Tailoring stability, catalytic activity and selectivity of covalent metal–organic frameworks via steric modification of metal nodes†
Haiyan
Duan‡
ab,
Xu
Chen‡
a,
Yi-Nan
Yang
a,
Jianping
Zhao
a,
Xiao-Chun
Lin
a,
Wen-Jing
Tang
a,
Qiang
Gao
c,
Guo-Hong
Ning
 *a and
Dan
Li
*a and
Dan
Li
 *a
*a
aCollege of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou 510632, China. E-mail: guohongning@jnu.edu.cn; danli@jnu.edu.cn
bInternational Joint Laboratory of Catalytic Chemistry, College of Sciences, Shanghai University, Shanghai 200444, China
cCAS Key Lab of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China
First published on 17th January 2023
Abstract
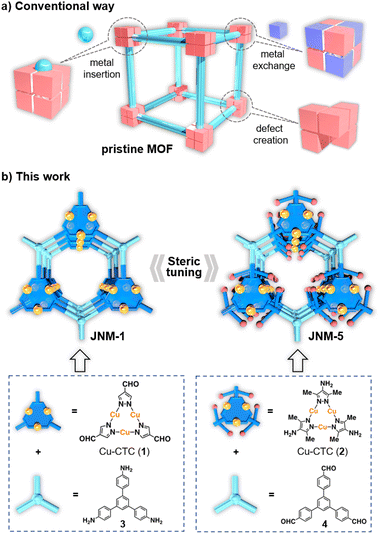
Although many efforts have been made to tune catalytic performance via the modification of MOF nodes including metal exchange, defect creation and metal insertion, steric tuning of MOF nodes via ligand modification remains challenging and unexplored. Herein, we have successfully fabricated two two-dimensional (2D) Cu(I) cyclic trinuclear unit (Cu-CTU)-based MOFs with similar structures, denoted as JNM-1 and JNM-5. JNM-1 has less steric hindrance on copper open sites, while JNM-5 incorporates bulky groups enhancing steric hindrance and partial coverage on copper open sites. Due to the steric effect, JNM-5 exhibited much higher crystallinity, porosity and chemical stability, but lower catalytic activity for hydroboration reactions than JNM-1. Interestingly, JNM-5 delivered much higher substrate selectivity and chemo-selectivity for hydroboration of olefins compared to JNM-1. Owing to its high chemical stability, JNM-5 can be reused for at least five cycles without losing catalytic performance and crystallinity, while the catalytic activity of JNM-1 is greatly decreased and it turns into an amorphous material after five cycles.
10th anniversary statementJournal of Materials Chemistry A is one of the most reputable journals for chemists in the field of materials chemistry. The development of novel metal- and covalent-organic frameworks (MOFs and COFs) with advanced functions has been a worthwhile pursuit in materials science and has grown with Journal of Materials Chemistry A. By combining the chemistry of MOFs and COFs, we have recently developed coinage-metal-based cyclic trinuclear unit (CTU)-based covalent metal–organic frameworks (CMOFs), which have become an emerging platform for materials development, due to their excellent features such as designable synthetic approaches, open active sites, high stability and structural periodicity. We believe that there are great opportunities in the development of advanced CTU-based CMOFs with diverse structures and advanced applications. We sincerely celebrate the 10th anniversary of Journal of Materials Chemistry A and would contribute further to the significant advancement of the journal. |
Introduction
The discovery and exploration of catalysts with superior performance including high activity, stability and selectivity is not only a fundamental challenge but also highly desired for industrial applications.1,2 Conventionally, compared to homogeneous catalysts, heterogeneous catalysts are more often used in industrial processes because of their easy separation, excellent recyclability and high stability.3–11 However, many traditional heterogeneous catalysts are lacking well-defined and periodic structures as well as finely tunable structural parameters; thus, their discovery usually relied on intuitive design or trial-and-error rather than hypothesis-guided, rational design and screening.12,13Metal–organic frameworks (MOFs) are a class of crystalline porous materials composed of metal ions/clusters and organic linkers,14–18 and they have been considered as promising heterogeneous catalysts due to their atomically precise and periodic structures, the presence of coordinatively unsaturated sites or open metal sites, and intrinsic porosity.19–24 More importantly, the chemical designability and tunability of MOFs potentially allowed chemists to explore the design principle for tuning catalytic performance along with molecular understanding.25–28 Specifically, the catalytic performance of MOFs can be effectively mediated at a molecular level through several strategies including the modification of metal nodes, the introduction of organic linkers with functional groups, and the encapsulation of other active species. It is well known that the modulation of steric effects on metal centres can greatly impact the catalytic activity and selectivity of homogeneous catalysts. So, it can be envisioned that the catalytic performance of MOFs can be mediated via the steric modification of metal nodes.
