Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Natural cationic polymer-derived injectable hydrogels for targeted chemotherapy
Sabya Sachi
Das†
 *a,
Devanshi
Sharma†
bc,
Balaga Venkata Krishna
Rao†
*a,
Devanshi
Sharma†
bc,
Balaga Venkata Krishna
Rao†
 d,
Mandeep Kumar
Arora
d,
Mandeep Kumar
Arora
 a,
Janne
Ruokolainen
e,
Mukesh
Dhanka
b,
Hemant
Singh
b and
Kavindra Kumar
Kesari
a,
Janne
Ruokolainen
e,
Mukesh
Dhanka
b,
Hemant
Singh
b and
Kavindra Kumar
Kesari
 *ef
*ef
aSchool of Pharmaceutical and Population Health Informatics, DIT University, Dehradun-248009, Uttarakhand, India. E-mail: ss.das@dituniversity.edu.in
bDiscipline of Biological Engineering, Indian Institute of Technology Gandhinagar, Gandhinagar, Palaj 382355, Gujrat, India
cInstitute of Science, Nirma University, S G Highway, Ahmedabad-382481, Gujarat, India
dSchool of Pharmacy, Suresh Gyan Vihar University, Mahal Road, Jagatpura, Jaipur-302017, Rajasthan, India
eDepartment of Applied Physics, School of Science, Aalto University, 00076 Espoo, Finland. E-mail: kavindra.kesari@aalto.fi
fResearch and Development Cell, Lovely Professional University, Phagwara, Punjab, India
First published on 24th October 2023
Abstract
Injectable hydrogels have the potential to revolutionize therapeutics. Therapeutic hydrogels exhibit distinctive physicochemical properties, including flexible porous structure, binding affinity for biological fluids, porous structural configuration, higher water content, high flexibility, biodegradability, and biocompatibility. These technologies have had tremendous clinical implications, specifically for the site-specific and sustained delivery of chemotherapeutic drugs. Drug-encapsulated injectable hydrogels showcase significant superiority over conventional therapeutics, such as minimized adverse effects, enhanced therapeutic efficacy, augmented pharmacological profile, and superior patient compliance. Conventional approaches mainly include intravenous chemotherapy, which can potentially cause adverse effects such as myelosuppression, nephro- or hepatic dysfunction, and neurotoxicity. The injectable hydrogel is a potent approach to overcome these impediments by releasing the chemotherapeutic drugs at specific tumor sites after topical administration. Moreover, the therapeutic efficiency of cancer immunotherapy is majorly dependent upon the tumor microenvironment, which can be targeted with chemotherapeutic drug-loaded injectable hydrogels for improved cancer therapy. In addition, natural cationic polymers such as chitosan, cyclodextrins, gelatin, cellulose, dextran, and others have received substantial attention from investigators in drug delivery due to their easy obtainability, high encapsulation efficiency, improved bioavailability, sustained drug release properties, biodegradability, and biocompatibility. This review summarizes the mainstream approaches for synthesizing injectable hydrogels and the biological properties of different natural cationic polymers. We have also focused on the notable studies of cationic polymers used definitively to fabricate hydrogel-mediated systems for anticancer drug delivery. Further, the therapeutic approaches, molecular insights, pharmacological actions, and clinical significance have been discussed.
1. Introduction
Cancer is one of the leading causes of social, clinical, and economic burden compared to all other various human diseases. With 18 million newly reported cancer cases, the most recurrently occurring cancers are lung, breast, and prostate cancers.1 Cancer's increasing frequency, prevalence, and morbidity indicate the burden of malignant diseases for a prolonged time.2 Breakthrough technological and scientific advancements in targeted delivery for cancer treatment hold the potential for revolutionizing cancer care worldwide.3 Environmental and lifestyle factors are the leading causes of cancer. Early identification of various causes of cancer and the proposal of a multi-stage model of cancer by epidemiologists have paved the way for extraordinary advances in the treatment and identification of cancers through various cellular and molecular approaches.4 Long suspected risks which are minor in nature are now being estimated more precisely due to various advancements in theranostics and drug delivery approaches. The impact of the COVID-19 pandemic across various regions around the globe has resulted in delay in terms of diagnosis and treatment thereby resulting in an overall increase in mortality caused by cancer.1,5Elementary results of clinical studies suggest combining combinational therapies with the standard conventional therapies for cancer treatment. The mode of delivery used during combinational therapy can enhance treatment efficacy and influence disease progression due to prolonged time.6 Research has suggested using molecular and immunological factors to inhibit cancer progression at later stages, which is effective.7 Studies have demonstrated that cancer chemotherapy usually causes nausea and vomiting. However, present treatments to regulate and monitor acute chemotherapy-induced nausea and vomiting (CINV) are practically effective in most patients, but deferred CINV is more vital and challenging.8 The consolidative perception associated with the various hallmarks of cancer has helped to refine the complexities of cancer and re-occurrence. The understanding can lead to the establishment of the mechanisms of cancer development and malignant progression, and to the development of effective cancer therapies with negligible toxicity possiblities.9,10 Apart from this, one of the most critical limitations related to chemotherapy is their inability to sensitivity to currently accessible chemotherapeutic drugs and occurrence of drug resistance. In addition, precise information and understanding of various mechanisms associated with chemotherapeutic effects is highly needed to establish significant findings or outcomes.11
Conventional therapies for cancer, such as chemotherapy, radiation therapy, and surgery, have several side effects. Thus, various targeted and non-conventional ways are being researched to treat cancer.7,12 One of them is the use of exosomes. Exosomes can be categorized as diagnostic markers and therapeutic agents in cancer therapy due to their ability to have high biocompatibility, stability, immunogenicity, pharmacokinetics, biodistribution, and a cellular uptake mechanism. Due to their size and heterogenicity, exosomal delivery is still being researched.13 Certain limitations of various conventional therapies, like immunosuppression, modulation of tumor microenvironment's expression of tumor antigens, etc., are still being researched, leading to surpassing these limitations.14 Studies have shown that chemotherapy has demonstrated disruption of the various suppressive pathways and lymphodepletion post administration of chemotherapy.15
Even injectable biomaterials have several challenges for the design of optimal therapies, including optimization of the material form, method of injection, and the mechanisms of action of the same.16,17 Injectable hydrogels with desired response to pH and self-healing ability can be used for anti-cancer drug-delivery.18 Making a pH-responsive injectable hydrogel is crucial for efficient drug release in the targeted acidic environment. The self-healing property of an injectable hydrogel can prolong the life during the implantation and provide the benefit of minimally invasive surgery.19 In addition, it has been reported that the functionalized-fluorescent nanoparticle conjugated hydrogel systems can simultaneously exhibit fluorescence properties and can be tagged with therapeutics to accomplish their therapeutic efficacy, leading to improved theranostic applications.20 The intelligent hydrogel drug delivery system that released doxorubicin for hepatocellular carcinoma enhanced anti-cancer response generation. Injectable hydrogel's self-healing ability can be confirmed by forming Schiff's base.21 In addition, recent studies have reported that fluorescent nanoparticle conjugated hydrogels have been extensively explored for drug delivery, biosensing and imaging applications.20
As an alternative approach, injectable hydrogels for localized chemotherapy have shown diminishing effects of systemic chemotherapy and provide the sustained release of chemotherapeutics at the targeted tumor site.22 Injectable hydrogels formed in situ include thermosensitive hydrogels, photosensitive hydrogels, active targeting hydrogels, etc. the systemic administration of various chemotherapeutics is dose limited and shows off-target toxicity. Smart injectable hydrogel delivery systems for localized chemotherapeutic administration are promising ways to combat the side effects and toxicity.23 Injectable hydrogels possess a sol–gel transition phase dependent on the concentration of the polymer and crosslinker used. This makes them physically responsive to various body-specific factors like pH and temperature. Synthetic and natural polymers have been studied based on their structure, chemical bonding, and mechanical properties for making controlled drug release systems to enhance therapeutic efficacy.23
The injectable hydrogel's efficacy greatly depends on the polymeric properties and what kind of anti-cancer drugs are being used. Thus, in this review, we have summarized the method of preparation and characterization studies essential for hydrogels. Further, we have discussed the therapeutic potential of various natural cationic polymeric injectable hydrogels to deliver chemotherapeutic agents in cancer therapy effectively. Finally, we have listed various reported or ongoing clinical/pre-clinical studies associated with anticancer drug-encapsulated polymeric injectable hydrogels for treating multiple cancers.
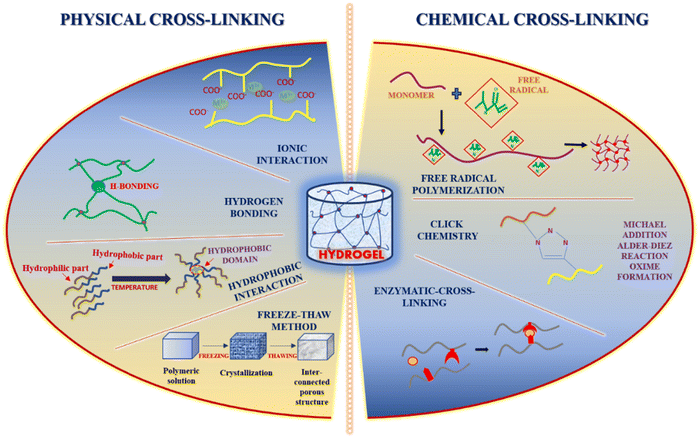
2. Injectable hydrogels: methods of preparation and characterization
Owing to their high water content and mechanical strength as in the case of natural tissues, hydrogels have emerged as promising sources for biomedical applications.24 Hydrogels can be formed as an injectable material to fulfill the criteria of non-invasiveness, thereby significantly decreasing the costs of surgery and recovery.25 Self-healing hydrogels in liquid form are injected inside the body and rapidly form a gel eliminating the risks of the crosslinker used. Injectable hydrogels can surpass phase-1 of drug metabolism.26 Specific requirements should be considered while forming the injectable hydrogel for biomedical/clinical applications, such as viscosity, mechanical properties, biocompatibility, etc.26 The two widely used approaches for the crosslinking of polymers include physical cross-linking and chemical cross-linking for the application of drug delivery (Fig. 1). However, many laboratories that developed injectable hydrogels still face significant challenges regarding translation into clinical use.25 Hydrogels are attractive delivery systems for localized and targeted therapy due to their sustained delivery. In addition, unlike active and passive targeting techniques, hydrogels work well regardless of tumor blood supply and microvasculature.27,28 Moreover, they can enhance the physical stability of the therapeutic drugs inhibiting drug precipitation.29In the recent era, in situ stimuli-responsive hydrogel-based systems, also known as smart hydrogels, have shown immense importance for delivering chemotherapeutic agents with no or negligible systemic toxicity.19 These smart hydrogels exhibit properties such as superior injectability, biocompatibility, and sensitivity to various stimuli, including pH, heat, enzyme, light, electric potential, and magnetic field (Fig. 2a). Interestingly, the behavior of drug release is well regulated in several types of smart hydrogels as a response to different stimuli such as enzymes, electric impulses, magnetic field, and glutathione (Fig. 2b). In particular, the pH-responsive hydrogel exhibits enhanced antitumor activity by enhancing the acidity within the tumor microenvironment and again neutralizing to normal pH leading to suppression of tumor growth (Fig. 2c).30
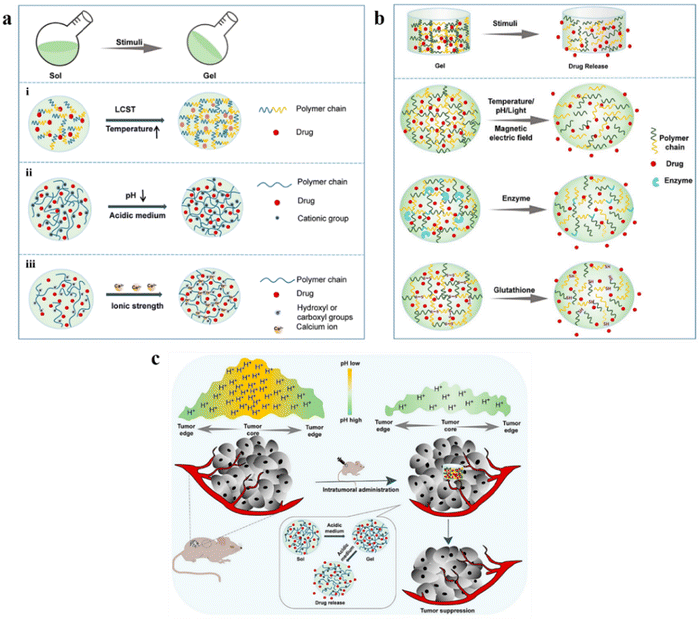 | ||
| Fig. 2 (a) Schematic phase transition of representative hydrogels: (i) temperature-responsive hydrogel; (ii) pH-responsive hydrogel; (iii) ionic strength-responsive hydrogel. (b) Schematic presentation of drug release from representative smart hydrogels upon various stimuli. (c) In situ pH-responsive hydrogel alleviates the tumor's acidic microenvironment and inhibits tumor growth. Reproduced from ref. 30 with permission from MDPI, copyright 2022. | ||
The several mechanisms currently used for preparing injectable hydrogels are physical cross-linking, chemical cross-linking, ionic cross-linking, i.e., self-assembly, and enzyme-initiated cross-linking 25. Injectable hydrogels that are physically cross-linked have the gelation triggered by temperature, pH, etc. However, it is a one-phase system; during delivery, there is a significant chance of a higher burst release of drug from the hydrogel.31 Chemical cross-linking results in enhanced elasticity properties but has the drawback of toxic precursors used for cross-linking.25 Aldehyde chemistry used during chemical cross-linking lacks specificity even if paired cross-linking occurs.25 Enzyme-initiated crosslinking is highly specific and depends mainly on the enzyme concentration.25 Injectable tissue engineering constructs are well-structured cell carriers that showcase the potential of the minimally invasive techniques of delivery.25 In physically cross-linked injectable hydrogels, the gelation occurs after the injection, and the most significant aspect to drive the gelation is the body temperature.32 Hence, the crosslinking properties of the polymeric precursors through physical or chemical medium and their response to external stimuli such as temperature and ionic concentration control injectable hydrogel formation.26 The self-healing behavior of the injectable hydrogel is governed by non-covalent interactions and dynamic covalent bonds or sometimes both.33 Hydrogels that show shear-thinning can also be categorized as injectable hydrogels due to the adequate control of gelation kinetics.34
2.1. Physical cross-linking approaches
The safest crosslinking method showcasing non-toxic behavior, high biocompatibility, and intense self-healing ability is through physical non-covalent polymerization by the bonds.35 The structure formed by the physical cross-linking method depends on the type of interaction between the molecules.35 Several types of interactions can occur, i.e. ionic interaction (based on the negative charges present in different functional groups or via metal–ligand interaction),36 hydrogen bond formation resulting in enhanced self-recovery property of the hydrogel and high efficacy in terms of self-repair,37 and crystallization (via freeze-thawing), hydrophobic interactions (mainly for the hydrophobic water-soluble polymers) and lastly through protein interaction and conjugation38 where the proteins are synthesized rationally, e.g. T4 lysozyme mutant which has several free amine groups on the surface. These customized covalent interactions can increase the strength of the hydrogel network, constitutively exhibiting specific binding affinity for different metal ions such as zinc and magnesium.39 Cross-linking through UV (ultraviolet) irradiation has also been extensively explored for the various kinds of hydrogel.402.2. Chemical cross-linking approaches
The structural linkages formed through chemical cross-linking are stronger than the physically cross-linked hydrogels. The cross-linking is induced through the induction of free radical polymerization, a “click” reaction known as the Diels–Alder reaction, a reaction involving the formation of Schiff base, a Michael type-addition reaction, and the formation of oximes.41 One of the significant advantages of using the chemical crosslinking method is its controllable degradation behavior.42 Enzymatic cross-linking has paved the way for actively manipulating the kinetics of the in situ gel formation by varying the concentration of enzymes which results in covalent solid bond formation and interaction, thereby causing gelation rapidly.41 Transglutaminase has been widely used as the enzymatic crosslinker for several injectable hydrogels due to its formation of amide linkages.43The widely recognized click chemistry has proven advantageous due to high yield even under milder conditions, high selectivity and specificity, and less by-product formation.44 Cross-linking through the Diels–Alder reaction, which involves cycloaddition between a dienophile and a diene, is highly selective in nature but has the significant advantage of the reaction being a one-step process without using any catalysts, initiators, or coupling reagents; however, it requires the modification of the hydrogel polymers with furan derivatives or furan alone.45 Another reaction involving click-chemistry that results in the formation of Schiff base occurs between amino and aldehyde groups that generate imine linkage under physiological conditions. This reaction helps enhance the hydrogel's self-healing capacity, and the self-healing behavior is highly dependent on the pH of the surrounding medium.46
Hydrogels cross-linked by oximes and through Michael's addition reaction result in self-healing hydrogels with good mechanical strength. The oxime bond formation exhibits elevated hydrolytic stability. Oxime crosslinking occurs between the hydroxylamine/aminooxy group and a ketone/aldehyde, group showcasing high specificity, and also with few other functional groups.47 Michael's addition reaction tends to be a more simplistic response involving nucleophiles and electrophilic olefins/alkynes in an activated form, which are added across C–C multiple bonds. The advantage of the Michael addition reaction is the favourable reaction rates and the reaction occurring even under mild conditions.48
As the functionality and properties of hydrogels depend on the density of cross-linking, polymeric composition, strength, internal structure, and the water holding capacity, these parameters can be judged by the physicochemical and mechanochemical characterization to provide both quantitative and qualitative data.49 Hydrogel properties such as mechanical strength, viscosity, swelling, and elasticity highly depend on the polymer chain's dimensions, the fibers' orientation and the chemical bonds present.50 The characterization methods are based on rheology, scattering, microscopy, composition, and strength measurements.51 The microscopy methods successfully provide real-space images of the hydrogel structure. Hydrogels are broadly characterized in two ways, i.e., dried (freeze-dried or in the air) or hydrated (according to the water content present).52 Various kinds of hydrogels require different instrumental settings.52 For mechanochemical characterization, rheology is the most appropriate method because it is sensitive, quick, and has a small sample size requirement and the potential to reveal the degree to which the cross-linking has been done, homogeneity/heterogeneity of structure, etc.53 Rheology involves the characterization of hydrogels via small-amplitude oscillatory shear (SAOS).
