Open Access Article
Open Access ArticleThermal cyclodimerization of isoprene for the production of high-performance sustainable aviation fuel†
Josanne-Dee
Woodroffe
 and
Benjamin G.
Harvey
and
Benjamin G.
Harvey
 *
*
US NAVY, NAWCWD, Research Department, Chemistry Division, China Lake, California 93555, USA. E-mail: benjamin.g.harvey@navy.mil
First published on 8th April 2022
Abstract
Isoprene was converted to a jet fuel blendstock through thermal cyclodimerization followed by hydrogenation. The dimerization was performed at moderate temperature (200 °C) and completed in 90 min. The product distribution consisted of six isomers produced via Diels–Alder [4+2]-cycloadditions as well as [4+4]-cycloadditions. The hydrogenation reactions were conducted with Pt, Pd, and Ni-based catalysts. The Pt and Ni catalysts yielded >99% saturated C10H20 products, while significant quantities of p- and m-cymene were generated with the Pd-catalyst. The hydrogenated isoprene dimers (HID) exhibited outstanding fuel properties including a gravimetric net heat of combustion (NHOC) of 43.34 MJ kg−1, a density of 0.806 g mL−1, and a −20 °C kinematic viscosity of 3.10 mm2 s−1. These values are 1.3% higher, 4% higher, and 61% lower, respectively, compared to the Jet-A specifications. These properties suggest that HID can be used as a blendstock with either conventional jet fuel or bio-based synthetic paraffinic kerosenes (SPKs) to enhance the operability and performance of the final fuel blend. The catalyst- and solvent-free dimerization method is amenable to a high-throughput process for fuel production. In combination with biosynthetic generation of isoprene or isoprene precursors, the approach described herein holds great promise for the formulation of drop-in sustainable aviation fuels.
Introduction
The growing impacts of global warming have heightened the need to develop sustainable methods for the production of transportation fuels.1,2 Although a number of ground vehicles can be powered by electricity, the enormous power requirements of commercial and military jet aircraft suggest that the development of energy-dense, hydrocarbon-based sustainable aviation fuels (SAF) will be critical for supporting global commerce while reducing net greenhouse gas emissions.1,3 Current SAFs are based on acyclic synthetic paraffinic kerosenes (SPKs) that have outstanding gravimetric net heats of combustion (NHOCs), but low densities, and low volumetric NHOCs.4,5 Furthermore, these SPKs are not effective at swelling nitrile rubber engine seals, which requires them to be blended with conventional jet fuel to ensure engine integrity and compatibility with fuel storage and delivery systems. Conventional jet fuel contains a significant concentration of aromatic compounds (8–25%) that are highly effective at swelling elastomeric O-rings. Recent studies have shown that some cycloalkanes are also effective at swelling O-rings,6–8 which suggests that zero-aromatic SAF blends could be prepared that meet the requirements of conventional jet fuel. The elimination of aromatic compounds results in a number of enhanced properties including increased gravimetric NHOC, improved combustion properties, and reduced particulate formation.9–11 To realize these optimized blends, efficient routes for the production of bio-based cycloalkanes are required.A number of selective methods for the production of bio-based cycloalkanes have been developed.3 For example, our group studied alkyl cyclobutane fuels generated by [Fe]-catalyzed [2+2]-cycloaddition of unactivated alkenes.12 Multicyclic hydrocarbons like tetrahydrodicyclopentadiene (JP-10) have been synthesized from furfural13 while dimethyltetrahydrodicyclopentadiene (RJ-4) has been synthesized from bio-based substrates including linalool,14 and cellulose-derived platform chemicals.15–17 Cyclic monoterpenes are an important class of bio-based cycloalkenes that can be derived from turpentine or produced via fermentation of biomass sugars.18–20 Simple hydrogenation of these feedstocks can generate monocyclic and bicyclic hydrocarbons,4 while chemoselective hydrogenation can afford bicyclic alkanes containing ring-strained cyclopropane groups.21 The densities and volumetric NHOCs of terpenes can be further enhanced through cyclopropanation reactions.22–25
Isoprene is a compelling substrate for the preparation of sustainable jet fuel blendstocks. It can be directly produced from biomass sugars via fermentation,26 by dehydration of biosynthetic 3-methyl-3-buten-1-ol,27 or from other bio-based substrates including mevalonolactone28,29 and mesaconic/itaconic acid.30–32 In addition, isoprene and related dienes can be produced by the dehydra-decyclization of bio-based tetrahydrofurans.32–34 The conjugated diene in isoprene allows for the controlled synthesis of dimers. For example, dimerization of isoprene with palladium catalysts can yield various acyclic C10 trienes.35 More recently, Ottosson and co-workers reported a photochemical route to cyclic isoprene dimers.36 Isoprene can also be effectively dimerized via a [4+4]-cycloaddition catalyzed by an iron pyridineimine (Fe-PDI) catalyst to generate 2,6-dimethyl-1,5-cyclooctadiene (DMCOD).37,38 As an alternative to these approaches, isoprene can undergo a [4+2]-cycloaddition to generate substituted cyclohexenes. This reaction can be catalyzed by low valent iron catalysts39,40 or conducted under high temperature/pressure conditions.41
To evaluate the potential to generate a high-performance jet fuel blendstock from isoprene using an economical and high-throughput approach, the thermal cyclodimerization of isoprene was conducted followed by hydrogenation with three different heterogeneous catalysts. The fuel properties of the resulting blend were then measured and compared to related bio-based fuels as well as conventional petroleum-based jet/rocket fuels.