Surprisingly, although many examples have been reported for investigating the alteration of MOFs' catalytic performance via the modification of metal nodes such as metal exchange,29–32 defect creation,33–35 and metal insertion (Scheme 1a),36–41 the steric tuning of MOF nodes via ligand modification is less explored. This may be because, unlike homogeneous catalysts, the modification of organic linkers usually induces the structural transformation of metal nodes and produces completely different MOF structures,42 leading to difficulty in comparing their catalytic performance and hampering further understanding of molecular design principles.
Recently, we have prepared copper(I) cyclic trinuclear unit (Cu-CTU)-based two-dimensional (2D) MOFs by combining the chemistry of MOFs and COFs.43–45 These CMOFs (covalent metal–organic frameworks, constructed by metalated secondary building units and organic linkers via dynamic covalent bonds) possessed advanced features such as porous structures, favourable crystallinity, good stability, and satisfactory catalytic performance. Importantly, the introduction of bulky substitutes (i.e., methyl groups) on Cu-CTUs still produced a 2D honeycomb structure.41,43 We envisioned that the 2D Cu-CTU-based CMOFs would be a promising platform to investigate the relationship between steric effects of metal nodes and catalytic performance. Herein, a Cu-CTU-based CMOF, namely JNM-5 (JNM represents Jinan material), was fabricated through an imine condensation reaction between a copper(I) cyclic trinuclear complex (Cu-CTC, 2) and 1,3,5-tris(4-formylphenyl)benzene (4) (Scheme 1b). For comparison, JNM-1 with less steric hindrance was synthesized from Cu-CTC 1 and 1,3,5-tris(4-aminophenyl)benzene (3) (Scheme 1b).43JNM-5 featured much higher crystallinity, stability and porosity than JNM-1 due to the partially protected open metal sites by decorating the ligands with methyl groups. Due to the increase of steric hindrance on the metal nodes, JNM-5 exhibited decreased catalytic activities for hydroboration reactions compared to JNM-1. However, JNM-5 delivered superior substrate selectivity and chemo-selectivity, and it preferred catalyzing conjugated olefins rather than non-conjugated ones. In addition, JNM-5 exhibited much higher cycling stability and reusability than JNM-1. Our work demonstrated that the chemical stability and catalytic performance of MOFs can be easily altered by the simple introduction of steric hindrance (methyl groups) on the organic linkers, which would be beneficial for the design of novel MOFs with superior catalytic performance.
Experimental method
Synthesis of Cu-CTC (2)
A mixture of ligand 3,5-dimethyl-1H-pyrazol-4-amine (HL) (50.0 mg, 0.45 mmol), Cu2O (21.5 mg, 0.15 mmol), 2 mL ethanol, and 0.2 mL pyridine were placed in an 8 mL Pyrex tube, and heated in an oven at 120 °C for 72 h. The colorless needle crystals of Cu-CTC (2) formed were filtered and collected under a microscope manually. The yield of Cu-CTC (2): 37.7 mg (72.3%, based on Cu2O).Synthesis of JNM-5
Cu-CTC (2) (26.1 mg, 0.05 mmol) and 1,3,5-tris(4-formylphenyl)benzene (4) (19.5 mg, 0.05 mmol) were added to a 10 mL Schlenk tube. 1,4-Dioxane (0.5 mL), mesitylene (0.5 mL), and 6 M aqueous acetic acid (0.1 mL) were added to the above mixture. Then the tube was flash frozen at 77 K in a liquid nitrogen bath and degassed with three freeze–pump–thaw cycles. After being warmed to room temperature, the mixture was heated at 120 °C for 72 h, yielding a tawny solid. The solid was separated by filtration and washed with EtOH, DMF and acetone. The resulting solid was dried under vacuum at 100 °C for 12 h. Yield: 36.3 mg (84.6%, based on 2). Elemental analysis calc. (%): C 58.7, H 4.1, N 14.6; found: C 53.1, H 4.2, N 13.1.JNM-5-catalyzed hydroboration of styrene
A solution of styrene (5a) (57.5 μL, 0.5 mmol), B2pin2 (254.2 mg, 1.0 mmol), Cs2CO3 (325.8 mg, 1.0 mmol), and JNM-5 (6.4 mg, 0.0075 mmol) in CH3CN (AR) (4 mL) was added to a 10 mL Pyrex tube. The mixture was stirred under a N2 atmosphere for 3 h at room temperature. After the evaporation of the solvent, the crude residue was purified by flash column chromatography on silica gel (petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 100
ethyl acetate = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford product 6a (107.7 mg, 0.456 mmol) in 93% yield.
1) to afford product 6a (107.7 mg, 0.456 mmol) in 93% yield.