Physicochemical characterization involves phase analysis through XRD, and for chemical description, FTIR is done. For morphological analysis and to view the microstructure and pore size, characterization of the lyophilized hydrogel samples is done through FESEM.54 Density measurement is also an important factor for the characterization of hydrogels, which is estimated by attaining integrated function of density. However, it requires a desiccator before analysis.55 DLS-zeta potential is used to determine the surface charge, thereby determining the stability of the nano-formulation.56 Each type of technique requires a precise methodology for sample preparation resulting in accurate and efficient qualitative data generation.57
3. Natural cationic polymers: preparation methods and anticancer mechanism
Cationic polymer-mediated hydrogels are synthesized using cationic monomers and/or polymers isolated from natural, synthetic, and semi-synthetic sources. In the last few decades, such biomaterials have gained much interest due to diverse therapeutics and biomedical applications. These hydrogel systems are biocompatible, biodegradable, and bio-responsive, accelerate tissue regeneration, exhibit antimicrobial activity, and enable controlled release of drugs/biomolecules, leading to their high applicability in therapeutics.58 The pH of the hydrogel plays a crucial role in deciding if the behavior of the hydrogel would be hydrophobic or hydrophilic. Also, charged groups over the polymeric backbone affect the osmotic equilibrium between the hydrogel and the adjacent medium.59 In addition, cellular adhesion enhancement and heparin immobilization are significantly affected by the cationic hydrogels.58 Natural cationic polymers are obtained from natural biodegradable sources exhibiting low toxicity and immunogenicity. Modifying external reactive sites can alter most of their physicochemical properties.58,60 This aids in their wide biomedical applications, including drug and gene delivery and other tissue engineering applications. Cationic polymeric hydrogels are composed of various cationic polymers; however, the most used naturally occurring cationic polymers include chitosan, dextran, cellulose, and gelatin alone or in combination.Chitosan, a cationic polymer, comprises randomly distributed D-glucosamine and N-acetyl glucosamine units (Fig. 3a). It is a potential carrier for drug delivery applications due to its biodegradability, biocompatibility, and mucoadhesive properties.62 Cationic chitosan polymer particles can be obtained by various methods, including emulsion crosslinking,63 polyelectrolyte complexation,64 and the most widely used ionic gelation method.65 Studies have shown that chitosan strongly attracts the sialic acid (negatively charged) residues over the red blood cells, leading to severe hemagglutination.66 Additionally, chitosan has been demonstrated to augment the function of fibroblasts, macrophages, and leukocytes, leading to significant improvement in granulation and tissue regeneration.60 Lu et al. prepared a lanthanum-doped chitosan hydrogel. When evaluated on mouse melanoma cells (B-16) and skin fibroblast cells (L929), it prevented the proliferation of B-16 melanoma cells and further reduced the accumulation of toxic side effects for L929 skin fibroblast cells.67 Similarly, in another study by Highton et al., the chitosan hydrogel was evaluated on CD8+ T cells in a mouse model and it was found that vaccination with the chitosan hydrogel was equally effective as dendritic cell vaccination in terms of tumor protection.68
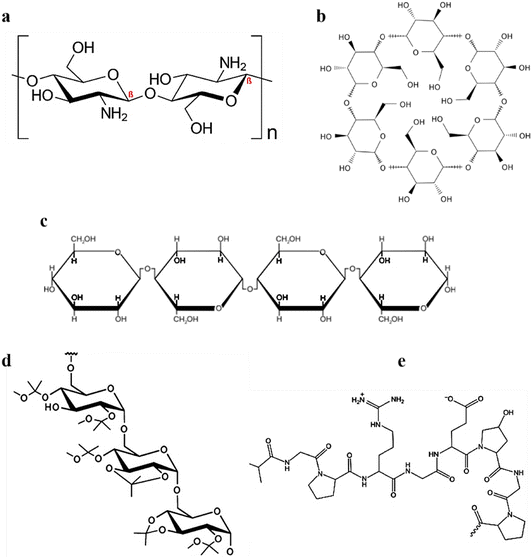 | ||
| Fig. 3 Chemical configurations of (a) chitosan, (b) cyclodextrin/s, (c) cellulose, (d) acetalated dextran, and (e) gelatin. An image of gelatin reproduced from ref. 61 with permission from MDPI, copyright 2021. | ||
Cyclodextrins are a class of cyclic oligosaccharides formed by D-(+)-glucopyranoses linked by a 1,4-α-glucosidic bond (Fig. 3b). Industrially they are produced via amylose zymolysis in the presence of glucose transferase. α-, β-, γ-forms of cyclodextrins are the most common forms, and they mainly differ owing to the presence of glucopyranose units. α-, β-, γ-forms contain 6, 7, and 8 glucopyranose units, respectively.69 Although they differ in their internal diameter, owing to stable intramolecular hydrogen bonding, they have the same depth of 7.9 Å.
Cellulose, the main constituent of the plant cell wall, is one of the most abundant organic materials. It consists of ringed β-1,4-D-glucan molecules that are arranged linearly (Fig. 3c). Although numerous preparative strategies exist, enzymatic hydrolysis70 and acid hydrolysis71 are the most widely used. The anticancer effects of doxorubicin-loaded carboxymethylcellulose hydrogel was reported against HEK 293T cells, and A375 melanoma cancer cells with improved cytotoxicity.72
Gelatin, a biodegradable and inexpensive polymer of natural origin, is obtained from collagen and has wide biomedical applications.73–75 The cationic property of gelatin is inherited by the arginine and lysine residues (Fig. 3d). Along with lysine and arginine, it consists of 18 amino acids dispersed in a non-uniform manner. Gelatin is obtained from a variety of methods, including green extraction technologies,61 emulsification solvent evaporation,76 coacervation phase separation,77 reverse phase microemulsion,77 and desolvation.78
Dextran is a hydrophilic homopolysaccharide of glucose (Fig. 3e) and possesses exceptional properties such as biodegradability, bioavailability, and hydrophilicity.79 Doxorubicin-loaded dextrin composite hydrogels substantially suppressed tumor cells when evaluated for skin cancer on mouse myoblast cells (C2C12) and human liver cells (HL7702).80
3.1. Cationic polymers targeting hallmarks of cancer
Various physiological events regulate the divergence, apoptosis, proliferation, and cell arrest which further control homeostasis and activities of cells/tissues. Any irregularity amongst these consecutive events changes the proportion between cell death, cell variation, and proliferation leading to the formation of carcinogenic cells and/or tumors.81,82 Furthermore, various macromolecular transport pathways within the tumor vessels appear through open gaps, vesicular organelles, and apertures. The physicochemical and physiological properties of the interstitium and the physicochemical characteristics of the chemotherapeutics play a crucial role in governing the transportation of anticancer drugs.83 Thus, successful delivery of a chemotherapeutic drug to cancer or tumor cells in vivo can be attained by overcoming the various physiological barriers of the tumor microenvironment at the cellular level. The cationic polymeric hydrogel systems have shown immense progress as carriers for chemotherapeutic drugs at targeted carcinogenic sites with negligible cytotoxicity.84,85Immunotherapy enhances the antitumor immune response by activating or suppressing immune system components, resulting in a highly active long-lasting T-cell response against the tumor and forming a stable immune memory. However, immunosuppressive TME limits the clinical application of immunotherapy. For instance, suppressive molecule expressions are affected by cancer cells, which can compromise immune system surveillance, further reducing the effectiveness of immunotherapeutic methods and eventually making mono-immunotherapy ineffective against tumor cells.
Preclinical evidence suggests that chemotherapeutic and immunotherapeutic agents work in tandem to modulate various targets' immune reactions throughout the cancer immune cycle by stimulating or suppressing different cellular and molecular pathways (Fig. 4).88 Combination therapy can potentially increase antitumor activity, promoting autoimmune system rebuilding while minimizing toxicity and long-term effects. Cancer vaccines, adoptive cell therapies, immune-checkpoint blockade, and cytokine methods are some of the cancer immunotherapy approaches that have been successfully developed.89 Chemotherapy continues to be the most effective option in cancer treatment. Owing to the TME's synergistic effects, it has been demonstrated that chemotherapy and immunotherapy, when combined, are potentially helpful in cancer therapy.90 Simply using chemotherapy and immunotherapy simultaneously does not result in chemoimmunotherapy, as the mechanisms of these therapeutic modalities may interact. Most chemotherapeutic agents (agents with no specific targets) are cytotoxic that can influence the majority of cell types in the human body's reproduction and growth in addition to killing cancer cells.90 In conclusion, combining immunotherapy and chemotherapeutic agents can improve cancer therapy by increasing the antitumor response in the TME.
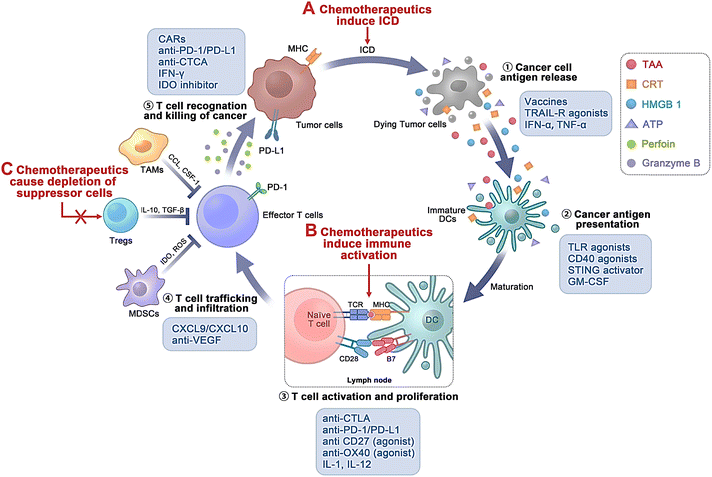 | ||
| Fig. 4 Various biological targets of chemo-immunotherapeutic agents during the cancer immune process. Chemotherapeutics assist in immunomodulatory effects mainly through (A) inducing immunogenic cell death (ICD); (B) inducing immune activation; and (C) triggering reduction of suppressor cells. Int. J. Nanomed., 2022, 17, 5209–5227 ref. 88. Originally published by and used with permission from Dove Medical Press Ltd. | ||
Cancer therapy has been immensely reformed through immune checkpoint blockade (ICB) immunotherapy, particularly antibodies against PD-1, CTLA-4, and PD-L1.91 However, ICB immunotherapy exhibits inadequate efficiency in most patients and may lead to substantial toxicity.92 Thus, more efficient, safer combinatorial therapeutic approaches including ICB are underway. PD-L1 is expressed over the tumor surface, and the antigen-representing cells can interrelate with PD-1 expressed over the stimulated T cells, causing T-cell apoptosis, inactivation, and depletion.93,94 Thus, hindering the PD-1/PD-L1 pathway using anti-PD-1 and/or anti-PD-L1 antibodies has established potential therapeutic efficiency in various cancer types, including melanoma.95–98 Furthermore, repetitive administration of anti-PD-1 antibodies can lead to severe immune-mediated adverse effects.99,100
Additionally, tumor-associated macrophages (TAMs), a vital constituent of the tumor microenvironment, play a crucial role in tumor development and progression.101 TAMs are broadly categorized as M1 macrophages (M1-TAMs) and M2 macrophages (M2-TAMs). Foreign antigens and tumor cell eradication can be attained by M1-TAMs, which express higher levels of IL-12 and IL-23.102 The stability of M1-TAM and M2-TAMs has been related to angiogenesis, drug resistance, and immunosuppression within the tumor cells.103 Additionally, CSF-1/CSF- and CCL2/CCR2-1R-targeting approaches often lead to monocyte and macrophage formation, increasing neo-angiogenesis and metastasis.104
Qing et al.107 developed bortezomib (BZ), luteolin (LT) and indocyanine green (ICG) co-loaded pH-mediated supramolecular mPEG-based (BZ/LT-ICN@mPEG) hydrogels for the effective management of colorectal cancer through the combination of chemo-photothermal-photodynamic therapy.107 In another study, Kong et al.108 reported the anticancer potential of a fabricated injectable thermosensitive liposomal hydrogel system using a chemo (gemcitabine, a chemotherapeutic drug)-photothermal (DPP-BTz, a NIR-II photothermal agent) combined approach. The hydrogels significantly reduced the treated tumors by generating heat under the irradiation of a 1064 nm laser, breaching the liposomal layer and releasing the drug leading to death of tumor cells.108 Costa et al.109 developed polymeric (hyaluronic acid-conjugated with thiol groups/deacetylated chitosan grafted with maleimide groups) hydrogels for treating breast cancer through combined chemo-photothermal therapy. On the other hand, doxorubicin-loaded dopamine-condensed graphene oxide (DDGO) was synthesized for accomplishing NIR-responsive chemo-photothermal nanocarriers. Further, the polymeric hydrogel was simply mixed with DDGO to form a stable chemo-photothermal agent. These hybrid hydrogels significantly reduced the viability of breast cancer cells, showing improved combinatorial effects.109
The combinatorial strategy has also assisted in overcoming the limitations of multi-drug resistance which is a complex cellular defensive mechanism of tumorous/carcinogenic cells to resist chemotherapeutic drugs leading to chemotherapy failure.110 In a study researchers reported the potential activity of injectable hydrogels composed of doxorubicin (chemotherapeutic drug) conjugated with gold-manganese oxide nanoparticles which were further loaded with liposome-based self-assembled micelles against MDR hepatocellular carcinoma. The hydrogel significantly released the drug in a sustained manner under the presence of NIR laser irradiation (808 nm; 1 W cm−2; 10 min.) with enhanced antitumor efficiency against MDR HCC. Moreover, the in vivo results confirmed that the hydrogel system downregulated the P-glycoprotein, p53 and Bcl-2 level, while upregulated the Bax and caspase-3 level.110
Postsurgical treatment has exhibited significant importance for combating tumor reappearance and metastasis. Chen et al.112 synthesized an in situ gel implant using pullulan and crosslinking chitosan for postsurgical care. They co-loaded cyclopamine (Cyc) with aCD47 for gel chemoimmunotherapy. The gels showed sequential drug release kinetics, with nanotherapeutics killing residual tumor cells and releasing tumor antigens. To restore macrophage functionality and activate anti-tumor immune responses, ACD47 was released over time in a sustained manner. Further investigations on 4T1 mouse breast cancer models concluded that in situ chemoimmunotherapy was effective, effectively augmenting anti-tumor effects and establishing a long-term immune memory to combat tumor metastasis.112 The combinatorial effect of chemotherapy and immunotherapy for treating tumour or cancer has been well-explored. Han et al.113 explored a unique chemoimmunotherapy technique for targeting cancer cells that uses hydrogels as a localized drug delivery system. Chemoimmunotherapeutic agents such as doxorubicin (DOX) and vaccinia virus vaccine expressing Sig/E7/LAMP-1 (Vac-Sig/E7/LAMP-1) were loaded into chitosan hydrogels (CH-DOX). Co-administration of vaccinia virus-based vaccine and CH-DOX resulted in a synergistic antitumor effect as the hydrogel inhibited tumor growth. Moreover, it also elevated the CD8(+) T lymphocytes that are tumor-specific, extending the antitumor effects up to 60 days compared to monotherapy alone. This led to the foundation to rationally explore chemoimmunotherapy's antitumor efficacy.113
Similarly, Seo et al.114 used a biodegradable hydrogel platform to simultaneously administer an immunoadjuvant and an anti-cancer medication to patients for chemoimmunotherapy. The effect of the chitosan hydrogel (CH), loaded with a cancer drug and GMCSF, on TC-1 cervical tumor growth in mice was assessed. The TC-1 tumor growth was decreased post-administration of the hydrogel containing cancer drug (DOX, cisplatin, or cyclophosphamide (CTX)) and GMCSF. While CTX was found to be a more potent anti-cancer agent, intra-tumoral treatment of CH, a cancer medication, in combination with GM-CSF elicited a significant immunological response in E7-specific CD8(+) T cells.114 Gu et al.115 developed a silk-chitosan composite scaffold encapsulating the drug DOX and JQ1 (a small chemical inhibitor of the protein BRD4 and its bromodomain) for localized delivery in the acidic TME. The DOX-JQ1@Gel contains a pH-degradable group, which triggers an antitumor immunity response. Antitumor immunity was associated with chemotherapy-induced antigen release and JQ1-mediated PD-L1 checkpoint blockade. Local DOX-JQ1@Gel injection is anticipated to reduce systemic side effects while increasing immunotherapy's objective response rate.115 Wang et al.116 created twin-like core–shell nanoparticles (TCNs) composed of sorafenib and IMD-0354 (a TAM repolarization drug) focused on tumor-targeted chemoimmunotherapy. The in vivo investigations in Hepa1–6 tumor-bearing mice and phenotype analysis revealed that TCNs had superior effects to sorafenib alone. The combination treatment revealed enhanced and synergistic anticancer effects and superior polarisation capacities towards M2-type TAMs.116
The effectiveness of the available therapy options for cancer treatment is currently limited. A novel chemo-immunotherapy system combining DOX, IL-2 (interleukin-2), and IFN-γ (interferon-gamma) offers promise for improved treatment outcomes. It was developed for the local treatment of melanoma xenografts. The system showed short-term bursts and long-term sustained releases, with the hydrogel degrading completely. Within 3 weeks, the chemo-immunotherapy system including DOX, IL-2, and IFN-γ demonstrated effectiveness without inducing inflammatory reactions. In B16F10 cells, the DOX/IL-2/IFN-γ co-loaded hydrogel increased cell apoptosis and induced G2/S phage cell cycle arrest. On the other hand, in an in vivo nude mouse model, the combined method improved therapy against B16F10 melanoma xenografts while causing no systemic adverse effects. Overall, using polypeptide hydrogels for localized DOX/IL-2/IFN-γ co-delivery offers a promising approach for efficient melanoma therapy.117
Chen et al.118 developed a hydrogel for localized chemoimmunotherapy. A polypeptide hydrogel with thermo-gelling capabilities was produced, including anti-cytotoxic T-lymphocyte-associated protein 4 (aCTLA-4), immune checkpoint blockade antibodies (anti-programmed cell death protein 1, aPD-1) and DOX. In vitro results showed that the hydrogel displayed sustained release of DOX and IgG model antibodies for more than 12 days. The DOX/aCTLA-4/aPD-1 co-loaded hydrogel dramatically increased tumor suppression, boosted anticancer immune response, and extended the survival time in mice with B16F10 melanoma. Furthermore, after surgical site injection, the hydrogel-based chemo-immunotherapy method substantially prevented tumor recurrence, demonstrating its promise for anti-tumor therapy and prevention.118 Akbari et al.119 loaded macrophage colony-stimulating factor (GM-CSF) and paclitaxel (PTX) into a hyaluronic acid (HA) hydrogel for cancer therapy. Further, tocopheryl polyethylene glycol (TPGS) and pluronic F127 were used to prepare micelles which were later loaded with PTX. In vitro and in vivo immunological activities were also assessed. The optimized formulation was tested in a mouse model of subcutaneous melanoma using the B16 F10 cell line. The hydrogel exhibited prolonged PTX release when compared to GM-CSF. Moreover, in melanoma-affected mice, the optimized formulation exhibited a potent anti-tumor effect compared to GM-CSF and PTX alone, post intra-tumoral administration.119 A melittin-RADA32 hybrid peptide hydrogel encapsulating doxorubicin (DOX) was developed by Jin et al.120 for treating melanoma. The synthesized hydrogel exhibited an interweaving nanofiber structure and excellent biocompatibility, offered controlled drug release properties, and enhanced the killing efficiency of melanoma cells. A single-dose injection of the MRD hydrogel retarded primary melanoma tumor growth by over 95%, recruiting activated natural killer cells. In addition, the hydrogel therapy efficiently activated M2-like tumor-associated macrophages (TAMs), promoting the generation of cytotoxic T cells to attack the residual tumors. Furthermore, successive injections of the MRD hydrogel resulted in the eradication of 50% of primary tumors and the induction of a robust immunological memory response, protecting against tumor recurrence after eradication.120
4. Chemotherapeutic applications of various natural cationic polymer-derived injectable hydrogels
A significant challenge in delivering therapeutic agents to cancer tissues is the dynamic and complex tumor microenvironment (TME), often resulting in the off-target delivery of associated drugs.121 Therefore, drug delivery systems should be able to deliver the therapeutic payload in a controlled and targeted manner. Conventional approaches mainly aim to reduce toxicity and associated adverse effects and improve hydrophilicity, circulation time, and control of the release profile. Targeted and localized delivery is a reliable solution.122,123 Although advanced drug delivery systems such as microspheres and nanocarriers are developed, these fail to provide the initial burst release, jeopardizing their efficiency.124 Another challenge is passive targeting by the enhanced permeation and retention (EPR) effect, which mainly relies on the tumor vascular permeability. This often fails in clinical settings due to tumor heterogenicity, variable tumour cell density, and tissue barriers.125–127 In addition, some limitations of active targeting include high interstitial fluid pressure in tumors and rapid elimination of the therapeutic payload by reticuloendothelial systems.128 Therefore, nanoformulations may be less efficient as carriers for anticancer drugs owing to their small size, which aids in their rapid elimination, and ability to interact non-specifically with normal cells, which results in low penetration into tumor sites.129 According to Wilhelm et al., among the applied nanoparticles, the fraction of nanoparticles that penetrate tumor cells is less than 0.7%.130Hydrogels are attractive delivery systems for localized and targeted therapy due to their sustained delivery. In addition, unlike active and passive targeting techniques, hydrogels work well regardless of tumor blood supply and microvasculature.27,28 Hydrogels have improved clinical applications by significantly improving RNAi delivery systems.129,131 Moreover, they can enhance the physical stability of the therapeutic drugs inhibiting drug precipitation.29 Injectable hydrogels for cancer therapy are another hot topic that has demonstrated excellent properties of hydrogels;132,133 mechanisms and strategies of the same are illustrated in Fig. 6a and b.