Experimental
General
All glassware was dried in an oven at 140 °C prior to use. Isoprene was obtained from Millipore Sigma and distilled from CaH2 under nitrogen. 4,6-Dinitro-o-cresol was obtained from Eastman Organic Chemicals and was used as received. 1H and 13C NMR spectra were obtained as described previously.22 Fuel characterization details (GC-FID, GC-MS, kinematic viscosity, density and heat of combustion measurements) are included in the ESI.†Thermal dimerization of isoprene
A Parr reactor was charged with freshly distilled isoprene (524.4 g, 7.7 mol), 4,6-dinitro-o-cresol (0.524 g, 1000 ppm), and swept with nitrogen. The reactor was then heated to 200 °C and stirred for 90 min. To reduce the formation of heavy oligomers, the reactor was rapidly cooled in a salt/ice bath to ambient temperature and the reaction mixture was then transferred to a round bottom flask fitted with a short-path distillation apparatus. The product was distilled under reduced pressure to obtain three fractions. The first fraction contained residual isoprene (47.44 g, 9.0%). Fraction 2 (75–79 °C, 44.6 mm Hg) contained a mixture of isoprene dimers (375.98 g, 71.7%), and fraction 3, the residual material, contained trimers and heavier oligomers (67.74 g, 12.9%). 1H NMR of the dimer fraction (500 MHz, CDCl3): δ 6.49–6.42 (m), 5.86–5.78 (m), 5.78–5.41 (m), 5.21–5.17 (bd), 5.09–5.06 (bd), 5.01–4.91 (m), 4.72 (s), 2.36–1.64 (m), 1.57–1.34 (m), 1.00 (s). 13C NMR (125 MHz, CDCl3) δ 150.11, 150.06, 147.7, 147.6, 135.8, 133.7, 133.5, 132.9, 122.7, 122.5, 120.8, 120.1, 119.6, 110.3, 110.2, 108.6, 108.5, 41.7, 41.2, 36.9, 35.6, 34.1, 33.5, 30.9, 30.7, 28.0, 27.8, 27.4, 25.8, 25.7, 23.8, 23.5, 23.5, 20.84, 20.79. MS data: molecular ion peaks at 136 m/z for the dimer fraction.Hydrogenation of isoprene dimers with Pt
PtO2 (0.5 g, 2.2 mmol) and glacial acetic acid (10 mL, 0.17 mol) were added to isoprene dimers (57.12 g, 0.42 mol) in a Parr reactor. The reaction vessel was charged with hydrogen 3 times via pump/pressurize cycles, and then pressurized to 45 psi and stirred for 24 h at ambient temperature. The reaction mixture was filtered through Celite with diethyl ether, and the filtrate was then washed with a 10% aqueous solution of Na2CO3 (2 × 20 mL) and brine (2 × 20 mL). The organic extracts were dried with MgSO4 and concentrated under reduced pressure. The crude product was then distilled under reduced pressure (50–52 °C, 12.2 mm Hg) to yield 44.22 g (75.1%) of hydrogenated dimers as a complex mixture of isomers with 0.44% (by GC-FID) aromatic compounds (p- and m-cymene). 1H NMR (500 MHz, CDCl3) δ 1.89–1.84 (m), 1.78–1.57 (m), 1.62–1.57 (m), 1.56–1.19 (m), 1.18–1.02 (m), 0.96–0.59 (m). MS molecular ion for saturated hydrocarbons: 140 m/z. MS molecular ion for aromatic compounds: 134 m/z. Anal. calc. for C10H20: C, 85.63; H, 14.37. Found: C, 85.48; H, 14.27.Hydrogenation of isoprene dimers with RANEY® Ni