Results and discussion
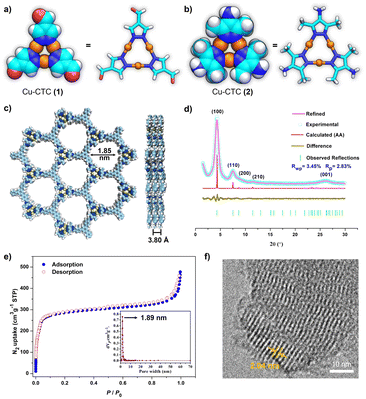
Cu-CTC 1 and JNM-1 were prepared according to our reported procedures.43 To introduce steric hindrance, Cu-CTC 2 was newly designed and synthesized from 3,5-dimethyl-1H-pyrazol-4-amine (Fig. S1†). The single crystal structure of 2 clearly reveals that the copper active sites are partially protected by methyl groups compared to 1 (Fig. 1a and b), giving rise to the significantly enhanced chemical and thermal stability (Fig. S2–S4†) of 2. Afterwards, an imine condensation reaction to link 2 and organic linker 4 in a mixed solution of mesitylene, 1,4-dioxane, and 6 M aqueous acetic acid obtained highly crystalline powders of JNM-5 (Fig. S5†). In addition, JNM-5 can also be obtained from Cu2O, pyrazole ligand, and 4 through one-pot synthesis (Fig. S6†). Similar to JNM-1, the Fourier transform infrared (FTIR) spectrum of JNM-5 showed CN stretching bands located at 1602 cm−1, while no peaks appear at around 3382 cm−1 and 1690 cm−1 corresponding to the stretching vibrations of N–H and CO groups of starting compounds 2 and 4, suggesting the formation of imine bonds and successful preparation of JNM-5 (Fig. S7†). The formation of imine linkages can be further proved by characteristic resonance peaks of imine carbons at 148 ppm in solid-state 13C CP/MAS NMR (Fig. S8†). The scanning electron microscopy (SEM) images taken on the surface verified that the as-formed JNM-5 presents well-developed particle-like morphology (Fig. S9†). The uniform distribution of elements C, Cu and N was also demonstrated by energy dispersive X-ray spectroscopy (EDS) (Fig. S10†).The crystal structure of JNM-5 was determined by powder X-ray diffraction (PXRD) analysis (Fig. 1d). The PXRD pattern of JNM-5 show reflections at 4.29, 7.48, 8.69, 11.45, and 26.16° corresponding to the diffractions of (100), (110), (200), (210) and (001), respectively. In addition, the structure simulation and geometry optimization were carried out through Materials Studio (see the ESI† for details). The comparison between the experimental curve and the simulated PXRD profiles including eclipsed stacking (AA), and staggered stacking (AB and ABC) models manifests that JNM-5 has an AA stacking mode and crystallizes in the P3 space group (Fig. S11–S14 and Tables S2–S4†). The Pawley refinements of JNM-5 yield the optimized unit cell parameters (a = b = 23.5308 Å and c = 3.8053 Å) with good residual factors of Rp = 2.83% and Rwp = 3.45%. The negligible difference plot in Fig. 1d suggests that the refined PXRD patterns are in good agreement with the experimental data. The porosity of JNM-5 was evaluated by nitrogen adsorption–desorption measurement (Fig. 1e), which exhibited type-IV isotherms with a dominant mesoporous structure. The Brunauer–Emmett–Teller (BET) surface area is calculated to be 1027 m2 g−1 (Fig. 1e). The pore size distribution calculated from nonlocal density functional theory suggests a narrow pore size distribution of ∼1.89 nm (Fig. 1e, inset), which is highly consistent with the simulated values from the eclipsed AA (∼1.85 nm). The much larger BET surface area of JNM-5 than that of JNM-1 (∼534 m2 g−1)43 can be attributed to the higher crystallinity of JNM-5. Transmission electron microscopy (TEM) was also conducted to further investigate the structure of JNM-5. In high resolution TEM, the micrometer-size layered structure of JNM-5 (Fig. S15†) with a lattice spacing of 2.04 nm can be observed (Fig. 1f), which was assigned to the (100) atomic plane, further verifying the eclipsed AA stacking mode. These results unambiguously revealed that JNM-5 features a similar 2D honeycomb structure to that of JNM-1, which allowed us to further study and compare their catalytic performance.
Thermal gravimetric analyses (TGA) and various temperature PXRD patterns under a N2 atmosphere were both performed to probe the stability of JNM-5. JNM-5 clearly demonstrated higher stability up to 390 °C than JNM-1 (Fig. 2a and b).43 It is also worth noting that JNM-5 has better crystallinity under the same synthesis conditions (Fig. S16†) compared with JNM-1. In addition, when being exposed to air for six months and to boiling water for 1 month (Fig. S17 and S18†), the PXRD patterns of JNM-5 did not show noticeable changes, suggesting high robustness and chemical stability of JNM-5. In contrast, the crystallinity of JNM-1 almost vanished after only 7 days in boiling water (Fig. S19†). X-ray photoelectron spectroscopy of JNM-5 showed an intense sharp and symmetrical Cu(I) 2p3/2 peak at 933.1 eV, indicating that only Cu(I) was found in JNM-5. These features remained unchanged after being exposed to air or immersed in boiling water for one month (Fig. 2d and S20†). Furthermore, JNM-5 exhibited comparable chemical stability with JNM-1 toward various organic solvents, water, and even 12 M NaOH solution for 3 days, while it was unstable in 1 M HCl solution (Fig. 2c). These results illustrated that the thermal and chemical stability can be largely enhanced by the tuning of steric effects on the MOF nodes.
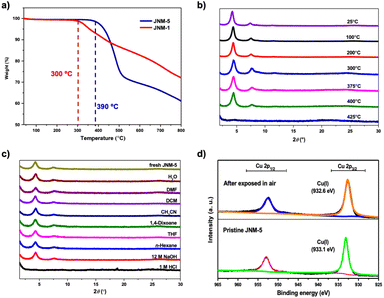 | ||
| Fig. 2 (a) TGA curves of JNM-5 and JNM-1 (ref. 43) under a N2 atmosphere. (b) In situ variable-temperature PXRD patterns of JNM-5 under a N2 atmosphere. (c) PXRD patterns of JNM-5 after treatment with different solvents for 3 days. (d) XPS spectra of JNM-5 before and after exposing to air for one month. | ||
With similar structures but differing steric hindrance on copper nodes, the catalytic performance including activity and selectivity of JNM-1 and JNM-5 were investigated and compared. In our previous work, JNM-1 delivered excellent catalytic activity for the Sonogashira cross-coupling reaction.40 However, the cross-coupling reaction of phenylacetylene and iodobenzene catalyzed by JNM-5 produced 1,2-diphenylethyne as the coupling product with only 52% conversion under the same reaction condition (Fig. S21†). Such a lower catalytic activity of JNM-5 than JNM-1 (∼99% conversion) can be attributed to the steric hindrance on the copper nodes, which hampered the formation of copper(I) vinyl intermediates.
The transition metal-catalyzed hydroboration of olefins is a versatile and straightforward method to access functionalized organoboron compounds, and the resulting alkylboronates are versatile intermediates for a wide range of applications in organic synthesis.46,47JNM-1 and JNM-5 were employed to test the catalytic performance of hydroboration of olefins. Initially, the hydroboration of styrene (5a) was chosen as the model reaction for optimizing the reaction conditions. As presented in Table 1, the hydroborylation of styrene (5a) and B2pin2 can be achieved in 93% isolated yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity at room temperature (rt) with 1.5 mol% JNM-5 as the catalyst and Cs2CO3 as the base (Table 1, entry 1), which is slightly lower than that of JNM-1 (Table 1, entry 15). The indispensable role of JNM-5 or Cs2CO3 was strongly verified by the control experiments, which did not show any hydroboration products in the absence of JNM-5 or Cs2CO3 (Table 1, entries 2 and 3). The alteration of Cs2CO3 into triethylamine, t-BuOK or Na2CO3 led to lower yields and decreased regioselectivities (Table 1, entries 4–6). The exploration of solvent effects demonstrated that acetonitrile is the most favorable solvent for achieving high catalytic performance compared to other solvents including CH3CN/H2O (9
1 regioselectivity at room temperature (rt) with 1.5 mol% JNM-5 as the catalyst and Cs2CO3 as the base (Table 1, entry 1), which is slightly lower than that of JNM-1 (Table 1, entry 15). The indispensable role of JNM-5 or Cs2CO3 was strongly verified by the control experiments, which did not show any hydroboration products in the absence of JNM-5 or Cs2CO3 (Table 1, entries 2 and 3). The alteration of Cs2CO3 into triethylamine, t-BuOK or Na2CO3 led to lower yields and decreased regioselectivities (Table 1, entries 4–6). The exploration of solvent effects demonstrated that acetonitrile is the most favorable solvent for achieving high catalytic performance compared to other solvents including CH3CN/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1,4-dioxane and DMF (Table 1, entries 7–9). Decreasing the catalyst loading to 1.0 mol% resulted in lower yield (Table 1, entry 10). Increasing the catalyst loading to 2.5 mol% gave products in 99% yield and >99
1), 1,4-dioxane and DMF (Table 1, entries 7–9). Decreasing the catalyst loading to 1.0 mol% resulted in lower yield (Table 1, entry 10). Increasing the catalyst loading to 2.5 mol% gave products in 99% yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity (Table 1, entry 11). When Cu2O, Cu-CTC (1), Cu-CTC (2) or organic linker (4) were used as catalysts instead of JNM-5, low yields of 41%, 12%, 81% or <1% were observed (Table 1, entries 12–14 and Fig. S22†). In addition, the gram-scale experiments under the same reaction conditions were conducted. Specifically, 1.15 g of 6a can be eventually obtained in 82% isolated yield (Table 1, entry 16 and Fig. S23†), and the TOF was estimated to be 394 h−1 (Table S5†). The catalytic kinetics of JNM-1 and JNM-5 were estimated by the plot of conversion versus reaction time. In the case of JNM-1, product 6a was detected at 5 min with 21% conversion, which further elevated to 95% at 45 min (Fig. S24†), while JNM-5 required a longer reaction time to complete the reaction (Fig. S25†). Initial rate experiments indicated that the hydroboration reaction was the first order for both 5a and B2pin2. The measurement of rate constants resulted in kJNM-1/kJNM-5 = 2.67 (Fig. S24 and S25†), suggesting that the introduction of a methyl group on the Cu center decreased catalytic activity for hydroboration of styrene.