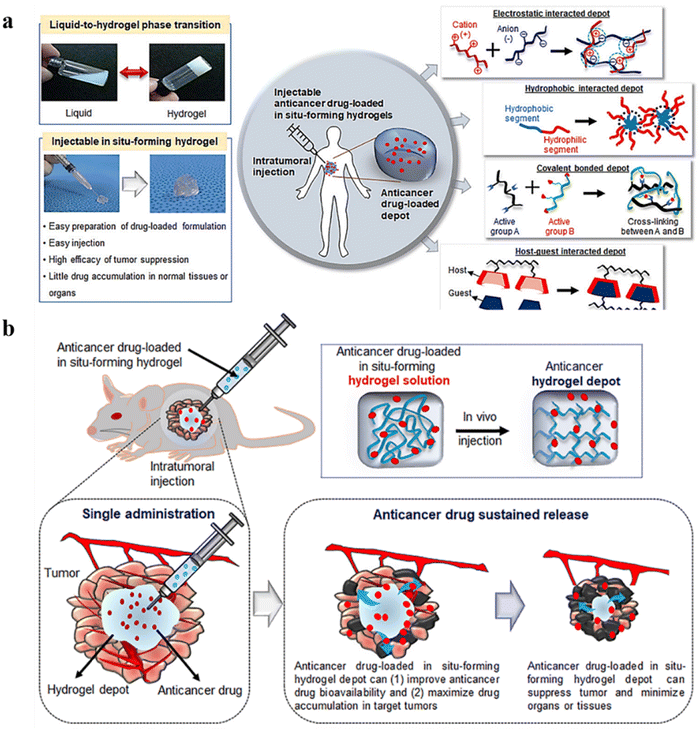 | ||
| Fig. 6 (a) Schematic representation of an injectable in situ forming hydrogel for intratumoral injection. (b) Schematic illustration of intratumoral injection of anticancer drug-loaded injectable hydrogels. Reproduced from ref. 133 with permission from MDPI, copyright 2021. | ||
Hydrogels are also capable of delivering multiple therapeutic payloads at once. Often due to the heterogenic nature of tumors and the presence of malignant cells at distinct stages of growth and divisions, a single drug might not be efficient in inhibiting tumor growth.134 Concurrent delivery of multiple medications can be a promising strategy in such cases.135 This strategy further improves the treatment efficacy by lowering associated adverse effects. For instance, Hu et al. prepared a thermosensitive injectable hydrogel for the codelivery of lapatinib and paclitaxel, which upon peritumoral injection resulted in a synergistic effect.136
Moreover, concurrent delivery of therapeutic agents with DNA or RNAi can yield promising results in overcoming angiogenesis and tumor resistance to drugs by inhibiting efflux pumps associated with multidrug resistance.135 In a study by Strong et al., concurrent delivery of doxorubicin and DNA enclosed within a poly(N-isopropyl acrylamide-co-acrylamide) hydrogel with near-infrared absorbing silica–gold nano-shells was achieved.137 In another study by Guo et al., an injectable linoleic acid-coupled pluronic hydrogel carrying paclitaxel and protein kinase B (AKT)-targeted gene therapy exhibited a synergistic anticancer effect by downregulating AKT signaling further inducing apoptosis.121
Furthermore, hydrogels can also be used for the codelivery of radioisotopes and chemotherapeutic agents for cancer therapy. For instance, Huang et al. developed a macroscale thermosensitive injectable micellar hydrogel for the co-delivery of iodine-131 labeled hyaluronic acid (131I-HA) and doxorubicin for enhanced in situ synergistic chemoradiotherapy.138 The therapeutic application of multiple drugs for synergistic effects often requires separate encapsulation of the therapeutic payload. Wang et al. developed PEGylated hydrocarbon nanoparticles with PEGylated fluoro-carbon nanoparticles for encapsulating doxorubicin and paclitaxel into different hydrogel compartments.134 Another proposed approach for controlled cargo release from the hydrogel is incorporating nanoparticles. In a study, a gold nanoparticle enclosed within a dextran-based implantable dendrimer hydrogel was reported for the co-delivery of cisplatin and miRNAs.139 Many engineered injectable hydrogels prepared from cationic polymers of natural origin are briefly discussed in this section.
4.1. Chitosan-based injectable hydrogels for targeted chemotherapy
Chitosan, a natural polymer discovered in 1859 by C. Roget, is the second most abundant polysaccharide after cellulose. It is usually obtained by alkaline deacetylation of chitin mounds, mostly in crustacean shells. Structurally chitosan is like glycosaminoglycan, consisting of β-(1–4) linked D-glucosamine and N-acetyl-D-glucosamine units arranged randomly.140,141 Chitosan has high biocompatibility, low immunogenicity, and better intrinsic bacteriostatic activity and thus is widely used for various biomedical applications.138,142 In another study,143 researchers developed thermo- and pH-responsive hydrogels by conjugating poly(N-isopropylacrylamide-co-itaconic acid) and chitosan via ionic crosslinking using glycerophosphate for effective delivery of doxorubicin against breast cancer. Recent advancements in chitosan-based injectable hydrogels are discussed hereafter.A chitosan-based thermo-responsive hydrogel was prepared by Ahsan et al. for efficient and sustained delivery of disulfiram (CS-DS) (Fig. 7a). The drug was firmly distributed in the injectable thermo-responsive hydrogels, confirmed by SEM micrographs (Fig. 7b). The cumulative drug release profile showed a better release of DS from injectable hydrogels (Fig. 7c). Moreover, CS-DS injectable hydrogels exhibited better cellular uptake in the treated SMMC-7721 cell line (Fig. 7d), ensuing significant anticancer activity against hepatocellular carcinoma.144 Similarly, dialdehyde-functionalized polyethylene glycol (DF-PEG) and β-glycerophosphate (GP) cross-linked chitosan (CS) hydrogels were prepared by Han et al. for sustained delivery of doxorubicin hydrochloride (DOX) for antitumor therapy via intratumoral injection. In Heps tumor-bearing mice, the resultant hydrogel exhibited a superior tumor inhibition rate.145 Moreover, for localized delivery of an anticancer drug, 5-fluorouracil, for breast cancer treatment, Abdellatif et al. prepared a chitosan hydrogel and investigated its efficacy in the MCF-7 cell line both in vitro and in vivo. Reduction in tumor volume and tumor marker levels in blood showed that injectable hydrogels are potential drug delivery systems for anticancer drugs.146
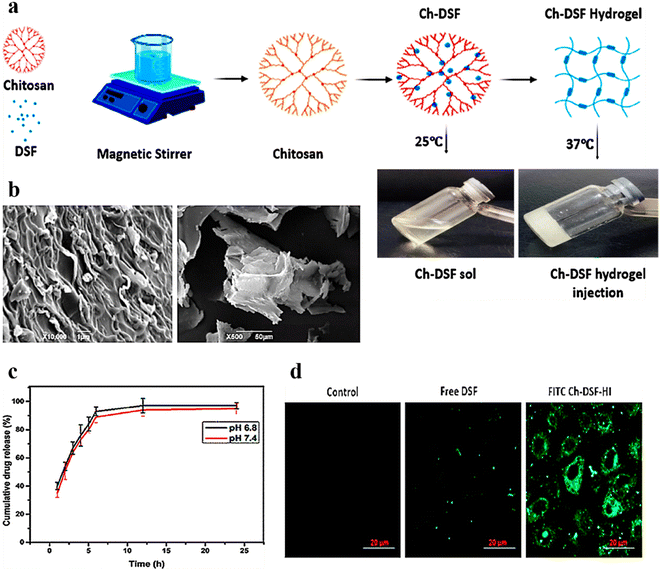 | ||
| Fig. 7 (a) Schematic illustration of the steps involved in the formulation of thermo-responsive CS-DS injectable hydrogels. (b) SEM micrographs of thermo-responsive CS-DS injectable hydrogels at various magnifications and cross sections. (c) Cumulative release (%) of DS from thermo-responsive CS-DS injectable hydrogels over time at varying pH (n = 3). (d) Cell uptake of free DS and FITC-tagged CS-DS injectable hydrogels in the hepatocellular carcinoma SMMC-7721 cell line after 4 h incubation; scale bar: 20 μm. Reproduced from ref. 144 with permission from American Chemical Society, copyright 2020. | ||
Wu et al. constructed a crosslinked chemical and physical composite injectable gel for co-delivery of doxorubicin, recombinant human interferon-gamma (IFN-γ), and the protein cytokine recombinant human interleukin-2 (IL-2).147 When administered to the xenograft tumor-bearing mice, this exhibited a synergistic anticancer effect by downregulating Janus kinase/signal transducer and activating JAK/STAT pathways. Further, a pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan was prepared by Qu et al. for hepatocellular carcinoma therapy.21 Wang et al. prepared an injectable chitosan-based hydrogel for antitumor and antimetastatic effects on hepatocarcinoma using Bel-7402 cells.148 Belali et al. prepared a cell-specific and pH-sensitive nanostructured chitosan hydrogel as a potential photosensitizer carrier for selective photodynamic therapy.149 To improve intraperitoneal chemotherapy in colon carcinoma, Chen et al. constructed a thermosensitive poly(N-isopropyl acrylamide)-based hydrogel (HACPN) loaded with doxorubicin and investigated its effects on CT-26 cells in vitro.150
4.2. Cyclodextrin-based injectable hydrogels for targeted chemotherapy
Injectable hydrogels can be employed for dual loading of both hydrophilic and hydrophobic drugs, as their primary (6-OH) and secondary surfaces (2-OH, 3-OH) are formed by hydroxy hydrophilic medicines whereas ether along with carbon–hydrogen accounts for the hydrophobic cavities.151 Due to their good solubility and permeability, along with specific recognition and bonding abilities with numerous inorganic, organic, and biological substrates and polymer chains,152–154 cyclodextrins can be efficiently used as a carrier for drug delivery applications.Kuang et al. synthesized A-PEG-A and -PEG-T (Fig. 8a), and further conjugated with α-cyclodextrin (α-CD) for developing injectable hydrogels (Fig. 8b and c) for sustained release of antitumor drugs. Initially, the in vivo development of the G2 hydrogel was performed (Fig. 8d). Later on, in vivo, experiments employing U14 cancer cell xenograft-bearing mice (Fig. 8e) showed that the intratumoral injection of a DOX-loaded A-PEG-A/T-PEG-T/a-CD gel inhibited tumor growth more effectively than that of free DOX.155 In another study, Fiorica et al. investigated the penetration (in solid tumors) and release profile of anticancer drug DOX, embedded in hyaluronic-((2-aminoethyl)-carbamate) acid (HA-EDA) conjugated with sulfone functionalized β-cyclodextrins (HA-EDA/β-CD-VS(DOX)). The complex hydrogels significantly inhibited the growth of colorectal carcinoma micro-masses cultured under 3D conditions. In vivo, studies have validated that tumor mass was reduced considerably without inducing any localized or systematic side effects.156 Similarly, Fu et al. prepared a paclitaxel-loaded injectable micellar supramolecular hydrogel composed of α-cyclodextrin (α-CD) and monomethoxy poly(ethylene glycol)-b-poly(caprolactone). This resulted in enhanced biological activity of paclitaxel over free drug. In addition, it was shown that by altering the composition of α-CD in the hydrogel, the release profile of the drug could be modified.157
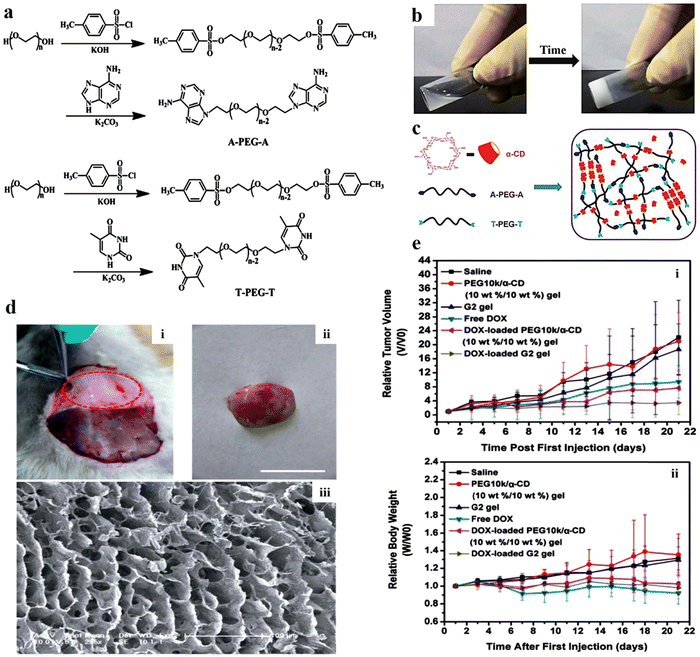 | ||
| Fig. 8 (a) Scheme for the synthesis of adenine-terminated poly(ethylene glycol) (A-PEG-A) and thymine-terminated poly(ethylene glycol) (T-PEG-T). (b) Images of an A-PEG-A/T-PEG-T/α-CD aqueous solution and G2 (B-PEG10k-B: 10% w/w) sample injectable hydrogel. (c) The mechanism associated with the gelation of the supramolecular hydrogel. (d) Photographs of (i) in vivo development of the G2 hydrogel within subcutaneous tissue post-treatment of 30 min (marked with red dots); (ii) G2 hydrogel-separated skin of a treated animal (rat); (iii) the equivalent SEM images of the hydrogel. (e) Graphs highlighting the alterations in (i) relative tumor volume and (ii) relative body weight of varying samples that were injected intratumorally into the xenograft-bearing mice (U14) after showing an initial tumor volume of 150–250 mm3. Reproduced from ref. 155 with permission from Royal Society of Chemistry, copyright 2014. | ||
4.3. Cellulose-based injectable hydrogels for targeted chemotherapy
Cellulose, one of the most frequently found polymers in nature, consists of anhydrous-D-glucopyranose units linked by 1,4 linkages.158 Its unique physicochemical properties and wide applications have invoked a greater interest among researchers. Biocompatibility, cost-efficiency, and high thermal and mechanical stability are among the numerous factors that promote its use for broad biomedical applications. Cellulose is insoluble in water and most organic solvents due to strong bonds and intermolecular hydrogen bonding between chains; as a result, chemical modifications are done.159 Examples of modified cellulose include ethylcellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, oxidized cellulose, carboxymethyl cellulose, cellulose acetate, and cellulose triacetate, the common derivatives of cellulose obtained through chemical modification processes such as etherification, esterification, and oxidation.160 Cellulose hydrogels are predominantly used in drug delivery applications owing to their highly porous structure and mechanical properties. A biodegradable, multicompartmental, thermo-responsive hydrogel of cellulose and N-isopropyl acrylamide was prepared for dual loading of doxorubicin and niclosamide by Andrade et al. The drug release profile of both drugs was retarded (only 4% of doxorubicin and 30% of niclosamide were released after 1 week) due to the presence of cellulose, promoting cell death in cell lines HCT116 and OVCAR-3.161You et al. prepared a quaternized cellulose (QC) and cationic cellulose nanocrystals (CCNs) crosslinked with β-glycerophosphate (β-GP) (Fig. 9a) with rigid rod-like (Fig. 9b) structure for localized and sustained drug delivery of doxorubicin (DOX). The administration of the hydrogel showed a specific site location (Fig. 9c(i)–(iii)), with significant results confirmed through histological images observed at varying time intervals (0–16 days) (Fig. 9c(iv)–(ix)). The administration of the DOX-loaded optimized hydrogel significantly suppressed the progression of cancer cells and also the rate of survival augmented vividly (Fig. 9d), signifying an enhanced chemotherapeutic efficacy due to the sustained release of DOX.162 In another study, a thermo-reversible hydrogel of methyl cellulose was prepared for the controlled release of docetaxel. The formulation exhibited sustained drug release over 25 days, lowering tumor growth and promoting survival in B16F10 melanoma-induced mouse models.163 Weng et al. prepared an in situ forming carboxymethyl cellulose hydrogel for sustained doxorubicin delivery, which exhibited a mere 30% release over 78 hours.164 Ding et al. prepared a hydroxypropyl methylcellulose injectable hydrogel for the co-delivery of paclitaxel and temozolomide for glioma treatment.165 An alginate and sodium carboxymethyl cellulose hydrogel for dual drug delivery of aspirin and methotrexate was developed by sheng et al. for colorectal cancer treatment.166 Balahura et al. designed 5-fluorouracil-loaded cellulose nanofiber hydrogels for promoting pyroptosis activation in breast cancer cells.167 Bollareddy et al. prepared a transferosome hydrogel containing a 5-fluorouracil and etodolac combination for synergistic oral cancer treatment.168 A self-healing cellulose injectable hydrogel for sustained cancer therapy was developed by Jiang et al.169 In addition, Capanema et al. acquired doxorubicin-loaded bioengineered carboxymethyl cellulose hydrogels for topical chemotherapy of melanoma skin cancer.170