In a similar manner to the procedure described for platinum, activated RANEY® nickel in ethanol (3.13 g. 4 mol%) was added to isoprene dimers (64.99 g, 0.48 mol) in a Parr reactor, which was then pressurized to 500 psi H2 and heated to 150 °C with vigorous stirring for 24 h. The reaction mixture was transferred to a flask with diethyl ether, filtered through Celite, dried with MgSO4, and concentrated under reduced pressure. The crude product was then distilled under N2 at 154–156 °C (atmospheric pressure) to yield 59.4 g (88.8%) of a mixture of isomers with 0.68% (by GC-FID) aromatic compounds (p- and m-cymene). Spectroscopic data were similar to those obtained from the Pt-catalyzed hydrogenation.Hydrogenation of isoprene dimers with Pd/C
In a similar manner to the procedure described for platinum, 10% Pd/C (1.64 g, ∼0.164 g Pd) was added to isoprene dimers (123.6 g, 0.91 mol) and acetic acid (3.5 mL) in a Parr reactor, which was then pressurized to 500 psi and stirred for 24 h. A salt-ice bath was used to control the exotherm and the temperature of the reactor did not increase above 29 °C. The reaction mixture was then diluted with diethyl ether, filtered through Celite, and the filtrate was then washed with a 10% aqueous solution of Na2CO3 (2 × 40 mL) and brine (2 × 40 mL). The organic extracts were dried with MgSO4 and concentrated under reduced pressure. The crude product was then distilled (62–64 °C, 17.6 mm Hg,) to yield 104.5 g (82.2%) of a mixture of isomers with 6.82% (by GC-FID) aromatic compounds (p- and m-cymene). Spectroscopic data were similar to those obtained from the Pt-catalyzed hydrogenation.Results and discussion
Recent work on the selective dimerization of isoprene showed that an iron pyridineimine (Fe-PDI) catalyst could be used for the selective transformation of isoprene into 1,6-dimethyl-1,5-cyclooctadiene (DMCOD).37,38 DMCOD could subsequently be hydrogenated to generate 1,4-dimethylcyclooctane, a high-performance jet fuel blendstock exhibiting a higher density and gravimetric heat of combustion compared to conventional jet fuel.37 Although the dimerization reaction proceeded with yields greater than 90% and high selectivity, it was of interest to study other approaches for the preparation of jet fuel blendstocks from isoprene. A number of literature examples describe the use of low-valent iron catalysts for the [4+2]-Diels–Alder cycloaddition of isoprene.39,40 In contrast to these approaches, the catalyst-free cycloaddition of isoprene was studied with an eye toward a high-throughput, low-cost method that would potentially allow for the production of jet fuel surrogates on a commercial scale.Using a similar approach to that described by Colvin,41 we thermally dimerized isoprene at 200 °C in the presence of a radical inhibitor, 4,6-dinitro-o-cresol, to reduce the formation of heavy oligomers. Fractional distillation of the product resulted in a 71.7% isolated yield of isoprene dimers, 9.0% unreacted isoprene, and 12.9% trimers and heavier oligomers, with a mass balance of 93.7%. Although the oligomers were not extensively studied in this work, the trimers have potential applications as high-density turbine or diesel fuels42–44 while the heavier oligomers can be used in heating oil or heavy diesel for ship propulsion.45–47 Residual isoprene can be recycled for further conversion to dimers, and losses due to distillation or the volatility of isoprene can be minimized in an industrial environment, resulting in near quantitative conversion of isoprene to fuel products.
