1O2 generation.147 As shown in Fig. 19, using 1,2-bis(2-methyl-5-(pyridin-4-yl)thiophen-3-yl)cyclopent-1-ene (BPDTE) as a photochromic switch, they designed two MOFs by solvothermal reaction of BPDTE, TCPP or tetratopic carboxylate linker DBTCB and Zn2+ to obtained SO-PCN and PC-PCN. Meanwhile, SO-PCN has been demonstrated to regulate 1O2 generation under UV/visible irradiation. Recently, Zhang et al. utilized Mn(III) as a sealer to quench the fluorescence of TCPP and inhibit ROS generation, designing an “inert” Mn(III)-TCPP MOFs.159 The as-prepared MOFs could be activated by the overexpression of GSH in tumor cells, and consumed GSH to release Mn(II) ions and free TCPP. So, the unlocking MOFs exhibited Mn(II)-based MRI and TCPP-based OI. On the other hand, the consumption of GSH could effectively control ROS production and enhance the efficacy of TCPP-based PDT (Fig. 20).
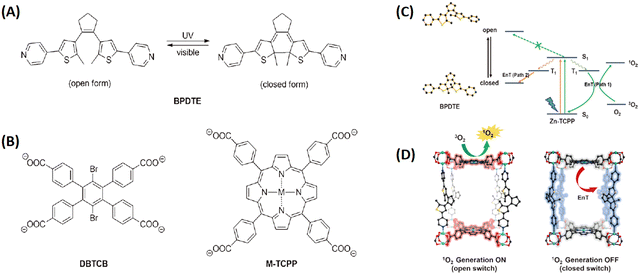 | ||
| Fig. 19 Photochromic porphyrin-based MOFs used to reversibly control 1O2 generation. (A) Photoisomerization of BPDTE under UV and visible light. (B) Structures of ligands consist of two-dimensional layers in PC-PCN and SO-PCN, respectively. (C) Proposed mechanism of energy transfer (EnT) in SO-PCN. (D) Illustration of switching operation in SO-PCN.147 Copyright 2015, Angew. Chem.-Int. Edit. | ||
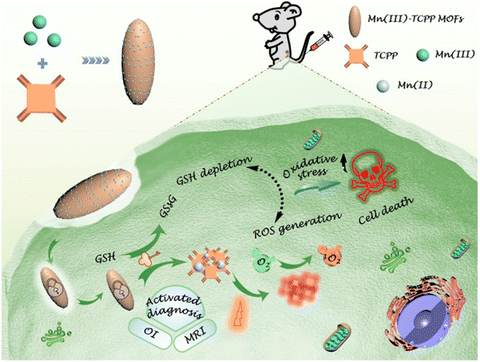 | ||
| Fig. 20 Schematic illustration of an endocytosis Mn(III)-TCPP MOF nanosystem for MRI- and OI-guided PDT by controlled ROS generation and GSH depletion after being unlocked by overexpressed GSH in tumor cells.159 Copyright 2019, ACS Nano. | ||
3.2. Active metals
Some active metal centers have also been explored for therapy in recent years, such as Ag+, Cu+, Co2+, and Zn2+ ions are known for their antibacterial activities. Therefore, these active metal ions in MOFs/NCPs exhibited corresponding therapeutic effects.Due to the excellent antibacterial activity of Ag+ ions, Ag-based MOFs/NCPs have been synthesized to deliver Ag+ ions for antibacterial therapy. Fromm and coworkers formulated a nanostructured silver coordination polymer compounds, and deposited the as-prepared compounds onto the surface of a gold–titanium alloy as an antimicrobial agent for dental implant and restorative materials.163 They further demonstrated the biochemical and molecular mechanisms of the bactericidal activity of Ag+ ions, which could be attributed to the ability of Ag+ ions to inactivate enzymes by binding thiol groups in amino acids, and promote iron release with ˙OH production and subsequent DNA damage.164 Jaffrès et al. synthesized Ag3(3-phosphonobenzoate) MOF for the sustainable release of Ag+ with high bactericidal activity against 6 bacterial strains.165 Afterwards, several new silver MOFs were constructed for antimicrobial therapy.166,167
Recently, transition metal ions (Cu+, Co2+, Fe3+, etc.) as metal centers in coordination polymers (CPs) presented good bactericidal action and anticancer activities.168 The Zamaro group synthesized a Cu-based MOF, named HKUST-1, and successfully applied HKUST-1 to inhibit the growth of S. cerevisiae and G. candidum because of the release of Cu+ ions during the progressive degradation.169 Liu et al. designed a novel Co-based MOF (Co-TDM) with high bactericidal activity, which was constructed from Co2+ ions and octa-topic carboxylate ligand, tetrakis [(3,5-dicarboxyphenyl)-oxamethyl] methane (TDM8−).170 As shown in Fig. 21, the mechanism of the bactericidal activities could be observed in six steps: (1) diffusion-directed lipid-oxidation, (2) direct interaction, (3) reaction-oxygen species generation, (4) cation transport interruption, (5) chelation effects, and (6) membrane depolarization. Co2+ ions served as active sites to catalyze lipid peroxidation. Thus, the bacterial membrane ruptured and the bacteria were inactivated. A few Co-based MOFs also have been developed to combat bacteria.171,172
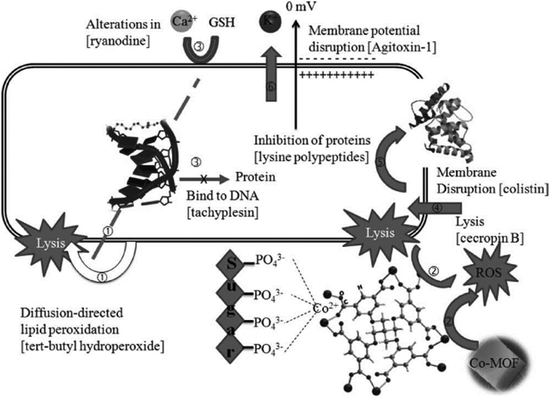 | ||
| Fig. 21 The mechanism of bactericidal activities using Co-TDM single crystals as disinfectants: six steps synergistically contribute to the bactericidal efficacy.170 Copyright 2012, Advanced Healthcare Materials. | ||
High atomic (Z) number elements, such as Au and HfO2, with high X-ray absorption coefficients, have been deemed as promising radioenhancers for anticancer therapy. Inspired by this, Lin and coworkers synthesized Hf-based MOFs as highly effective radioenhancers for radiotherapy (RT). Importantly, when combined with the anti-programmed death-ligand 1 (anti-PD-L1) antibody, the Hf-based MOF-mediated low-dose RT with immunotherapy achieved local therapeutic effects of RT and distant tumor therapy via immunity.173 As shown in Fig. 22, Hf12-DBA could efficiently generate ˙OH upon X-ray irradiation to enhance RT for local tumor therapy. The immunogenic cell death of RT led to tumor antigen release, and when used in combination with the anti-PD-L1 antibody, caused regression in distant tumors via systemic antitumor immunity.