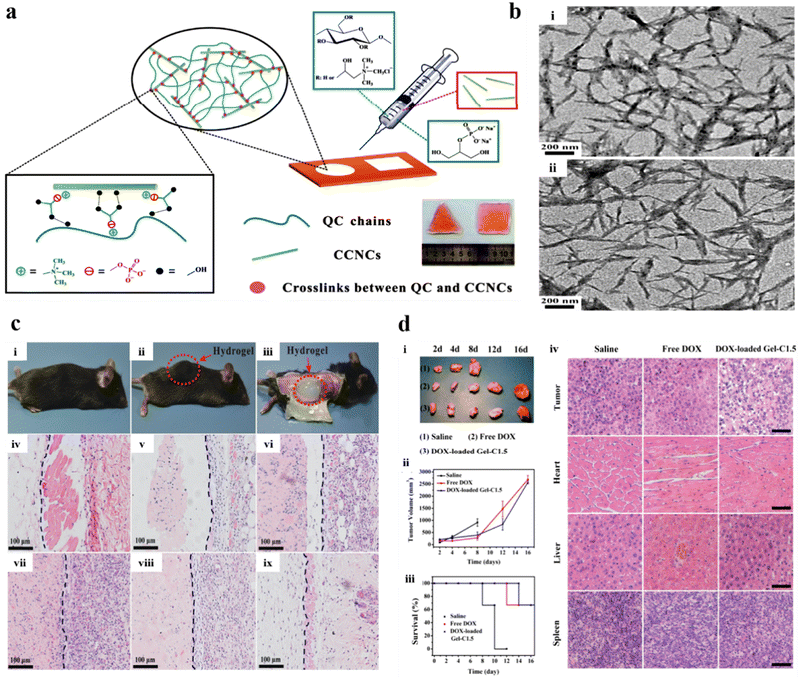 | ||
| Fig. 9 (a) Schematic illustration of hydrogel synthesis using hydrogel precursors and injectable QC/CCN/β-GP nanocomposite hydrogels. (b) TEM images of (i) CN and (ii) CCN. (c) Comprehensive view: (i) before and (ii) after s.c injection of the hydrogel (optimized), and (iii) subsequent segmentation after 10 min of post-injection. Histological micrographs of s.c implantation of the optimized hydrogel treated within nude mice at (iv) 0, (v) 2, (vi) 4, (vii) 8, (viii) 12, and (ix) 16 days. The hydrogels are situated on the left side of the blue lines. (d) The illustrative images of (i) the tumors after various treatments, (ii) the tumor volume, and (iii) the rate of survival of each treated group. (iv) Histological micrographs of the H&E stained sections of different organs of treated mice after various treatments at 8 days. Reproduced from ref. 162 with permission from American Chemical Society, copyright 2011. | ||
4.4. Dextran-based injectable hydrogels for targeted chemotherapy
Dextran is a natural polymer that owing to its high availability, low cost, and ability to undergo easy chemical modifications is used widely for wide biomedical applications. It consists of glucose monomers linked by α-1,6 glycosidic bonds. Dextran has invoked greater interest as a drug delivery carrier due to its high stability, absence of toxicity, hydrophilicity, and biodegradability,171 and it enables enhanced penetration of chemotherapeutic agents in tumor masses.172 This has allowed the fabrication of effective delivery vehicles for cancer treatment.173 Chemical modifications such as oxidation,80,174 conjugation to thiol,175 and acrylic129 group of dextran yielded effective carriers for the delivery of genes129 and cytotoxic drugs.80,174Liu et al. developed a sericin (isolated from Bombyx mori)/dextran (SC/DX)-based hydrogel (Fig. 10a and b) encapsulated with DOX for real-time in vivo monitoring and delivery of the therapeutic payload for malignant melanoma treatment (Fig. 10c). The hydrogel exhibited superior gelation time, biodegradability, and biocompatibility with improved drug loading and controlled release of both small-molecular and macro-molecular drug entities with better storage abilities (Fig. 10d–f). In addition, drug distribution was confirmed through morphological studies using SEM (Fig. 10g). Furthermore, the in vivo results showed that SC/DX-loaded DOX hydrogels exhibited superior anticancer effects by significantly reducing the tumour size and improving the survival rate of treated B16-F10-induced mice (Fig. 10h–m).80
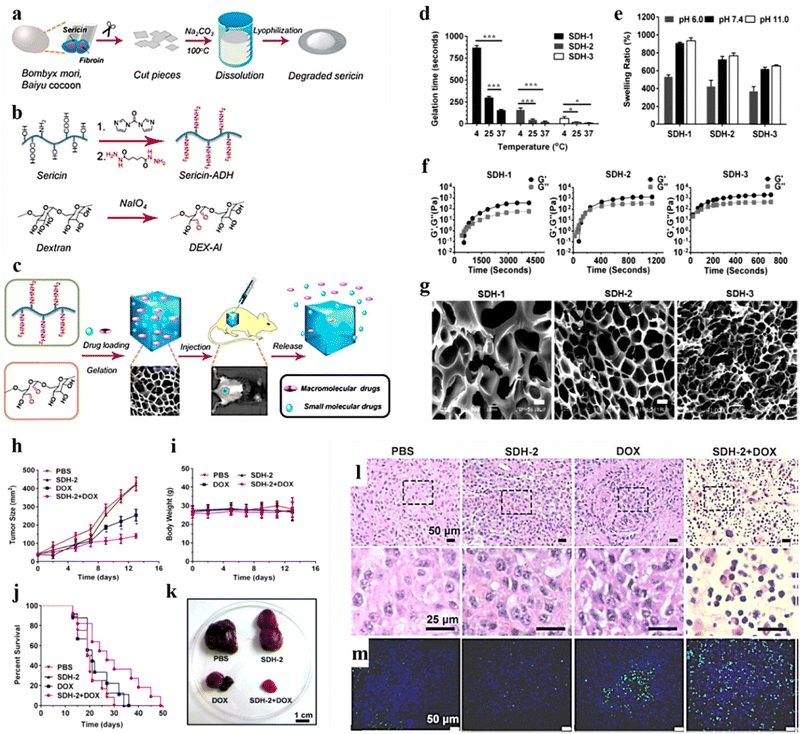 | ||
| Fig. 10 Schematic illustration representing preparation of the sericin/dextran-based (SC/DX) composite hydrogel: (a) extraction steps involved in isolating sericin from Bombyx mori, Baiyu cocoons (wild-type). (b) Chemical modifications (oxidation) of SC and DX. (c) Illustration showing the usage of the SC/DX cross-linked hydrogel as an injectable and photoluminescence-detectable drug delivery system. (d) Gelation time of the composite hydrogels (SDH-1, SDH-2, and SDH-3) formed at 4, 25, and 37 °C (mean ± SD, n = 3; *P < 0.05, **P < 0.01, ***P < 0.001; and Student's t-tests). (e) Time evolution of the storage modulus (G′) and loss modulus (G′′) of SDH-1, SDH-2, and SDH-3 at 15 °C. (f) SEM images of SDH-1, SDH-2, and SDH-3 [scale bars: 10 μm]. (g) In vivo antitumor effects of the DOX-loaded SDH-2 hydrogel. Quantification of (h) tumor size, (i) body weight, and (j) the survival rate in B16-F10-induced mice treated with various samples. (k) Images of the isolated tumors from the mice administered with mentioned treatments (day 14). (l) H&E histological staining of the tumors of mentioned treatments (day 14). (m) TUNEL staining of the isolated tumors in (l). Reproduced from ref. 80 with permission from American Chemical Society, copyright 2011. | ||
Luo et al. constructed an injectable hydrogel to act as a depot system for increasing drug concentration at the tumor site.176 The nanocomposite hydrogel consisting of oxaliplatin (OXA)-conjugated G5 polyamidoamine (G5-OXA) and oxidized dextran (Dex-CHO) exhibited increased drug retention and tumor penetration via active transcytosis. In vivo studies performed on the 4T1 tumor model revealed inhibited primary tumor growth and metastasis.176 Similarly, dextran methacrylate hydrogel microneedles loaded with doxorubicin and trametinib for continuous transdermal administration of melanoma were prepared by Huang et al.177 Further, van Es et al. prepared a dextran hydrogel and holmium-poly(L-lactic acid) microspheres and investigated them by radionuclide and histological pilot study through tumor embolization in a Vx2 rabbit head and neck cancer model.178 Solomevich et al. designed biodegradable pH-sensitive dextran phosphate hydrogels loaded with prospidine (DP-PrH) for local tumor therapy. The hydrogels inhibited the propagation of Hep-2 and HeLa cells in a dose-dependent manner. In addition, the hydrogels demonstrated superior antitumor effects than pure drugs.179 Saboktakin et al. encapsulated 5-,10-,15-,20-tetrakis(meso-hydroxyphenyl)porphyrin (mTHPP), a porphyrin-based PS agent, into hydrogels for photodynamic treatment of cancer.180
4.5. Gelatin-based injectable hydrogels for targeted chemotherapy
Gelatin is a naturally occurring biodegradable polymer obtained from acid/alkali hydrolysis of collagen. It is non-toxic, non-irritant, consists of denatured proteins, and has been widely used in pharmaceutical and biomedical industries due to its biodegradability.181–184 It comprises 18 different amino acids, arranged randomly with ample free carboxyl and amino groups that pave the way for electrostatic interactions. Further, increased free carboxyl and amino groups in alkali and acid-treated gelatin make it a suitable carrier for the controlled release of drugs and peptides.185Laser-triggered injectable gelatin hydrogels were prepared by Li et al., for combinatorial up-conversion of fluorescence imaging and antitumor chemo-photothermal therapy.187 Ding et al. prepared a gelatin hydrogel containing cisplatin loaded with gelatin (CDPP)/poly(acrylic acid) nanoparticles (Fig. 11a and b). It was observed that due to body temperature, the CDDP-NP-Jelly (CNJ) coating over the tumour progressively converted into a gelatinous sol in vivo (Fig. 11c). Results of in vitro cytotoxicity studies for 48 h showed significant findings for free CDDP, CNPs, and CNJ against treated adenocarcinoma (MKN-28) cells and mouse hepatoma (H22) cells (Fig. 11d–f). Further, significant in vivo findings were noticed for the samples isolated from the treated mouse model using typical fluorescence microscopy (Fig. 11g), dark-field microscopy images (Fig. 11h), and immunohistochemical assay (Fig. 11i). The implantation of the jellies comprising CDDP-loaded nanoparticles over the tumor tissue hindered tumor growth. It extended the lifetime of the treated animals (mice) compared to animals treated with i.v. injection of CDDP-loaded nanoparticles in a murine hepatoma H22 cancer model.186 In another study, Yamashita et al. reported the effectiveness of cisplatin-loaded gelatin hydrogels and anti-tumor activity in the peritoneal metastasis murine model of the human gastric cancer MKN45-Luc cell line.188 The formulation further prolonged the survival time (p = 0.0012) and suppressed the in vivo tumor growth (p = 0.02), additionally releasing cisplatin in a controlled manner (30% drug remaining in the murine abdominal cavity after 7 days). An interleukin-12 (IL-12) loaded gelatin hydrogel was prepared by Liu et al., and was investigated for sustained release of IL-12 in transplanted colon carcinoma C57BC/6 N mice.189
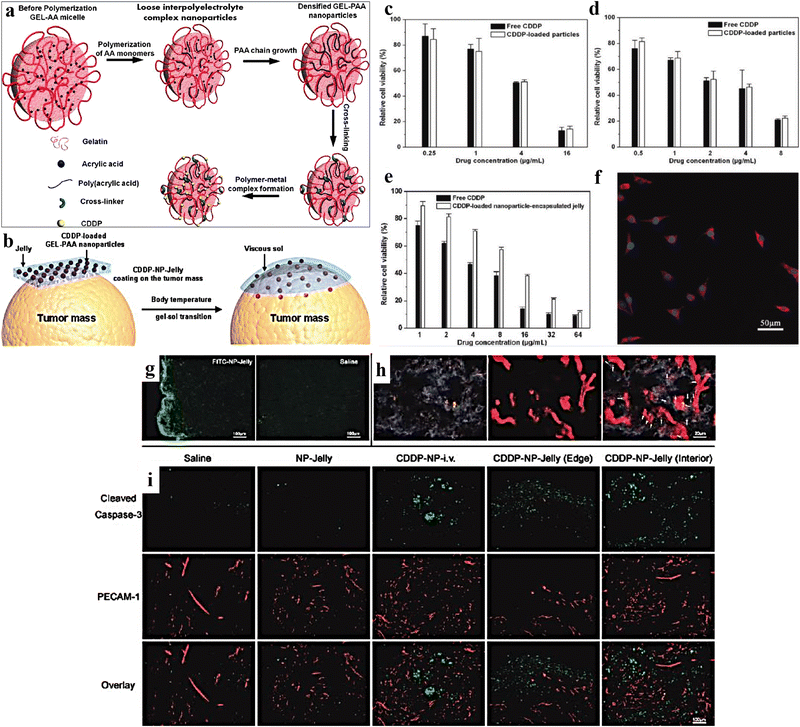 | ||
| Fig. 11 (a) Schematic representation of the synthesis of CDDP-loaded gelatin-poly(acrylic acid) nanoparticles (CNPs). (b) Schematic representation of CDDP-NP-Jelly (CNJ) coating over the tumor, which progressively converts into a gelatinous sol in vivo, because of body temperature. In vitro cytotoxicity (48 h) of (c) free CDDP and CNPs against adenocarcinoma (MKN-28) cells (48 h); (d) free CDDP and CNPs against mouse hepatoma (H22) cells; (e) free CDDP and CNJ against MKN-28 cells. (f) LSCM image of MKN-28 cells incubated with CNPs, labeled by RBITC (37 °C, 2 h). (g) Typical fluorescence microscopy micrographs of tumor slices isolated from mentioned FITC-sample-treated mouse models. (h) Illustrative dark-field microscopy image of tumor slices isolated from sample-treated mice. Dark-field microscopy image overlapped with vasculature fluorescence, displaying the spreading of the groups of GEL-PAAAu hybrid NPs (bright spots) related to the blood vessels (red) in the internal portions of the tumor. The arrows indicate the site of bright spots. (i) Characteristic photos of immunohistochemical samples comprising sliced caspase-3 (green) and PECAM-1 (red) in diverse groups specified. Reproduced from ref. 186 with permission from American Chemical Society, copyright 2011. | ||
Studies have also reported a few other cationic polymeric hydrogel-based systems for effectively delivering chemotherapeutic agents. In a study, hyaluronic acid (HA), a non-sulfated glycosaminoglycan, predominantly found in joints and connective tissue and which is highly biocompatible was used to fabricate numerous injectable hydrogels.190–193 Injectable hydrogels of HA suitable for delivery of doxorubicin for breast cancer194 and colon cancer195 or co-delivery of doxorubicin and docetaxel for treatment of colon carcinoma196 were prepared in the presence of PF127. Another polysaccharide alginate, a biopolymer consisting of units of guluronic acid and mannuronic acid in irregular blocks,197 owing to its biocompatibility and hydrophobicity is widely used in the biomedical field. Moreover, its hydroxyl and carboxyl groups can be chemically altered to achieve the desired properties.198 Cisplatin dendrimers to breast and lung cancer cells were delivered efficiently through an injectable hydrogel prepared via ionic gelation.199 Moreover, incorporating moieties such as N-isopropyl acrylamide led to the formation of thermo-responsive hydrogels to deliver doxorubicin micelles200 and genes201 for osteosarcoma and prostate cancer.
4.6. Polypeptide-based injectable hydrogels for targeted chemotherapy
Traditional chemotherapeutic drugs have significant drawbacks. Tumors, for example, can develop resistance to treatment, higher relapse chances post-treatment, and secondary malignancies due to drugs used against metastatic cancer.202 Drugs that may specifically eliminate cancer cells are still in high demand, effectively treating slow-growing and dormant cells while avoiding chemoresistance mechanisms. A steadily increasing amount of research suggests that peptides may help discover and develop cancer drugs.202 Peptides are ideal candidates for cancer treatment due to their excellent tissue penetrability, low immunogenicity, and low manufacturing costs, and modification is simple for enhancing in vivo stability and biological activity.202 Due to secondary conformations that are unique, variable functions, and, most significantly, stimuli-enhanced therapeutic efficacy and minimizing adverse effects, stimuli-responsive polypeptide nano-assemblies have great potential for cancer nanomedicines.203 Owing to the above advantages, various endogenous stimuli (e.g., pH, reduction, reactive oxygen species, adenosine triphosphate, enzymes, etc.) and exogenous light stimuli (e.g., UV and near-infrared light) that are biologically relevant are used to create stimulus-responsive polypeptide nanoassemblies which are currently widely used.203Peptide-based and peptide-conjugated delivery systems often comprise peptides produced from viral protein molecules that help viruses transfer their genome into host cells and have a high delivery efficiency.204 Targeting tumor microenvironments (TME) and improving aspects such as cellular absorption and lysosomal degradation pathways were also inhibited, and controlled and sustained therapeutic release were provided through peptide-based systems.205 Peptides, irrespective of their origin, possess specific biochemical properties, based on which they are classified as cell-penetrating, fusogenic, and targeting peptides. These all classes of peptides are widely used as therapeutic modalities (peptide vaccines), and as drug carriers for cancer therapy.206 Furthermore, PGmatrix, a peptide hydrogel 3D scaffolding technology for cell culture, has recently been reported to grow organoid-like spheroids physiologically mimicking the 3D microenvironment that can be used as an in vitro 3D model for investigating cell activities, which is expected to improve the prediction rate.207
To successfully block the Arginase 1 (ARG1) pathway, Ren et al.208 synthesized an injectable hydrogel loaded with an L-norvaline-based immunomodulating gelator. The gelator, a diblock copolymer, consists of an L-norvaline-based polypeptide block, which ensures high drug loading of L-norvaline and controlled release in tumor microenvironments via responsive peptide bond cleavage. The hydrogel and DOX were effective immunotherapies for primary tumor removal, abscopal tumor suppression, and pulmonary metastasis inhibition. This novel approach offers a strong injectable hydrogel technology that effectively reverses ARG1 immunosuppressive conditions, resulting in enhanced immunotherapy.208 Jin et al.209 synthesized an injectable hydrogel using genetically engineered polypeptide and hollow gold nanoshells (HAuNS) for treating HepG2 tumors. The hydrogel was created by layering DOX and PC10A, with DOX having a positive charge and PC10A having a negative charge, further coating negatively charged HAuNS. The hybrid PC10A/DOX/HAuNS nanogel was dissolved in polypeptide PC10A to create the multifunctional hydrogel PC10A/DOX/HAuNS. DOX was absorbed by the HAuNS and then incorporated in the PC10A hydrogel, allowing sequential drug release for sustainable chemotherapy. Furthermore, tumor-bearing mouse experiments in vitro and in vivo revealed a significant improvement in tumor inhibition by the combination of chemo-photothermal therapy or chemotherapy alone.209
Garrett et al.210 developed a diblock co-polypeptide hydrogel (DCH) to test injectable hydrogel-based carrier systems for chemotherapeutics in glioblastoma treatment. The DCH could carry and release paclitaxel, a highly potent compound against primary gliomasphere cells. The DCH showed minimal tissue reactivity in the immune-competent mouse brain and was well tolerated. Moreover, Cremaphor-taxol or hydrogel caused less tissue damage, cellular inflammation, and reactive astrocytes. In vivo studies revealed that an injection of the paclitaxel-loaded hydrogel resulted in local tumor control and enhanced survival in the immunosuppressive mouse xenograft model of glioblastoma.210 Liu et al.211 developed a supramolecular injectable hydrogel for local delivery of the DPPA-1 peptide and DOX. DOX can kill tumor cells and induce immunogenic cell death, while the DPPA-1 peptide blocks the PD-1/PD-L1 pathway, potentiating T-cell-mediated responses and minimizing side effects. This hydrogel's local injection demonstrated a synergistic cancer therapeutic effect, improving immunotherapy's objective response rate while minimizing systemic side effects.211
Song et al.212 used an injectable poly(L-valine) hydrogel as an antigen delivery vehicle and immunopotentiator for DC modulation in cancer immunotherapy. Further, in vitro and in vivo investigations revealed that the vaccine formulation of poly(I:C), TLR3 agonist, tumor cell lysates (TCL), and polypeptide hydrogel effectively recruits, activates, and matures DCs. Furthermore, melanoma-induced mice treated with subcutaneously injected hydrogel-based vaccine elicited a cytotoxic solid T-lymphocyte immune response. This demonstrates that the hydrogel vaccine stimulates the generation of CD8+ T cells in draining lymph nodes and tumor-infiltrating T-lymphocytes.212 The multifunctional polypeptide hydrogel has excellent potential as a green manufacturing and engineering material for cancer vaccines and anticancer applications. Hou et al.213 developed a genetically engineered polypeptide hydrogel PC10ARGD for mammary carcinoma treatment. As a photosensitizer, the hydrogel contains a near-infrared silver sulfide (Ag2S) QD and the water-soluble drugs DOX and Bestatin. The photothermal effect of the hydrogel resulted in continuous delivery of DOX, which can be used for in situ vaccination. In vivo tests revealed that laser irradiation of the Ag2S QD/DOX/Bestatin@PC10ARGD hydrogel activated anti-tumor immune effects, inhibiting tumor growth and distal lung metastatic nodules. A safer lower-temperature treatment strategy with multiple laser irradiation demonstrated more effective tumor-killing performance and increased immune cell penetration into tumor tissue.213
Jin et al.214 developed an injectable multifunctional hydrogel based on modified coiled-coil polypeptide and Ag2S quantum dots (QDs) for long-term chemo-photothermal treatment and PTX. The preparation of hydrogels was done by dissolution of oil-soluble Ag2S-QDs and PTX in PC10A hydrogels. The combination treatment effectively suppressed the development of SKOV3 ovarian cancer tumors. Real-time monitoring of in vivo degradation was achieved using near-infrared fluorescence and photoacoustic imaging. These results revealed that the developed multifunctional injectable hydrogel could be a potential theranostic platform for sustained cancer treatments.214 Liu et al.215 synthesized a thermosensitive supramolecular hydrogel consisting of iodine-131 (I131)-labeled injectable thermosensitive methoxy PEG-b-poly(tyrosine) (PETyr-I131) for brachytherapy. The PETyr-I131 radioactive source was immobilized at the injection site and monitored in real-time with single-photon emission computed tomography. The SmacN7 peptide coupled with cell membrane-permeable oligosarginine (SmacN7-R9) therapy had no negative effects. The combination improved radiosensitivity in cancer cells. Because of its proximity to the primary tumor or postoperative cavity site, the thermosensitive supramolecular hydrogel platform conformally immobilized radionuclides and delivered radiosensitizers, offering a high chance of producing synergistic therapy outcomes while minimizing radiation-related negative effects.215
Shi et al.216 synthesized an injectable, biocompatible polypeptide hydrogel to deliver along with DOX an immune checkpoint inhibitor antibody that targets programmed death-ligand 1 (aPD-L1). DOX significantly induced immunogenic tumorand promoted an antitumor immune response. Furthermore, the simultaneous release of aPD-L1 at the tumor site increased the tumour inhibitory effect by inhibiting the PD-1/PD-L1 pathway and restoring the cytotoxic T cell tumor-killing effect. The aPD-L1 and Dox co-loaded hydrogel treatment of the B16F10 melanoma model resulted in remarkable tumor progression inhibition and animal survival prolongation.216 Jin et al.217 developed a multifunctional PC10A/DOX/MoS2 hydrogel for chemotherapy, photothermal therapy, and photodynamic therapy of 4T1 tumors. The hydrogel was prepared using positively charged DOX and negatively charged PC10A, and the 2D MoS2 nanosheet was used as both photothermal and photodynamic agent. In vivo tumor-bearing mouse experiments showed that combining chemo-photothermal-photodynamic therapy significantly enhanced tumor inhibition compared to photothermal therapy, photodynamic therapy, or chemotherapy alone. The PC10A/DOX/MoS2 hydrogel inhibited primary 4T1 breast tumours as well as distal lung metastatic nodules by activating antitumor immune actions.217 Further, some significant effects of various natural cationic polymeric hydrogel systems are summarized in Table 1.
| Composition | Cancer model | Major outcomes | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Hydrogel | Major components | Therapeutic agent | Type | In vitro | In vivo | ||
| Abbreviations: CS: chitosan; TA-ZnPc: tetra-aldehyde functionalized zinc phthalocyanine; β-GP: β-glycerophosphate; HA: hyaluronic acid; CNT: carbon nanotubes; DA: dihydrocaffeic acid; oxPLN: oxidized pullulan; oxHA: oxidized HA; Tyr: tyramine; CD: cyclodextrin; Gln: glutamine substrate peptide; PEG-8-SH: 8-arm PEG; PEG: poly(ethylene glycol); Lys: lysine; others, PF127 (PEO/PPO balance 70/30); PEO: poly(ethylene oxide); PPO: poly(propylene oxide); oxALG: oxidized alginate; PEI: poly(ethylene imine); QCL: quaternized cellulose; CCNCs: cationic cellulose nanocrystals; CL: cellulose; ALG: alginate; oxDEX: oxidized dextran; SRC: sericin; DEX: dextran; MADEX: methacrylated DEX; MAHA: methacrylated HA; AGR: agarose; GG: gellan gum; HPMCL: hydroxypropyl methyl cellulose; NIPAAm: N-isopropyl acrylamide; Sn: Tin; MSNs: mesoporous silica nanoparticles; AuBNs: gold nanobipyramids; PLA: polylactide; PLGA: poly(lactide-co-glycolide); BPNs: black phosphorus nanosheets; PAMAM: polyamidoamine dendrimer; Bi NPs: bismuth nanoparticles; CuS NPs: copper sulphate nanoparticles; DEX: dextran; DEX-SH: dextran sulphate; MPEG: monomethoxy poly(ethylene glycol); DPPE: dipalmitoylphosphatidyle-thanoiamine; HRP: horseradish peroxidase; DOX: doxorubicin; GT: gelatin; PEG-PEGdA: polyethylene glycol (PEG)–diacrylate (PEGdA); AuP: gold(III) porphyrin; PVA: polyvinyl alcohol; CisP: cisplatin; SBP: sugar beet pectin; GM-CSF: granulocyte-macrophage colony-stimulating factor; IL-12: interleukin-12. | |||||||
| CS | TA-ZnPc | TA-ZnPc | Breast | MDA-MB-231 | — | The TA-ZnPc was directly released in a sustained manner for 8 days evading the circulation system in the tumour acidic environment. | 218 |
| CS/β-GP/HA | — | DOX | Cervix | HeLa | — | The hydrogel adhered to the tumor site promoting site specific release. | 219 |
| Reinforced mechanical strength helped in reduction of initial burst release from the hydrogel. | |||||||
| CS/β-GP | Liposome | DOX | Ovarian | A 2780 | — | A thermoresponsive hydrogel for localized therapy, activated by the external thermal stimuli which resulted in on-demand scheduled dosing of medicaments. | 220 |
| CS/β-GP/CNT | — | Methotrexate | Breast | MCF-7 | — | The hydrogel enhanced the anti-tumour effects of methotrexate by releasing the drug in a controlled manner. | 221 |
| CS/β-GP | Sn | DOX | Liver | N1-S1 | N1-S1 | The C/GP/Dox/Re-188-Tin colloids significantly inhibited tumors when compared with the control group post treatment. | 222 |
| CS–DA/oxPLN | — | DOX | Colon | HCT116 | — | The release profile of DOX was enhanced by hydrogels effectively killing colon tumor cells. | 223 |
| oxHA | — | Anti-2B11 | Breast | MDA-MB-231 | MDA-MB-231 | The hydrogel system induced cell apoptosis, and further reduced the invasive ability of cells by reducing the mitochondrial membrane potential of MCF-7 and MDA-MB-231 cells. | 224 |
| HA | MSNs | DOX | Breast | SKBR3 | — | The hydrogel nanocomposite was able to provide a microenvironment with rich anticancer drugs for a prolonged period. | 225 |
| HA-Tyr | — | Interferon-α | Liver | HAK-1B | HAK-1B | HA-Tyr hydrogels effectively inhibited tumor growth with lower cell density and proliferating cells, and with more apoptotic cells. | 226 |
| HA-αCD | AuBNs-MSNs | DOX | Squamous carcinoma | SCC | — | The hydrogel nanocomposite was able to provide a microenvironment with rich anticancer drugs for a prolonged period. | 227 |
| HA-Gln/PEG-8-SH-Lys | — | — | Breast | MCF7 | — | — | 228 |
| PF127/HA | PF127_PL121 | DOX | Colorectal | — | CT26 | Co-delivery enhanced the efficacy of tumor inhibition | 196 |
| oxALG-PEI | PLGA-PLA | Cisplatin and paclitaxel | Breast | MDA-MB-231 | — | Programmed cell death was promoted by significant accumulation of mitochondrial ROS upon treatment. | 229 |
| oxALG-PEI | PLGA-PLA | Cisplatin and paclitaxel | Liver | HepG2 | — | The premature release of drugs was prevented by the hydrogel which provided an additional diffusion barrier against Cis-DDP. | 230 |
| QCL-CCNCs | — | DOX | Murine Liver | — | H22 | The hydrogel acts as a depot system for anticancer therapy | 162 |
| CL | BPNs | — | Murine melanoma | B16 | — | The hydrogel exhibited excellent photothermal response and enhanced stability and flexibility by possessing strong cellulosic walls and 3D networks with irregular micrometer-sized pores. | 231 |
| ALG | PAMAM | Cisplatin | Breast | MFC7 | — | The hydrogel/DEP ts-mediated repeated photothermal therapy suppressed tumor growth efficiently. | 199 |
| oxDEX-SRC | — | HRP and DOX | Melanoma | B16F10 | The hydrogel achieved efficient drug loading and released the encapsulated drugs in a controlled manner suppressing tumour growth. | 80 | |
| oxDEX | PAMAM | Platinum | Breast | MDA-MB-231 | MDA-MB-231 | For repeated photothermal therapy, the hydrogel was able to remain in tumors for prolonged periods leading to complete tumor regression. | 174 |
| MADEX-SH/MAHA | BiNPs | DOX | Murine breast | 4T1 | 4T1 | The macroporous hydrogels aided in the sequential release of Bi NPs and DOX significantly exhibiting synergistic antitumor effects in vitro. | 175 |
| GG | Liposome | Paclitaxel | Bladder | T24 | — | The LP-Gel exhibited enhanced adhesion on the urothelium, and increased bladder wall penetration, along with appreciable cytotoxicity in rat and human bladder cancer cells. | 232 |
| GG | CuS NPs | DOX | Murine breast | 4T1 | 4T1 | — | 233 |
| AGR | DEX-SH | DOX | Breast | MDA-MB-231 | — | The entrapment efficiency of DOX was enhanced by the addition of divalent metal ions in the complex further prolonging the DOX release profile. | 234 |
| HPMCL/PF127/ALG | MPEG-DPPE | Paclitaxel and temozolomide | Murine glioma | C6 | — | The gel exhibited superior antitumor performance by inducing autophagy. | 165 |
| ALG-NIPAAm | — | DOX | Prostate | AT3B-1N | — | The hydrogels released the drug in sustained manner enhancing its cellular uptake in drug resistant AT3B-1N cells achieving better killing efficiency. | 200 |
| GT | PEG-PEGdA | AuP | Lung cancer | NCI-H460 | A549 | The AuP loaded formulation exhibited 60% tumour inhibition after 14 days. | 235 |
| GT | PVA | CisP | Ovarian cancer | A2780/CP70 | A2780/CP70 | The hydrogel containing low dose CisP (30 μg) is as effective as 150 μg of CisP administered intraperitoneally in inhibiting tumour growth. | 236 |
| GT | SBP | DOX | Melanoma | — | B16F1 | The therapeutic evaluation over 10 days indicated that Dox-loaded hydrogels successfully suppressed the increase in tumor size. | 237 |
| GT | — | IL-12 and GM-CSF | Lung carcinoma | HEK293 | C57BL/6 | Prolonged immune response with co-delivery of IL-12 and GM-CSF. | 238 |
5. Clinical and pre-clinical status of various natural cationic polymer-derived injectable hydrogels for targeted chemotherapy
Hydrogels have exhibited diversified applications in cancer therapy owing to their versatility and flexibility. While conventional hydrogels are not sensitive to environmental changes,239,240 smart hydrogels could be affected by pH, temperature, and photoelectricity225,241,242 resulting in temporal and spatial control over the releasing rate, thus improving the therapeutic index of commonly used chemotherapeutics.243 This section provides the various cationic polymer-based injectable hydrogels widely used in cancer therapy. Further, a list of clinical trials (Table 2) related to various hydrogel formulations gives an idea about the current research being conducted in this area.| Hydrogel characteristics | Drug | Pre-clinical/clinical studies | Major outcomes | Ref. | ||
|---|---|---|---|---|---|---|
| Component(s) | Stimuli responsiveness | In vitro | In vivo | |||
| Abbreviations: CS: chitosan; GB: glycerophosphate; ICG: indocyanine green; HCC: hepatocellular carcinoma; PLGA: polylactic-co-glycolic acid; PTX: paclitaxel; DOX: doxorubicin; PF127: pluronic F127; DOC: docetaxel; CS-DA: chitosan dihydrocaffeic acid; PPM: hyperbranched polyprodrug; DPC: DNA polyacrylamide conjugate; MMP: matrix metalloproteinase. | ||||||
| CS/GB | Thermosensitive | ICG | HCC | — | The hydrogel was feasible for drug delivery and fluorescence imaging. | 244 |
| PLGA | Thermosensitive | PTX | M234-p | Mammary tumour | The hydrogel exhibits a four fold increase in efficacy over existing marketed formulations. | 245 |
| CS/GB | Thermosensitive | DOX | H22 and SMMC7721 | Hepatoma | The thermosensitive hydrogel delivered DOX to the tumour site efficiently and constantly. | 246 |
| Hyaluronic acid (HA) and PF127 | Thermosensitive | DOX and DOC | CT26 | Colorectal carcinoma | The hydrogel was efficient in co-delivery of DOX and DOC further decreasing associated side effects and improving cancer management. | 196 |
| CS and GP | pH-sensitive | DOX | MCF-7 | Breast cancer | The hydrogel exhibited pH dependent drug release at pH 5.5 | 143 |
| CS-DA and oxidized pullulan | pH-sensitive | DOX and Amoxicillin | HCT-116 | Colon tumour | Ideal for management of mucosal localised tumour and infection | 223 |
| CS/HA/GP | pH-sensitive | DOX | HeLa | Cervical cancer | The hydrogel was successful in cervical cancer management. | 219 |
| PPM | Photosensitive | PPM | A549 | Lung cancer | The hydrogel was ideal for management of mucosal localised tumour and infection | 247 |
| DPC | Photosensitive | DOX and DNA | CEM | Lymphocytic leukaemia | The photosensitive hydrogel crosslinked with DNA helped in controlled release of DOX. | 248 |
| HA | Photosensitive | MMP | MDA-MB-231 | — | The HA hydrogel proved to be an ideal biomimetic cell culture model for breast cancer research. | 249 |
6. Conclusion and future perspectives
The peculiar characteristics of hydrogels make them efficient carriers for drug delivery. Elementary results of clinical studies suggest combining combinational therapies with the standard conventional therapies for cancer treatment. The mode of delivery used during combinational therapy can enhance treatment efficacy and influence disease progression due to prolonged drug release time.6 This review brings forward the various therapeutic applications of cationic polymer-mediated injectable hydrogels. Different preparative strategies for synthesizing cationic polymers with desired properties and transport mechanisms for effective and specific delivery were discussed. Certain limitations of various conventional cancer therapies, like immunosuppression, modulation of tumor microenvironment's expression of tumor antigens, etc., are still being researched, leading to surpassing these limitations.14 Studies have shown that chemotherapy has demonstrated disruption of the various suppressive pathways and lymphodepletion post administration of chemotherapy.15Furthermore, the development of biodegradable cationic polymers with reduced toxicity and massive growth in polymer science have led to numerous therapeutic applications. Continuous research in multi-disciplinary areas of cationic polymers has further elucidated their role in cellular processes and established a guide for different designs. The bottleneck for designing cationic polymers lies in surpassing the subcellular barrier, endosomal escape, and nuclear translocation. However, non-degradability and toxicity hindered the success of cationic polymers traditionally. Surface and structure modifications and novel carriers have been developed over the past few years to overcome these drawbacks. Incorporating more biodegradable cationic polysaccharides and natural cationic polymers may be widely used. The use of injectable hydrogels for anticancer therapy is widely recognized among researchers, but to effectively replace conventional therapies, continuous innovations and developments in the field of polymer science and injectables concerning structural aspects and design strategies are required. To effectively translate injectable hydrogels into clinical reality, future research should explore and emphasize combination therapy, utilizing chemotherapy, immunotherapy, and radiotherapy, by selecting suitable polymers tested both in vitro and in vivo, evaluating their cellular and molecular mechanisms.
Author contributions
SSD, MKA and KKK: conceptualization of the work. SSD, DS, BVKR, HS, and MKA: data collection and drafting. SSD and DS: made illustrations. JR, SSD, MD and KKK: finalized and reviewed the manuscript.Conflicts of interest
The authors declare no conflict of interest for this work.Acknowledgements
This research received no external funding.References
- C. Mattiuzzi and G. Lippi, J. Epidemiol. Global Health, 2019, 9, 217–222 CrossRef PubMed.
- J. Ferlay, M. Colombet, I. Soerjomataram, D. M. Parkin, M. Pineros, A. Znaor and F. Bray, Int. J. Cancer, 2021 DOI:10.1002/ijc.33588.
- S. S. Das, S. K. Singh, P. R. P. Verma, R. Gahtori, B. Z. Sibuh, K. K. Kesari, N. K. Jha, S. Dhanasekaran, V. K. Thakur, L. S. Wong, S. Djearamane and P. K. Gupta, Biomed. Pharmacother., 2022, 154, 113654 CrossRef PubMed.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, CA Cancer J. Clin., 2021, 71, 209–249 CrossRef PubMed.
- V. S. Sivasankarapillai, A. M. Pillai, A. Rahdar, A. P. Sobha, S. S. Das, A. C. Mitropoulos, M. H. Mokarrar and G. Z. Kyzas, Nanomaterials, 2020, 10, 852 CrossRef CAS PubMed.
- P. M. Arlen, R. A. Madan, J. W. Hodge, J. Schlom and J. L. Gulley, Update Cancer Ther., 2007, 2, 33–39 CrossRef PubMed.
- N. K. Jha, S. Arfin, S. K. Jha, R. Kar, A. Dey, R. Gundamaraju, G. M. Ashraf, P. K. Gupta, S. Dhanasekaran, M. M. Abomughaid, S. S. Das, S. K. Singh, K. Dua, S. Roychoudhury, D. Kumar, J. Ruokolainen, S. Ojha and K. K. Kesari, Semin. Cancer Biol., 2022, 86, 1086–1104 CrossRef CAS PubMed.
- K. Nurgali, R. T. Jagoe and R. Abalo, Front. Pharmacol., 2018, 9, 245 CrossRef PubMed.
- L. A. Diaz and L. C. Cantley, Cancer Discovery, 2023, 13, 797–798 CrossRef PubMed.
- D. Hanahan, Cancer Discovery, 2022, 12, 31–46 CrossRef CAS PubMed.
- J. J. Marin, M. R. Romero, A. G. Blazquez, E. Herraez, E. Keck and O. Briz, Anticancer Agents Med. Chem., 2009, 9, 162–184 CrossRef CAS PubMed.
- Harshita, M. A. Barkat, S. S. Das, F. H. Pottoo, S. Beg and Z. Rahman, Curr. Pharm. Des., 2020, 26, 1167–1180 CrossRef CAS PubMed.
- G. H. Nam, Y. Choi, G. B. Kim, S. Kim, S. A. Kim and I. S. Kim, Adv. Mater., 2020, 32, e2002440 CrossRef PubMed.
- Y. Yan, A. B. Kumar, H. Finnes, S. N. Markovic, S. Park, R. S. Dronca and H. Dong, Front. Immunol., 2018, 9, 1739 CrossRef PubMed.
- R. Ramakrishnan and D. I. Gabrilovich, Cancer Immunol. Immunother., 2013, 62, 405–410 CrossRef CAS PubMed.
- T. D. Johnson and K. L. Christman, Expert Opin. Drug Delivery, 2013, 10, 59–72 CrossRef CAS PubMed.
- M. Dhanka, V. Pawar, D. S. Chauhan, N. K. Jain, R. S. Prabhuraj, C. Shetty, M. K. Kumawat, R. Prasad and R. Srivastava, Colloids Surf., B, 2021, 201, 111597 CrossRef CAS PubMed.
- Z. Sun, C. Song, C. Wang, Y. Hu and J. Wu, Mol. Pharm., 2020, 17, 373–391 CAS.
- S. S. Das, P. Bharadwaj, M. Bilal, M. Barani, A. Rahdar, P. Taboada, S. Bungau and G. Z. Kyzas, Polymers, 2020, 12, 1397 CrossRef CAS PubMed.
- A. Sood, S. S. Das, A. Dev, D. Bhardwaj, A. Kumar, G. Agrawal and S. S. Han, Eur. Polym. J., 2023, 196, 112323 CrossRef CAS.
- J. Qu, X. Zhao, P. X. Ma and B. Guo, Acta Biomater., 2017, 58, 168–180 CrossRef CAS PubMed.
- V. S. Sivasankarapillai, S. S. Das, F. Sabir, M. A. Sundaramahalingam, J. C. Colmenares, S. Prasannakumar, M. Rajan, A. Rahdar and G. Z. Kyzas, Mater. Today Chem., 2021, 19, 100382 CrossRef CAS.
- M. Norouzi, B. Nazari and D. W. Miller, Drug Discovery Today, 2016, 21, 1835–1849 CrossRef CAS PubMed.
- S. Alfei, A. Zorzoli, D. Marimpietri, A. M. Schito and E. Russo, Pharmaceutics, 2022, 14, 2444 CrossRef CAS PubMed.
- D. J. Overstreet, D. Dutta, S. E. Stabenfeldt and B. L. Vernon, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 881–903 CrossRef CAS.
- A. P. Mathew, S. Uthaman, K. H. Cho, C. S. Cho and I. K. Park, Int. J. Biol. Macromol., 2018, 110, 17–29 CrossRef CAS PubMed.
- J. Conde, N. Oliva, Y. Zhang and N. Artzi, Nat. Mater., 2016, 15, 1128–1138 CrossRef CAS PubMed.
- N. Huebsch, C. J. Kearney, X. Zhao, J. Kim, C. A. Cezar, Z. Suo and D. J. Mooney, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 9762–9767 CrossRef CAS PubMed.
- Z. Lin, W. Gao, H. Hu, K. Ma, B. He, W. Dai, X. Wang, J. Wang, X. Zhang and Q. Zhang, J. Controlled Release, 2014, 174, 161–170 CrossRef CAS PubMed.
- J. Zhao, L. Wang, H. Zhang, B. Liao and Y. Li, Pharmaceutics, 2022, 14, 2028 CrossRef CAS PubMed.
- T. Thambi, Y. Li and D. S. Lee, J. Controlled Release, 2017, 267, 57–66 CrossRef CAS PubMed.
- L. Yu and J. Ding, Chem. Soc. Rev., 2008, 37, 1473–1481 RSC.
- P. Bertsch, M. Diba, D. J. Mooney and S. C. G. Leeuwenburgh, Chem. Rev., 2023, 123, 834–873 CrossRef CAS PubMed.
- J. M. Alonso, J. Andrade Del Olmo, R. Perez Gonzalez and V. Saez-Martinez, Polymers, 2021, 13, 650 CrossRef CAS PubMed.
- J. Su, J. Li, J. Liang, K. Zhang and J. Li, Life, 2021, 11, 1016 CrossRef CAS PubMed.
- L. Ye, Y. Zhang, Q. Wang, X. Zhou, B. Yang, F. Ji, D. Dong, L. Gao, Y. Cui and F. Yao, ACS Appl. Mater. Interfaces, 2016, 8, 15710–15723 CrossRef CAS PubMed.
- X. Li, X. Peng, R. Li, Y. Zhang, Z. Liu, Y. Huang, S. Long and H. Li, Macromol. Rapid Commun., 2020, 41, e2000202 CrossRef PubMed.
- A. Deng, Y. Yang, S. Du, X. Yang, S. Pang, X. Wang and S. Yang, Mater. Sci. Eng., C, 2021, 119, 111555 CrossRef CAS PubMed.
- X. Chen, B. Tan, S. Wang, R. Tang, Z. Bao, G. Chen, S. Chen, W. Tang, Z. Wang, C. Long, W. W. Lu, D. Yang, L. Bian and S. Peng, Biomaterials, 2021, 274, 120895 CrossRef CAS PubMed.
- M. Ozeki and Y. Tabata, J. Biomater. Sci., Polym. Ed., 2005, 16, 549–561 CrossRef CAS PubMed.
- W. Hu, Z. Wang, Y. Xiao, S. Zhang and J. Wang, Biomater. Sci., 2019, 7, 843–855 RSC.
- M. D. Konieczynska and M. W. Grinstaff, Acc. Chem. Res., 2017, 50, 151–160 CrossRef CAS PubMed.
- C. Echalier, L. Valot, J. Martinez, A. Mehdi and G. Subra, Mater. Today Commun., 2019, 20, 100536 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS PubMed.
- C. M. Nimmo, S. C. Owen and M. S. Shoichet, Biomacromolecules, 2011, 12, 824–830 CrossRef CAS PubMed.
- Y. Zhang, L. Tao, S. Li and Y. Wei, Biomacromolecules, 2011, 12, 2894–2901 CrossRef CAS PubMed.
- G. N. Grover, J. Lam, T. H. Nguyen, T. Segura and H. D. Maynard, Biomacromolecules, 2012, 13, 3013–3017 CrossRef CAS PubMed.
- B. D. Mather, K. Viswanathan, K. M. Miller and T. E. Long, Prog. Polym. Sci., 2006, 31, 487–531 CrossRef CAS.
- V. S. Raghuwanshi and G. Garnier, Adv. Colloid Interface Sci., 2019, 274, 102044 CrossRef CAS PubMed.
- S. H. Hsu, Y. L. Leu, J. W. Hu and J. Y. Fang, Chem. Pharm. Bull., 2009, 57, 453–458 CrossRef CAS PubMed.
- J. Skubiszewska-Zięba, S. Khalameida and V. Sydorchuk, Colloids Surf., A, 2016, 504, 139–153 CrossRef.
- J. Hurler, A. Engesland, B. Poorahmary Kermany and N. Škalko-Basnet, J. Appl. Polym. Sci., 2012, 125, 180–188 CrossRef CAS.
- J. M. Zuidema, C. J. Rivet, R. J. Gilbert and F. A. Morrison, J. Biomed. Mater. Res., Part B, 2014, 102, 1063–1073 CrossRef PubMed.
- H. Goodarzi, K. Jadidi, S. Pourmotabed, E. Sharifi and H. Aghamollaei, Int. J. Biol. Macromol., 2019, 126, 620–632 CrossRef CAS PubMed.
- L. W. Wong, P. Pasbakhsh, W. T. Cheng, C. B. S. Goh and J. B. L. Tan, Appl. Clay Sci., 2023, 232, 106812 CrossRef CAS.
- S. Kumar, M. Prasad and R. Rao, Mater. Sci. Eng., C, 2021, 119, 111605 CrossRef CAS PubMed.
- C. Fiorica, G. Pitarresi, F. S. Palumbo, N. Mauro, S. Federico and G. Giammona, Carbohydr. Polym., 2020, 236, 116033 CrossRef CAS PubMed.
- S. K. Samal, M. Dash, S. Van Vlierberghe, D. L. Kaplan, E. Chiellini, C. van Blitterswijk, L. Moroni and P. Dubruel, Chem. Soc. Rev., 2012, 41, 7147–7194 RSC.
- A. K. Bajpai, S. K. Shukla, S. Bhanu and S. Kankane, Prog. Polym. Sci., 2008, 33, 1088–1118 CrossRef CAS.
- S. Huang and X. Fu, J. Controlled Release, 2010, 142, 149–159 CrossRef CAS PubMed.
- N. Q. I. M. Noor, R. S. Razali, N. K. Ismail, R. A. Ramli, U. H. M. Razali, A. R. Bahauddin, N. Zaharudin, A. Rozzamri, J. Bakar and S. M. Shaarani, Processes, 2021, 9, 2227 CrossRef CAS.
- S. S. Das, S. Kar, S. K. Singh, P. R. P. Verma, A. Hussain and S. Beg, Chitosan in Drug Delivery, 2022, pp. 23–53 DOI:10.1016/b978-0-12-819336-5.00009-1.
- Z. Zhou, F. Jiang, T.-C. Lee and T. Yue, J. Alloys Compd., 2013, 581, 843–848 CrossRef CAS.
- N. P. Birch and J. D. Schiffman, Langmuir, 2014, 30, 3441–3447 CrossRef CAS PubMed.
- Y. Dong, W. K. Ng, S. Shen, S. Kim and R. B. Tan, Carbohydr. Polym., 2013, 94, 940–945 CrossRef CAS PubMed.
- S. Y. Ong, J. Wu, S. M. Moochhala, M. H. Tan and J. Lu, Biomaterials, 2008, 29, 4323–4332 CrossRef CAS PubMed.
- J. W. Lu, Y. Miao, C. X. Guo, Q. F. Ke, J. H. Yin, S. M. Zhou and Y. P. Guo, ACS Appl. Bio Mater., 2018, 1, 1468–1477 CrossRef CAS PubMed.
- A. J. Highton, T. Kojarunchitt, A. Girardin, S. Hook and R. A. Kemp, Immunol. Cell Biol., 2015, 93, 634–640 CrossRef CAS PubMed.
- W. F. Lai, A. L. Rogach and W. T. Wong, Chem. Soc. Rev., 2017, 46, 6379–6419 RSC.
- B. L. Peng, N. Dhar, H. L. Liu and K. C. Tam, Can. J. Chem. Eng., 2011, 89, 1191–1206 CrossRef CAS.
- N. Duran, A. P. Lemes and A. B. Seabra, Recent Pat. Nanotechnol., 2012, 6, 16–28 CrossRef CAS PubMed.
- S. M. Carvalho, A. A. P. Mansur, N. S. V. Capanema, I. C. Carvalho, P. Chagas, L. C. A. de Oliveira and H. S. Mansur, J. Mol. Liq., 2018, 266, 425–440 CrossRef CAS.
- C. Y. Li, W. Yuan, H. Jiang, J. S. Li, F. J. Xu, W. T. Yang and J. Ma, Bioconjugate Chem., 2011, 22, 1842–1851 CrossRef CAS PubMed.
- Y. W. Won, S. M. Yoon, C. H. Sonn, K. M. Lee and Y. H. Kim, ACS Nano, 2011, 5, 3839–3848 CrossRef CAS PubMed.
- G. K. Zorzi, L. Contreras-Ruiz, J. E. Parraga, A. Lopez-Garcia, R. R. Bello, Y. Diebold, B. Seijo and A. Sanchez, Mol. Pharm., 2011, 8, 1783–1788 CrossRef PubMed.
- J. Choubey and A. K. Bajpai, J. Mater. Sci.: Mater. Med., 2010, 21, 1573–1586 CrossRef CAS PubMed.
- S. Patra, P. Basak and D. N. Tibarewala, Mater. Sci. Eng., C, 2016, 59, 310–318 CrossRef CAS PubMed.
- C. Park, C. L. Vo, T. Kang, E. Oh and B. J. Lee, Eur. J. Pharm. Biopharm., 2015, 89, 365–373 CrossRef CAS PubMed.
- M. Alibolandi, M. Mohammadi, S. M. Taghdisi, M. Ramezani and K. Abnous, Carbohydr. Polym., 2017, 155, 218–229 CrossRef CAS PubMed.
- J. Liu, C. Qi, K. Tao, J. Zhang, J. Zhang, L. Xu, X. Jiang, Y. Zhang, L. Huang, Q. Li, H. Xie, J. Gao, X. Shuai, G. Wang, Z. Wang and L. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 6411–6422 CrossRef CAS PubMed.
- H. A. Barkat, S. S. Das, M. A. Barkat, S. Beg and H. A. Hadi, Future Oncol., 2020, 16, 2959–2979 CrossRef CAS PubMed.
- P. Bharadwaj, S. S. Das, S. Beg and M. Rahman, in Nanoformulation Strategies for Cancer Treatment, ed. S. Beg, M. Rahman, H. Choudhry, E. B. Souto and F. J. Ahmad, Elsevier, Amsterdam, Netherlands, 2021, ch. 16, pp. 277–289 DOI:10.1016/b978-0-12-821095-6.00020-3.
- H. A. Barkat, M. A. Barkat, M. Taleuzzaman, S. S. Das, M. Rizwanullah and H. A. Hadi, Handbook of Research on Advancements in Cancer Therapeutics, 2021, ch. 11, pp. 339–355 DOI:10.4018/978-1-7998-6530-8.ch011.
- P. Chytil, T. Etrych, C. Konak, M. Sirova, T. Mrkvan, B. Rihova and K. Ulbrich, J. Controlled Release, 2006, 115, 26–36 CrossRef CAS PubMed.
- A. Mitra, T. Coleman, M. Borgman, A. Nan, H. Ghandehari and B. R. Line, J. Controlled Release, 2006, 114, 175–183 CrossRef CAS PubMed.
- T. Wang, Y. Suita, S. Miriyala, J. Dean, N. Tapinos and J. Shen, Pharmaceutics, 2021, 13, 520 CrossRef CAS PubMed.
- D. S. Chen and I. Mellman, Immunity, 2013, 39, 1–10 CrossRef CAS PubMed.
- S. Liu, J. Li, L. Gu, K. Wu and H. Xing, Int. J. Nanomed., 2022, 17, 5209–5227 CrossRef CAS PubMed.
- Q. Jin, Z. Liu and Q. Chen, J. Controlled Release, 2021, 329, 882–893 CrossRef CAS PubMed.
- V. Schirrmacher, Int. J. Oncol., 2019, 54, 407–419 CrossRef CAS PubMed.
- P. Sharma and J. P. Allison, Science, 2015, 348, 56–61 CrossRef CAS PubMed.
- R. Kuai, W. Yuan, S. Son, J. Nam, Y. Xu, Y. Fan, A. Schwendeman and J. J. Moon, Sci. Adv., 2018, 4, eaao1736 CrossRef PubMed.
- V. A. Boussiotis, N. Engl. J. Med., 2016, 375, 1767–1778 CrossRef CAS PubMed.
- W. Zou, J. D. Wolchok and L. Chen, Sci. Transl. Med., 2016, 8, 328rv324 Search PubMed.
- J. R. Brahmer, S. S. Tykodi, L. Q. Chow, W. J. Hwu, S. L. Topalian, P. Hwu, C. G. Drake, L. H. Camacho, J. Kauh, K. Odunsi, H. C. Pitot, O. Hamid, S. Bhatia, R. Martins, K. Eaton, S. Chen, T. M. Salay, S. Alaparthy, J. F. Grosso, A. J. Korman, S. M. Parker, S. Agrawal, S. M. Goldberg, D. M. Pardoll, A. Gupta and J. M. Wigginton, N. Engl. J. Med., 2012, 366, 2455–2465 CrossRef CAS PubMed.
- O. Hamid, C. Robert, A. Daud, F. S. Hodi, W. J. Hwu, R. Kefford, J. D. Wolchok, P. Hersey, R. W. Joseph, J. S. Weber, R. Dronca, T. C. Gangadhar, A. Patnaik, H. Zarour, A. M. Joshua, K. Gergich, J. Elassaiss-Schaap, A. Algazi, C. Mateus, P. Boasberg, P. C. Tumeh, B. Chmielowski, S. W. Ebbinghaus, X. N. Li, S. P. Kang and A. Ribas, N. Engl. J. Med., 2013, 369, 134–144 CrossRef CAS PubMed.
- T. Powles, J. P. Eder, G. D. Fine, F. S. Braiteh, Y. Loriot, C. Cruz, J. Bellmunt, H. A. Burris, D. P. Petrylak, S. L. Teng, X. Shen, Z. Boyd, P. S. Hegde, D. S. Chen and N. J. Vogelzang, Nature, 2014, 515, 558–562 CrossRef CAS PubMed.
- C. Robert, G. V. Long, B. Brady, C. Dutriaux, M. Maio, L. Mortier, J. C. Hassel, P. Rutkowski, C. McNeil, E. Kalinka-Warzocha, K. J. Savage, M. M. Hernberg, C. Lebbe, J. Charles, C. Mihalcioiu, V. Chiarion-Sileni, C. Mauch, F. Cognetti, A. Arance, H. Schmidt, D. Schadendorf, H. Gogas, L. Lundgren-Eriksson, C. Horak, B. Sharkey, I. M. Waxman, V. Atkinson and P. A. Ascierto, N. Engl. J. Med., 2015, 372, 320–330 CrossRef CAS PubMed.
- C. Boutros, A. Tarhini, E. Routier, O. Lambotte, F. L. Ladurie, F. Carbonnel, H. Izzeddine, A. Marabelle, S. Champiat, A. Berdelou, E. Lanoy, M. Texier, C. Libenciuc, A. M. Eggermont, J. C. Soria, C. Mateus and C. Robert, Nat. Rev. Clin. Oncol., 2016, 13, 473–486 CrossRef CAS PubMed.
- D. B. Johnson, J. M. Balko, M. L. Compton, S. Chalkias, J. Gorham, Y. Xu, M. Hicks, I. Puzanov, M. R. Alexander, T. L. Bloomer, J. R. Becker, D. A. Slosky, E. J. Phillips, M. A. Pilkinton, L. Craig-Owens, N. Kola, G. Plautz, D. S. Reshef, J. S. Deutsch, R. P. Deering, B. A. Olenchock, A. H. Lichtman, D. M. Roden, C. E. Seidman, I. J. Koralnik, J. G. Seidman, R. D. Hoffman, J. M. Taube, L. A. Diaz Jr., R. A. Anders, J. A. Sosman and J. J. Moslehi, N. Engl. J. Med., 2016, 375, 1749–1755 CrossRef PubMed.
- L. Cassetta and J. W. Pollard, Nat. Rev. Drug Discovery, 2018, 17, 887–904 CrossRef CAS PubMed.
- M. Xu, I. Mizoguchi, N. Morishima, Y. Chiba, J. Mizuguchi and T. Yoshimoto, Clin. Dev. Immunol., 2010, 2010, 832454 Search PubMed.
- S. Musetti and L. Huang, ACS Nano, 2018, 12, 11740–11755 CrossRef CAS PubMed.
- D. A. Hume and K. P. MacDonald, Blood, 2012, 119, 1810–1820 CrossRef CAS PubMed.
- X. Huang, J. Wu, M. He, X. Hou, Y. Wang, X. Cai, H. Xin, F. Gao and Y. Chen, Mol. Pharmaceutics, 2019, 16, 2172–2183 CrossRef CAS PubMed.
- Z. Wang, X. Xue, Y. He, Z. Lu, B. Jia, H. Wu, Y. Yuan, Y. Huang, H. Wang, H. Lu, K. S. Lam, T.-Y. Lin and Y. Li, Adv. Funct. Mater., 2018, 28, 1802159 CrossRef PubMed.
- W. Qing, X. Xing, D. Feng, R. Chen and Z. Liu, Photodiagn. Photodyn. Ther., 2021, 36, 102521 CrossRef CAS PubMed.
- Y. Kong, Y. Dai, D. Qi, W. Du, H. Ni, F. Zhang, H. Zhao, Q. Shen, M. Li and Q. Fan, ACS Appl. Bio Mater., 2021, 4, 7595–7604 CrossRef CAS PubMed.
- F. J. P. Costa, M. Nave, R. Lima-Sousa, C. G. Alves, B. L. Melo, I. J. Correia and D. de Melo-Diogo, Int. J. Pharm., 2023, 635, 122713 CrossRef CAS PubMed.
- S. Huang, Z. Ma, C. Sun, Q. Zhou, Z. Li, S. Wang, Q. Yan, C. Liu, B. Hou and C. Zhang, J. Mater. Chem. B, 2022, 10, 2828–2843 RSC.
- D. Y. Fan, Y. Tian and Z. J. Liu, Front. Chem., 2019, 7, 675 CrossRef CAS PubMed.
- Q. Chen, Y. Li, S. Zhou, D. Chen, M. Zhou, Q. Chen, Y. Lu, N. Cai, C. Liu, Y. Guo, Z. Qiu, X. Hou, J. Tu, W. Shen and C. Sun, J. Controlled Release, 2022, 350, 803–814 CrossRef CAS PubMed.
- H. D. Han, C. K. Song, Y. S. Park, K. H. Noh, J. H. Kim, T. Hwang, T. W. Kim and B. C. Shin, Int. J. Pharm., 2008, 350, 27–34 CrossRef CAS PubMed.
- S. H. Seo, H. D. Han, K. H. Noh, T. W. Kim and S. W. Son, Clin. Exp. Metastasis, 2009, 26, 179–187 CrossRef CAS PubMed.
- J. Gu, G. Zhao, J. Yu, P. Xu, J. Yan, Z. Jin, S. Chen, Y. Wang, L. W. Zhang and Y. Wang, J Nanobiotechnol., 2022, 20, 372 CrossRef CAS PubMed.
- T. Wang, J. Zhang, T. Hou, X. Yin and N. Zhang, Nanoscale, 2019, 11, 13934–13946 RSC.
- Q. Lv, C. He, F. Quan, S. Yu and X. Chen, Bioact. Mater., 2018, 3, 118–128 Search PubMed.
- Z. Chen, Y. Rong, J. Ding, X. Cheng, X. Chen and C. He, Pharmaceutics, 2023, 15, 428 CrossRef CAS PubMed.
- V. Akbari, E. Hejazi, M. Minaiyan, J. Emami, A. Lavasanifar and M. Rezazadeh, J. Biomater. Appl., 2022, 37, 551–562 CrossRef CAS PubMed.
- H. Jin, C. Wan, Z. Zou, G. Zhao, L. Zhang, Y. Geng, T. Chen, A. Huang, F. Jiang, J. P. Feng, J. F. Lovell, J. Chen, G. Wu and K. Yang, ACS Nano, 2018, 12, 3295–3310 CrossRef CAS PubMed.
- D. D. Guo, S. H. Hong, H. L. Jiang, J. H. Kim, A. Minai-Tehrani, J. E. Kim, J. Y. Shin, T. Jiang, Y. K. Kim, Y. J. Choi, C. S. Cho and M. H. Cho, Biomaterials, 2012, 33, 2272–2281 CrossRef CAS PubMed.
- Y. Brudno, E. A. Silva, C. J. Kearney, S. A. Lewin, A. Miller, K. D. Martinick, M. Aizenberg and D. J. Mooney, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 12722–12727 CrossRef CAS PubMed.
- J. Conde, N. Oliva, M. Atilano, H. S. Song and N. Artzi, Nat. Mater., 2016, 15, 353–363 CrossRef CAS PubMed.
- R. Liu, O. V. Khullar, A. P. Griset, J. E. Wade, K. A. V. Zubris, M. W. Grinstaff and Y. L. Colson, Ann. Thorac. Surg., 2011, 91, 1077–1084 CrossRef PubMed.
- J. W. Nichols and Y. H. Bae, J. Controlled Release, 2014, 190, 451–464 CrossRef CAS PubMed.
- D. Simberg, ACS Nano, 2015, 9, 8647–8650 CrossRef CAS PubMed.
- C. von Roemeling, W. Jiang, C. K. Chan, I. L. Weissman and B. Y. S. Kim, Trends Biotechnol., 2017, 35, 159–171 CrossRef CAS PubMed.
- J. Wan, S. Geng, H. Zhao, X. Peng, Q. Zhou, H. Li, M. He, Y. Zhao, X. Yang and H. Xu, J. Controlled Release, 2016, 235, 328–336 CrossRef CAS PubMed.
- K. Nguyen, P. N. Dang and E. Alsberg, Acta Biomater., 2013, 9, 4487–4495 CrossRef CAS PubMed.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nat. Rev. Mater., 2016, 1, 16014 CrossRef CAS.
- M. Kulkarni, U. Greiser, T. O'Brien and A. Pandit, Trends Biotechnol., 2010, 28, 28–36 CrossRef CAS PubMed.
- S. A. Bencherif, R. Warren Sands, O. A. Ali, W. A. Li, S. A. Lewin, T. M. Braschler, T. Y. Shih, C. S. Verbeke, D. Bhatta, G. Dranoff and D. J. Mooney, Nat. Commun., 2015, 6, 7556 CrossRef CAS PubMed.
- G. R. Shin, H. E. Kim, J. H. Kim, S. Choi and M. S. Kim, Pharmaceutics, 2021, 13, 1953 CrossRef CAS PubMed.
- W. Wang, H. Song, J. Zhang, P. Li, C. Li, C. Wang, D. Kong and Q. Zhao, J. Controlled Release, 2015, 203, 57–66 CrossRef CAS PubMed.
- X. Dai and C. Tan, Adv. Drug Delivery Rev., 2015, 81, 184–197 CrossRef CAS PubMed.
- H. Hu, Z. Lin, B. He, W. Dai, X. Wang, J. Wang, X. Zhang, H. Zhang and Q. Zhang, J. Controlled Release, 2015, 220, 189–200 CrossRef CAS PubMed.
- L. E. Strong, S. N. Dahotre and J. L. West, J. Controlled Release, 2014, 178, 63–68 CrossRef CAS PubMed.
- P. Huang, Y. Zhang, W. Wang, J. Zhou, Y. Sun, J. Liu, D. Kong, J. Liu and A. Dong, J. Controlled Release, 2015, 220, 456–464 CrossRef CAS PubMed.
- A. Gilam, J. Conde, D. Weissglas-Volkov, N. Oliva, E. Friedman, N. Artzi and N. Shomron, Nat. Commun., 2016, 7, 12868 CrossRef PubMed.
- M. Hamidi, A. Azadi and P. Rafiei, Adv. Drug Delivery Rev., 2008, 60, 1638–1649 CrossRef CAS PubMed.
- I. Younes and M. Rinaudo, Mar. Drugs, 2015, 13, 1133–1174 CrossRef CAS PubMed.
- H. Ding, C. J. Zhao, X. Cui, Y. F. Gu, W. T. Jia, M. N. Rahaman, Y. Wang, W. H. Huang and C. Q. Zhang, PLoS One, 2014, 9, e85472 CrossRef PubMed.
- M. Fathi, M. Alami-Milani, M. H. Geranmayeh, J. Barar, H. Erfan-Niya and Y. Omidi, Int. J. Biol. Macromol., 2019, 128, 957–964 CrossRef CAS PubMed.
- A. Ahsan, M. A. Farooq and A. Parveen, ACS Omega, 2020, 5, 20450–20460 CrossRef CAS PubMed.
- X. Han, X. Meng, Z. Wu, Z. Wu and X. Qi, Mater. Sci. Eng.: C, 2018, 93, 1064–1072 CrossRef CAS PubMed.
- A. A. H. Abdellatif, A. M. Mohammed, I. Saleem, M. Alsharidah, O. Al Rugaie, F. Ahmed and S. K. Osman, Pharmaceutics, 2022, 14, 661 CrossRef CAS PubMed.
- X. Wu, C. He, Y. Wu, X. Chen and J. Cheng, Adv. Funct. Mater., 2015, 25, 6744–6755 CrossRef CAS.
- H. Wang, F. Song, Q. Chen, R. Hu, Z. Jiang, Y. Yang and B. Han, J. Biomed. Mater. Res., Part A, 2015, 103, 3879–3885 CrossRef CAS PubMed.
- S. Belali, A. R. Karimi and M. Hadizadeh, Int. J. Biol. Macromol., 2018, 110, 437–448 CrossRef CAS PubMed.
- C.-H. Chen, C.-Y. Kuo, S.-H. Chen, S.-H. Mao, C.-Y. Chang, K. Shalumon and J.-P. Chen, Int. J. Mol. Sci., 2018, 19, 1373 CrossRef PubMed.
- D. Prochowicz, A. Kornowicz and J. Lewinski, Chem. Rev., 2017, 117, 13461–13501 CrossRef CAS PubMed.
- I. V. Kolesnichenko and E. V. Anslyn, Chem. Soc. Rev., 2017, 46, 2385–2390 RSC.
- B.-w Liu, H. Zhou, S.-t Zhou and J.-y Yuan, Eur. Polym. J., 2015, 65, 63–81 CrossRef CAS.
- B. Schmidt and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2017, 56, 8350–8369 CrossRef CAS PubMed.
- H. Kuang, H. He, Z. Zhang, Y. Qi, Z. Xie, X. Jing and Y. Huang, J. Mater. Chem. B, 2014, 2, 659–667 RSC.
- C. Fiorica, F. S. Palumbo, G. Pitarresi, R. Puleio, L. Condorelli, G. Collura and G. Giammona, Int. J. Pharm., 2020, 589, 119879 CrossRef CAS PubMed.
- C. Fu, X. Lin, J. Wang, X. Zheng, X. Li, Z. Lin and G. Lin, J. Mater. Sci.: Mater. Med., 2016, 27, 73 CrossRef PubMed.
- M. Jorfi and E. J. Foster, J. Appl. Polym. Sci., 2015, 132, 41719 CrossRef.
- B. Medronho, A. Romano, M. G. Miguel, L. Stigsson and B. Lindman, Cellulose, 2012, 19, 581–587 CrossRef CAS.
- F. Joubert, O. M. Musa, D. R. Hodgson and N. R. Cameron, Chem. Soc. Rev., 2014, 43, 7217–7235 RSC.
- F. Andrade, M. M. Roca-Melendres, M. Llaguno, D. Hide, I. Raurell, M. Martell, S. Vijayakumar, M. Oliva, S. Schwartz Jr., E. F. Duran-Lara, D. Rafael and I. Abasolo, Carbohydr. Polym., 2022, 295, 119859 CrossRef CAS PubMed.
- J. You, J. Cao, Y. Zhao, L. Zhang, J. Zhou and Y. Chen, Biomacromolecules, 2016, 17, 2839–2848 CrossRef CAS PubMed.
- J. K. Kim, Y. W. Won, K. S. Lim, E. J. Park and Y. H. Kim, J. Controlled Release, 2011, 152(Suppl 1), e44–e45 CrossRef CAS PubMed.
- L. Weng, N. Rostambeigi, N. D. Zantek, P. Rostamzadeh, M. Bravo, J. Carey and J. Golzarian, Acta Biomater., 2013, 9, 8182–8191 CrossRef CAS PubMed.
- L. Ding, Q. Wang, M. Shen, Y. Sun, X. Zhang, C. Huang, J. Chen, R. Li and Y. Duan, Autophagy, 2017, 13, 1176–1190 CrossRef CAS PubMed.
- Y. Sheng, J. Gao, Z. Z. Yin, J. Kang and Y. Kong, Carbohydr. Polym., 2021, 269, 118325 CrossRef CAS PubMed.
- L. R. Balahura, S. Dinescu, M. Balas, A. Cernencu, A. Lungu, G. M. Vlasceanu, H. Iovu and M. Costache, Pharmaceutics, 2021, 13, 1189 CrossRef CAS PubMed.
- S. R. Bollareddy, V. Krishna, G. Roy, D. Dasari, A. Dhar and V. V. K. Venuganti, AAPS PharmSciTech, 2022, 23, 70 CrossRef CAS PubMed.
- X. Jiang, F. Zeng, X. Yang, C. Jian, L. Zhang, A. Yu and A. Lu, Acta Biomater., 2022, 141, 102–113 CrossRef CAS PubMed.
- N. S. V. Capanema, A. A. P. Mansur, S. M. Carvalho, I. C. Carvalho, P. Chagas, L. C. A. de Oliveira and H. S. Mansur, Carbohydr. Polym., 2018, 195, 401–412 CrossRef CAS PubMed.
- O. Vittorio, G. Cirillo, F. Iemma, G. Di Turi, E. Jacchetti, M. Curcio, S. Barbuti, N. Funel, O. I. Parisi, F. Puoci and N. Picci, Pharm. Res., 2012, 29, 2601–2614 CrossRef CAS PubMed.
- O. Vittorio, M. Brandl, G. Cirillo, K. Kimpton, E. Hinde, K. Gaus, E. Yee, N. Kumar, H. Duong, C. Fleming, M. Haber, M. Norris, C. Boyer and M. Kavallaris, Oncotarget, 2016, 7, 47479–47493 CrossRef PubMed.
- A. Agarwal, U. Gupta, A. Asthana and N. K. Jain, Biomaterials, 2009, 30, 3588–3596 CrossRef CAS PubMed.
- L. Li, C. Wang, Q. Huang, J. Xiao, Q. Zhang and Y. Cheng, J. Mater. Chem. B, 2018, 6, 2474–2480 RSC.
- J. Deng, X. Xun, W. Zheng, Y. Su, L. Zheng, C. Wang and M. Su, J. Mater. Chem. B, 2018, 6, 7966–7973 RSC.
- F. Q. Luo, W. Xu, J. Y. Zhang, R. Liu, Y. C. Huang, C. Xiao and J. Z. Du, Acta Biomater., 2022, 147, 235–244 CrossRef CAS PubMed.
- S. Huang, H. Liu, S. Huang, T. Fu, W. Xue and R. Guo, Carbohydr. Polym., 2020, 246, 116650 CrossRef CAS PubMed.
- R. J. van Es, J. F. Nijsen, H. F. Dullens, M. Kicken, A. van der Bilt, W. Hennink, R. Koole and P. J. Slootweg, J. Cranio-Maxillofac. Surg., 2001, 29, 289–297 CrossRef CAS PubMed.
- S. O. Solomevich, P. M. Bychkovsky, T. L. Yurkshtovich, N. V. Golub, P. Y. Mirchuk, M. Y. Revtovich and A. I. Shmak, Carbohydr. Polym., 2019, 226, 115308 CrossRef CAS PubMed.
- M. R. Saboktakin, R. M. Tabatabaie, P. Ostovarazar, A. Maharramov and M. A. Ramazanov, Int. J. Biol. Macromol., 2012, 51, 544–549 CrossRef CAS PubMed.
- B. Balakrishnan and A. Jayakrishnan, Biomaterials, 2005, 26, 3941–3951 CrossRef CAS PubMed.
- Y. Ikada and Y. Tabata, Adv. Drug Delivery Rev., 1998, 31, 287–301 CrossRef PubMed.
- K. Kawai, S. Suzuki, Y. Tabata, Y. Ikada and Y. Nishimura, Biomaterials, 2000, 21, 489–499 CrossRef CAS PubMed.
- M. Yamamoto, Y. Ikada and Y. Tabata, J. Biomater. Sci., Polym. Ed., 2001, 12, 77–88 CrossRef CAS PubMed.
- A. Gaowa, T. Horibe, M. Kohno, K. Sato, H. Harada, M. Hiraoka, Y. Tabata and K. Kawakami, J. Controlled Release, 2014, 176, 1–7 CrossRef CAS PubMed.
- D. Ding, Z. Zhu, R. Li, X. Li, W. Wu, X. Jiang and B. Liu, ACS Nano, 2011, 5, 2520–2534 CrossRef CAS PubMed.
- P. Li, W. Chen, Y. Yan, B. Chen, Y. Wang and X. Huang, ACS Appl. Bio Mater., 2019, 2, 3722–3729 CrossRef CAS PubMed.
- K. Yamashita, S. Tsunoda, S. Gunji, T. Murakami, T. Suzuki, Y. Tabata and Y. Sakai, Surg. Today, 2019, 49, 785–794 CrossRef CAS PubMed.
- L. Liu, T. Sakaguchi, T. Kanda, J. Hitomi, Y. Tabata and K. Hatakeyama, Cancer Chemother. Pharmacol., 2003, 51, 53–57 CrossRef CAS PubMed.
- J. A. Burdick, Biomed. Mater., 2012, 7, 020201 CrossRef PubMed.
- J. A. Burdick and G. D. Prestwich, Adv. Mater., 2011, 23, H41–H56 CrossRef CAS PubMed.
- S. Mitragotri, P. A. Burke and R. Langer, Nat. Rev. Drug Discovery, 2014, 13, 655–672 CrossRef CAS.
- D. Seliktar, Science, 2012, 336, 1124–1128 CrossRef CAS PubMed.
- Y. Y. Chen, H. C. Wu, J. S. Sun, G. C. Dong and T. W. Wang, Langmuir, 2013, 29, 3721–3729 CrossRef CAS PubMed.
- H. J. Jhan, J. J. Liu, Y. C. Chen, D. Z. Liu, M. T. Sheu and H. O. Ho, Nanomedicine, 2015, 10, 1263–1274 CrossRef CAS PubMed.
- M. T. Sheu, H. J. Jhan, C. Y. Su, L. C. Chen, C. E. Chang, D. Z. Liu and H. O. Ho, Colloids Surf., B, 2016, 143, 260–270 CrossRef CAS PubMed.
- J. Wroblewska-Krepsztul, T. Rydzkowski, I. Michalska-Pozoga and V. K. Thakur, Nanomaterials, 2019, 9, 404 CrossRef CAS PubMed.
- J.-S. Yang, Y.-J. Xie and W. He, Carbohydr. Polym., 2011, 84, 33–39 CrossRef CAS.
- C. Wang, X. Wang, K. Dong, J. Luo, Q. Zhang and Y. Cheng, Biomaterials, 2016, 104, 129–137 CrossRef CAS PubMed.
- M. Liu, X. Song, Y. Wen, J. L. Zhu and J. Li, ACS Appl. Mater. Interfaces, 2017, 9, 35673–35682 CrossRef CAS PubMed.
- M. J. Chalanqui, S. Pentlavalli, C. McCrudden, P. Chambers, M. Ziminska, N. Dunne and H. O. McCarthy, Mater. Sci. Eng., C, 2019, 95, 409–421 CrossRef CAS.
- B. Yavari, R. Mahjub, M. Saidijam, M. Raigani and M. Soleimani, Curr. Protein Pept. Sci., 2018, 19, 759–770 CrossRef CAS PubMed.
- Y. Song, Y. Ding and C. M. Dong, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2022, 14, e1742 CAS.
- Y. Wan, W. Dai, R. J. Nevagi, I. Toth and P. M. Moyle, Acta Biomater., 2017, 59, 257–268 CrossRef CAS PubMed.
- S. Dissanayake, W. A. Denny, S. Gamage and V. Sarojini, J. Controlled Release, 2017, 250, 62–76 CrossRef CAS PubMed.
- T. Samec, J. Boulos, S. Gilmore, A. Hazelton and A. Alexander-Bryant, Mater. Today Bio, 2022, 14, 100248 CrossRef CAS PubMed.
- J. Xu, G. Qi, W. Wang and X. S. Sun, npj Sci. Food, 2021, 5, 14 CrossRef PubMed.
- X. Ren, N. Wang, Y. Zhou, A. Song, G. Jin, Z. Li and Y. Luan, Acta Biomater., 2021, 124, 179–190 CrossRef CAS PubMed.
- R. Jin, J. Yang, D. Zhao, X. Hou, C. Li, W. Chen, Y. Zhao, Z. Yin and B. Liu, J. Nanobiotechnol., 2019, 17, 99 CrossRef PubMed.
- M. C. Garrett, T. M. O'Shea, A. L. Wollenberg, A. M. Bernstein, D. Hung, B. Staarman, H. Soto, T. J. Deming, M. V. Sofroniew and H. I. Kornblum, PLoS One, 2020, 15, e0219632 CrossRef CAS PubMed.
- M. Liu, Z. Cao, R. Zhang, Y. Chen and X. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 33874–33884 CrossRef CAS PubMed.
- H. Song, P. Huang, J. Niu, G. Shi, C. Zhang, D. Kong and W. Wang, Biomaterials, 2018, 159, 119–129 CrossRef CAS PubMed.
- X. L. Hou, X. Dai, J. Yang, B. Zhang, D. H. Zhao, C. Q. Li, Z. Y. Yin, Y. D. Zhao and B. Liu, J. Mater. Chem. B, 2020, 8, 8623–8633 RSC.
- R. Jin, X. Yang, D. Zhao, X. Hou, C. Li, X. Song, W. Chen, Q. Wang, Y. Zhao and B. Liu, Nanoscale, 2019, 11, 16080–16091 RSC.
- J. Liu, Y. Zhang, Q. Li, Z. Feng, P. Huang, W. Wang and J. Liu, Acta Biomater., 2020, 114, 133–145 CrossRef CAS PubMed.
- Y. Shi, D. Li, C. He and X. Chen, Macromol. Biosci., 2021, 21, e2100049 CrossRef PubMed.
- R. Jin, J. Yang, P. Ding, C. Li, B. Zhang, W. Chen, Y. D. Zhao, Y. Cao and B. Liu, Nanotechnology, 2020, 31, 205102 CrossRef CAS PubMed.
- A. R. Karimi, A. Khodadadi and M. Hadizadeh, RSC Adv., 2016, 6, 91445–91452 RSC.
- W. Zhang, X. Jin, H. Li, R. R. Zhang and C. W. Wu, Carbohydr. Polym., 2018, 186, 82–90 CrossRef CAS PubMed.
- A. Lopez-Noriega, C. L. Hastings, B. Ozbakir, K. E. O'Donnell, F. J. O'Brien, G. Storm, W. E. Hennink, G. P. Duffy and E. Ruiz-Hernandez, Adv. Healthcare Mater., 2014, 3, 854–859 CrossRef CAS PubMed.
- L. Saeednia, L. Yao, K. Cluff and R. Asmatulu, ACS Omega, 2019, 4, 4040–4048 CrossRef CAS PubMed.
- F.-Y. J. Huang, G.-Y. Gan, W.-Y. Lin, L.-K. Huang, T.-Y. Luo, J.-J. Hong and B.-T. Hsieh, J. Radioanal. Nucl. Chem., 2013, 299, 31–40 CrossRef.
- Y. Liang, X. Zhao, P. X. Ma, B. Guo, Y. Du and X. Han, J. Colloid Interface Sci., 2019, 536, 224–234 CrossRef CAS PubMed.
- Y. Zhao, H. Yan, S. Qiao, L. Zhang, T. Wang, Q. Meng, X. Chen, F. H. Lin, K. Guo, C. Li and W. Tian, J. Mater. Chem. B, 2016, 4, 6183–6191 RSC.
- X. Chen and Z. Liu, Macromol. Rapid Commun., 2016, 37, 1533–1539 CrossRef CAS PubMed.
- K. Xu, F. Lee, S. J. Gao, J. E. Chung, H. Yano and M. Kurisawa, J. Controlled Release, 2013, 166, 203–210 CrossRef CAS PubMed.
- X. Chen, Z. Liu, S. G. Parker, X. Zhang, J. J. Gooding, Y. Ru, Y. Liu and Y. Zhou, ACS Appl. Mater. Interfaces, 2016, 8, 15857–15863 CrossRef CAS PubMed.
- A. Ranga, M. P. Lutolf, J. Hilborn and D. A. Ossipov, Biomacromolecules, 2016, 17, 1553–1560 CrossRef CAS PubMed.
- P. Davoodi, W. C. Ng, M. P. Srinivasan and C. H. Wang, Biotechnol. Bioeng., 2017, 114, 2931–2946 CrossRef CAS PubMed.
- P. Davoodi, W. C. Ng, W. C. Yan, M. P. Srinivasan and C. H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 22785–22800 CrossRef CAS PubMed.
- C. Xing, S. Chen, M. Qiu, X. Liang, Q. Liu, Q. Zou, Z. Li, Z. Xie, D. Wang, B. Dong, L. Liu, D. Fan and H. Zhang, Adv. Healthcare Mater., 2018, 7, e1701510 CrossRef PubMed.
- S. GuhaSarkar, P. More and R. Banerjee, J. Controlled Release, 2017, 245, 147–156 CrossRef CAS PubMed.
- Y. Zheng, Y. Liang, D. Zhang, Z. Zhou, J. Li, X. Sun and Y. N. Liu, Chem. Commun., 2018, 54, 13805–13808 RSC.
- X. Niu, Z. Zhang and Y. Zhong, Mater. Sci. Eng., C, 2017, 77, 888–894 CrossRef CAS PubMed.
- P. Lee, C. N. Lok, C. M. Che and W. J. Kao, Pharm. Res., 2019, 36, 61 CrossRef PubMed.
- R. Oun, J. A. Plumb and N. J. Wheate, J. Inorg. Biochem., 2014, 134, 100–105 CrossRef CAS PubMed.
- T. Takei, K. Sugihara, M. Yoshida and K. Kawakami, J. Biomater. Sci., Polym. Ed., 2013, 24, 1333–1342 CrossRef CAS PubMed.
- E. Oh, J. E. Oh, J. Hong, Y. Chung, Y. Lee, K. D. Park, S. Kim and C. O. Yun, J. Controlled Release, 2017, 259, 115–127 CrossRef CAS PubMed.
- V. Castelletto, C. J. C. Edwards-Gayle, F. Greco, I. W. Hamley, J. Seitsonen and J. Ruokolainen, ACS Appl. Mater. Interfaces, 2019, 11, 33573–33580 CrossRef CAS PubMed.
- T. Kerdsirichairat, A. K. Narang, E. Thompson, S. H. Kim, A. Rao, K. Ding and E. J. Shin, Gastroenterology, 2019, 157, 933–935 CrossRef PubMed.
- G. Milcovich, S. Lettieri, F. E. Antunes, B. Medronho, A. C. Fonseca, J. F. J. Coelho, P. Marizza, F. Perrone, R. Farra, B. Dapas, G. Grassi, M. Grassi and S. Giordani, Adv. Colloid Interface Sci., 2017, 249, 163–180 CrossRef CAS PubMed.
- A. Sood, A. Dev, S. S. Das, H. J. Kim, A. Kumar, V. K. Thakur and S. S. Han, Int. J. Biol. Macromol., 2023, 232, 123283 CrossRef CAS PubMed.
- Y. Tu, N. Chen, C. Li, H. Liu, R. Zhu, S. Chen, Q. Xiao, J. Liu, S. Ramakrishna and L. He, Acta Biomater., 2019, 90, 1–20 CrossRef CAS PubMed.
- A. Salis, G. Rassu, M. Budai-Szucs, I. Benzoni, E. Csanyi, S. Berko, M. Maestri, P. Dionigi, E. P. Porcu, E. Gavini and P. Giunchedi, Expert Opin. Drug Delivery, 2015, 12, 1583–1596 CrossRef CAS PubMed.
- J. I. Pesoa, M. J. Rico, V. R. Rozados, O. G. Scharovsky, J. A. Luna and L. N. Mengatto, J. Pharm. Pharmacol., 2018, 70, 1494–1502 CrossRef CAS PubMed.
- S. Ren, Y. Dai, C. Li, Z. Qiu, X. Wang, F. Tian, S. Zhou, Q. Liu, H. Xing, Y. Lu, X. Chen and N. Li, Eur. J. Pharm. Sci., 2016, 92, 137–145 CrossRef CAS PubMed.
- D. Guo, S. Xu, Y. Huang, H. Jiang, W. Yasen, N. Wang, Y. Su, J. Qian, J. Li, C. Zhang and X. Zhu, Biomaterials, 2018, 177, 67–77 CrossRef CAS PubMed.
- H. Kang, H. Liu, X. Zhang, J. Yan, Z. Zhu, L. Peng, H. Yang, Y. Kim and W. Tan, Langmuir, 2011, 27, 399–408 CrossRef CAS PubMed.
- R. Y. Tam, L. J. Smith and M. S. Shoichet, Acc. Chem. Res., 2017, 50, 703–713 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |