 Open Access Article
Open Access ArticleLayered double hydroxides: where should research stress on for massive scaling up?
Claire
Dazon
 *a,
Christine
Taviot-Guého
*a,
Christine
Taviot-Guého
 b and
Vanessa
Prévot
b
b and
Vanessa
Prévot
b
aESPCI – PSL – Paris – Institute of Porous Materials, 10 rue Vauquelin, 75005 Paris, France. E-mail: dazonclaire@gmail.com
bClermont-Ferrand Chemical Institute, Campus des Cézeaux 24, avenue Blaise Pascal TSA 6002663178, Aubière, France
First published on 5th October 2023
Abstract
For more than half a century, layered double hydroxides (LDHs) have attracted great attention from the scientific community. These materials belong to a rare anionic clay group and have demonstrated amazing physicochemical properties, leading to disruptive, cost-effective and eco-friendly applications for health, environment and agriculture. However, LDHs are not widely used in daily items, nor are they massively applied in industry despite their very good potential for large-scale development. This study looks at the current shortcomings preventing the industrial implementation of LDH powders while evidencing and discussing the strategies to encourage research to bridge the gaps on this topic.
Introduction
Health, environment, and agriculture are essential areas that have been made significantly fragile by human activities. The recent COVID-19 pandemic and global warming are signs of a pessimistic outlook for the future. As mentioned in the latest NIC report about the global trends in 2040, “avoidance” strategies addressing the aforementioned domain issues are not the best ones, and our societies should turn to “adapting” behaviours to fight against numerous troubles.1 In this view, there is good news to highlight; in particular, materials science could be a great resource to bring smart, efficient, and maybe transcendent solutions, provided the applications have available raw materials, eco-friendly design criteria, and are cost-effective. From this perspective, layered double hydroxides (LDHs) are undoubtedly materials of choice for development and expansion.LDH particles belong to a natural and rare anionic clay group, but several synthesis methods are relatively well controlled today to compensate for this natural scarcity and allow vast possibilities of chemical compositions and textural properties for numerous applications in strategic fields.2–4 LDHs have great potential for use as drug delivery systems, controlled release pesticides for agriculture, adsorbents of heavy metals and pollutants for water decontamination, and promising applications in the controlled release of fertilizers, CO2 capture, CO2 conversion and green electrochemical energy storage devices are being seriously considered.3–9 Notably, LDHs display a 2D structure, allowing them to be insertion systems for active molecules, pollutants, and so forth, promising numerous smart host–guest materials. For each of these fields, considerable progress has been made recently as highlighted by Kameliya et al.10 with eco-friendly LDH synthesized today for application as catalysts, and in photoelectrocatalysis with amazing results11 with a strong proof of concept for water decontamination,12,13 and the sensor-biosensor perspectives are also stemming from these long years of research. Another field where LDHs can be the original material for good solutions is in corrosion protection.14 Co-precipitation and mechanosynthesis are well-known LDH synthesis methods that can produce several kg of LDH powder using available precursors.15–18 We did not notice a massive spread of LDH in applications and at the industrial scale in the aforementioned sectors, even if some reduced-scale studies have emerged, especially in the environmental field19,20 and for new buildings in the case of concretes reinforced with LDH particles.21 Since LDHs encompass the required criteria for sustainable applicative development and industrial scale production (the availability of the precursors and their relative competitive prices, cost-effective production through proven methods, and a non-toxic nature for numerous chemical compositions), one can wonder finally, why these particles remain insufficiently applied, despite a significant number of patents (226 patents registered at the beginning of 2022 since the 1960s22). Looking closely, most of them indicate numerous processes to scale up LDH (nano)composites, with these being less versatile for the applications than the powder form, and hardly 16% of them directly address the raw LDH powder synthesis. None of these patents mention the use of LDH in their dry form (supposing the powder) for further implementation. Nonetheless, the LDH powders could provide great opportunities for disruptive applications, using them as produced, such as aerosol therapy for drug delivery, fillers for innovating textiles or material buildings, and fertilizers for smart agriculture, to quote a few. The LDH powders are practical for packaging and transportation, weighing, or handling at the industrial scale, and can no doubt pop up, concerning the highly pragmatic features they offer. So, what are the shortcomings that prevent their large-scale application? Where should research make efforts to overcome them? Given the urgency of issues our societies should solve in health, the environment, agriculture, or energy areas, maybe it is time to boost the scalability of LDH powder. This article compares co-precipitation and mechanosynthesis for the massive production of raw LDH powder and explores the possibilities that could be proposed to unlock the agglomeration/aggregation issues of LDH particles as synthesized, which we have pointed out as an important one hindering their further scale-up in items or industry. The ideas we propose cover the raw LDH, with their (nano)composites being out of our scope.
Co-precipitation and mechanosynthesis methods: the relevant industrial production of LDH powders
Anionic clays are rare natural inorganic materials that are, organized in 2D structures, resulting in superposed hydroxide sheets between which the anion inserted is exchangeable. layered double hydroxides (LDH) belong to the anionic clay group and can be described as brucite (Mg(OH)2)-like structured materials surrounded by water molecules, with the general formula| [MII1−xMIIIx(OH)2]x+(An−x/n)·yH2O |
| M(+II) | Be, Mg, Cu, Ni, Co, Zn, Fe, Mn, Cd, Ca |
| M(+III) | Al, Si, Ga, Ni, Co, Fe, Mn, Cr, Ti, Ce, Eu, La, Ru, Y |
| Anion | Organic species (sulfate, acetate, sulfonate, phosphate), NO3−, CO32−, SO42−, F−, Cl−, Br−, I−, ClO4−, OH−, anionic complexes, macromolecules, polymers… |
For more than half a century, chemists have managed to rebuild LDH materials with available precursors and involving different synthesis methods, mainly soft chemistry as co-precipitation, anionic exchange, and the urea method, but other synthesis routes are possible, such as mechanosynthesis (solid-state chemistry). The large-scale and sustainable production of LDH requires a quite long list of criteria, depending on the chemical composition. However, for any LDH composition given, one can mention general requirements inspired by the work of Patino-Ruiz et al.28 regarding the composite production of Fe3O4 magnetite nanoparticles: (i) the availability of the precursors, (ii) satisfying production yield by batch, (iii) an excellent control process at any stage of the synthesis, and (iv) eco-friendly design both on the material and the process (possibility of recycling, limited waste, reduced occupational and environmental risks). Regarding these criteria, co-precipitation and mechanosynthesis are relevant candidates for comparison and discussion for large-scale LDH production. Most of the patents related to LDH synthesis involve co-precipitation, but mechanosynthesis is within reach on a larger scale. Note that the other non conventional routes (urea, hydrothermal, sonochemical, sol–gel methods) all involve a co-precipitation step during synthesis. That is why it is worth taking a deep look at the purpose of industrial scalability for the general co-precipitation approach and mechanosynthesis. They are significantly different from each other in the whole material production cycle regarding cost, energy demand, precursors, solvent, and water consumption to quote a few. To begin this comparison, in Fig. 1, we display the pros and cons for each of these methods regarding key parameters for the scale-up process.
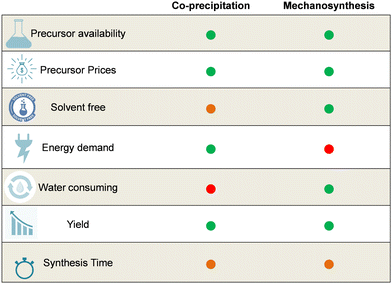 | ||
| Fig. 1 Comparison of co-precipitation and mechanosynthesis in view of the large-scale production of LDH powders, taking into account the basic principles of green chemistry.29 | ||
Co-precipitation and mechanosynthesis have common points that are advantageous for the industrial-scale development of LDH powders, which also fall within the green chemistry concepts. Co-precipitation implies the dissolution of salts (sulfate, nitrate, chloride, etc.) containing the MII/MIII cations, most of the time in aqueous solutions, and the continuous mixing of the precursors, with anions for intercalation or proton scavengers.2,4 The LDH is immediately formed in solution and is, therefore, ready to be used, except that it requires several washing and centrifugation cycles with water or sometimes other solvents. Mechanosynthesis consists of grinding the hydroxide (Mg(OH)2, Al(OH)3) or salt precursors in a planetary ball mill (mortar and pestle otherwise), sometimes assisted by the addition of a reduced solvent (water or organic solvent). Post-treatment can be considered after synthesis but generally, the LDH powder composition is directly obtained after short milling times, less than 60 min. Washing steps can be implemented but with a limited quantity of water or solvent regarding the co-precipitation. Both processes described here are valid for raw LDH compositions as well as hybrid materials,3,4,16 even if in this latter case, adjustments can be made to produce intercalated LDH with organic molecules.27 Taking a closer look at the advantages and shortcomings of each of the methods based on Fig. 1, one can see that both are ready for industrial scalability with a clear attractiveness of mechanosynthesis to reduce water consumption and the generation of basic liquid effluents.
Co-precipitation and mechanosynthesis are well-known approaches for the important production of other types of inorganic (nano)materials,16,30–34 and yields of several kilograms (>10 kg) are achievable goals for satisfying LDH production,9–18,30 taking into account the continuous improvement of reactor design and controlled parameters benefiting from past feedback. One can also note the relatively reduced energy demand involved during the first step of co-precipitation as compared to mechanosynthesis, the first occurring generally at low temperature (typically at room temperature), contributing to a cost-effective approach.16,34 Mechanosynthesis requires much more energy to start the solid-state reaction.
Another point in favour of the large-scale development of LDH is the availability of the salts or hydroxide precursors. This does not seem problematic regarding the chemical compositions considered in the different studies. The majority of LDH materials synthesized for health, environmental and agricultural perspectives show a clear predominance of common salts precursors, with hydroxide, nitrate, chloride, sulfate, carbonate compounds (Mg(OH)2, Mg(NO3)2, MgCl2, Mg(SO4), and their aluminium equivalents for instance…) for co-precipitation as well as for mechanosynthesis. For the latter, 45% of the studies chose cheap precursors that were aluminium based, 30% Mg-based, the rest being shared among iron, zinc, calcium, nickel, and copper, which are more expensive precursors.16,30 Moreover, given the reduced costs of primary materials, it is possible to use raw metal oxides, as well as recycled effluents containing the metallic precursors.35
The main drawbacks in co-precipitation and mechanosynthesis generally include more than four synthesis parameters to control.2,4,9–17 Synthesis times for co-precipitation vary between a few minutes to more than 8 h, depending on the LDH composition, the nature of intercalation compounds and above all, the crystallization degree expected. The synthesis time for mechanosynthesis is longer, typically more than 12 hours, although studies have reported milling times of less than 1 hour. For co-precipitation, temperature, pH, solvent, concentration, surfactant addition, speed of precursors addition, and stirring are the crucial parameters to be controlled, whereas mechanosynthesis calls for meticulous attention to materials, such as beads and reactors for grinding, milling speed, the nature of solvent added, if any, and the atmosphere of the synthesis.9,15–17 Post-treatment may be necessary for LDH since this step strengthens good crystallization, achieves the targeted particle sizes, and eliminates residual impurities remaining after washing. The post-treatment can take different forms, typically with thermal sequences like hydrothermal treatments, calcination, or supplementary grinding and washing. This adds extra time and energy costs for the final LDH product but when considering the whole synthesis cycle, co-precipitation remains less consuming in terms of energy demand. Nonetheless, on looking closer at the apparent shortcomings of co-precipitation and mechanosynthesis, these are not specific to these compounds. For several years, the industry has managed to synthesize numerous other inorganic and hybrid (nano)particles with these methods with good production yields, and pure materials were obtained, thanks to cost-effective strategies stemming from continuously adapted procedures. LDH should not be specific in this view. However, one major point, and not the least, separates the two approaches and could increase LDH powder production. Mechanosynthesis promotes a solvent-free LDH method or a limited solvent use compared to co-precipitation that uses an aqueous phase, and sometimes other solvents as mentioned before in the case of hybrid synthesis.15,16,36,37 In this case, the quantity of water (or solvent) is much higher as compared to mechanosynthesis. With the worrisome management of water worldwide today, mechanosynthesis appears to be a more suitable method for LDH industrial eco-scalability and should attract and motivate research to develop and scale up the benchtop mechanosynthesis that is currently available. More room should be provided for such work. Indeed, papers dealing with LDH powders produced by mechanosynthesis at the laboratory are fewer than those on co-precipitation, however, very promising LDH compounds are ready to be scaled up massively with mechanosynthesis, such as pigments or intercalated LDH, as reviewed recently.16 Concerning the pilot plant studies, according to our knowledge, none have been carried out to date for LDH produced by mechanosynthesis. Co-precipitation, carried out using separate nucleation and aging steps method (SNAS), sometimes followed by hydrothermal treatment is more in advance on this, with emerging pilot plants or meso scale studies for the continuous production of LDH.9,17,33,37–40 However, interestingly, production plants are already underway in China in Shandong Vansinvena Material Technology Co., Ltd.,38 which use both co-precipitation and mechanosynthesis with production capacities of 1500 t.a−1 and 3000 t.a−1, respectively, without more information on the LDH chemical composition, purity and other physicochemical properties for supposedly confidential reasons. Other high-production sites located in the same country are implementing patented co-precipitation methods.41 Also, a patent assigned to Sud-Chemie42 describes a method for the synthesis and manufacture of hydrotalcite where one of the starting compounds or both are not used in the form of a solution. The mixture is subjected to intensive wet grinding, and further processing can lead to either a crystalline phase or an amorphous phase of a high-quality hydrotalcite. The patent suggests starting compounds including unreactive MgO, Al(OH)3 and CO2 in the form of carbonated water, gas or dry ice. Sigma-Aldrich has proposed a synthetic hydrotalcite (product No. 652288), supposedly obtained by co-precipitation, but official information on this cannot be obtained due to confidential reasons. The scaled-up LDH diversity is low, whereas a myriad of compositions make it possible to go from the benchtop to the industrial scale. On the other hand, for mechanosynthesis, the diversity of LDH studied is not as important as for co-precipitation. Mechanosynthesis remains attractive for direct LDH powder production, eventually followed by post-treatment and/or the insertion of organic molecules, with the whole process interestingly organized with the same site production of powders, post-treatment and direct packaging for further transportation or use. The variety of LDH studied through mechanosynthesis is limited but it can be supposed that the production of pure LDH with mechanosynthesis (i) does not gather all the energetic conditions to start the chemical reactions between the precursors, (ii) works in a dry state most of the time, preventing the intimate mixing of the precursors and calling for longer synthesis time, and (iii) the set-up is not easy. However, some compositions can be obtained with very good purity, such as the Cu-Al LDH explored by Qu et al.,30 leading to the exploration of more LDH structures, including the design of inorganic–organic compounds. The MgAl43,44 and CaAl35 LDH are also studied compositions. Isupov et al.43 used a planetary mill to perform the mechanochemical synthesis of magnesium–aluminium double hydroxides starting with a mixture of magnesium hydroxide and aluminium salts, also from magnesium hydroxide, aluminium hydroxide and sodium bicarbonate.44 Ferencz et al.45 used a mixer mill with 20 mm diameter stainless steel grinding balls and a minute and controlled amount of water to produce Ca-Al LDH.
Thus, apart from a lack of studies on the meso/industrial scale production of LDH powders by mechanosynthesis, and thinking about improving water management for co-precipitation, both methods can be readily considered in this view, increasing the large deployment of applications with LDH powders.
So, what prevents this large LDH scale-up worldwide if the powder production is operational? Why do we not notice more use in the applications? A possible answer (but not a unique one) could be found in the systematic agglomeration/aggregation phenomenon of LDH powders, which is likely to degrade their intrinsic properties and very often entails re-dispersion in liquids before use. This adds extra steps in the application processes or impedes them due to solvent compatibility with the particles and/or unwanted interactions. As will be explained in the next section, the control of particle size (well separated in the media, agglomerated or aggregated) is one of the key parameters likely to unlock industrial-scale development.
Insight into the LDH agglomeration/aggregation
The literature exploration of LDH processed by co-precipitation and mechanosynthesis presents a common phenomenon for raw LDH powders or their hybrids, namely the agglomeration/aggregation of the particles. This disturbing element entails a loss of the key properties provided by LDH in its applications. An agglomerate is defined as a non-rigid assembly of the smallest pieces of matter often designed as unit, individual or primary particles. An aggregate refers to a strong link created between the unit particles, making the clear identification of the individual components difficult.46 Agglomerates and aggregates are composed of at least two individual particles. To clearly illustrate both definitions with the simplest shape (spherical), the typical case of SiO2 particles produced by the co-precipitation method is displayed in Fig. 2.47 This agglomeration/aggregation phenomenon is exacerbated when small particles (typically the nanosized ones) are synthesized. Even if considerable efforts are made to limit this well-known behaviour, an important parameter to control is the particle size distribution (the unit particles, or their agglomerates/aggregates) and well characterize this as explained by Weibel et al.48 If the size distribution is well known, it is possible to consider the particles in appropriate applications providing they are stable.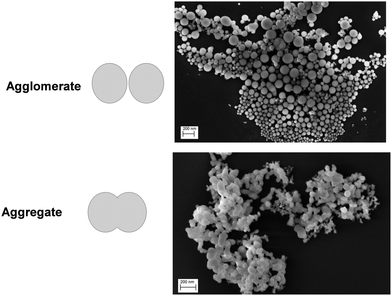 | ||
| Fig. 2 Illustration of the agglomerate and aggregate definition in the case of SiO2 powder particles obtained by a co-precipitation method at the industrial scale (scanning electron microscopy micrographs from Dazon et al.47). | ||
LDH produced by co-precipitation in solution at room temperature follows the well-known nucleation-growth mechanism encountered for bottom-up synthesis approaches. The necessary aging step subsequent to co-precipitation leads to agglomeration/aggregation of the LDH particles.49 To avoid this, one approach is to consider coprecipitation on the surface of silica beads. However, although interesting, the transposition of the conditions used on the industrial scale seems difficult. For mechanosynthesis, LDH production involves a top-down approach, sometimes assisted by solvent to improve the chemical reaction resulting in agglomeration/aggregation, depending on the nature of the intrinsic forces between the particles (van der Waals, electrostatic, capillary forces).15,16 Even if considerable efforts were made to limit it, the final dry particles remain strongly agglomerated. However, the main issue is still the size distribution control of the agglomerates as explained by Xu et al.50 and Zhou et al.51 In an attempt to understand what rules the agglomeration process of LDH, the authors51 highlighted that zeta potential, crystallinity and surface defects are important parameters to monitor, given LDH scalability. In any case, the LDH agglomeration results in dramatic consequences for the applications using dry powders for which it is necessary to have a well-known size distribution (painting formulation, composite elaboration). For instance, in the building field, LDH materials demonstrated very good potential to preserve structures from corrosion phenomena estimated at several millions of US$ annually, representing between 2.5% and 3.5% of the growth domestic product (GDP).21,52 When agglomerated, typical Mg–Al–CO3 – LDH dispersed in concrete does not affect concrete durability against corrosion, which is particularly accelerated in the presence of chloride species,21 with a loss of anti-corrosion properties varying between – 21% and −14% (most of the time, due to a lack of size distribution control of the raw LDH powder (agglomerated) and concentration in the concrete). The compressive strength can also be impacted, with LDH giving better mechanical properties to cementitious structures. The LDH particles are used as received, i.e., in a dry state and agglomerated, and are hardly dispersed in the concrete; no supplementary steps were implemented to improve the dry particle dispersion before incorporation into the final bulk, and there was no size control at any step. Another example of bad results can be highlighted when LDH particles are used in fire retardancy.53,54 The use of dry LDH particles leads to the dispersion of polydisperse agglomerates randomly in the polymer matrix, and the temperature degradation is the same as that of the raw polymer. A good LDH dispersion results in the homogeneous occupation of the particles in the whole polymer matrix, leading to better insulation and mechanical strength. Inhomogeneous LDH agglomeration causes “holes” in the polymer matrix, disequilibrating the properties achieved. Therefore, in the case of dry LDH powder use, an initial size distribution control is required even if the agglomeration/aggregation phenomenon cannot be limited.
