Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of complex aryl MIDA boronates by Rh-catalyzed [2+2+2] cycloaddition†‡
John M.
Halford-McGuff
,
David B.
Cordes
 and
Allan J. B.
Watson
and
Allan J. B.
Watson
 *
*
EaStCHEM, School of Chemistry, University of St Andrews, North Haugh, St Andrews, KY16 9ST, UK. E-mail: aw260@st-andrews.ac.uk
First published on 26th May 2023
Abstract
A Rh-catalyzed [2+2+2] cycloaddition approach for the synthesis of BMIDA-functionalized arenes is reported. The developed method overcomes the long-standing reactivity problems associated with internal alkynes and allows access to highly functionalized borylated benzene scaffolds. The method is broadly functional group tolerant and generally high yielding. The utility of the products is demonstrated through elaboration of the BMIDA motif, and in the synthesis of fused heterocycles via intramolecular Chan–Lam etherification and amination. The dominance of sterically controlled reactivity is described.
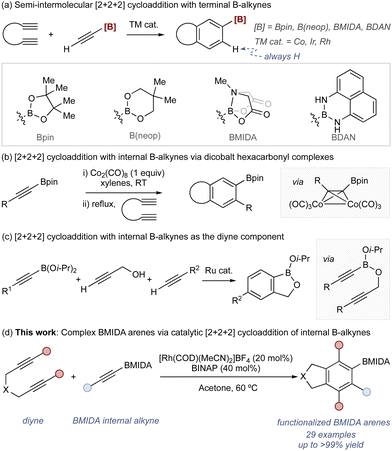
Arylboronic acids and their derivatives are fundamental components in contemporary organic synthesis.1,2 These reagents underpin many of the most widely used catalytic reactions, including the Suzuki–Miyaura,3,4 Chan–Lam,5,6 and Hayashi reactions.7,8 Methods to access arylboronic acids and esters are therefore similarly important and find widespread utility: exemplar processes range from nucleophilic addition reactions using stoichiometric metal complexes and boron electrophiles (e.g., B(Oi-Pr)3)9 to catalytic borylation using transition metal complexes.10,11
These widely used methods involve the borylation of an established aryl motif and are therefore constrained by starting material availability. This impacts the available regiochemistry of the borylated arene product, with some more difficult to access than others. An alternative approach to borylated arenes is via annulation through [2+2+2] cycloaddition.12–23 This allows modular assembly of a borylated arene from simple alkyne building blocks and can provide access to products with regiochemistry and/or functionality that is complementary to arene borylation strategies.
The use of borylated terminal alkynes (i.e., acetylene boronic acid derivatives) in the versatile semi-intermolecular [2+2+2] cycloaddition has seen good success with several catalytic approaches using boron motifs including Bpin,24,25 B(neop),26–28 BMIDA,29,30 and BDAN (Scheme 1a).25,31
A key limitation of this strategy is associated with a long-standing problem in semi-intermolecular [2+2+2] cycloaddition where internal alkynes are not tolerated unless they have coordinating groups.32–34 With borylated internal alkynes, there are very few examples. Aubert has shown that dicobalt hexacarbonyl complexes undergo cycloaddition to deliver the expected products (Scheme 1b),24,25 and Yamamoto has used borylated internal alkynes as the diene component through a tethering approach (Scheme 1c).35,36 Catalytic approaches using borylated internal alkynes as the monoalkyne component remain underdeveloped.
Here we provide a method for Rh-catalyzed [2+2+2] cycloadditions using unactivated internal borylated alkynes that allows access to highly functionalized borylated arenes (Scheme 1d). The process generates a range of arene products bearing the valuable and synthetically powerful BMIDA motif.37,38 The utility of this approach is demonstrated via downstream manipulation including a range of C–X bond formation processes, including formation of fused heterocycles.
Exploration of the internal BMIDA alkyne cycloaddition was based on a model system using diyne 1 and propyne BMIDA 2 (Table 1).
| Entry | Deviation from ‘standard conditions’ | Yielda (%) |
|---|---|---|
| Reactions performed on 0.1 mmol scale.a Determined by 1H NMR using an internal standard as an average of two experiments.b Isolated yield. | ||
| 1 | — | 75 (73)b |
| 2 | 10 mol% catalyst, 20 mol% ligand | 39 |
| 3 | PPh3 as ligand | <5 |
| 4 | dppp as ligand | 10 |
| 5 | RT | 32 |
| 6 | 80 °C | 70 |
| 7 | Diyne added at outset (no slow addition) | 22 |
Optimization provided a system that delivered BMIDA product 3 in good yield (entry 1; for full details, see ESI‡). Importantly, control reactions with a complementary Bpin (i.e., pentyne Bpin) were unsuccessful and delivered a complex mixture of products derived from C–B activation, principally protodeboronation. Decreasing the catalyst loading was not tolerated (entry 2). This is directly related to the steric footprint of the BMIDA unit, which restricts catalytic turnover as previously described (vide infra).32 BINAP was the optimal ligand, with other ligands significantly less effective (e.g., entries 2 and 3). Similarly, other catalyst systems, which have shown utility in other [2+2+2] cycloadditions,12–23 were less effective (see ESI‡). Increases or decreases in temperature offered no improvement (entries 5 and 6). Finally, slow addition of the diyne was necessary to control competing reaction kinetics (entry 7): diyne reactivity is significantly greater than the internal alkyne leading to dimer- and trimerisation pathways.32 Slow addition effectuates control allowing productive engagement of the less reactive internal alkyne. Reactions stop upon consumption of the diyne, which is fully consumed. Throughout, no issues were observed with the integrity of the BMIDA unit.
Under the optimized conditions, a series of BMIDA alkynes were successfully accommodated to generate a small library of BMIDA arene products in good to excellent yield (Scheme 2).39 In some cases, chromatographic purification was difficult and further purification by crystallization was required (see ESI‡).
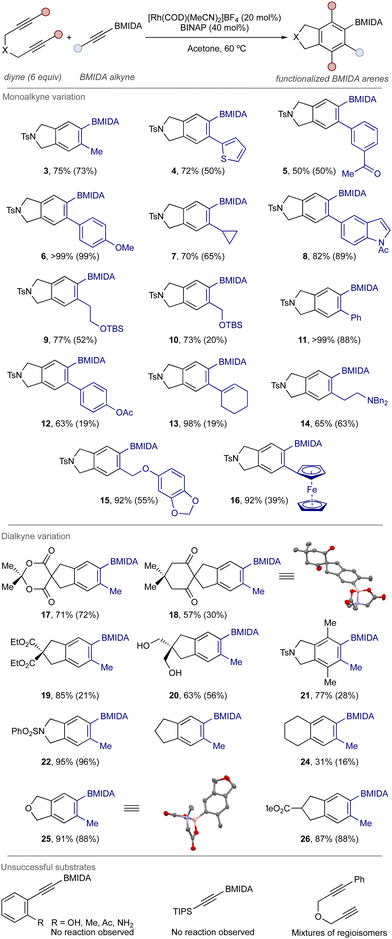 | ||
| Scheme 2 Example scope of the cycloaddition process. Monoalkyne component indicated in blue in products. Yields determined by 1H NMR, isolated yields (chromatography and/or crystallization) in brackets. See ESI‡ for full details. | ||
Using the benchmark diyne 1 (products 3–16), aryl- and heteroaryl BMIDA alkynes were broadly successful. An alkene-substituted BMIDA alkyne was also tolerated, giving product 13 in high yield and without any issues of alkene migration. Reactivity issues were noted with ortho-substituted aryl alkynes.
Lastly, alkyl BMIDA alkynes were readily accommodated, delivering the expected products in good yield. Changes to the tether and substitution of the diyne component was effective (compounds 17–26), with compound 21 perhaps the most notable result – a fully substituted borylated arene obtained in high yield, illustrating the utility of this method towards the generation of densely functionalized arene products. Mono-substituted diynes led to mixtures of product regioisomers. Structural detail was provided through X-ray crystal structure of representative compounds 18 and 25.
Regarding practical accessibility, the relevant BMIDA alkyne starting materials were prepared either through metalation-borylation (alkyl BMIDA alkynes) or via Sonogashira coupling using acetylene BMIDA (aryl BMIDA alkynes) (see ESI‡).40
The BMIDA product can be manipulated using a variety of standard reactions to provide a range of functionalized arene scaffolds (Scheme 3a). This includes Brown oxidation (27), direct Chan–Lam amination (28) and etherification (31),5,6,41 Suzuki–Miyaura cross-coupling (29), formation of the BF3K derivative (30), and direct Hayashi conjugate addition (32). Protected phenethyl alcohol derivative 9 and phenethyl amine derivative 14 could be deprotected and undergo direct intramolecular Chan–Lam etherification and amination to deliver fused heterocycle products (Scheme 3b).41 For 14, the expected indoline 34 was isolated in low yield, with the major product the indole 35, arising from the desired Chan–Lam followed by Cu-mediated oxidation.42–44 The utility of other protected alkynyl orgnoborons was evaluated through the use of BDAN alkyne 36 (Scheme 3c), which underwent the cycloaddition using the standard conditions (Table 1), displaying good reactivity.
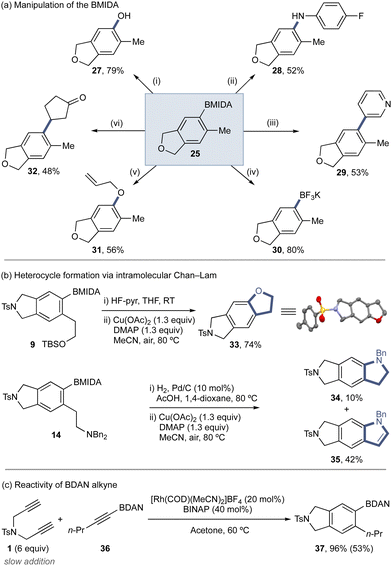 | ||
| Scheme 3 (a) Product diversification. Conditions from: (i) H2O2 (30% aq., 10 equiv.), K3PO4 (3 equiv.), THF, RT, N2; (ii) 4-fluoroaniline (2.1 equiv.), Cu(OAc)2 (1.3 equiv.), DMAP (1.3 equiv.), MeCN, air, 80 °C; (iii) 3-boromopyridine (1.5 equiv.), Pd(dppf)Cl2 (4 mol%), K3PO4 (3 equiv.), H2O (3 equiv.), THF, 90 °C, N2; (iv) KHF2 (4.1 equiv.), MeOH/H2O, 70 °C; (v) allyl alcohol (2.1 equiv.), Cu(OAc)2 (1.3 equiv.), DMAP (1.3 equiv.), MeCN, air, 80 °C; (vi) [Rh(COD)(MeCN)2]BF4 (5 mol%), K3PO4 (2 equiv.), PhMe/H2O, 100 °C. (b) Heterocycle formation via intramolecular Chan–Lam. (c) Utility of BDAN alkyne in the cycloaddition under standard conditions. Yields determined by 1H NMR, isolated yields in brackets. See ESI‡ for full details. | ||
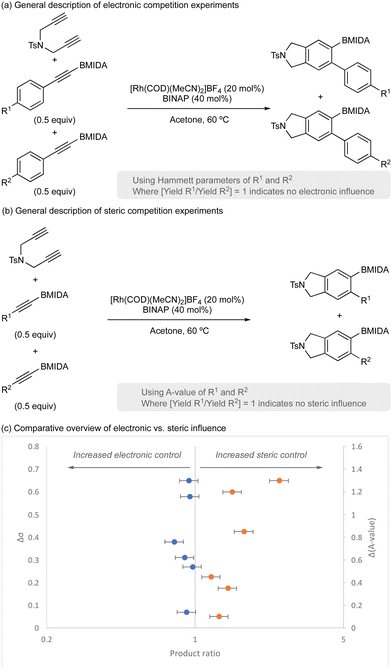
Finally, the importance of steric parameters vs. electronic parameters was explored using a series of competition experiments (Scheme 4). A range of aryl acetylene BMIDA compounds with electronically diverse functional groups (variation of R1 and R2) were prepared and used in competition experiments to assess electronic influence (Scheme 4a). Similarly, a range of functionalized acetylene BMIDA reagents with sterically diverse functional groups (variation of R1 and R2) were prepared and used in competition experiments to assess steric influence (Scheme 4b). Analysis of product distribution (product 1/product 2) in each dataset, would report on the influence of electronics or sterics, with deviation from 1 indicating some selectivity. An overview of the dataset is shown in Scheme 4c.
