Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal-coordinated distibene and dibismuthene dications – isoelectronic analogues of butadiene dications†
Hanns M.
Weinert
 a,
Yannick
Schulte
a,
Alexander
Gehlhaar
a,
Yannick
Schulte
a,
Alexander
Gehlhaar
 a,
Christoph
Wölper
a,
Gebhard
Haberhauer
a,
Christoph
Wölper
a,
Gebhard
Haberhauer
 b and
Stephan
Schulz
b and
Stephan
Schulz
 *ac
*ac
aInstitute of Inorganic Chemistry, University of Duisburg-Essen, Universitätsstr. 5–7, Essen 45141, Germany. E-mail: stephan.schulz@uni-due.de
bInstitute of Organic Chemistry, University of Duisburg-Essen, Universitätsstr. 5–7, Essen 45141, Germany
cCenter for Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, Carl-Benz-Str. 199, Duisburg 47057, Germany
First published on 17th May 2023
Abstract
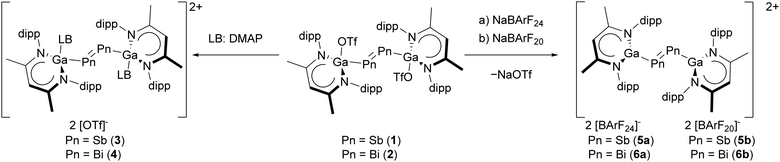
We report the synthesis and solid-state structures of DMAP-coordinated ([L(DMAP)GaPn]2[OTf]2; Pn = Sb 3, Bi 4) and base-free dipnictene dications ([LGaPn]2[BArFx]2, Pn = Sb: x = 24, 5a; 20, 5b; Bi: x = 24, 6a; 20, 6b). Quantum chemical calculations indicate that the dications 52+ and 62+ represent isoelectronic analogues of the butadiene dication.
With the structural characterisation of the first room temperature-stable diphosphene [Mes*P]2 (Mes* = 2,4,6-t-Bu3-C6H2)1 and disilene [Mes2Si]2 (Mes = 2,4,6-Me3-C6H2)2 in the early 1980s, the synthesis of multi-bonded compounds of heavy p-block elements became a rapidly developing field of research.3 Neutral dipnictenes4 have been kinetically stabilised using sterically demanding aryl,5 amide,6,7 boryle,7,8 ferrocenyl,9 and phosphanide ligands,10 whereas electropositive L(X)M units (L = HC[C(Me)N(2,6-i-Pr2C6H3)]2, M = Al,11 Ga12–14) have only recently been introduced as stabilising ligands.
Cationic dipnictenes recently received increasing interest.15 Dipnictene dications were synthesised by oxidation of NHC- (I) and NHO-coordinated (II) dipnictenes (Scheme 1),16–18 In contrast, distibene and dibismuthene cations stabilised by neutral carbenes are unknown,19,20 although the neutral carbene-coordinated distibene IV21 and dipnictene dications coordinated by anionic carbenes ((WCA-IDipp)2E2, E = P-Bi, V) have been reported.22 In addition, carbene-coordinated bismuthinidenes (III, VI) were structurally characterised.23 The reduced stability of carbene-coordinated low valent heavy pnictogen compounds most likely results from the decreasing (2p–np)π interactions (n = 5 (Sb), 6 (Bi)).19,24,25 However, we demonstrated that L(X)Ga ligands stabilise Sb- and Bi-centred radicals and π-bonded compounds26 including L(X)Ga-substituted cations and dipnictene radical anions.27 We report here the synthesis of base-coordinated ([L(DMAP)GaPn]2[OTf]2 (Pn = Sb 3, Bi 4)) and base-free ([LGaPn]2[BArFx]2 (x = 24, Pn = Sb 5a, Bi 6a; x = 20, Pn = Sb 5b, Bi 6b) dipnictene dications using triflate-coordinated dipnictenes [L(TfO)GaPn]2 (Pn = Sb, Bi).