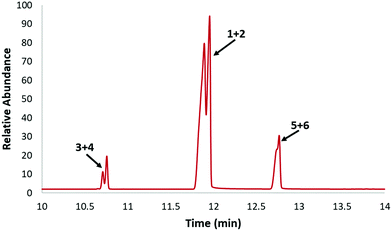
Six different dimer isomers were expected from a combination of [4+2]-Diels–Alder and [4+4]-cycloaddition of isoprene (Fig. 1). The composition of the dimer fraction was first studied by GC-MS, which revealed three poorly resolved sets of peaks (Fig. 2). A careful evaluation of the mass spectra and comparison of retention times with authentic samples of limonene and 1,6-dimethyl-1,5-cyclooctadiene (5) showed that 1 and 2 comprised 79% of the product with 5 and 6 comprising 15% and 3 and 4 accounting for only 6% of the total. Compounds 1–4 are produced via [4+2] Diels Alder cycloadditions, while 5 and 6 are generated via [4+4] cycloadditions. 1 and 2 are present in the highest concentrations due to reduced steric hindrance when the primary alkene of isoprene serves as the dienophile. These values can be compared to those obtained by Colvin (29.1% 1, 33.6% 2, 18.6% 3, 9.2% 4, 5.2% 5, 4.2% 6).41 The difference in the product distribution reported by Colvin is likely due to the lower temperature conditions used in that work (175 °C). Other factors could include isoprene purity, heating/cooling rates, or even the reactor used for the cycloaddition. [4+4]-Cycloadditions are forbidden in the ground state, so it's possible that interactions with the reactor surface may facilitate the formation of cyclooctadienes. The higher concentration of cyclooctadienes in the current work is beneficial due to the higher density and heat of combustion of the hydrogenated products.
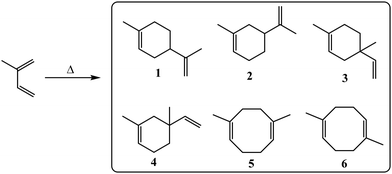 | ||
| Fig. 1 Structures of isoprene dimers generated by Diels–Alder (1–4) and [4+4]-cycloaddition (5 and 6). | ||
To generate a jet fuel blendstock from the isoprene dimers, the mixture was hydrogenated with three different commercial catalysts: PtO2, 10% Pd/C, and RANEY® Ni. Hydrogenation with PtO2 was accomplished at ambient temperature and low pressure (45 psi). These conditions were selected to minimize the dehydrogenation of compounds 1 and 2, which would lead to formation of p- and m-cymene, respectively. The resulting product distribution was composed of a complex mixture of C10H20 isomers, along with a trace (0.44%) of aromatic products (cymenes). Hydrogenation with RANEY® Ni required a much higher temperature and pressure (150 °C, 500 psi), but resulted in a similar product distribution, including 0.68% cymenes. The hydrogenation with 10% Pd/C was conducted with careful temperature control. Similar reactions with pure limonene are highly exothermic, with the Pd catalyst facilitating both isomerization and dehydrogenation.48,49 In the current work, the reactor was maintained at or below room temperature with the aid of an ice bath. Despite this precaution, the resulting hydrogenated product contained substantially more aromatic compounds (6.82%) compared to the other catalysts. Increased production of aromatic compounds could be desirable for fuel formulations due to the seal swelling property of this class of molecules,50 which is important for maintaining engine integrity. However, aromatic compounds also exhibit lower gravimetric net heats of combustion (NHOC) and are more prone to incomplete combustion and particulate emissions.9–11 Recent work has also shown that many cycloalkanes can effectively swell legacy nitrile rubber o-rings,6–8 while nearly all synthetic kerosenes are compatible with modern fluorosilicone or Viton™-based engine seals.
RANEY® nickel is an order of magnitude cheaper than Pt or Pd-based catalysts and only generates slightly more cymenes compared to PtO2. Therefore, nickel-based catalysts are likely preferred for the hydrogenation of the isoprene cyclodimers.51 However, the higher pressures and temperatures required to obtain complete conversion may partially offset the lower catalyst cost.
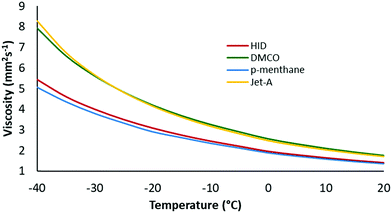
After successfully demonstrating the preparative-scale synthesis of saturated jet fuel blendstocks from isoprene, it was of interest to measure key fuel properties including density, heat of combustion, low-temperature viscosity, and flashpoint (Table 1). The density of the mixture was slightly higher than p-menthane (hydrogenated limonene), due to the presence of the higher density cyclooctane isomers. The density also falls within the specification range of commercial Jet-A, while it was 2.6% lower than 1,4-dimethylcyclooctane (DMCO). The gravimetric NHOC was slightly higher than p-menthane, well within the specification range for Jet-A, but lower than DMCO, due to the modest concentration of ring-strained (cyclooctane-based) structures in the mixture. The −40 °C viscosity of the HID mixture was intermediate between that of p-menthane and DMCO (Fig. 3) while the flashpoint was within the specification for Jet-A. These preliminary fuel screening results were promising and suggest that HID can be blended with Jet-A in high concentrations while imparting enhanced energy density (gravimetric NHOC) and lower viscosity. Although not studied in the current work, HID blends with other synthetic paraffinic kerosenes (SPKs) could be prepared to generate full-performance SAFs with broader distillation profiles.
| Fuel | NHOC (MJ kg−1) | NHOC (MJ L−1) | ρ [15 °C, g mL −1] | η [−20 °C, mm2 s−1] | η [−40 °C, mm2 s−1] | Flash point (°C) |
|---|---|---|---|---|---|---|
| HID | 43.34 | 34.94 | 0.806 | 3.10 | 5.45 | 42 |
| p-Menthane4 | 43.20 | 34.72 | 0.804 | 2.98 | 5.19 | NM |
| DMCO37 | 43.82 | 36.22 | 0.827 | 4.17 | 7.95 | 50 |
| Jet-A | >42.8 | >33.17 | >0.775 | <8.0 | <12.0 | >38 |
| RP-152 | 43.37 | 34.96 | 0.806 | — | — | 57 |
In addition to comparisons to conventional jet fuel, the similarities between the properties of the HID mixture and petroleum-derived rocket propellant (RP-1) are striking (Table 1). Both fuels have the same density, gravimetric NHOC, and similar H/C ratios (∼2). This suggests the potential to use HID as a sustainable surrogate for kerosene-based rocket fuels.
Conclusions
Isoprene is a versatile platform chemical that can be readily converted to jet fuel blendstocks with outstanding properties including, high energy density and low viscosity. In the current work, thermal dimerization of isoprene was shown to be a viable pathway for the generation of a high-performance jet fuel blendstock. Catalyst-free cyclodimerization holds great promise for the low-cost, high-throughput production of cyclic monoterpenes. Interestingly, this approach affords both cyclohexenes as well as dimethylcyclooctadienes. The formation of dimethylcyclooctadienes via [4+4]-cycloaddition in the absence of a conventional transition metal catalyst is surprising and warrants further study. Hydrogenation of the terpene mixture can be accomplished with both precious and first-row transition metals and the process tailored to generate significant amounts of p- and m-cymene, if desired.Large-scale production of jet fuels from biosynthetic isoprene will require significant fermentation advances including increased titers, more efficient direction of carbon flux toward isoprene, and the utilization of crude biomass sources (lignocellulosic) as feedstocks. Alternative approaches may include derivation of isoprene and related dienes from cyclic ethers generated through valorization of biomass. If efficient large-scale production of biosynthetic isoprene can be realized, hydrogenated isoprene dimers may become a key component in SAF blends used to power commercial and military jet aircraft in the coming years.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Dr Patrick Fedick and Mr Christopher Walkling for collecting GC-MS and GC-FID data, respectively. Financial support for this work was provided by the Office of Naval Research and the Naval Air Warfare Center, Weapons Division NISE-219 program.References
- H. Wei, W. Liu, X. Chen, Q. Yang, J. Li and H. Chen, Renewable bio-jet fuel production for aviation: A review, Fuel, 2019, 254, 115599 CrossRef CAS.
- S. S. Doliente, A. Narayan, J. F. D. Tapia, N. J. Samsatli, Y. Zhao and S. Samsatli, Front. Energy Res., 2020, 8, 110 CrossRef.
- J. A. Muldoon and B. G. Harvey, Bio-based cycloalkanes: The missing link to high-performance sustainable jet fuels, ChemSusChem, 2020, 13, 5777–5807 CrossRef CAS PubMed.
- J.-D. Woodroffe and B. G. Harvey, High-performance, bio-based jet fuel blends containing hydrogenated monoterpenes and synthetic paraffinic kerosenes, Energy Fuels, 2020, 34, 5929–5937 CrossRef CAS.
- E. Corporan, T. Edwards, L. Shafer, M. J. DeWitt, C. Klingshirn, S. Zabarnick, Z. West, R. Striebich, J. Graham and J. Klein, Chemical thermal stability, seal swell, and emissions studies of alternative jet fuels, Energy Fuels, 2011, 25, 955–966 CrossRef CAS.
- S. Kosir, J. Heyne and J. Graham, A machine learning framework for drop-in volume swell characteristics of sustainable aviation fuel, Fuel, 2020, 274, 117832 CrossRef CAS.
- J. L. Graham, T. F. Rahmes, M. C. Kay, J.-P. Belieŕes, J. D. Kinder, S. A. Millett, J. Ray, W. L. Vannice and J. A. Trela, Impact of alternative jet fuel and fuel blends on non-metallic materials used in commercial aircraft fuel systems, Federal Aviation Administration Report DOT/FAA/AEE/2014-10, pp. 1–95.
- Y. Liu and C. W. Wilson, Investigation into the impact of ndecane, decalin, and isoparaffinic solvent on elastomeric sealing materials, Adv. Mech. Eng., 2012, 4, 127430 CrossRef.
- S. Richter, T. Kathrotia, C. Naumann, S. Scheuermann and U. Riedel, Investigation of the sooting propensity of aviation fuel mixtures, CEAS Aeronaut. J., 2021, 12, 115–123 CrossRef.
- R. Tian, Y. Zhang, S. Kook, K. S. Kim and C.-B. Kweon, Effect of jet fuel aromatics on in-flame soot distribution and particle morphology in a small-bore compression ignition engine, Fuel, 2021, 305, 121582 CrossRef CAS.
- C. Voigt, et al., Cleaner burning aviation fuels can reduce contrail cloudiness, Commun. Earth Environ., 2021, 2, 114 CrossRef.
- D. M. Morris, R. L. Quintana and B. G. Harvey, High performance jet fuels derived from bio-based alkenes by iron catalyzed [2+2] cycloaddition, ChemSusChem, 2019, 12, 1646–1652 CrossRef CAS PubMed.
- G. Li, B. Hou, A. Wang, X. Xin, Y. Cong, X. Wang, N. Li and T. Zhang, Making JP-10 superfuel affordable with a lignocellulosic platform compound, Angew. Chem., Int. Ed., 2019, 58, 12154–12158 CrossRef CAS PubMed.
- H. A. Meylemans, R. L. Quintana, B. R. Goldsmith and B. G. Harvey, Solvent-free conversion of linalool to methylcyclopentadiene dimers: A route to renewable high-density fuels, ChemSusChem, 2011, 4, 465–469 CrossRef CAS PubMed.
- J.-D. Woodroffe and B. G. Harvey, Synthesis of bio-based methylcyclopentadiene from 2,5-hexanedione: A sustainable route to high energy density jet fuels, ChemSusChem, 2021, 14, 339–343 CrossRef CAS PubMed.
- Y. Liu, R. Wang, H. Qi, X. Y. Liu, g. Li, A. Wang, X. Wang, Y. Cong, T. Zhang and N. Li, Synthesis of bio-based methylcyclopentadiene via direct hydrodeoxygenation of 3-methylcyclopent-2-enone derived from cellulose, Nat. Commun., 2021, 12, 46 CrossRef CAS PubMed.
- G. Nie, C. Shi, Y. Dai, Y. Liu, Y. Liu, C. Ma, Q. Liu, L. Pan, X. Zhang and J.-J. Zou, Producing methylcyclopentadiene dimer and trimer based high-performance jet fuels using 5-methylfurfural, Green Chem., 2020, 22, 7765–7768 RSC.
- Z. Zebec, J. Wilkes, A. J. Jervis, N. S. Scrutton, E. Takano and R. Breitling, Towards synthesis of monoterpenes and derivatives using synthetic biology, Curr. Opin. Chem. Biol., 2016, 34, 37–43 CrossRef CAS PubMed.
- L. Walls and L. Rios-Solis, Sustainable production of microbial isoprenoid derived advanced biojet fuels using different generation feedstocks: A review, Front. Bioeng. Biotechnol., 2020, 8, 599560 CrossRef PubMed.
- W. Wu and C. T. Maravelias, Synthesis and techno-economic assessment of microbial-based processes for terpenes production, Biotechnol. Biofuels, 2018, 11, 294 CrossRef CAS PubMed.
- J.-D. Woodroffe and B. G. Harvey, Chemoselective hydrogenation of ring-strained monoterpenes: A route to high-performance sustainable aviation fuels, Energy Technol., 2021, 9, 2100221 CrossRef CAS.
- J.-D. Woodroffe, D. V. Lupton, M. D. Garrison, E. M. Nagel, M. J. Siirila and B. G. Harvey, Synthesis and fuel properties of high-energy density cyclopropanated monoterpenes, Fuel Process. Technol., 2021, 222, 106952 CrossRef CAS.
- Y. Liu, C. Ma, C. Shi, L. Pan, J. Xie, S. Gong, Y.-C. Zhang, G. Nie, X. Zhang and J.-J. Zou, Synthesis of strained high-energy rocket bio-kerosene via cyclopropanation of myrcene, Fuel Process. Technol., 2020, 201, 106339 CrossRef CAS.
- Y. Liu, C. Shi, L. Pan, X. Zhang and J.-J. Zou, Synthesis and performance of cyclopropanated pinanes with high density and high specific impulse, Fuel, 2022, 307, 121906 CrossRef CAS.
- A. Langlois and O. Lebel, To cyclopropanate or not to cyclopropanate? A look at the effect of cyclopropanation on the performance of biofuels, Energy Fuels, 2010, 24, 5257–5263 CrossRef CAS.
- L. Ye, X. Lv and H. Yu, Engineering microbes for isoprene production, Metab. Eng., 2016, 38, 125–138 CrossRef CAS PubMed.
- K. W. George, M. G. Thompson, A. Kang, E. Baidoo, G. Wang, L. J. G. Chan, P. D. Adams, C. J. Petzold, J. D. Keasling and T. S. Lee, Metabolic engineering for the high-yield production of isoprenoid-based C5 alcohols in E. coli, Sci. Rep., 2015, 5, 11128 CrossRef PubMed.
- D. Dugar and B. Nelter, US10208008, Visolis Inc., Berkeley, CA, 2019.
- E. Heracleous, E. Pachatouridou, L. Louie, D. Dugar and A. A. Lappas, Efficient route for the production of isoprene via decarboxylation of bioderived mevalonolactone, ACS Catal., 2020, 10, 9649–9661 CrossRef CAS.
- D. J. Lundberg and P. J. Dauenhauer, Hybrid routes to biofuels, Nature, 2007, 447, 914–915 CrossRef PubMed.
- D. J. Lundberg, D. J. Lundberg, K. Zhang and P. J. Dauenhauer, Process design and economic analysis of renewable isoprene from biomass via mesaconic acid, ACS Sustainable Chem. Eng., 2019, 7(5), 5576–5586 CrossRef CAS.
- O. A. Abdelrahman, D. S. Park, K. P. Vinter, C. S. Spanjers, L. Ren, H. J. Cho, K. Zhang, W. Fan, M. Tsapatsis and P. J. Dauenhauer, Renewable isoprene by sequential hydrogenation of itaconic acid and dehydra-decyclization of 3-methyl-tetrahydrofuran, ACS Catal., 2017, 7(2), 1428–1431 CrossRef CAS.
- M. D. Kumbhalkar, J. S. Buchanan, G. W. Huber and J. A. Dumesic, Ring opening of biomass-derived cyclic ethers to dienes over silica/alumina, ACS Catal., 2017, 7, 5248–5256 CrossRef CAS.
- O. A. Abdelrahman, D. S. Park, K. P. Vinter, C. S. Spanjers, L. Ren, H. J. Cho, D. G. Vlachos, W. Fan, M. Tsapatsis and P. J. Dauenhauer, Biomass-derived butadiene by dehydra-decyclization of tetrahydrofuran, ACS Sustainable Chem. Eng., 2017, 5, 3732–3736 CrossRef CAS.
- D. Kellner, M. Weger, A. Gini and O. G. Mancheño, Pd(OAc)2/Ph3P-Catalyzed dimerization of isoprene and synthesis of monoterpenic heterocycles, Beilstein J. Org. Chem., 2017, 13, 1807–1815 CrossRef CAS PubMed.
- A. Rana, L. C. Gomes, J. S. Rodrigues, H. Arrou-Vignod, J. Sjölander, N. P. Vedin, O. E. Bakouri, K. Stensjö, P. Lindblad, L. Andersson, M. Berglund, P. Lindberg and H. Ottosson, A combined photobiological–photochemical route to C10 cycloalkane jet fuels from carbon dioxide via isoprene, ChemRxiv, 2021 DOI:10.26434/chemrxiv.14054564.
- K. E. Rosenkoetter, C. R. Kennedy, P. J. Chirik and B. G. Harvey, [4+4]-Cycloaddition of isoprene for the production of high-performance bio-based jet fuel, Green Chem., 2019, 21(20), 5616–5623 RSC.
- C. R. Kennedy, H. Zhong, R. L. Macaulay and P. J. Chirik, Regio- and diastereoselective iron-catalyzed [4+4]-cycloaddition of 1,3-dienes, J. Am. Chem. Soc., 2019, 141, 8557–8573 CrossRef CAS PubMed.
- R. A. Ligabue, J. Dupont and R. F. de Souza, J. Mol. Catal. A: Chem., 2001, 169, 11–17 CrossRef CAS.
- D. J. Strope, US4144278, Phillips Petroleum, 1979.
- H. A. Colvin, Process for the thermal dimerization of isoprene, US Pat., 4973787, 1990 Search PubMed.
- K. W. Harrison and B. G. Harvey, Renewable high density fuels containing tricyclic sesquiterpanes and alkyl diamondoids, Sustainable Energy Fuels, 2017, 1, 467–473 RSC.
- B. G. Harvey, W. W. Merriman and T. A. Koontz, High density renewable diesel and jet fuels prepared from multicyclic sesquiterpanes and a 1-hexene derived synthetic paraffinic kerosene, Energy Fuels, 2015, 29, 2431–2436 CrossRef CAS.
- B. G. Harvey, H. A. Meylemans, R. V. Gough, R. L. Quintana, M. D. Garrison and T. J. Bruno, High-density biosynthetic fuels: The intersection of heterogeneous catalysis and metabolic engineering, Phys. Chem. Chem. Phys., 2014, 16, 9448–9457 RSC.
- H. A. Meylemans, L. C. Baldwin and B. G. Harvey, Low-temperature properties of renewable high-density fuel blends, Energy Fuels, 2013, 27, 883–888 CrossRef CAS.
- H. A. Meylemans, R. L. Quintana and B. G. Harvey, Efficient conversion of pure and mixed terpene feedstocks to high density fuels, Fuel, 2012, 97, 560–568 CrossRef CAS.
- B. G. Harvey, M. E. Wright and R. L. Quintana, High density renewable fuels based on the selective dimerization of pinenes, Energy Fuels, 2010, 24, 267–273 CrossRef CAS.
- R. J. Grau, P. D. Zgolicz, C. Gutierrez and H. A. Taher, Liquid phase hydrogenation, isomerization and dehydrogenation of limonene and derivatives with supported palladium catalysts, J. Mol. Catal. A: Chem., 1999, 148, 203–214 CrossRef CAS.
- P. Lesage, J. P. Candy, C. Hirigoyen, F. Humblot and J. M. Basset, Selective dehydrogenation of dipentene (R-(+)-limonene) into p-cymene on silica supported palladium assisted by α-olefins as hydrogen acceptor, J. Mol. Catal. A: Chem., 1996, 112, 431–435 CrossRef CAS.
- J. L. Graham, R. Striebich, K. J. Myers, D. K. Minus and W. E. Harrison, Swelling of nitrile rubber by selected aromatics blended in a synthetic jet fuel, Energy Fuels, 2006, 20, 759–765 CrossRef CAS.
- N. R. Baral, M. Yang, B. G. Harvey, B. Simmons, A. Mukhopadhyay, T. S. Lee and C. Scown, Production cost and carbon footprint of biomass-derived dimethylcyclooctane as a high performance jet fuel blendstock, ACS Sustainable Chem. Eng., 2021, 9, 11872–11882 CrossRef CAS.
- T. J. Edwards, Liquid fuels and propellants for aerospace propulsion: 1903–2003, J. Propuls. Power, 2003, 19, 1089–1107 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ya00017b |
| This journal is © The Royal Society of Chemistry 2022 |