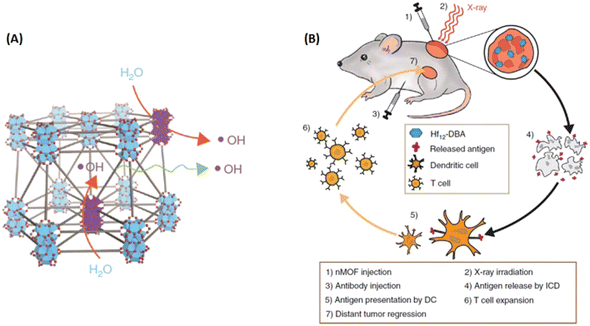 | ||
| Fig. 22 Hf-based MOFs served as highly efficient radiation enhancers for radiotherapy (RT). (A) Illustration of efficient hydroxyl radical generation upon X-ray irradiation and diffusion through porous Hf12-DBA nanoplates; (B) Abscopal effect of nMOF-mediated RT and immune checkpoint blockade using fractionated X-rays.173 Copyright 2018, Nature Communications. | ||
3.3. The combination of active metals and ligands
Nowadays, combination/synergistic therapy is widely used to boost the therapeutic effect. In this regard, MOFs combining bioactive cations with therapeutic ligands could achieve a highly effective synergistic treatment.Spencer et al. synthesized a series of bismuth-dicarboxylate-deferiprone coordination networks, wherein both the ligand (deferiprone) and metal center (Bi3+ ions) in the coordination networks played their therapy roles for the inhibition of H. pylori growth.174 Blanco-Prieto and colleagues constructed a novel BioMIL-5 based on Zn2+ ions and azelaic acid via hydrothermal method, and both components of BioMIL-5 exhibited high antibacterial activity and interesting dermatological property.175 The progressive simultaneous release of active Zn2+ and azelaic acid from BioMIL-5, in both water and culture media, not only have continuous antibacterial properties for as long as 7 days to inhibit the growth of Gram-positive S. epidermidis, but also provide complementary beneficials for skin disorders treatment.
Besides the combined antimicrobial therapy, the synergistic and combined therapy of cancer has also been developed using MOFs/NCPs built up from active metal and therapeutic ligands. For example, we constructed NCPs based on NO donor and iron ions for synergistic NO and chemodynamic therapy (CDT) of liver cancer.176 As shown in Fig. 23, a GSH-sensitive NO donor with two carboxyl groups coordinated with iron ions to form NCP via simple precipitation and partial ion exchange. The NO donors in Fe(II)-BNCP released NO and the Fe2+ ions exerted Fenton activity to generate ROS in tumor microenvironments triggered by GSH and induced by H2O2, respectively. In addition, the synergistic NO-CDT effect of Fe(II)-BNCP has been applied to retard the tumor growth in Heps xenograft ICR mouse models. Liu et al. synthesized a carrier-free MOFs system based on Hf4+ and TCPP that was used for TCPP-mediated PDT and Hf4+-enabled radiotherapy (RT).177 Lin and colleagues engineered a Cu-porphyrin MOF for synergistic hormone-triggered CDT and light-triggered PDT.178 As shown in Fig. 24, pH-triggered MOF decomposed to release Cu2+ ions and porphyrins for estradiol-induced CDT and light-driven PDT, respectively. Furthermore, with the combination of MOF-mediated CDT/PDT with anti-PD-L1 immunotherapy, the local and distant tumors effectively regressed. In addition, the combination of porphyrinic MOF-based PDT and active metal ions for synergistic therapy have been described in the part of porphyrinic MOFs.
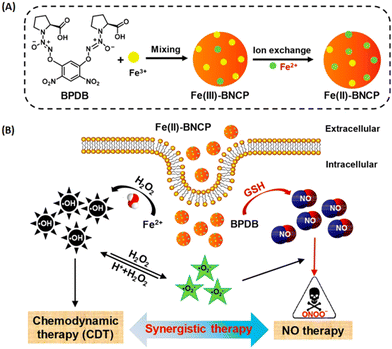 | ||
| Fig. 23 NO donor-constructed Fe-based NCPs for NO-CDT synergistic antitumor therapy. (A) Schematic illustration of Fe(III)-BNCP and Fe(II)-BNCP preparation; (B) NO-CDT synergistic therapy of Fe(II)-BNCP in tumor cells.176 Copyright 2019, Nano Letters. | ||
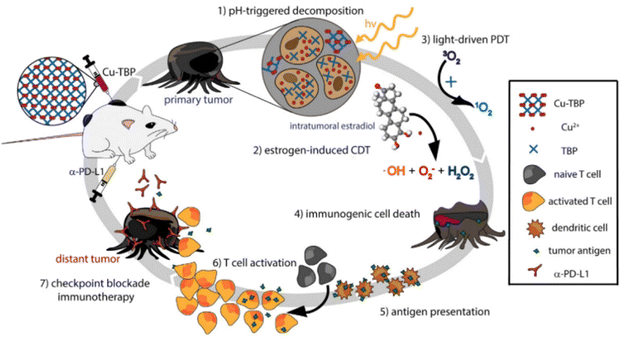 | ||
| Fig. 24 Synergy of checkpoint blockade immunotherapy and MOF-mediated CDT/PDT triggered by both hormone and light stimulation.178 Copyright 2019, Chemistry. | ||
4. Conclusion and outlooks
We have summarized the main applications of nanoscale MOFs or NCPs in the field of therapy, which has achieved growing development. In the early state, MOF-based porous materials were extensively used as carriers to deliver a variety of drugs, including small molecule chemical drugs, proteins, nucleic acids, saccharides, antigens, adjuvants, and gasotransmitters. Compared with other nanocarriers, MOF-based delivery systems have shown some intrinsic advantages of biodegradability, high porosity, adjustability of components, the flexibility of modification and controlled release. Although MOF-based nanocarriers have made enormous progress in the field of drug delivery, their therapeutic applications are still in their infancy. MOFs or NCPs were then derived from therapeutic ligands, active metal ions or their combination, developing as therapeutic agents, which greatly facilitated the therapy of various diseases.In recent years, MOFs have achieved tremendous advances and indicated great prospects in the field of biomedicine. However, there still remain critical challenges: (1) the biological safety of MOFs is worrying. (2) The stability of MOFs in biological systems is not satisfactory. (3) The synthesis conditions are not friendly. (4) The metabolic pathway is ambiguous. (5) The structure and druggability of MOFs need to be improved. (6) The strategies for large-scale fabrication of MOFs are rare. (7) Most targeting modification approaches are tedious and complicated. These existing problems greatly hinder the clinical transformation of MOFs.
Taken together, the challenges of MOFs need to be overcome urgently before realizing their clinical applications. We still have much to do to achieve the safe and efficient application of MOF-based therapy. To achieve this, we call for more researchers to devote attention toward advancing and translating MOF-based nanodrugs into clinic. We also hope this review will help readers understand the current development of MOF-based therapy, including their advantages and challenges, and will drive chemists, materials scientists, biologists, pharmacists and clinicians to participate in this research and to benefit patients for achieving clinical application of MOF-based therapy as soon as possible.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82001953, 82103527, 82203064), Shanghai Municipal Health Commission (20214Y0022), and Original Basic Research Project of Tongji University (22120210583).References
- K. Zhang, A. G. Mikos, R. L. Reis and X. Zhang, Bioact. Mater., 2022, 18, 337–338 CrossRef PubMed.
- D. H. Kohn, Matter, 2019, 1, 1114–1115 CrossRef PubMed.
- X. Guo, H. Xu, W. Li, Y. Liu, Y. Shi, Q. Li and H. Pang, Adv. Sci., 2023, 10, e2206084 CrossRef PubMed.
- T. Chen, F. Wang, S. Cao, Y. Bai, S. Zheng, W. Li, S. Zhang, S. X. Hu and H. Pang, Adv. Mater., 2022, 34, e2201779 CrossRef PubMed.
- P. Geng, L. Wang, M. Du, Y. Bai, W. Li, Y. Liu, S. Chen, P. Braunstein, Q. Xu and H. Pang, Adv. Mater., 2022, 34, e2107836 CrossRef PubMed.
- X. Joseph, V. Akhil, A. Arathi and P. V. Mohanan, Mater. Sci. Eng., B, 2022, 279, 115680–115695 CrossRef CAS.
- Y. Li, Curr. Org. Chem., 2016, 20, 1754–1755 CrossRef CAS.
- T. V. Safronova, Inorg. Mater., 2021, 57, 443–474 CrossRef CAS.
- P. Horcajada, C. Serre, M. Vallet-Regi, M. Sebban, F. Taulelle and G. Ferey, Angew. Chem., Int. Ed., 2006, 45, 5974–5978 CrossRef CAS PubMed.
- P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regi, M. Sebban, F. Taulelle and G. Ferey, J. Am. Chem. Soc., 2008, 130, 6774–6780 CrossRef CAS PubMed.
- J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2009, 131, 8376–8377 CrossRef CAS PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- T. Chalati, P. Horcajada, P. Couvreur, C. Serre, M. Ben Yahia, G. Maurin and R. Gref, Nanomedicine, 2011, 6, 1683–1695 CrossRef CAS PubMed.
- V. Agostoni, T. Chalati, P. Horcajada, H. Willaime, R. Anand, N. Semiramoth, T. Baati, S. Hall, G. Maurin, H. Chacun, K. Bouchemal, C. Martineau, F. Taulelle, P. Couvreur, C. Rogez-Kreuz, P. Clayette, S. Monti, C. Serre and R. Gref, Adv. Healthcare Mater., 2013, 2, 1630–1637 CrossRef CAS PubMed.
- S. Rojas, E. Quartapelle-Procopio, F. J. Carmona, M. A. Romero, J. A. R. Navarro and E. Barea, J. Mater. Chem. B, 2014, 2, 2473–2477 RSC.
- E. Bellido, T. Hidalgo, M. V. Lozano, M. Guillevic, R. Simón-Vázquez, M. J. Santander-Ortega, Á. González-Fernández, C. Serre, M. J. Alonso and P. Horcajada, Adv. Healthcare Mater., 2015, 4, 1246–1257 CrossRef CAS PubMed.
- V. Rodriguez-Ruiz, A. Maksimenko, R. Anand, S. Monti, V. Agostoni, P. Couvreur, M. Lampropoulou, K. Yannakopoulou and R. Gref, J. Drug Targeting, 2015, 23, 759–767 CrossRef CAS PubMed.
- M. Al Haydar, H. R. Abid, B. Sunderland and S. Wang, Drug Des., Dev. Ther., 2017, 11, 2685–2695 CrossRef CAS PubMed.
- T. Simon-Yarza, M. Gimenez-Marques, R. Mrimi, A. Mielcarek, R. Gref, P. Horcajada, C. Serre and P. Couvreur, Angew. Chem., Int. Ed., 2017, 56, 15565–15569 CrossRef CAS PubMed.
- A. Golmohamadpour, B. Bahramian, M. Khoobi, M. Pourhajibagher, H. R. Barikani and A. Bahador, Photodiagn. Photodyn. Ther., 2018, 23, 331–338 CrossRef CAS PubMed.
- I. Ghaffar, M. Imran, S. Perveen, T. Kanwal, S. Saifullah, M. F. Bertino, C. J. Ehrhardt, V. K. Yadavalli and M. R. Shah, Mater. Sci. Eng., C, 2019, 105, 110111–110120 CrossRef CAS PubMed.
- D. Cunha, C. Gaudin, I. Colinet, P. Horcajada, G. Maurin and C. Serre, J. Mater. Chem. B, 2013, 1, 1101–1108 RSC.
- C. He, K. Lu, D. Liu and W. Lin, J. Am. Chem. Soc., 2014, 136, 5181–5184 CrossRef CAS PubMed.
- X. Zhu, J. Gu, Y. Wang, B. Li, Y. Li, W. Zhao and J. Shi, Chem. Commun., 2014, 50, 8779–8782 RSC.
- L. L. Tan, H. Li, Y. Zhou, Y. Zhang, X. Feng, B. Wang and Y. W. Yang, Small, 2015, 11, 3807–3813 CrossRef CAS PubMed.
- M. Filippousi, S. Turner, K. Leus, P. I. Siafaka, E. D. Tseligka, M. Vandichel, S. G. Nanaki, I. S. Vizirianakis, D. N. Bikiaris, P. Van der Voort and G. Van Tendeloo, Int. J. Pharm., 2016, 509, 208–218 CrossRef CAS PubMed.
- I. A. Lazaro, S. Haddad, S. Sacca, C. Orellana-Tavra, D. Fairen-Jimenez and R. S. Forgan, Chem, 2017, 2, 561–578 Search PubMed.
- I. A. Lazaro, S. Haddad, J. M. Rodrigo-Munoz, C. Orellana-Tavra, V. del Pozo, D. Fairen-Jimenez and R. S. Forgan, ACS Appl. Mater. Interfaces, 2018, 10, 5255–5268 CrossRef PubMed.
- F. Ke, Y.-P. Yuan, L.-G. Qiu, Y.-H. Shen, A.-J. Xie, J.-F. Zhu, X.-Y. Tian and L.-D. Zhang, J. Mater. Chem., 2011, 21, 3843–3848 RSC.
- K. Lu, T. Aung, N. Guo, R. Weichselbaum and W. Lin, Adv. Mater., 2018, 30, e1707634 CrossRef PubMed.
- Y. A. Li, X. D. Zhao, H. P. Yin, G. J. Chen, S. Yang and Y. B. Dong, Chem. Commun., 2016, 52, 14113–14116 RSC.
- C. Y. Sun, C. Qin, X. L. Wang, G. S. Yang, K. Z. Shao, Y. Q. Lan, Z. M. Su, P. Huang, C. G. Wang and E. B. Wang, Dalton Trans., 2012, 41, 6906–6909 RSC.
- L. He, T. Wang, J. An, X. Li, L. Zhang, L. Li, G. Li, X. Wu, Z. Su and C. Wang, CrystEngComm, 2014, 16, 3259–3263 RSC.
- J. Zhuang, C. H. Kuo, L. Y. Chou, D. Y. Liu, E. Weerapana and C. K. Tsung, ACS Nano, 2014, 8, 2812–2819 CrossRef CAS PubMed.
- C. Adhikari, A. Das and A. Chakraborty, Mol. Pharmaceutics, 2015, 12, 3158–3166 CrossRef CAS PubMed.
- M. R. di Nunzio, V. Agostoni, B. Cohen, R. Gref and A. Douhal, J. Med. Chem., 2014, 57, 411–420 CrossRef CAS PubMed.
- S. Nagata, K. Kokado and K. Sada, Chem. Commun., 2015, 51, 8614–8617 RSC.
- L.-L. Tan, N. Song, S. X.-A. Zhang, H. Li, B. Wang and Y.-W. Yang, J. Mater. Chem. B, 2016, 4, 135–140 RSC.
- X. G. Wang, Z. Y. Dong, H. Cheng, S. S. Wan, W. H. Chen, M. Z. Zou, J. W. Huo, H. X. Deng and X. Z. Zhang, Nanoscale, 2015, 7, 16061–16070 RSC.
- H. Zhang, Q. Li, R. Liu, X. Zhang, Z. Li and Y. Luan, Adv. Funct. Mater., 2018, 28, 1802830–1802839 CrossRef.
- B. P. Mugaka, S. Zhang, R.-Q. Li, Y. Ma, B. Wang, J. Hong, Y.-H. Hu, Y. Ding and X.-H. Xia, ACS Appl. Mater. Interfaces, 2021, 13, 11195–11204 CrossRef CAS PubMed.
- K. M. Taylor-Pashow, J. Della Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261–14263 CrossRef CAS PubMed.
- S. Javanbakht, A. Hemmati, H. Namazi and A. Heydari, Int. J. Biol. Macromol., 2019, 155, 876–882 CrossRef PubMed.
- Y. Li, X. Li, Q. Guan, C. Zhang, T. Xu, Y. Dong, X. Bai and W. Zhang, Int. J. Nanomed., 2017, 12, 1465–1474 CrossRef CAS PubMed.
- S. Li, K. Wang, Y. Shi, Y. Cui, B. Chen, B. He, W. Dai, H. Zhang, X. Wang, C. Zhong, H. Wu, Q. Yang and Q. Zhang, Adv. Funct. Mater., 2016, 26, 2715–2727 CrossRef CAS.
- I. B. Vasconcelos, T. G. d Silva, G. C. G. Militão, T. A. Soares, N. M. Rodrigues, M. O. Rodrigues, N. B. d Costa, R. O. Freire and S. A. Junior, RSC Adv., 2012, 2, 9437–9442 RSC.
- H. Zheng, Y. Zhang, L. Liu, W. Wan, P. Guo, A. M. Nystrom and X. Zou, J. Am. Chem. Soc., 2016, 138, 962–968 CrossRef CAS PubMed.
- M. Zheng, S. Liu, X. Guan and Z. Xie, ACS Appl. Mater. Interfaces, 2015, 7, 22181–22187 CrossRef CAS PubMed.
- A. Tiwari, A. Singh, N. Garg and J. K. Randhawa, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- H. Zhang, W. Jiang, R. Liu, J. Zhang, D. Zhang, Z. Li and Y. Luan, ACS Appl. Mater. Interfaces, 2017, 9, 19687–19697 CrossRef CAS PubMed.
- X. Chen, R. Tong, Z. Shi, B. Yang, H. Liu, S. Ding, X. Wang, Q. Lei, J. Wu and W. Fang, ACS Appl. Mater. Interfaces, 2018, 10, 2328–2337 CrossRef CAS PubMed.
- Z. Shi, X. Chen, L. Zhang, S. Ding, X. Wang, Q. Lei and W. Fang, Biomater. Sci., 2018, 6, 2582–2590 RSC.
- X. Zhang, L. Liu, L. Huang, W. Zhang, R. Wang, T. Yue, J. Sun, G. Li and J. Wang, Nanoscale, 2019, 11, 9468–9477 RSC.
- F.-M. Zhang, H. Dong, X. Zhang, X.-J. Sun, M. Liu, D.-D. Yang, X. Liu and J.-Z. Wei, ACS Appl. Mater. Interfaces, 2017, 9, 27332–27337 CrossRef CAS PubMed.
- K. Xing, R. Fan, F. Wang, H. Nie, X. Du, S. Gai, P. Wang and Y. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 22746–22756 CrossRef CAS PubMed.
- P. P. Bag, D. Wang, Z. Chen and R. Cao, Chem. Commun., 2016, 52, 3669–3672 RSC.
- F. R. Lucena, L. C. de Araujo, D. Rodrigues Mdo, T. G. da Silva, V. R. Pereira, G. C. Militao, D. A. Fontes, P. J. Rolim-Neto, F. F. da Silva and S. C. Nascimento, Biomed. Pharmacother., 2013, 67, 707–713 CrossRef CAS PubMed.
- L. Q. Wei, Q. Chen, L. L. Tang, C. Zhuang, W. R. Zhu and N. Lin, Dalton Trans., 2016, 45, 3694–3697 RSC.
- Z. Wu, N. Hao, H. Zhang, Z. Guo, R. Liu, B. He and S. Li, Biomater. Sci., 2017, 5, 1032–1040 RSC.
- Q. Hu, J. Yu, M. Liu, A. Liu, Z. Dou and Y. Yang, J. Med. Chem., 2014, 57, 5679–5685 CrossRef CAS PubMed.
- T. Kundu, S. Mitra, P. Patra, A. Goswami, D. D. Diaz and R. Banerjee, Chem. – Eur. J., 2014, 20, 10514–10518 CrossRef CAS PubMed.
- K. Jiang, L. Zhang, Q. Hu, D. Zhao, T. Xia, W. Lin, Y. Yang, Y. Cui, Y. Yang and G. Qian, J. Mater. Chem. B, 2016, 4, 6398–6401 RSC.
- W. Lin, Q. Hu, J. Yu, K. Jiang, Y. Yang, S. Xiang, Y. Cui, Y. Yang, Z. Wang and G. Qian, ChemPlusChem, 2016, 81, 804–810 CrossRef CAS PubMed.
- Y. Yang, Q. Hu, Q. Zhang, K. Jiang, W. Lin, Y. Yang, Y. Cui and G. Qian, Mol. Pharmaceutics, 2016, 13, 2782–2786 CrossRef CAS PubMed.
- M. H. Teplensky, M. Fantham, P. Li, T. C. Wang, J. P. Mehta, L. J. Young, P. Z. Moghadam, J. T. Hupp, O. K. Farha, C. F. Kaminski and D. Fairen-Jimenez, J. Am. Chem. Soc., 2017, 139, 7522–7532 CrossRef CAS PubMed.
- G. N. Lucena, R. C. Alves, M. P. Abucafy, L. A. Chiavacci, I. C. da Silva, F. R. Pavan and R. C. Galvao Frem, J. Solid State Chem., 2018, 260, 67–72 CrossRef CAS.
- L. L. Tan, H. Li, Y. C. Qiu, D. X. Chen, X. Wang, R. Y. Pan, Y. Wang, S. X. Zhang, B. Wang and Y. W. Yang, Chem. Sci., 2015, 6, 1640–1644 RSC.
- M. Kotzabasaki, I. Galdadas, E. Tylianakis, E. Klontzas, Z. Cournia and G. E. Froudakis, J. Mater. Chem. B, 2017, 5, 3277–3282 RSC.
- B. Yang, M. Shen, J. Liu and F. Ren, Pharm. Res., 2017, 34, 2440–2450 CrossRef CAS PubMed.
- G. Tan, Y. Zhong, L. Yang, Y. Jiang, J. Liu and F. Ren, Chem. Eng. J., 2020, 390, 124446–124458 CrossRef CAS.
- X. Du, R. Fan, L. Qiang, K. Xing, H. Ye, X. Ran, Y. Song, P. Wang and Y. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 28939–28948 CrossRef CAS PubMed.
- B. Liu, H. Li, X. Xu, X. Li, N. Lv, V. Singh, J. F. Stoddart, P. York, X. Xu, R. Gref and J. Zhang, Int. J. Pharm., 2016, 514, 212–219 CrossRef CAS PubMed.
- K. J. Hartlieb, D. P. Ferris, J. M. Holcroft, I. Kandela, C. L. Stern, M. S. Nassar, Y. Y. Botros and J. F. Stoddart, Mol. Pharmaceutics, 2017, 14, 1831–1839 CrossRef CAS PubMed.
- H. Li, N. Lv, X. Li, B. Liu, J. Feng, X. Ren, T. Guo, D. Chen, J. Fraser Stoddart, R. Gref and J. Zhang, Nanoscale, 2017, 9, 7454–7463 RSC.
- J.-Q. Sha, X.-H. Zhong, L.-H. Wu, G.-D. Liu and N. Sheng, RSC Adv., 2016, 6, 82977–82983 RSC.
- T. J. Pisklak, M. Macías, D. H. Coutinho, R. S. Huang and K. J. Balkus, Top. Catal., 2006, 38, 269–278 CrossRef CAS.
- V. Lykourinou, Y. Chen, X. S. Wang, L. Meng, T. Hoang, L. J. Ming, R. L. Musselman and S. Ma, J. Am. Chem. Soc., 2011, 133, 10382–10385 CrossRef CAS PubMed.
- F. Lyu, Y. Zhang, R. N. Zare, J. Ge and Z. Liu, Nano Lett., 2014, 14, 5761–5765 CrossRef CAS PubMed.
- K. Liang, R. Ricco, C. M. Doherty, M. J. Styles, S. Bell, N. Kirby, S. Mudie, D. Haylock, A. J. Hill, C. J. Doonan and P. Falcaro, Nat. Commun., 2015, 6, 7240–7247 CrossRef CAS PubMed.
- F. K. Shieh, S. C. Wang, C. I. Yen, C. C. Wu, S. Dutta, L. Y. Chou, J. V. Morabito, P. Hu, M. H. Hsu, K. C. Wu and C. K. Tsung, J. Am. Chem. Soc., 2015, 137, 4276–4279 CrossRef CAS PubMed.
- X. Wu, J. Ge, C. Yang, M. Hou and Z. Liu, Chem. Commun., 2015, 51, 13408–13411 RSC.
- J. Cui, Y. Feng, T. Lin, Z. Tan, C. Zhong and S. Jia, ACS Appl. Mater. Interfaces, 2017, 9, 10587–10594 CrossRef CAS PubMed.
- S. Y. Li, H. Cheng, B. R. Xie, W. X. Qiu, J. Y. Zeng, C. X. Li, S. S. Wan, L. Zhang, W. L. Liu and X. Z. Zhang, ACS Nano, 2017, 11, 7006–7018 CrossRef CAS PubMed.
- T.-T. Chen, J.-T. Yi, Y.-Y. Zhao and X. Chu, J. Am. Chem. Soc., 2018, 140, 9912–9920 CrossRef CAS PubMed.
- G. Cheng, W. Li, L. Ha, X. Han, S. Hao, Y. Wan, Z. Wang, F. Dong, X. Zou, Y. Mao and S.-Y. Zheng, J. Am. Chem. Soc., 2018, 140, 7282–7291 CrossRef CAS PubMed.
- L. Zhang, Z. Wang, Y. Zhang, F. Cao, K. Dong, J. Ren and X. Qu, ACS Nano, 2018, 12, 10201–10211 CrossRef CAS PubMed.
- C. Zhang, S. Hong, M. D. Liu, W. Y. Yu, M. K. Zhang, L. Zhang, X. Zeng and X. Z. Zhang, J. Controlled Release, 2020, 320, 159–167 CrossRef CAS PubMed.
- D. Feng, T. F. Liu, J. Su, M. Bosch, Z. Wei, W. Wan, D. Yuan, Y. P. Chen, X. Wang, K. Wang, X. Lian, Z. Y. Gu, J. Park, X. Zou and H. C. Zhou, Nat. Commun., 2015, 6, 5979–5986 CrossRef PubMed.
- S. Jung, Y. Kim, S. J. Kim, T. H. Kwon, S. Huh and S. Park, Chem. Commun., 2011, 47, 2904–2906 RSC.
- Y.-H. Shih, S.-H. Lo, N.-S. Yang, B. Singco, Y.-J. Cheng, C.-Y. Wu, I. H. Chang, H.-Y. Huang and C.-H. Lin, ChemPlusChem, 2012, 77, 982–986 CrossRef CAS.
- W.-L. Liu, S.-H. Lo, B. Singco, C.-C. Yang, H.-Y. Huang and C.-H. Lin, J. Mater. Chem. B, 2013, 1, 928–932 RSC.
- C. Marti-Gastaldo, D. Antypov, J. E. Warren, M. E. Briggs, P. A. Chater, P. V. Wiper, G. J. Miller, Y. Z. Khimyak, G. R. Darling, N. G. Berry and M. J. Rosseinsky, Nat. Chem., 2014, 6, 343–351 CrossRef CAS PubMed.
- P. A. Sontz, J. B. Bailey, S. Ahn and F. A. Tezcan, J. Am. Chem. Soc., 2015, 137, 11598–11601 CrossRef CAS PubMed.
- J. B. Bailey, L. Zhang, J. A. Chiong, S. Ahn and F. A. Tezcan, J. Am. Chem. Soc., 2017, 139, 8160–8166 CrossRef CAS PubMed.
- Y. Chen, S. Han, X. Li, Z. Zhang and S. Ma, Inorg. Chem., 2014, 53, 10006–10008 CrossRef CAS PubMed.
- Y. Chen, V. Lykourinou, T. Hoang, L.-J. Ming and S. Ma, Inorg. Chem., 2012, 51, 9156–9158 CrossRef CAS PubMed.
- Y. Chen, V. Lykourinou, C. Vetromile, T. Hoang, L. J. Ming, R. W. Larsen and S. Ma, J. Am. Chem. Soc., 2012, 134, 13188–13191 CrossRef CAS PubMed.
- X. Lian, A. Erazo-Oliveras, J.-P. Pellois and H.-C. Zhou, Nat. Commun., 2017, 8, 2075–2084 CrossRef PubMed.
- B. B. Mendes, J. Conniot, A. Avital, D. Yao, X. Jiang, X. Zhou, N. Sharf-Pauker, Y. Xiao, O. Adir, H. Liang, J. Shi, A. Schroeder and J. Conde, Nat. Rev. Methods Primers, 2022, 2, 24–69 CrossRef CAS PubMed.
- R. Nishiyabu, N. Hashimoto, T. Cho, K. Watanabe, T. Yasunaga, A. Endo, K. Kaneko, T. Niidome, M. Murata, C. Adachi, Y. Katayama, M. Hashizume and N. Kimizuka, J. Am. Chem. Soc., 2009, 131, 2151–2158 CrossRef CAS PubMed.
- M. Zhang, W. Lu, J.-R. Li, M. Bosch, Y.-P. Chen, T.-F. Liu, Y. Liu and H.-C. Zhou, Inorg. Chem. Front., 2014, 1, 159–162 RSC.
- V. Agostoni, R. Anand, S. Monti, S. Hall, G. Maurin, P. Horcajada, C. Serre, K. Bouchemal and R. Gref, J. Mater. Chem. B, 2013, 1, 4231–4242 RSC.
- Q. Chen, M. Xu, W. Zheng, T. Xu, H. Deng and J. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 6712–6724 CrossRef CAS PubMed.
- W. Morris, W. E. Briley, E. Auyeung, M. D. Cabezas and C. A. Mirkin, J. Am. Chem. Soc., 2014, 136, 7261–7264 CrossRef CAS PubMed.
- H. Wang, Y. Chen, H. Wang, X. Liu, X. Zhou and F. Wang, Angew. Chem., Int. Ed., 2019, 58, 7380–7384 CrossRef CAS PubMed.
- S. Peng, B. Bie, Y. Sun, M. Liu, H. Cong, W. Zhou, Y. Xia, H. Tang, H. Deng and X. Zhou, Nat. Commun., 2018, 9, 1293–1302 CrossRef PubMed.
- Y. Zhang, F. Wang, E. Ju, Z. Liu, Z. Chen, J. Ren and X. Qu, Adv. Funct. Mater., 2016, 26, 6454–6461 CrossRef CAS.
- F. Duan, X. Feng, X. Yang, W. Sun, Y. Jin, H. Liu, K. Ge, Z. Li and J. Zhang, Biomaterials, 2017, 122, 23–33 CrossRef CAS PubMed.
- Y. Zhang, C. Liu, F. Wang, Z. Liu, J. Ren and X. Qu, Chem. Commun., 2017, 53, 1840–1843 RSC.
- W. Ning, Z. Di, Y. Yu, P. Zeng, C. Di, D. Chen, X. Kong, G. Nie, Y. Zhao and L. Li, Small, 2018, 14, e1703812 CrossRef PubMed.
- K. Ni, T. Luo, G. Lan, A. Culbert, Y. Song, T. Wu, X. Jiang and W. Lin, Angew. Chem., Int. Ed., 2020, 59, 1108–1112 CrossRef CAS PubMed.
- H. Zhang, W. Chen, K. Gong and J. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 31519–31525 CrossRef CAS PubMed.
- B. Xiao, P. S. Wheatley, X. Zhao, A. J. Fletcher, S. Fox, A. G. Rossi, I. L. Megson, S. Bordiga, L. Regli, K. M. Thomas and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 1203–1209 CrossRef CAS PubMed.
- P. K. Allan, P. S. Wheatley, D. Aldous, M. I. Mohideen, C. Tang, J. A. Hriljac, I. L. Megson, K. W. Chapman, G. De Weireld, S. Vaesen and R. E. Morris, Dalton Trans., 2012, 41, 4060–4066 RSC.
- A. C. McKinlay, J. F. Eubank, S. Wuttke, B. Xiao, P. S. Wheatley, P. Bazin, J. C. Lavalley, M. Daturi, A. Vimont, G. De Weireld, P. Horcajada, C. Serre and R. E. Morris, Chem. Mater., 2013, 25, 1592–1599 CrossRef CAS.
- D. Cattaneo, S. J. Warrender, M. J. Duncan, C. J. Kelsall, M. K. Doherty, P. D. Whitfield, I. L. Megson and R. E. Morris, RSC Adv., 2016, 6, 14059–14067 RSC.
- S. R. Miller, E. Alvarez, L. Fradcourt, T. Devic, S. Wuttke, P. S. Wheatley, N. Steunou, C. Bonhomme, C. Gervais, D. Laurencin, R. E. Morris, A. Vimont, M. Daturi, P. Horcajada and C. Serre, Chem. Commun., 2013, 49, 7773–7775 RSC.
- M. Ma, H. Noei, B. Mienert, J. Niesel, E. Bill, M. Muhler, R. A. Fischer, Y. Wang, U. Schatzschneider and N. Metzler-Nolte, Chem, 2013, 19, 6785–6790 CrossRef CAS PubMed.
- M. J. Ingleson, R. Heck, J. A. Gould and M. J. Rosseinsky, Inorg. Chem., 2009, 48, 9986–9988 CrossRef CAS PubMed.
- S. A. Baudron, CrystEngComm, 2010, 12, 2288–2295 RSC.
- C. Kim, S. Diring, S. Furukawa and S. Kitagawa, Dalton Trans., 2015, 44, 15324–15333 RSC.
- S. Diring, A. Carne-Sanchez, J. Zhang, S. Ikemura, C. Kim, H. Inaba, S. Kitagawa and S. Furukawa, Chem. Sci., 2017, 8, 2381–2386 RSC.
- Z. Jin, P. Zhao, J. Zhang, T. Yang, G. Zhou, D. Zhang, T. Wang and Q. He, Chem. – Eur. J., 2018, 24, 11667–11674 CrossRef CAS PubMed.
- Q. Guan, L. L. Zhou, Y. A. Li and Y. B. Dong, Chem. Commun., 2019, 55, 14898–14901 RSC.
- S. Diring, D. O. Wang, C. Kim, M. Kondo, Y. Chen, S. Kitagawa, K.-I. Kamei and S. Furukawa, Nat. Commun., 2013, 4, 917–935 Search PubMed.
- V. V. Vinogradov, A. S. Drozdov, L. R. Mingabudinova, E. M. Shabanova, N. O. Kolchina, E. I. Anastasova, A. A. Markova, A. A. Shtil, V. A. Milichko, G. L. Starova, R. L. M. Precker, A. V. Vinogradov, E. Hey-Hawkins and E. A. Pidko, J. Mater. Chem. B, 2018, 6, 2450–2459 RSC.
- L.-C. Yu, Z.-F. Chen, C.-S. Zhou, H. Liang and Y. Li, J. Coord. Chem., 2005, 58, 1681–1687 CrossRef CAS.
- K. M. P. William, J. Rieter, K. M. L. Taylor and W. Lin, J. Am. Chem. Soc., 2008, 130, 11584–11585 CrossRef PubMed.
- R. C. Huxford, W. S. Boyle, D. Liu and W. Lin, Chem. Sci., 2012, 3, 198–204 RSC.
- D. Liu, S. A. Kramer, R. C. Huxford-Phillips, S. Wang, J. Della Rocca and W. Lin, Chem. Commun., 2012, 48, 2668–2670 RSC.
- R. C. Huxford-Phillips, S. R. Russell, D. Liu and W. Lin, RSC Adv., 2013, 3, 14438–14443 RSC.
- D. Liu, C. Poon, K. Lu, C. He and W. Lin, Nat. Commun., 2014, 5, 4182–4192 CrossRef CAS PubMed.
- C. He, D. Liu and W. Lin, Biomaterials, 2015, 36, 124–133 CrossRef CAS PubMed.
- C. He, D. Liu and W. Lin, ACS Nano, 2015, 9, 991–1003 CrossRef CAS PubMed.
- C. Poon, C. He, D. Liu, K. Lu and W. Lin, J. Controlled Release, 2015, 201, 90–99 CrossRef CAS PubMed.
- C. He, C. Poon, C. Chan, S. D. Yamada and W. Lin, J. Am. Chem. Soc., 2016, 138, 6010–6019 CrossRef CAS PubMed.
- Y. Yang, Y. Chao, J. Liu, Z. Dong, W. He, R. Zhang, K. Yang, M. Chen and Z. Liu, NPG Asia Mater., 2017, 9, e344 CrossRef CAS.
- K. Ni, G. Lan, S. S. Veroneau, X. Duan, Y. Song and W. Lin, Nat. Commun., 2018, 9, 4321–4333 CrossRef PubMed.
- C. Chan, N. Guo, X. Duan, W. Han, L. Xue, D. Bryan, S. C. Wightman, N. N. Khodarev, R. R. Weichselbaum and W. Lin, Biomaterials, 2019, 210, 94–104 CrossRef CAS PubMed.
- X. Duan, C. Chan, W. Han, N. Guo, R. R. Weichselbaum and W. Lin, Nat. Commun., 2019, 10, 1899–1913 CrossRef PubMed.
- Y. Yang, J. Liu, C. Liang, L. Feng, T. Fu, Z. Dong, Y. Chao, Y. Li, G. Lu and M. Chen, ACS Nano, 2016, 10, 2774–2781 CrossRef CAS PubMed.
- J. Wu, Y. Hu, H. Ye, S. Zhang, J. Zhu, D. Ji, Y. Zhang, Y. Ding and Z. Huang, JACS Au, 2022, 2, 2339–2351 CrossRef CAS PubMed.
- K. Lu, C. He and W. Lin, J. Am. Chem. Soc., 2014, 136, 16712–16715 CrossRef CAS PubMed.
- K. Lu, C. He and W. Lin, J. Am. Chem. Soc., 2015, 137, 7600–7603 CrossRef CAS PubMed.
- K. Lu, C. He, N. Guo, C. Chan, K. Ni, R. R. Weichselbaum and W. Lin, J. Am. Chem. Soc., 2016, 138, 12502–12510 CrossRef CAS PubMed.
- G. Lan, K. Ni, Z. Xu, S. S. Veroneau, Y. Song and W. Lin, J. Am. Chem. Soc., 2018, 140, 5670–5673 CrossRef CAS PubMed.
- J. Park, D. Feng, S. Yuan and H.-C. Zhou, Angew. Chem., Int. Ed., 2015, 54, 430–445 CrossRef CAS PubMed.
- J. Park, Q. Jiang, D. Feng, L. Mao and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 3518–3525 CrossRef CAS PubMed.
- S.-Y. Li, H. Cheng, B.-R. Xie, W.-X. Qiu, J.-Y. Zeng, C.-X. Li, S.-S. Wan, L. Zhang, W.-L. Liu and X.-Z. Zhang, ACS Nano, 2017, 11, 7006–7018 CrossRef CAS PubMed.
- W. Liu, Y.-M. Wang, Y.-H. Li, S.-J. Cai, X.-B. Yin, X.-W. He and Y.-K. Zhang, Small, 2017, 13, 1603459–1603466 CrossRef PubMed.
- Y. Ma, X. Li, A. Li, P. Yang, C. Zhang and B. Tang, Angew. Chem., Int. Ed., 2017, 56, 13752–13756 CrossRef CAS PubMed.
- B. Li, X. Wang, L. Chen, Y. Zhou, W. Dang, J. Chang and C. Wu, Theranostics, 2018, 8, 4086–4096 CrossRef CAS PubMed.
- J. Wang, Y. Fan, Y. Tan, X. Zhao, Y. Zhang, C. Cheng and M. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 36615–36621 CrossRef CAS PubMed.
- J.-Y. Zeng, M.-Z. Zou, M. Zhang, X.-S. Wang, X. Zeng, H. Cong and X.-Z. Zhang, ACS Nano, 2018, 12, 4630–4640 CrossRef CAS PubMed.
- K. Zhang, X. Meng, Y. Cao, Z. Yang, H. Dong, Y. Zhang, H. Lu, Z. Shi and X. Zhang, Adv. Funct. Mater., 2018, 28, 1804634–1804643 CrossRef.
- W. Zhang, J. Lu, X. Gao, P. Li, W. Zhang, Y. Ma, H. Wang and B. Tang, Angew. Chem., Int. Ed., 2018, 57, 4891–4896 CrossRef CAS PubMed.
- Y. Zhao, Y. Kuang, M. Liu, J. Wang and R. Pei, Chem. Mater., 2018, 30, 7511–7520 CrossRef CAS.
- X. Zheng, L. Wang, M. Liu, P. Lei, F. Liu and Z. Xie, Chem. Mater., 2018, 30, 6867–6876 CrossRef CAS.
- S.-S. Wan, Q. Cheng, X. Zeng and X.-Z. Zhang, ACS Nano, 2019, 13, 6561–6571 CrossRef CAS PubMed.
- C. Wang, F. Cao, Y. Ruan, X. Jia, W. Zhen and X. Jiang, Angew. Chem., Int. Ed., 2019, 58, 9846–9850 CrossRef CAS PubMed.
- X. Zhao, Z. Zhang, X. Cai, B. Ding, C. Sun, G. Liu, C. Hu, S. Shao and M. Pang, ACS Appl. Mater. Interfaces, 2019, 11, 7884–7892 CrossRef CAS PubMed.
- L. Zhang, J. Lei, F. Ma, P. Ling, J. Liu and H. Ju, Chem. Commun., 2015, 51, 10831–10834 RSC.
- T. V. Slenters, I. Hauser-Gerspach, A. U. Daniels and K. M. Fromm, J. Mater. Chem., 2008, 18, 5359–5362 RSC.
- O. Gordon, T. Vig Slenters, P. S. Brunetto, A. E. Villaruz, D. E. Sturdevant, M. Otto, R. Landmann and K. M. Fromm, Antimicrob. Agents Chemother., 2010, 54, 4208–4218 CrossRef CAS PubMed.
- M. Berchel, T. L. Gall, C. Denis, S. L. Hir, F. Quentel, C. Elléouet, T. Montier, J.-M. Rueff, J.-Y. Salaün, J.-P. Haelters, G. B. Hix, P. Lehn and P.-A. Jaffrès, New J. Chem., 2011, 35, 1000–1003 RSC.
- R. E. F. de Paiva, C. Abbehausen, A. F. Gomes, F. C. Gozzo, W. R. Lustri, A. L. B. Formiga and P. P. Corbi, Polyhedron, 2012, 36, 112–119 CrossRef CAS.
- S. W. Jaros, P. Smoleński, M. F. C. Guedes da Silva, M. Florek, J. Król, Z. Staroniewicz, A. J. L. Pombeiro and A. M. Kirillov, CrystEngComm, 2013, 15, 8060–8064 RSC.
- K. Wang, X. Ma, D. Shao, Z. Geng, Z. Zhang and Z. Wang, Cryst. Growth Des., 2012, 12, 3786–3791 CrossRef CAS.
- C. Chiericatti, J. C. Basilico, M. L. Zapata Basilico and J. M. Zamaro, Microporous Mesoporous Mater., 2012, 162, 60–63 CrossRef CAS.
- W. Zhuang, D. Yuan, J.-R. Li, Z. Luo, H.-C. Zhou, S. Bashir and J. Liu, Adv. Healthcare Mater., 2012, 1, 225–238 CrossRef CAS PubMed.
- S. Aguado, J. Quiros, J. Canivet, D. Farrusseng, K. Boltes and R. Rosal, Chemosphere, 2014, 113, 188–192 CrossRef CAS PubMed.
- S. A. Ahmed, D. Bagchi, H. A. Katouah, M. N. Hasan, H. M. Altass and S. K. Pal, Sci. Rep., 2019, 9, 19372–19382 CrossRef CAS PubMed.
- K. Ni, G. Lan, C. Chan, B. Quigley, K. Lu, T. Aung, N. Guo, P. La Riviere, R. R. Weichselbaum and W. Lin, Nat. Commun., 2018, 9, 1–12 CrossRef PubMed.
- A. D. Burrows, M. Jurcic, M. F. Mahon, S. Pierrat, G. W. Roffe, H. J. Windle and J. Spencer, Dalton Trans., 2015, 44, 13814–13817 RSC.
- C. Tamames-Tabar, E. Imbuluzqueta, N. Guillou, C. Serre, S. R. Miller, E. Elkaïm, P. Horcajada and M. J. Blanco-Prieto, CrystEngComm, 2015, 17, 456–462 RSC.
- Y. Hu, T. Lv, Y. Ma, J. Xu, Y. Zhang, Y. Hou, Z. Huang and Y. Ding, Nano Lett., 2019, 19, 2731–2738 CrossRef CAS PubMed.
- J. Liu, Y. Yang, W. Zhu, X. Yi, Z. Dong, X. Xu, M. Chen, K. Yang, G. Lu, L. Jiang and Z. Liu, Biomaterials, 2016, 97, 1–9 CrossRef CAS PubMed.
- K. Ni, T. Aung, S. Li, N. Fatuzzo, X. Liang and W. Lin, Chem, 2019, 5, 1892–1913 CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |