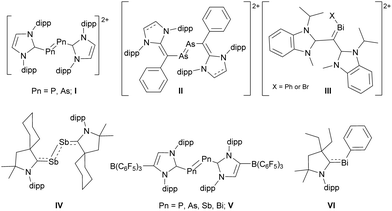 | ||
| Scheme 1 Structurally characterized carbene-coordinated dipnictene dications (I, II, V), carbene-coordinated IV, bismuth dication III and bismuthinidene VI. | ||
The synthesis of [L(TfO)GaBi]2 (2) by reaction of LGa and Bi(OTf)3 has been reported by Fischer et al.,12 however, we only isolated mixtures of LGa(H)OTf (7),28 Bi-centred radical [L(TfO)Ga]2Bi˙ (8) as was observed previously under similar reaction conditions for [L(X)Ga]2Bi˙ (X = Cl, I),29 and dibismuthene 2, which was isolated by fractional crystallization from 1,2-difluorobenzene. The analogous reaction of LGa and Sb(OTf)3 gave [L(TfO)GaSb]2 (1) in low yields (<20%), however, 1 was selectively formed in the reaction of MeOTf and [L(Me2N)GaSb]2. Room temperature 1H NMR spectra of 1 and 2 in CD2Cl2 showed broad resonances (Fig. S1 and S8, ESI†), indicating (partial) dissociation of the OTf group in solution. In contrast, sharp singlets for the γ-H atoms and the Me groups of the ligand backbone (ArNCCH3) as well as two septets and four doublets for the i-Pr groups were observed at temperatures below −20 °C (Fig. S39 and S40, ESI†) and in less polar solvents such as C6D6 (Fig. S4, ESI†).12 The reaction of 4-dimethylaminopyridine (DMAP) with 1 and 2 gave [L(DMAP)GaPn]2[OTf]2 (Pn = Sb 3, Bi 4) containing DMAP-coordinated dipnictene dications (Scheme 2). The OTf groups in radical 8 can be substituted analogously, but the product decomposed and only few crystals of [L(DMAP)Ga]2Bi[OTf] (9) were isolated and analysed (sc-XRD, Fig. S53, ESI†).
In contrast, reactions of 1 and 2 with Lewis base-free salts of weakly coordinating anions (WCAs), NaBArF24 and NaBArF20 (BArF24 = B(3,5-(CF3)2C6H3)4, BArF20 = B(C6F5)4), proceeded with elimination of NaOTf and formation of [LGaPn]2[BArF24]2 (Pn = Sb 5a; Bi 6a) and [LGaPn]2[BArF20]2 (Pn = Sb 5b; Bi 6b), respectively. Salts 3–6 are soluble in polar, non-coordinating solvents (e.g. CH2Cl2, 1,2-difluorobenzene) but insoluble in non-polar hydrocarbons (e.g. n-hexane, toluene, benzene) as well as fluorobenzene and bromobenzene (5a, 6a).
The 1H NMR spectra of DMAP-substituted dications 3 and 4 show singlets for the γ-H atoms and the Me groups and two septets and four doublets for the i-Pr groups. The rotation of the Ga-NDMAP bonds in 3 and 4 is restricted, resulting in magnetically inequivalent aromatic protons of the DMAP ligand (Table S1, ESI†). In contrast, the base-free dications in 5 and 6 show only one septet and two doublets for the i-Pr groups, indicating trigonal-planar coordinated Ga centres or rapid inversion processes in the case of a pyramidal coordination sphere in solution. The γ-H resonances are shifted to lower field (Table S1, ESI†) due to an increased Lewis acidity of the Ga centres within the base-free dications of 5 and 6 compared to 1–4. Electron density is therefore effectively pulled away from the ligand backbone and the dipnictene π system towards the Ga atoms. The coordination of an electropositive gallanediyl (LGa) ligand seems to be essential for the stabilisation of the dipnictene dications as was observed in the isolation of silylene-carbonyl complexes.30 The π → π* transition in the dications 5a and 6a is red-shifted compared to their neutral counterparts (UV/Vis: 431 (1) to 471 (5a); 505 (2) to 542 (6a) nm; Fig. S41–S44, S66, ESI†), indicating electronic changes within the π system.11b
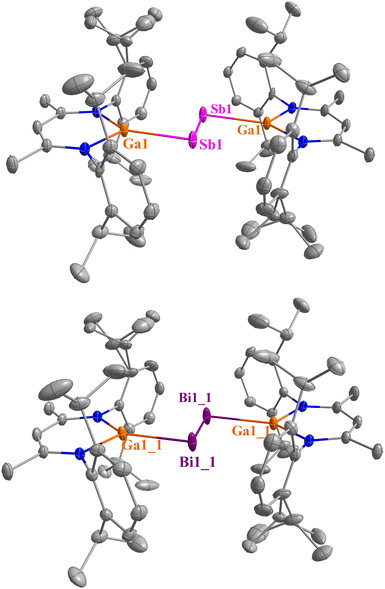
Single crystals of 1 and 2 (Fig. S45 and S46, ESI†) were obtained from saturated CH2Cl2 and fluorobenzene solutions upon storage at −30 °C, while those of 3, 4, 5a, and 6a (Fig. S47–S50, ESI†) were grown by slow diffusion of toluene or benzene into CH2Cl2 solutions of 3, 4, 5a, and 6a, respectively. The molecular units of 5a and 6a are disordered. Crystals of 5b and 6b were grown by layering their 1,2-difluorobenzene solutions with n-hexane (Fig. 1). Compounds 1 and 2 crystallise in the monoclinic space group C2/c, and those of 3, 4, 5b, and 6b in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively. The molecular units of all dipnictenes are located on a special position with a centre of inversion at the midpoint between both pnictogens atoms.
, respectively. The molecular units of all dipnictenes are located on a special position with a centre of inversion at the midpoint between both pnictogens atoms.
The Pn–Pn bonds of 5b (2.6530(3) Å) and 6b (2.8347(2) Å) are slightly elongated compared to the neutral dipnictenes 1 (2.6395(4) Å) and 2 (2.8145(2) Å), while the Ga–Pn bonds are slightly shortened (2.5578(3) 5b, 2.6492 (6) 6b, 2.5800(4) 1, 2.6587(3) 2 Å). In contrast, the Ga–Pn bond lengths in the DMAP-coordinated complexes (3, 4) are elongated due to the increased coordination number at the Ga atom and the steric demand of the DMAP ligand (Table 1). The Ga atoms in both 5b and 6b are nearly planar as indicated by the root-mean-square deviation of the C3N2Ga atoms from the least-squares planes defined by those within the LGa ring, which decreased to only 0.01 Å for 5b and 0.02 Å for 6b compared to 0.177 Å (1) and 0.160 Å (2), respectively. Carbene-coordinated diarsenes and diphosphenes dications16,18,31 showed significantly different C–Pn and Pn–Pn (Pn = P, As) bond lengths compared to their neutral counterparts, i.e., the C–As bond lengths in NHO-coordinated diarsene dication IV are shortened by 0.1 Å while the As–As bond is elongated by 0.1 Å,18 most likely caused by electronic changes in the conjugated ligand-Pn2-system upon oxidation.
| Pn | [L(TfO)GaPn]2 (Pn = Sb 1, Bi 2) | [L(DMAP)GaPn]22+ (Pn = Sb 3, Bi 4) | [LGaPn]22+ (Pn = Sb 5b, Bi 6b) | |
|---|---|---|---|---|
| NL = Nimine of the β-diketiminate ligand. | ||||
| Pn–Pn | Sb | 2.6395(4) (2.6262) | 2.6433(5) (2.6389) | 2.6530(3) (2.6523) |
| Bi | 2.8145(2) (2.7785) | 2.8193(3) (2.7913) | 2.8347(2) (2.8047) | |
| Ga–Pn | Sb | 2.5800(4) (2.5905/2.5777) | 2.6067(4) (2.5942/2.6060) | 2.5578(3) (2.5789/2.5856) |
| Bi | 2.6587(3) (2.6666/2.6626) | 2.6873(3) (2.6707/2.6798) | 2.6492(6) (2.6584/2.6585) | |
| Ga–NL | Sb | 1.913(3) (1.923/1.934), 1.924(2) (1.942/1.936) | 1.937(1) (1.941/1.938), 1.950(1) (1.945/1.945) | 1.865(1) (1.879/1.882), 1.869(1) (1.883/1.883) |
| Bi | 1.925(2) 1.936/1.934, 1932(2) (1.945/1.943) | 1.942(2), (1.946/1.947) 1.955(2) (1.951/1.951) | 1.874(2) (1.889/1.888), 1.877(2) (1.889/1.888) | |
To gain further insight into the electronic structure of the molecules 1–6, bond energy analyses,32 quantum theory of atoms in molecules (QTAIM),33 and interacting quantum atoms (IQA)34 analyses were carried out (Fig. S54–S65, ESI†).
A comparison of the bond energy analyses for the homolytic and heterolytic cleavage of the Ga–Sb and Ga–Bi bonds shows that in all cases they are electron sharing bonds (Fig. S54 and S55, ESI†). The difference in the Ga–Pn and Pn–Pn bond lengths is small overall (Table 1), whereas the charges on the pnictogen atoms differ significantly. The base-free dications 52+ and 62+ have significantly higher positive charges on the pnictogen atoms (Fig. S64 and S65, ESI†) compared to the neutral compounds (1, 2) and the DMAP-stabilised dications (32+, 42+), which have similar charges. In addition, the ellipticity at the bond critical points of the Pn–Pn double bonds is higher in dications 52+ and 62+, indicating an increase in the π bond character (Fig. S58 and S60, ESI†).
As mentioned above, dications I and II were synthesised by a two-step oxidation reaction of the corresponding neutral NHC-coordinated dipnictenes. Therefore, the corresponding neutral compounds 5 and 6 were calculated for comparison. The reduction of the dipnictene dications to the neutral analogues leads to an increase in both the ellipticity at the bond critical points and the bond order of the Ga–Pn bonds, while both the ellipticity and the bond order decrease for the Pn–Pn bonds (Fig. S58 and S60, ESI†), which is reflected in a decrease of the Ga–Pn and an increase of the Pn–Pn bond lengths by more than 0.1 Å. In addition, the IQA analyses show an increase in the covalent part of the total interatomic interaction energies for the Ga–Pn bond and a decrease for the Pn–Pn bond (Fig. S62 and S63, ESI†). The twofold reduction also leads to a greater increase in the electron density at the Pn atoms compared to the Ga atoms. In other words, the charge changes in oxidation and reduction essentially take place at the Pn centre.
Base-coordinated and base-free dipnictene dications were structurally characterised. Quantum chemical calculations and UV-vis spectroscopy studies reveal a weak π backbonding contribution from the Pn2 unit to the LGa ligand. Due to the large size of the frontier orbitals, this interaction is much smaller than expected for similar systems composed of lighter elements. The neutral molecules 5 and 6 can be considered as isoelectronic analogues of butadiene and the cations 52+ and 62+ represent analogues of the butadiene dication.
M. W. performed the experiments, C. W. the single crystal X-ray diffraction, and G. H. the quantum chemical calculations. Y. S. and A. G. assisted with the single crystal X-ray diffraction experiments. The work was supervised by S. S. The manuscript was written with contributions from all authors. All authors approved the final version of the manuscript.
Financial support by the Deutsche Forschungsgemeinschaft (DFG, SCHU 1069/22-3, INST 20876/282-1 FUGG) and the University of Duisburg-Essen is acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Yoshifuji, I. Shima, N. Inamoto, K. Hirotsu and T. Higuchi, J. Am. Chem. Soc., 1981, 103, 4587 CrossRef CAS.
- R. West, M. J. Fink and J. Michl, Science, 1981, 214, 1343 CrossRef CAS PubMed.
- (a) J. D. Protasiewicz, Comprehensive inorganic chemistry II. From elements to applications, (Hrsg.: J. Reedijk), Elsevier, Amsterdam, 2013, ch. 1.12, pp. 325–348 Search PubMed; (b) C. Präsang and D. Scheschkewitz, Chem. Soc. Rev., 2016, 45, 900 RSC; (c) P. P. Power, Organometallics, 2020, 39, 4127 CrossRef CAS; (d) S. Schulz, Chem. – Eur. J., 2010, 16, 6416 CrossRef CAS PubMed; (e) L. Weber, F. Ebeler and R. S. Ghadwal, Coord. Chem. Rev., 2022, 461, 214499 CrossRef CAS.
- (a) A. H. Cowley, J. G. Lasch, N. C. Norman and M. Pakulski, J. Am. Chem. Soc., 1983, 105, 5506 CrossRef CAS; (b) N. Tokitoh, Y. Arai, R. Okazaki and S. Nagase, Science, 1997, 277, 78 CrossRef CAS; (c) N. Tokitoh, Y. Arai, T. Sasamori, R. Okazaki, S. Nagase, H. Uekusa and Y. Ohashi, J. Am. Chem. Soc., 1998, 120, 433 CrossRef CAS.
- (a) P. K. Majhi, H. Ikeda, T. Sasamori, H. Tsurugi, K. Mashima and N. Tokitoh, Organometallics, 2017, 36, 1224 CrossRef CAS; (b) B. Twamley, C. d Sofield, M. M. Olmstead and P. P. Power, J. Am. Chem. Soc., 1999, 121, 3357 CrossRef CAS; (c) T. Sasamori and N. Tokitoh, Dalton Trans., 2008, 1395 RSC.
- R. J. Schwamm and M. P. Coles, Chem. – Eur. J., 2019, 25, 14183 CrossRef CAS PubMed.
- D. Dange, A. Davey, J. A. B. Abdalla, S. Aldridge and C. Jones, Chem. Commun., 2015, 51, 7128 RSC.
- C. Helling, J. Haak, C. Wölper, G. E. Cutsail and S. Schulz, Inorg. Chem., 2022, 61, 5124 CrossRef CAS PubMed.
- M. Sakagami, T. Sasamori, H. Sakai, Y. Furukawa and N. Tokitoh, Chem. – Asian J., 2013, 8, 690 CrossRef CAS PubMed.
- C. von Hänisch and D. Nikolova, Eur. J. Inorg. Chem., 2006, 4770 CrossRef.
- (a) L. Tuscher, C. Helling, C. Ganesamoorthy, J. Krüger, C. Wölper, W. Frank, A. S. Nizovtsev and S. Schulz, Chem. – Eur. J., 2017, 23, 12297 CrossRef CAS PubMed; (b) H. M. Weinert, C. Wölper and S. Schulz, Organometallics, 2021, 40, 3486 CrossRef CAS.
- G. Prabusankar, C. Gemel, P. Parameswaran, C. Flener, G. Frenking and R. A. Fischer, Angew. Chem., Int. Ed., 2009, 48, 5526 CrossRef CAS PubMed.
- L. Tuscher, C. Ganesamoorthy, D. Bläser, C. Wölper and S. Schulz, Angew. Chem., Int. Ed., 2015, 54, 10657 CrossRef CAS PubMed.
- L. Tuscher, C. Helling, C. Wölper, W. Frank, A. S. Nizovtsev and S. Schulz, Chem. – Eur. J., 2018, 24, 3241 CrossRef CAS PubMed.
- (a) C. Lichtenberg, Chem. Commun., 2021, 57, 4483 RSC; (b) K. Oberdorf, P. Grenzer, N. Wieprecht, J. Ramler, A. Hanft, A. Rempel, A. Stoy, K. Radacki and C. Lichtenberg, Inorg. Chem., 2021, 60, 19086 CrossRef CAS PubMed; (c) J. Ramler, K. Hofmann and C. Lichtenberg, Inorg. Chem., 2020, 59, 3367 CrossRef CAS PubMed; (d) J. Ramler and C. Lichtenberg, Chem. – Eur. J., 2020, 26, 10250 CrossRef CAS PubMed; (e) J. E. Walley, L. S. Warring, G. Wang, D. A. Dickie, S. Pan, G. Frenking and R. J. Gilliard, Angew. Chem., Int. Ed., 2021, 60, 6682 CrossRef CAS PubMed; (f) H. Steffenfauseweh, D. Rottschäfer, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, D. W. Szczepanik and R. Ghadwal, Angew. Chem., Int. Ed., 2023, 62, e202216003 CrossRef CAS PubMed.
- O. Back, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Nat. Chem., 2010, 2, 369 CrossRef CAS PubMed.
- M. Y. Abraham, Y. Wang, Y. Xie, R. J. Gilliard, P. Wei, B. J. Vaccaro, M. K. Johnson, H. F. Schaefer, P. V. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2013, 135, 2486 CrossRef CAS PubMed.
- M. K. Sharma, S. Blomeyer, B. Neumann, H.-G. Stammler, M. Gastel, A. Hinz and R. S. Ghadwal, Angew. Chem., Int. Ed., 2019, 58, 17599 CrossRef CAS PubMed.
- A. Doddi, M. Peters and M. Tamm, Chem. Rev., 2019, 119, 6994 CrossRef CAS PubMed.
- (a) Y. Wang and G. H. Robinson, Inorg. Chem., 2011, 50, 12326 CrossRef CAS PubMed; (b) Y. Wang and G. H. Robinson, Inorg. Chem., 2014, 53, 11815 CrossRef CAS PubMed; (c) S. K. Kushvaha, A. Mishra, H. W. Roesky and K. C. Mondal, Chem. – Asian J., 2022, 17, e202101301 Search PubMed; (d) P. Bellotti, M. Koy, M. N. Hopkinson and F. Glorius, Nat. Rev. Chem., 2021, 5, 711 CrossRef CAS PubMed; (e) B. Borthakur, B. Ghosh and A. K. Phukan, Polyhedron, 2021, 197, 115049 CrossRef CAS; (f) R. Deka and A. Orthaber, Dalton Trans., 2022, 51, 8540 RSC.
- R. Kretschmer, D. A. Ruiz, C. E. Moore, A. L. Rheingold and G. Bertrand, Angew. Chem., Int. Ed., 2014, 53, 8176 CrossRef CAS PubMed.
- (a) L. P. Ho, A. Nasr, P. G. Jones, A. Altun, F. Neese, G. Bistoni and M. Tamm, Chem. – Eur. J., 2018, 24, 18922 CrossRef CAS PubMed; (b) L. P. Ho and M. Tamm, Dalton Trans., 2021, 50, 1202 RSC.
- G. Wang, L. Freeman, D. Dickie, R. Mokrai, Z. Benkő and R. J. Gilliard, Chem. – Eur. J., 2019, 25, 4335 CrossRef CAS PubMed.
- V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt and S. Inoue, Chem. Rev., 2018, 118, 9678 CrossRef CAS PubMed.
- D. J. D. Wilson, S. A. Couchman and J. L. Dutton, Inorg. Chem., 2012, 51, 7657 CrossRef CAS PubMed.
- (a) C. Helling, G. E. Cutsail, H. Weinert, C. Wölper and S. Schulz, Angew. Chem., Int. Ed., 2020, 59, 7561 CrossRef CAS PubMed; (b) J. Krüger, C. Wölper, A. A. Auer and S. Schulz, Eur. J. Inorg. Chem., 2022, 1 Search PubMed; (c) J. Krüger, C. Wölper and S. Schulz, Angew. Chem., Int. Ed., 2021, 60, 3572 CrossRef PubMed; (d) J. Krüger, C. Wölper, L. John, L. Song, P. R. Schreiner and S. Schulz, Eur. J. Inorg. Chem., 2019, 1669 CrossRef; (e) J. Krüger, J. Schoening, C. Ganesamoorthy, L. John, C. Wölper and S. Schulz, Z. Anorg. Allg. Chem., 2018, 644, 1028 CrossRef; (f) J. Krüger, C. Ganesamoorthy, L. John, C. Wölper and S. Schulz, Chem. – Eur. J., 2018, 24, 9157 CrossRef PubMed; (g) C. Ganesamoorthy, J. Krüger, C. Wölper, A. S. Nizovtsev and S. Schulz, Chem. – Eur. J., 2017, 23, 2461 CrossRef CAS PubMed.
- (a) J. Krüger, J. Haak, C. Wölper, G. E. Cutsail, G. Haberhauer and S. Schulz, Inorg. Chem., 2022, 61, 5878 CrossRef PubMed; (b) B. Li, C. Wölper, G. Haberhauer and S. Schulz, Angew. Chem., Int. Ed., 2021, 60, 1986 CrossRef CAS PubMed; (c) J. Krüger, C. Wölper, G. Haberhauer and S. Schulz, Inorg. Chem., 2022, 61, 597 CrossRef PubMed; (d) H. M. Weinert, C. Wölper, J. Haak, G. E. Cutsail and S. Schulz, Chem. Sci., 2021, 12, 14024 RSC.
- 7 formed due to residual water in commercial Bi(OTf)3.
- (a) J. Krüger, C. Wölper and S. Schulz, Inorg. Chem., 2020, 59, 11142 CrossRef PubMed; (b) C. Ganesamoorthy, C. Helling, C. Wölper, W. Frank, E. Bill, G. E. Cutsail and S. Schulz, Nat. Commun., 2018, 9, 87 CrossRef PubMed.
- (a) J. Schoening, C. Ganesamoorthy, C. Wölper, E. Solel, P. R. Schreiner and S. Schulz, Dalton Trans., 2022, 51, 8249 RSC; (b) C. Ganesamoorthy, J. Schoening, C. Wölper, L. Song, P. R. Schreiner and S. Schulz, Nat. Chem., 2020, 12, 608 CrossRef CAS PubMed.
- (a) M. Y. Abraham, Y. Wang, Y. Xie, R. J. Gilliard, P. Wei, B. J. Vaccaro, M. K. Johnson, H. F. Schaefer, P. V. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2013, 135, 2486 CrossRef CAS PubMed; (b) A. Doddi, D. Bockfeld, M.-K. Zaretzke, T. Bannenberg and M. Tamm, Chem. – Eur. J., 2019, 25, 13119 CrossRef CAS PubMed; (c) M. K. Sharma, D. Rottschäfer, S. Blomeyer, B. Neumann, H.-G. Stammler, M. van Gastel, A. Hinz and R. S. Ghadwal, Chem. Commun., 2019, 55, 10408 RSC; (d) A. Doddi, D. Bockfeld, M.-K. Zaretzke, C. Kleeberg, T. Bannenberg and M. Tamm, Dalton Trans., 2017, 46, 15859 RSC.
- F. M. Bickelhaupt and E. J. Baerends, Rev. Comput. Chem., 2000, 15, 1–86 CAS.
- R. F. W. Bader, Atoms in Molecules: A Quantum Theory, Oxford University Press, Oxford, U.K., 1990 Search PubMed.
- M. A. Blanco, A. Martín Pendás and E. Francisco, J. Chem. Theory Comput., 2005, 8, 1096 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures and analytical data, NMR, IR, and UV-vis spectra, computational details and cif files. CCDC 2237145 (1), 2237146 (2), 2237147 (3), 2237148 (4), 2255817 (5a), 2237149 (5b), 2237151 (6a), 2237152 (6b), 2237153 (7), 2237154 (8), and 2237155 (9). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc01844j |
| This journal is © The Royal Society of Chemistry 2023 |