1 regioselectivity (Table 1, entry 11). When Cu2O, Cu-CTC (1), Cu-CTC (2) or organic linker (4) were used as catalysts instead of JNM-5, low yields of 41%, 12%, 81% or <1% were observed (Table 1, entries 12–14 and Fig. S22†). In addition, the gram-scale experiments under the same reaction conditions were conducted. Specifically, 1.15 g of 6a can be eventually obtained in 82% isolated yield (Table 1, entry 16 and Fig. S23†), and the TOF was estimated to be 394 h−1 (Table S5†). The catalytic kinetics of JNM-1 and JNM-5 were estimated by the plot of conversion versus reaction time. In the case of JNM-1, product 6a was detected at 5 min with 21% conversion, which further elevated to 95% at 45 min (Fig. S24†), while JNM-5 required a longer reaction time to complete the reaction (Fig. S25†). Initial rate experiments indicated that the hydroboration reaction was the first order for both 5a and B2pin2. The measurement of rate constants resulted in kJNM-1/kJNM-5 = 2.67 (Fig. S24 and S25†), suggesting that the introduction of a methyl group on the Cu center decreased catalytic activity for hydroboration of styrene.
| Entry | Change from the “standard conditions” | Yieldb (%) |
6a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7ab 7ab |
|---|---|---|---|
| a Reaction conditions: 5a (1.0 equiv.), B2pin2 (2 equiv.), Cs2CO3 (2 equiv.), JNM-5 (1.5 mol%), CH3CN (4 mL), room temperature (rt), N2. b Determined by GC-MS analysis of the reaction mixture. c Isolated yields. | |||
| 1 | None | 96 (93)c | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | No JNM-5 | <1 | — |
| 3 | No Cs2CO3 | <1 | — |
| 4 | Et3N, instead of Cs2CO3 | Trace | — |
| 5 | t-BuOK, instead of Cs2CO3 | 44 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 6 | Na2CO3, instead of Cs2CO3 | 64 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 7 | CH3CN/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), instead of CH3CN 1), instead of CH3CN |
84 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 8 | 1,4-Dioxane, instead of CH3CN | 60 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | DMF, instead of CH3CN | 87 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 1.0 mol% JNM-5 | 76 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 2.5 mol% JNM-5 | 99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | Cu2O, instead of JNM-5 | 41 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 2, instead of JNM-5 | 81 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 4, instead of JNM-5 | <1 | — |
| 15 | JNM-1, instead of JNM-5 | 99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | 1.15 g scale | 82c | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Stability and recyclability are other key factors for heterogeneous catalysts; thus, we examine the crystallinity and structural integrity of JNM-1 and JNM-5 after catalytic cycling. Interestingly, the PXRD pattern of recovered JNM-5 remained almost identical to the original one (Fig. 3b), and the reaction conversion slightly decreased from 97% to 90% after five catalytic cycles (Fig. 3a). In sharp contrast, JNM-1 turned into a completely amorphous structure (Fig. 3b) and the reaction conversion largely decreased from 98% to 82% after five cycles (Fig. 3a). In addition, the XPS spectra of JNM-5 revealed an unchanged Cu(I) 2p3/2 signal at 932.7 eV, the same as the original one, which verified the retrievable Cu(I) during recycling experiments (Fig. S26†). The Cu(II) 2p3/2 located at 934.1 eV was found in the XPS spectra of JNM-1 after the recyclability tests, which further confirmed the instability of JNM-1 during catalytic cycles (Fig. S27†). Based on our previous work47 and other reported examples, a reaction mechanism was also proposed, as shown in Fig. S28.†
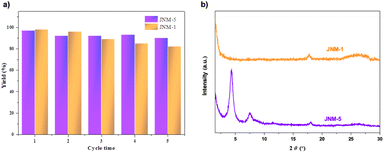 | ||
| Fig. 3 (a) Recyclability of JNM-catalyzed hydroboration of styrene. The reported yield is based on GC-MS analysis. (b) PXRD for JNMs after five catalytic cycles. | ||