Consistent with previous observations,32 this reaction is principally under steric control. Variation of electronics had little impact on product distribution; however, variation of steric footprint of the alkyne had a significant influence, with smaller alkynes more reactive and providing greater productive catalyst turnover. Since BMIDA has a relatively high steric component, this therefore necessitates higher catalyst loading for effective product formation.32
In summary, a Rh-catalyzed semi-intermolecular [2+2+2] cycloaddition of internal unactivated alkynes has been developed for the synthesis of functionalized BMIDA-substituted arenes. The process overcomes the key steric limitation, accommodating a diverse range of functionalized BMIDA alkyne and diyne components to deliver borylated arenes in good to excellent yield. The utility of the products has been highlighted through the diversification of the BMIDA unit and the synthesis of fused heterocycles.
J. M. H.-M. thanks the EPSRC Centre for Doctoral Training EaSI-CAT for a PhD studentship. A. J. B. W. thanks the Leverhulme Trust for a Research Fellowship (RF-2022-014) and the EPSRC Programme Grant “Boron: Beyond the Reagent” (EP/W007517/1) for support. We thank Dr Jamie Fyfe, Marek Varga, Dr Matthew West, and Dr George Bell for assistance with starting materials.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, ed. D. G. Hall, Wiley-VCH, Weinheim, 2005 Search PubMed.
- J. W. B. Fyfe and A. J. B. Watson, Chemistry, 2017, 3, 31–55 CrossRef CAS.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- A. J. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412–443 RSC.
- J. X. Qiao and P. Y. S. Lam, Synthesis, 2011, 829–856 CrossRef CAS.
- M. J. West, J. W. B. Fyfe, J. C. Vantourout and A. J. B. Watson, Chem. Rev., 2019, 119, 12491–12523 CrossRef CAS PubMed.
- P. Tian, H.-Q. Dong and G.-Q. Lin, ACS Catal., 2012, 2, 95–119 CrossRef CAS.
- T. Hayashi and K. Yamasaki, Chem. Rev., 2003, 103, 2829–2844 CrossRef CAS PubMed.
- H. C. Brown and T. E. Cole, Organometallics, 1983, 2, 1316–1319 CrossRef CAS.
- E. C. Neeve, S. G. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Chem. Rev., 2016, 116, 9091–9161 CrossRef CAS PubMed.
- J. S. Wright, P. J. H. Scott and P. G. Steel, Angew. Chem., Int. Ed., 2021, 60, 2796–2821 CrossRef CAS PubMed.
- W. Reppe, O. Schlichting, K. Klager and T. Toepel, Justus Liebigs Ann. Chem., 1948, 560, 1–92 CrossRef CAS.
- K. P. C. Vollhardt, Angew. Chem., Int. Ed. Engl., 1984, 23, 539–556 CrossRef.
- S. Kotha, E. Brahmachary and K. Lahiri, Eur. J. Org. Chem., 2005, 4741–4767 CrossRef CAS.
- V. Gandon, C. Aubert and M. Malacria, Chem. Commun., 2006, 2209–2217 RSC.
- P. R. Chopade and J. Louie, Adv. Synth. Catal., 2006, 348, 2307–2327 CrossRef CAS.
- N. Agenet, O. Buisine, F. Slowinski, V. Gandon, C. Aubert and M. Malacria, Org. React., 2007, 68, 1–302 Search PubMed.
- G. Domínguez and J. Pérez-Castells, Chem. Soc. Rev., 2011, 40, 3430–3444 RSC.
- Y. Shibata and K. Tanaka, Synthesis, 2012, 323–350 CAS.
- Transition-Metal-Mediated Aromatic Ring Construction, ed., K. Tanaka, Wiley, Hoboken, 2013, pp. 3–298 Search PubMed.
- S. Kotha, K. Lahiri and G. Sreevani, Synlett, 2018, 2342–2361 CrossRef CAS.
- P. Matton, S. Huvelle, M. Haddad, P. Phansavath and V. Ratovelomanana-Vidal, Synthesis, 2022, 4–32 CAS.
- B. R. Galan and T. Rovis, Angew. Chem., Int. Ed., 2009, 48, 2830–2834 CrossRef CAS PubMed.
- V. Gandon, D. Leca, T. Aechtner, K. P. C. Vollhardt, M. Malacria and C. Aubert, Org. Lett., 2004, 6, 3405–3407 CrossRef CAS PubMed.
- L. Iannazzo, K. P. C. Vollhardt, M. Malacria, C. Aubert and V. Gandon, Eur. J. Org. Chem., 2011, 3283–3292 CrossRef CAS.
- Y. Yamamoto and K. Hattori, Tetrahedron, 2008, 64, 847–855 CrossRef CAS.
- Y. Yamamoto, K. Hattori, J. Ishii, H. Nishiyama and K. Itoh, Chem. Commun., 2005, 4438–4440 RSC.
- A.-L. Auvinet, M. Ez-Zoubir, S. Bompard, M. R. Vitale, J. A. Brown, V. Michelet and V. Ratovelomanana-Vidal, ChemCatChem, 2013, 5, 2389–2394 CrossRef CAS.
- S. Melnes, A. Bayer and O. R. Gautun, Tetrahedron, 2013, 69, 7910–7915 CrossRef CAS.
- J. R. Struble, S. J. Lee and M. D. Burke, Tetrahedron, 2010, 66, 4710–4718 CrossRef CAS.
- R. W. Foster, C. J. Tame, H. C. Hailes and T. D. Sheppard, Adv. Synth. Catal., 2013, 355, 2353–2360 CrossRef CAS PubMed.
- J. M. Halford-McGuff, A. M. Z. Slawin and A. J. B. Watson, ACS Catal., 2023, 13, 3463–3470 CrossRef CAS.
- M. Amatore, D. Lebœuf, M. Malacria, V. Gandon and C. Aubert, J. Am. Chem. Soc., 2013, 135, 4576–4579 CrossRef CAS PubMed.
- A.-D. Manick, B. Salgues, J.-L. Parrain, E. Zaborova, F. Fages, M. Amatore and L. Commeiras, Org. Lett., 2020, 22, 1894–1898 CrossRef CAS PubMed.
- Y. Yamamoto, J. Ishii, H. Nishiyama and K. Itoh, J. Am. Chem. Soc., 2004, 126, 3712–3713 CrossRef CAS PubMed.
- Y. Yamamoto, J. Ishii, H. Nishiyama and K. Itoh, J. Am. Chem. Soc., 2005, 127, 9625–9631 CrossRef CAS PubMed.
- E. P. Gillis and M. D. Burke, Aldrichimica Acta, 2009, 42, 17–27 CAS.
- J. Li, A. S. Grillo and M. D. Burke, Acc. Chem. Res., 2015, 48, 2297–2307 CrossRef CAS PubMed.
- M. C. Kozlowski, Org. Lett., 2022, 40, 7247–7249 CrossRef PubMed.
- G. E. Bell, J. W. B. Fyfe, E. M. Israel, A. M. Z. Slawin, M. Campbell and A. J. B. Watson, Org. Lett., 2022, 24, 3024–3027 CrossRef CAS PubMed.
- J. M. Halford-McGuff, E. M. Israel, M. J. West, J. C. Vantourout and A. J. B. Watson, Eur. J. Org. Chem., 2022, e202200993 CAS.
- K. H. Vardhan, R. G. Satish, K. Ramesh, K. Karnakar and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 3, 3061–3065 Search PubMed.
- F. Peng, M. McLaughlin, Y. Liu, I. Mangion, D. M. Tschaen and Y. Xu, J. Org. Chem., 2016, 81, 10009–10015 CrossRef CAS PubMed.
- B. Huang, H. Tian, S. Lina, M. Xie, X. Yu and Q. Xu, Tetrahedron Lett., 2013, 54, 2861–2864 CrossRef CAS.
Footnotes |
| † The research data supporting this publication can be accessed at https://doi.org/10.17630/b45cb8f3-7047-4839-b413-df49ab7f3e7d. |
| ‡ Electronic supplementary information (ESI) available: Characterization data (PDF), copies of 1H and 13C NMR spectra (PDF), crystal structure data (PDF). CCDC 2181492 (Compound 19), 2181493 (Compound 25) and 2181494 (Compound 33) contains the supplementary crystallographic data for this study. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc00761h |
| This journal is © The Royal Society of Chemistry 2023 |