Another drawback in LDH agglomeration/aggregation is their inappropriate use as drug carriers, especially for aerosol therapy.55–57 Among the prerequisites for drug delivery by aerosol therapy is good particle dispersion, which should be managed with a narrow and stable particle size distribution of the unit/primary particles, and is more challenging in this type of application. Poor particle dispersion hinders the delivery efficiency of the therapeutic molecules due to unsuitable particle diameters passing through the respiratory tracts. Moreover, other parameters should be considered that can impact the LDH dispersion for aerosol therapy, such as the relative humidity or the design of aerosol delivery. Finally, depending on the applications, the LDH dispersion should be accurately checked, either in the agglomerated or unit particle state but with a stable and unique size distribution.
However, in an industrial approach, the powder form is practical and is a favoured way to produce, package, transport, and deliver raw LDH for further commercialisation and application. As described in recent works on inorganic nanoparticles massively encountered in industry (TiO2, SiO2, ZnO),47,58,59 the powder appears as the main form produced. It seems that the dry particle state could not be avoided but can be optimized in terms of the control of particle dispersion. Regarding what we described above, the main challenge lies in implementing co-precipitation and mechanosynthesis to produce relatively good dry LDH particles with stable size dispersion at the end of the process. According to Zhou et al.,51 the implementation of powder rheology requires a good understanding of the relationship between influencing parameters such as surface charge during synthesis, particle size, surface area, and processing equipment to achieve the desired particle size distribution in the dry state. This could lead to, promising ideas for LDH powder rheology.
Can powder rheology inspire LDH dispersion?
To face the agglomeration/aggregation phenomenon of LDH powders, efforts should be concentrated on laboratory and meso-scale studies aimed at LDH deagglomeration during or after synthesis, with a focus on the eco-friendliest LDH and their hybrids (magnesium, aluminum, calcium, and iron LDH based). To date, surfactants have largely been used to help LDH dispersion, but this requires meticulous and smart combinations of the chemical composition of the surfacing agent and those of the LDH, including the medium used. This approach does not offer great versatility for dry applications of LDH and adds complex processing, including the removal of surface agents. An interesting solution that is hardly explored today and directly inspired by powder rheology is using particles with a bigger size to “dilute” LDH powders with smaller diameters during synthesis or post-treatment. This concept is illustrated in Fig. 3.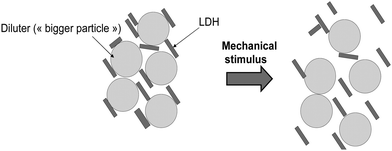 | ||
| Fig. 3 Schematic route to obtaining an efficient dispersion of LDH powder, “diluting” the LDH with smallest dimensions with bigger ones. | ||
Typically, the particles with the biggest sizes are mixed in the dry state with the smallest to disperse them, then, after a mechanical stimulus, the smallest particles are more isolated and the biggest ones are removed through a sieving step.55–57,60–62 To correctly achieve this dispersion, the bigger particles should stand against mechanical constraints imposed by the fluidizing device as well as being chemically inert. The smallest particles have less probability of interacting with others because of the presence of the bigger particles blocking their dynamic behaviour. In this case, the smallest particles maintain their high surface reactivity at the origin of their tendency to agglomerate and will be oriented around the biggest particles characterized by a lower surface tension. This approach is simple; reducing impurities, allowing the particles to disperse, and getting rid of the deagglomerating agents is easy due to the numerous sieving systems. Scattering the smallest LDH particles with micrometric ones has recently been explored for mechanosynthesis.63 SiO2 microparticles (5 μm sized) have been added to the reactor to improve the deagglomeration of the Zn–Al LDH formed during milling, on top of the zirconia beads. Adding ZnO or SiO2 improved the LDH adsorption capacity regarding methyl orange by more than 20%. The positive effect observed is attributed to the better dispersion of LDH, which is applicable in other areas such as fire retardancy.53,54 The nature and bead size can be directly exploited to also obtain good dispersed LDH as tested recently with zirconia or glass beads with different sizes ranging from 200 μm to more than 2 mm,64 followed by tri-sodium citrate treatment to improve the dispersion quality. In both cases, LDH is less agglomerated, even if there are some remaining clusters. Note that mechanosynthesis is appropriate for such exploration because the process itself involves “big particles” through the beads used but more studies should be proposed in this view. For co-precipitation, the possibility of adding a “diluter” during the nucleation and growth process is a harder task since a significant risk of a chemical reaction is possible in the co-precipitation medium. Nonetheless, co-precipitation can implement the powder rheology approach as a supplementary step leading to the final LDH powder. We can thus imagine setting fluidized bed equipment following the co-precipitation and washing. This adds an extra device cost but, results in an appropriate and controlled size distribution of the LDH powders. The fluidized bed pulses up the particles that settle on the bottom of a tank with the diluting particles, giving them extra mobility and very good dispersing behaviour. Given the quite simple strategy proposed by the sole sieving step to remove the diluters, this task should not prevent the scalability of LDH through co-precipitation synthesis, nor impose supplementary energy demand.
Are there other unlocking strategies for better LDH dispersion during synthesis? Some studies have indicated the possibilities for the accurate control of the co-precipitation parameters and post-treatment,65,66 favouring reduced temperature and aging time, and the use of the zeta potential as a key indicator of the agglomeration state to manage the process. However, these suggestions have already been considered for co-precipitation development without significant improvement on a large scale for LDH dispersion. Undoubtedly, it is worth inputting the very little studied LDH powder rheology at the laboratory and meso-scales due to the obvious economic, availability and already operational set-up it can offer. LDH-controlled powder dispersion is a big challenge, but inspiration from powder rheology is a potential smart response to address it and lead to massive scaling up, possibly making strides in laboratory studies, igniting efficient and sustainable solutions, and motivating more public–private partnerships to progress on this issue.
Conclusions
In the coming years, the world will face global cascading challenges regarding health, the environment agriculture, etc. Materials sciences should be one of the most relevant tools poised to bring about disruptive and resilient solutions for adapting and reorganizing our societies. There is a clear need for the development of eco-friendly, cost-effective and efficient materials as quickly as possible. Among the tremendous possibilities are layered double hydroxide (LDH) materials, a rare natural anionic clay group with amazing potential. However, we have not seen the large-scale use of LDH structures, even with the relatively good management suggested by co-precipitation and mechanosynthesis methods to produce significant amounts of LDH powder particles.Why so few LDH powder applications at the industrial scale? Are we ready to scale up LDH powders worldwide? Should research explore more strategic points to unlock the different shortcomings? Is it worth pursuing this development? The response is certainly “yes”. One of the big problems inherent to LDHs is their tendency to aggregate, which causes them to lose their powder or dry particle properties for certain applications. Here, research can offer considerable advances to overcome this drawback. This is a key point that public–private efforts should focus on. First, concentrating the efforts on strategic chemical compositions for raw LDH encompassing economic and ecological criteria should be favoured. Mg and Al LDH-based materials, as well as their hybrid derivatives combined with polymers or biomolecules for innovative applications, have proven their really good potential for future use in drug delivery, slow-release pesticides, as well as catalysis applications. More room should be given to accelerating the studies inspired by powder rheology to deagglomerate and control the particle size distribution and scale up the resulting process. As such, there should be new initiatives and experiments for co-precipitation and mechanosynthesis, diluting LDH particles in their medium or through a post-synthesis step by bigger-sized ones such as micrometric silica, which is a promising approach. It is important to systematically think about the transfer to the industrial scale during the development and study of new promising materials, suggesting a close exchange between private companies and academic structures. If this general concept is smartly organized for LDH, we can cultivate hope to see more and more of these materials integrating the industrial area, with sustainable development and numerous applications within reach.
Conflicts of interest
The author declares no conflicts of interest.Acknowledgements
This work was not funded by any grant or other financial support. In this view, the authors acknowledge sincerely all their colleagues for their cheerful encouragements for writing this article. The author thank also gratefully all the research teams worldwide feeding the references of this paper and letting the Layered Double Hydroxides being great materials to study, understand and apply for progress.References
- National Intelligence Council, NIC2021-02339, 2021, p. 156.
- F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 1991, 11, 173–301 CrossRef CAS.
- V. Prevot and Y. Tokudome, J. Mater. Sci., 2017, 52, 11229–11250 CrossRef CAS.
- C. Taviot-Guého, C. Forano, C. Mousty, V. Prevot, R. Renaudin and F. Leroux, Adv. Funct. Mater., 2018, 28, 1703868 CrossRef.
- X. Feng, R. Long, L. Wang., C. Liu, Z. Bai and X. Liu, Sep. Purif. Technol., 2021, 284, 120099 CrossRef.
- N. Kano and S. Zhang, Advanced Sorption Process Applications, IntechOpen, 2018, Available from: https://www.intechopen.com/chapters/63400 DOI:10.5772/intechopen.808.
- L. Yan, S. Gonca, G. Zhu, W. Zhang and X. Chen, J. Mater. Chem. B, 2019, 7, 5583 RSC.
- S. Mallakpour, M. Hatami and C. M. Hussain, Adv. Colloid Interface Sci., 2020, 283, 102216 CrossRef CAS PubMed.
- D. Tichit, G. Layrac and C. Gerardin, Chem. Eng. J., 2019, 369, 302–332 CrossRef CAS.
- J. Kameliya, A. Verma, P. Dutta, C. Arora, S. Vyas and R. S. Varma, Inorganics, 2023, 11, 121 CrossRef CAS.
- S.-F. Ng, M. Y. Ling Lau and W.-J. Ong, Sol. RRL, 2021, 5, 2000535 CrossRef CAS.
- M. Sajid, S. M. S. Jillani, N. Baig and K. Alhooshani, Chemosphere, 2022, 287, 132140 CrossRef CAS PubMed.
- E. M. Abd El-Monaem, H. M. Elshishini, S. S. Bakr, H. G. El-Aqapa and M. Hosny, et al. , Clean Water, 2023, 6, 34 CrossRef CAS.
- J. Tedim, T. L. P. Galvão, K. A. Yasakau, A. Bastos and J. R. B. Gomes, et al. , Front. Chem., 2022, 10 DOI:10.3389/fchem.2022.1048313.
- J. Qu, Q. Zhang, X. Li, X. He and S. Song, Appl. Clay Sci., 2016, 119, 185–192 CrossRef CAS.
- S. Intasa-ard, K. Imwiset, S. Bureekaew and M. Ogawa, Dalton Trans., 2018, 47, 2896 RSC.
- F. L. Theiss, G. A. Ayoko and R. L. Frost, Appl. Surf. Sci., 2016, 383, 200–213 CrossRef CAS.
- J. S. Valente, M. Sanchez-Cantu, E. Lima and F. Figueras, Chem. Mater., 2009, 21, 5809–5818 CrossRef CAS.
- M. Mohammadi, A. Mohammadi Torkashvand, P. Biparva and M. Esfandiari, Global J. Environ. Sci. Manage., 2021, 7(1), 59–78 CAS.
- D. Chaillot, S. Bennici and J. Brendlé, Environ. Sci. Pollut. Res., 2021, 28, 24375–24405 CrossRef CAS PubMed.
- Z. M. Mir, A. Bastos, D. Höche and M. L. Zheludkevich, Materials, 2020, 13, 1426 CrossRef CAS PubMed.
- https://data.inpi.fr/ (accessed on the 01/09/2023).
- S. Kim, J. Fahel, P. Durand, E. André and C. Carteret, Eur. J. Inorg. Chem., 2017, 669–678 CrossRef CAS.
- S. Mills, A. Christy, J. Génin, T. Kameda and F. Colombo, Mineral. Mag., 2012, 76(5), 1289–1336 CrossRef CAS.
- X. Duan, J. Lu and D. G. Evans, Chapter 18 – Functional Host–Guest Materials, in Modern Inorganic Synthetic Chemistry, Elsevier, 2nd edn, 2017, ch. 18, pp. 493–543 Search PubMed.
- M. Intissar, J.-C. Jumas, J.-P. Besse and F. Leroux, Chem. Mater., 2003, 15(24), 4625–4632 CrossRef CAS.
- E. S. Seliverstov, S. N. Golovin and O. E. Lebedeva, Front. Chem. Eng., 2022, 867615 CrossRef.
- D. A. Patiño-Ruiz, S. I. Meramo-Hurtado, M. Mehrvar, L. Rehmann, E. Quiñones-Bolaños, A. A. D. Gonzalez-Delgado and A. Herrera, ACS Omega, 2021, 6, 3644–3658 CrossRef PubMed.
- A. M. Borrero-López, A. Celzard and V. Fierro, ACS Sustainable Chem. Eng., 2022, 10, 16090–16112 CrossRef.
- J. Qu, X. He, M. Chen, H. Hu, Q. Zhang and X. Liu, Mater. Chem. Phys., 2017, 191, 173–180 CrossRef CAS.
- B. Prabowo, T. Khairunnisa and A. B. D. Nandiyanto, World Chem. Eng. J., 2018, 2, 1–4 Search PubMed.
- T. Jiang, H. Zhang, Y. Zhao, C. Qin, S. Wang and X. Ma, Fuel, 2021, 301, 121049 CrossRef CAS.
- P. Yaseneva, N. An, M. Finn, N. Tiedemann, N. A. Jose, A. Voutchkova-Kostal and A. Lapkin, Chem. Eng. J., 2019, 360, 190–199 CrossRef CAS.
- P. Matteazzi, D. Basset and E. Miani, Nanostruct. Mater., 1993, 2, 217–229 CrossRef CAS.
- L. Santamaría, S. A. Korili and A. Gil, J. Environ. Chem. Eng., 2022, 10(1), 106948 CrossRef.
- A. V. Karim, A. Hassan, P. Eghbali and P. V. Nidheesh, Curr. Opin. Solid State Mater. Sci., 2022, 26, 100965 CrossRef CAS.
- H. W. Olfs, L. O. Torres-Dorante, R. Eckelt and H. Kosslick, Appl. Clay Sci., 2009, 43, 459–464 CrossRef CAS.
- F. Mao, P. Hao, Y. Zhu, X. Kong and X. Duan, Chin. J. Chem. Eng., 2022, 41, 42–48 CrossRef CAS.
- I. Clark, R. L. Gomes, C. Crawshaw, L. Neve, R. Lodge and M. Fay, et al. , React. Chem. Eng., 2019, 4, 663 RSC.
- I. Clark, P. W. Dunne, R. L. Gomes and E. Lester, J. Colloid Interface Sci., 2017, 504, 492–499 CrossRef CAS PubMed.
- Y. Lin and G. Wang, Recent Pat. Nanotechnol., 2012, 6(3), 169–173 CrossRef CAS PubMed.
- M. Eisgruber, J. Ladebeck, J. Koy, H. Schiessling, W. Buckl and H. Ebert, Method for producing hydrotalcites, US Pat., 7211235B2, 2009 Search PubMed.
- V. P. Isupov, L. E. Chupakhina and R. P. Mitrofanova, J. Mater. Synth. Process., 2000, 8, 251–253 CrossRef CAS.
- V. P. Khusnutdinov and V. P. Isupov, Inorg. Mater., 2008, 44, 263–267 CrossRef CAS.
- Z. Ferencz, A. Kukovecz, Z. Konya, P. Sipos and I. Palinko, Appl. Clay Sci., 2015, 112–113, 94–99 CrossRef CAS.
- G. Nichols, S. Byard, M. J. Bloxham, J. Botterill, N. J. Dawson and A. Dennis, et al. , J. Pharm. Sci., 2002, 91(10), 2003–2019 CrossRef PubMed.
- C. Dazon, O. Witschger, S. Bau, V. Fierro and P. L. Llewellyn, Nanoscale Adv., 2019, 1(8), 3232–3242 RSC.
- A. Weibel, R. Bouchet, F. Boulc’h and P. Knauth, Chem. Mater., 2005, 17, 2378–2385 CrossRef CAS.
- A. P. Tathod and O. M. Gazit, Cryst. Growth Des., 2016, 16, 6709–6713 CrossRef CAS.
- Z. P. Xu, G. S. Stevenson, C.-Q. Lu, G. Q. Lu, P. F. Bartlett and P. P. Gray, J. Am. Chem. Soc., 2006, 128(1), 36–37 CrossRef CAS PubMed.
- Y. Zhou, X. Sun, K. Zhong, D. G. Evans, Y. Lin and X. Duan, Ind. Eng. Chem. Res., 2012, 51, 4215–4221 CrossRef CAS.
- L. de Arriba-Rodriguez, J. Villanueva-Balsera, F. Ortega-Fernandez and F. Rodriguez-Perez, Metals, 2018, 8, 334 CrossRef.
- M. Wang, G. Xiao, C. Chen, Z. Yang, F. Zhong and C. Chen, et al. , Colloids Surf., A, 2022, 638, 128315 CrossRef CAS.
- Z. Matusinovic, H. Lu and C. A. Wilkie, Polym. Degrad. Stab., 2012, 97, 1563–1568 CrossRef CAS.
- J. Dhanani, J. F. Fraser, H.-K. Chan, J. Rello, J. Cohen and J. A. Roberts, Crit. Care, 2016, 20, 269 CrossRef PubMed.
- K. AboulFotouh, Y. Zhang, M. Maniruzzaman, R. O. Williams III and Z. Cui, Int. J. Pharm., 2020, 587, 119711 CrossRef CAS PubMed.
- M. Matuszak, M. Ochowiak, S. Włodarczak, A. Krupinska and M. Doligalski, Int. J. Pharm., 2022, 614, 121432 CrossRef CAS PubMed.
- F. Babick, J. Mielke, W. Wohlleben, S. Weigel and V.-D. Hodoroaba, J. Nanopart. Res., 2016, 18(6), 158 CrossRef PubMed.
- C. Dazon, V. Fierro, A. Celzard and O. Witschger, Nanoscale Adv., 2020, 2(10), 4908–4917 RSC.
- G. Calvert, M. Ghadiri and R. Tweedie, Adv. Powder Technol., 2009, 20(1), 4–16 CrossRef CAS.
- N. Y. Chew, P. Tang, H. Chan and J. R. Raper, Pharm. Res., 2001, 22(1), 148–152 CrossRef PubMed.
- J. R. van Ommen, J. M. Valverde and R. Pfeffer, J. Nanopart. Res., 2012, 14, 737 CrossRef PubMed.
- Z. Ai, C. Liu, Q. Zhang, J. Qu, Z. Li and X. He, J. Alloys Compd., 2018, 763, 342–348 CrossRef CAS.
- T. Chantaramanee, et al. , IOP Conf. Ser.: Mater. Sci. Eng., 2019, 654, 012007 CAS.
- Z. Cao, L. Xue, M. Wu, B. He, J. Yu and M. Chen, Constr. Build. Mater., 2018, 178, 42–50 CrossRef CAS.
- L. D. Silva Neto, C. G. Anchieta, J. L. S. Duarte, L. Meili and J. T. Freire, ACS Omega, 2021, 6, 21819–21829 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |