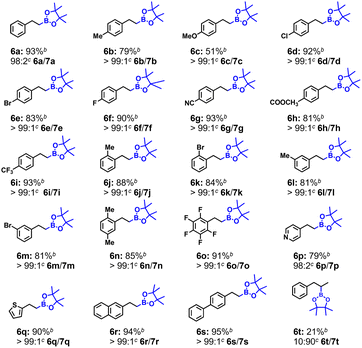
With the optimized conditions in hand, we explored the substrate scope for JNM-5-catalyzed hydroboration of olefins. As depicted in Table 2, aryl olefins possessing electron-withdrawing groups and electron-donating substituents could proceed with excellent yields (6b–6m). Moreover, the yield of aryl olefins with multiple electron-withdrawing groups and electron-donating substituents can also reach 85% (6n) and 91% (6o) respectively. The aryl olefins with heterocycles also achieved good yields, ranging from 79% (6p) to 90% (6q). These results confirmed that a variety of functional groups, despite their different substitution patterns and electronic properties, were all well tolerated, suggesting that JNM-5 is an excellent heterogeneous catalyst for hydroboration of aryl olefins.
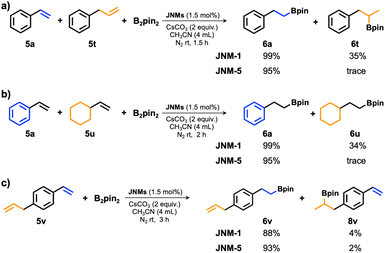
During the expansion of substrate scope, we found that olefins including naphthalene and biphenyl can both present a high yield of 94% (6r) and 95% (6s). However, a non-conjugated olefin, allylbenzene (6t), delivered a very low yield of 21%. Such results promoted us to further study the selectivity using JNM-5 as the catalyst. Initially, the k5a/k5t ratio estimated to be 11.4 by using the plot of reaction time versus conversion (Fig. S25 and S29†) indicates a remarkable decrease in the reaction rate with a non-conjugated olefin and potential high substrate selectivity between the conjugated olefin and non-conjugated one. A competition experiment was conducted to reveal clearer results: when a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5a and 5t was catalyzed with JNM-5 (Fig. 4a), hydroboration product 6t did not form before 90 min, resulting in excellent substrate selectivity for 5a (6a/6t = 95/trace) (Fig. 4a, S30 and Table S6†). In contrast, JNM-1 exhibited much lower selectivity, which led to a 99/35 mixture of 6a/6t under the same reaction conditions (Fig. 4a). In addition, we further verified substrate selectivity for a 1
1 mixture of 5a and 5t was catalyzed with JNM-5 (Fig. 4a), hydroboration product 6t did not form before 90 min, resulting in excellent substrate selectivity for 5a (6a/6t = 95/trace) (Fig. 4a, S30 and Table S6†). In contrast, JNM-1 exhibited much lower selectivity, which led to a 99/35 mixture of 6a/6t under the same reaction conditions (Fig. 4a). In addition, we further verified substrate selectivity for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 5a and non-conjugated olefin, ethenyl-cyclohexan (5u), using JNMs as catalysts. As shown in Fig. 4b and S31,† the products 6a and 6u can be obtained in a conversion of 95% and trace at 120 min catalyzed by JNM-5, respectively. In the same reaction time, a 6a/6u ratio of 99/34 can obtained using JNM-1 as the catalyst (Fig. 4b), indicating a much lower substrate selectivity compared to that of JNM-5. These results revealed that although JNM-5 exhibited lower catalytic activity, it delivered much higher substrate selectivity compared to JNM-1, further confirming that the steric tuning of MOF nodes could largely control their catalytic performance.
1 mixture of 5a and non-conjugated olefin, ethenyl-cyclohexan (5u), using JNMs as catalysts. As shown in Fig. 4b and S31,† the products 6a and 6u can be obtained in a conversion of 95% and trace at 120 min catalyzed by JNM-5, respectively. In the same reaction time, a 6a/6u ratio of 99/34 can obtained using JNM-1 as the catalyst (Fig. 4b), indicating a much lower substrate selectivity compared to that of JNM-5. These results revealed that although JNM-5 exhibited lower catalytic activity, it delivered much higher substrate selectivity compared to JNM-1, further confirming that the steric tuning of MOF nodes could largely control their catalytic performance.
To further explore the chemo-selectivity of olefins, 1-allyl-4-vinylbenzene (5v) containing conjugated and non-conjugated olefins have been prepared (Fig. 4c). When JNM-5 was employed as the catalyst, the corresponding product 6v with a yield of 93% was obtained. More importantly, a conversion ratio between 6v and 8v of 46.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 suggests that JNM-5 exhibited excellent chemo-selectivity for conjugated olefins. In sharp contrast, under the same reaction conditions, the yield of 6v is 88% and conversion ratio between 6v and 8v is 22/1 using JNM-1 as the catalyst. These results suggest that the chemo-selectivity for conjugated olefins using JNM-5 as the catalyst is twice higher than that of JNM-1.
1 suggests that JNM-5 exhibited excellent chemo-selectivity for conjugated olefins. In sharp contrast, under the same reaction conditions, the yield of 6v is 88% and conversion ratio between 6v and 8v is 22/1 using JNM-1 as the catalyst. These results suggest that the chemo-selectivity for conjugated olefins using JNM-5 as the catalyst is twice higher than that of JNM-1.
Conclusions
In summary, we have prepared two 2D Cu-CTU-based CMOFs, denoted as JNM-1 and JNM-5, through imine condensation reactions. Although they have similar 2D honeycomb structures, JNM-5 features larger steric hindrance on open copper sites than JNM-1 due to the introduction of methyl groups on Cu-CTC. Interestingly, owing to the partially protected metal nodes, JNM-5 featured much higher crystallinity, stability and porosity than JNM-1. In addition, JNM-5 exhibited decreased catalytic activities for hydroboration reactions compared to JNM-1, because of steric effects. Unexpectedly, JNM-5 delivered superior substrate selectivity and chemo-selectivity for hydroboration of conjugated aryl olefins than JNM-1. Owing to its high chemical stability, JNM-5 can be reused at least for five cycles without losing catalytic performance and crystallinity, while the catalytic activity of JNM-1 greatly decreased and it turned into an amorphous material after five cycles. Our work provides further understanding for designing heterogeneous catalysts at a molecular level and paves a novel way to rationally tune the catalytic performance of MOFs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported financially by the National Natural Science Foundation of China (no. 21975104, 21731002, 2150004 and 22201102) and the Guangdong Major Project of Basic and Applied Research (no. 2019B030302009). G. H. N. is thankful for the financial support from the Guangdong Basic and Applied Basic Research Foundation (no. 2019B151502024), and Guangzhou Science and Technology Project (no. 202201020038). G. Q. is sponsored by the Shanghai Pujiang Program (20PJ1415200).References
- C. Adams, Top. Catal., 2009, 52, 924–934 CrossRef CAS.
- R. Breslow, Acc. Chem. Res., 1995, 28, 146–153 CrossRef CAS.
- C. M. Friend and B. Xu, Acc. Chem. Res., 2017, 50, 517–521 CrossRef CAS PubMed.
- J. M. Thomas, Angew. Chem., Int. Ed., 1988, 27, 1673–1691 CrossRef.
- T. W. Van Deelen, C. H. Mejía and K. P. De Jong, Nat. Catal., 2019, 2, 955–970 CrossRef CAS.
- M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072–1133 CrossRef CAS PubMed.
- A. Iemhoff, M. Vennewald and R. Palkovits, Angew. Chem., Int. Ed., 2022, e202212015 Search PubMed.
- K. Ralphs, C. Hardacre and S. L. James, Chem. Soc. Rev., 2013, 42, 7701–7718 RSC.
- Z. Xiao, Y. Zhou, X. Xin, Q. Zhang, L. Zhang, R. Wang and D. Sun, Macromol. Chem. Phys., 2016, 217, 599–604 CrossRef CAS.
- W. Ji, T. X. Wang, X. Ding, S. Lei and B. H. Han, Coord. Chem. Rev., 2021, 439, 213875–213904 CrossRef CAS.
- Y. M. Wang, X. C. Lin, K. M. Mo, M. Xie, Y. L. Huang, G. H. Ning and D. Li, Angew. Chem., Int. Ed., 2023, e202218369 Search PubMed.
- F. Zaera, Chem. Rev., 2022, 122, 8594–8757 CrossRef CAS PubMed.
- J. Greeley, Annu. Rev. Chem. Biomol. Eng., 2016, 7, 605–635 CrossRef PubMed.
- M. Ding, X. Cai and H.-L. Jiang, Chem. Sci., 2019, 10, 10209–10230 RSC.
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- L. Zhu, X.-Q. Liu, H.-L. Jiang and L.-B. Sun, Chem. Rev., 2017, 117, 8129–8176 CrossRef CAS PubMed.
- F. L. i Xamena, A. Abad, A. Corma and H. Garcia, J. Catal., 2007, 250, 294–298 CrossRef.
- Q. Wang and D. Astruc, Chem. Rev., 2019, 120, 1438–1511 CrossRef PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- W. Tu, Y. Xu, S. Yin and R. Xu, Adv. Mater., 2018, 30, 1707582 CrossRef PubMed.
- J. Luo, X. Luo, M. Xie, H. Z. Li, H. Y. Duan, H. G. Zhou, R. J. Wei, G. H. Ning and D. Li, Nat. Commun., 2022, 13, 7771–7780 CrossRef CAS PubMed.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS PubMed.
- Z. H. Syed, F. Sha, X. Zhang, D. M. Kaphan, M. Delferro and O. K. Farha, ACS Catal., 2020, 10, 11556–11566 CrossRef CAS.
- T. A. Goetjen, J. Liu, Y. Wu, J. Sui, X. Zhang, J. T. Hupp and O. K. Farha, Chem. Commun., 2020, 56, 10409–10418 RSC.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC.
- S. Horike, M. Dinca, K. Tamaki and J. R. Long, J. Am. Chem. Soc., 2008, 130, 5854–5855 CrossRef CAS PubMed.
- L. Alaerts, E. Séguin, H. Poelman, F. Thibault-Starzyk, P. A. Jacobs and D. E. De Vos, Eur. J. Chem., 2006, 12, 7353–7363 CrossRef CAS PubMed.
- X. M. Lin, T. T. Li, Y. W. Wang, L. Zhang and C. Y. Su, Chem.–Asian J., 2012, 7, 2796–2804 CrossRef CAS PubMed.
- M. Y. Masoomi, A. Morsali, A. Dhakshinamoorthy and H. Garcia, Angew. Chem., Int. Ed., 2019, 131, 15330–15347 CrossRef.
- S. S.-Y. Chui, S. M.-F. Lo, J. P. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- A. Corma, H. Garcia and F. L. i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed.
- S. Dissegna, K. Epp, W. R. Heinz, G. Kieslich and R. A. Fischer, Adv. Mater., 2018, 30, 1704501 CrossRef PubMed.
- B. Li, J. G. Ma and P. Cheng, Small, 2019, 15, 1804849 CrossRef PubMed.
- Q. Yang, Q. Xu and H.-L. Jiang, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC.
- L. Cheng, Q. Guo, K. Zhao, Y.-M. Li, H. Ren, C.-Y. Ji and W. Li, Catal. Lett., 2022, 185, 1–12 Search PubMed.
- I. Gumus, A. Ruzgar, Y. Karatas and M. Gülcan, Mol. Catal., 2021, 501, 111363 CrossRef CAS.
- P. Y. You, R. J. Wei, G. H. Ning and D. Li, Chem. Res. Chin. Univ., 2022, 38, 415–420 CrossRef CAS.
- Y.-Z. Chen, Y.-X. Zhou, H. Wang, J. Lu, T. Uchida, Q. Xu, S.-H. Yu and H.-L. Jiang, ACS Catal., 2015, 5, 2062–2069 CrossRef CAS.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang and T. Gentle III, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- R. J. Wei, H. G. Zhou, Z. Y. Zhang, G. H. Ning and D. Li, CCS Chem., 2020, 2, 2045–2053 Search PubMed.
- H. G. Zhou, R. Q. Xia, J. Zheng, D. Q. Yuan, G. H. Ning and D. Li, Chem. Sci., 2021, 12, 6280–6286 RSC.
- J. P. Zhao, J. Luo, Z. Lin, X. Chen, G. H. Ning, J. Liu and D. Li, Inorg. Chem. Front., 2022, 9, 4907–4912 RSC.
- K. Burgess and M. J. Ohlmeyer, Chem. Rev., 1991, 91, 1179–1191 CrossRef CAS.
- R. J. Wei, P. Y. You, H. Y. Duan, M. Xie, R. Q. Xia, X. Chen, X. Zhao, G. H. Ning, A. I. Cooper and D. Li, J. Am. Chem. Soc., 2022, 144, 17487–17495 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, DFT calculations, PXRD, TGA, FTIR, solid-state NMR, SEM, EDS, TEM, XPS, catalytic kinetics, reaction mechanisms, comparison of catalytic performance, and 1H and 13C NMR spectra for new compounds. CCDC 2100102. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ta08797a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |