Open Access Article
Open Access ArticleImprovement on the electrochemical performance by morphology control of nanostructured La0.6Sr0.4CoO3−δ-Gd0.1Ce0.9O1.95 cathodes for IT-SOFC†
Leandro Marcelo
Acuña
 *ab,
Marcelo Daniel
Cabezas
a and
Rodolfo Oscar
Fuentes
*ab,
Marcelo Daniel
Cabezas
a and
Rodolfo Oscar
Fuentes
 bc
bc
aDepartment of Functional Advanced Materials, UNIDEF, V. Lopez, B1603ALO Buenos Aires, Argentina. E-mail: lacuna@citedef.gob.ar
bCONICET, C1033AAJ Buenos Aires, Argentina
cINN, Department of Physics, CAC-CNEA, Gral. San Martín, B1650LWP Buenos Aires, Argentina
First published on 20th April 2022
Abstract
The effect of the particle's shape of Gd0.1Ce0.9O2−d (GDC) nanopowders on the electrochemical performance of La0.6Sr0.4CoO3−δ (LSC)–GDC composite cathodes was experimentally studied by electrochemical impedance spectroscopy (EIS). Composites were made of nanostructured LSC perovskite (average crystallite size D = 30 nm) mixed with nanostructured GDC powder (D = 8 nm) of irregular particle shape or with spherical particles of 200 nm diameter. Symmetrical cells (cathode/electrolyte/cathode) with different weight ratios of LSC:GDC (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 75
0, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50
25, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 25
50 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 wt%) thick cathodes were fabricated by the screen-printing method and tested by EIS in the temperature range of 500–700 °C under a synthetic air flow of 100 mL min−1. These nanostructured composite materials exhibited excellent performance (area-specific resistance – ASR – values in the range of 0.019–0.032 Ω cm2 for an operating temperature of 700 °C). The best performance was achieved at a weight ratio of 75
75 wt%) thick cathodes were fabricated by the screen-printing method and tested by EIS in the temperature range of 500–700 °C under a synthetic air flow of 100 mL min−1. These nanostructured composite materials exhibited excellent performance (area-specific resistance – ASR – values in the range of 0.019–0.032 Ω cm2 for an operating temperature of 700 °C). The best performance was achieved at a weight ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 wt% (LSC
25 wt% (LSC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GDC) when nanostructured GDC spheres were employed. This enhanced performance can be attributed to GDC morphology along with GDC anionic conduction, which prevent the blocking of LSC electrochemical reaction sites on surface, keeping them active by depleting its surface of adsorbed and reduced oxygen. The limiting processes were determined to take place on the LSC surface. New insight on cobalt's speciation under working-like conditions, determined by in situ X-ray Absorption Spectroscopy (XAS) experiments, suggests that the ease of cobalt atoms for changing their oxidation state is the key feature that rules the electrochemical activity of LSC and an atomistic model that explains it is proposed.
GDC) when nanostructured GDC spheres were employed. This enhanced performance can be attributed to GDC morphology along with GDC anionic conduction, which prevent the blocking of LSC electrochemical reaction sites on surface, keeping them active by depleting its surface of adsorbed and reduced oxygen. The limiting processes were determined to take place on the LSC surface. New insight on cobalt's speciation under working-like conditions, determined by in situ X-ray Absorption Spectroscopy (XAS) experiments, suggests that the ease of cobalt atoms for changing their oxidation state is the key feature that rules the electrochemical activity of LSC and an atomistic model that explains it is proposed.
Introduction
The solid oxide fuel cells (SOFCs) are energy conversion devices that convert the chemical energy of fuels (hydrogen, natural gas, and other hydrocarbons) directly into electrical energy with high efficiency and low emission of pollutants.1 However, at the high working temperature of SOFCs (800–1000 °C), several undesirable effects, such as further chemical reaction and diffusion, which affects the materials’ structure, take place and reduce the SOFC's lifespan and performance. So, there is great interest in developing new materials for intermediate-temperature SOFC (IT-SOFC) devices, which can be operated between 500 and 700 °C.2 In particular, materials with perovskite structure have long been investigated due to their excellent mixed ionic and electronic conduction (MIEC) properties, which make them suitable for application as electrodes in IT-SOFCs. La0.6Sr0.4CoO3−δ is one of most the promising cathode materials for IT-SOFCs due to its significantly high ionic conductivity and surface exchange kinetics, along with excellent electronic conduction.3–5Nanostructured materials have attracted attention due to the size dependence of the crystallographic parameters and electronic structure that may favour its physical and electrochemical properties.6–8 One of the common goals of many researchers is to prepare nanostructured electrodes with high surface area and controlled morphology. The nanostructured cathodes prepared by different methods have significantly increased surface area for the oxygen surface exchange reaction. In order to evaluate the effect of cathode morphology on the oxygen reduction reaction (ORR) performance, cathodes based in LSC nanotubes with different diameters were reported by Sacanell et al. and Bellino et al.9,10 The resulting ASR was significantly higher at 600 °C (1–2 Ω cm2) than that for nanostructured LSC powders reported by Acuña et al. (0.395 Ω cm2).7
Dieterle et al. reported an ORR with ASR of 0.023 Ω cm2 at 600 °C where a dip coating technique was employed to obtain nano-porous LSC thin films with the thickness in the range of 150–200 nm.11 The use of the thin-film deposition method is promising but there are several issues such as optimization of the electrode microstructure, substrate choice and component durability that need to be addressed before commercialization.12–14 One successful strategy to improve chemical stability of LSC thin films is to deposit several LSC/GDC bilayers with a thickness of tens of nm on a substrate of NdGaO3 by pulsed laser deposition (PLD).15
The presence of the solid electrolyte phase in the porous cathode should serve to enhance the thermomechanical compatibility of the electrode with the electrolyte by improving the adhesion and matching the thermal expansion coefficient (TEC) of the materials.16 Thus, composite cathodes are generally favoured over single-phase MIECs. In particular, Gd0.1Ce0.9O1.95 is considered to be one of the best electrolytes for IT SOFC applications,17 and it is known by its low reactivity with LSC, what made the LSC–GDC composite cathode extensively investigated.16,18–23 Moreover, GDC layers are employed to prevent chemical reaction and ion migration between LSC and YSZ or SSZ (Ytria or Scandia-Stabilized Zirconia, respectively) electrolytes.16,18,24 It is assumed that improved ionic diffusion and additional triple phase boundary (TPB) reaction lead to performance enhancement, but it is still not clear how cathode performance can be correlated with microstructure characteristics for the LSC–GDC composite cathode. In addition, there is no consensus about the processes and reactions that take place in the cathode and different models were proposed.25 This difficulty is based on the lack of information, coming from direct probes at atomic scale, on the chemical properties of cobalt atoms in LSC under working-like conditions of IT-SOFCs. The knowledge of the “oxygen diffusion mechanisms at an atomic scale is of vital importance” for the development of strategies for improving IT-SOFC performance.26
The use of indirect methods, such as neutron diffraction, X-ray diffraction or iodometric or coulometric titration, led to the conclusion that cobalt atom plays a secondary and passive role in LSC electrochemical activity and to identify vacancies as the main feature behind the electrochemical activity of LSC material.27–29 The last type of experiments is known by its high accuracy, but it only provides the average oxidation state of a sample. In addition, it is a destructive experiment and do not allow for reproducing special environment conditions as those found in SOFC's electrodes.
XAS experiments, comprising XANES and EXAFS regions (Near Edge and Extended X-ray Absorption spectrum, respectively), are direct probes for the chemical and local order properties of a selected element in a material, they are not destructive and can be performed under controlled environment conditions. Previous studies,13,30–34 were not able to analyse cobalt's speciation in LSC because of the lack of proper Co(IV) standard. Some of them studied the sample only at room temperature. In all cases, they made qualitative analyses, such as comparing the XANES profile of Co-K edge in LSC with some reference standards or following the Co-K edge energy shift as function of strontium content, sample's temperature or oxygen partial pressure. Those qualitative analyses only gave, in the best cases, the average oxidation state (near +3) of cobalt atom, leading to the same conclusion found in the others works that used indirect methods, namely, that the electrochemical activity of LSC (and also changes in the electronic and spin configuration of Co atoms) can be explained only by vacancy formation and crystal structure distortions, such as octahedron's tilting of oxygen atoms or phase transitions. “Most of the studies of LSC, however, did not discuss the correlations among MIEC, crystal structure parameters, electron densities, band structures, and spin configurations, quantitatively.”35 Some works that focused the attention on the spin state properties at low or room temperature, made assumptions on cobalt's oxidation state from oxygen vacancy concentration or changes in the pre-edge-energy region of the Co-K edge XANES.31–33
On the other hand, Takeda et al. stated that the electrochemical activity of LSC (adsorption, diffusion, dissociation and reduction of oxygen and delivering of oxygen anions to the electrolyte phase, GDC, for instance) would partially rest on the ability of Co atoms for changing their oxidation state according to environment conditions and the electrochemical potential in a cell.36 Experimental evidence of this was observed by Acuña et al.8 In this last approach, it was observed that Co atoms easily change their oxidation state in nanostructured LSC powders in response to increasing temperature from 20 to 500 °C under air flow. In the same way, Baskar and Adler pointed out the strong dependence of electron transport properties in LSC with spin states and oxidation states, which clearly justifies the use of XANES to address these issues.37 Finally, the lack of detailed fundamental studies of the chemical properties of active materials at the atomic scale has prevented breaking the performance limits through the rational development of advanced materials.
Therefore, in this study, the effect of the particle's shape of GDC nanostructured powders on the electrochemical performance of LSC–GDC composite cathodes were studied on symmetrical cells (cathode/electrolyte/cathode) by varying the relative content of nanostructured LSC (D = 30 nm) and nanostructured GDC powders (D = 8 nm) of irregular (standard) particle shape or spherical particle shape of 200 nm diameter. The analysis of EIS data (recorded in the temperature range of 500–700 °C) showed an enhanced performance of the composite made of spherical particles of GDC and that the observed improvement takes place in the LSC phase. EIS analysis also suggests that the rate limiting processes take place on LSC surface. In order to analyse the LSC activity at atomic scale, in situ XAS experiments were performed on LSC nanopowders under working-like conditions of IT-SOFC's cathodes. Novel information about cobalt speciation gave new insight on the chemical activity of cobalt atom in LSC, and a model at the atomic scale that describes the path of oxygen anions in the composite cathode is proposed. Additionally, we found evidence that, once the oxygen vacancies in the LSC reaches a maximum concentration, disproportionation reaction in cobalt atoms is triggered, possibly acting as a stabilizing mechanism that prevents nanostructured LSC from phase instability.
XANES analysis also offers a possible explanation on how GDC particle's shape can contribute to improve LSC performance. We offer in this work a hypothetical mechanism that integrates the results coming from every experiment performed in this study, by which GDC particle shape can contribute to this factual enhancement of LSC performance. Nevertheless, experiments contributing with conclusive evidence of the relationship between GDC particle shape and LSC performance are out of the boundaries of the present study. Despite this, we believe that the results presented in this work will encourage future defining works on this issue.
Experimental
In order to obtain different morphologies of nanostructured Gd0.1Ce0.9O1.95 solid solutions, two chemical methods, previously reported by the authors, were employed: cation complexation (CC) and microwave assisted hydrothermal homogeneous co-precipitation (MW).38,39 Nanostructured GDC powders obtained by CC method will be denoted as GDCCC and those obtained by MW, as GDCMW.Synthesis of nanostructured GDCCC powders
Ce(NO3)3·6H2O (99.99%, Alfa Aesar) and Gd(NO3)3·6H2O (99.9%, Alfa Aesar) were employed as precursors. Each nitrate was dissolved in deionized H2O separately and then the solutions were mixed to obtain nitrate solutions with molar ratios of Ce:Gd appropriate for the preparation of products. Citric acid (99.5%, Merck) was dissolved in d.i. water and this was added to the cation nitrate solution in appropriate amounts to give a molar ratio of final total oxide to citric acid of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. After homogenization of this solution, the temperature was raised to 80 °C, and the solution maintained under stirring to remove excess water and to convert it to a transparent gel. While raising the temperature, the solution became more viscous with evolution of foam, and finally it gelled without any visible precipitation or turbidity. During the dwell at 80 °C there was an increase in viscosity and simultaneous elimination of water and NOx. The initial thermal decomposition of the precursor was carried out at 250 °C for 1 H in air and the resulting ash-like material was calcined at 500 °C for 1 H in air.
2. After homogenization of this solution, the temperature was raised to 80 °C, and the solution maintained under stirring to remove excess water and to convert it to a transparent gel. While raising the temperature, the solution became more viscous with evolution of foam, and finally it gelled without any visible precipitation or turbidity. During the dwell at 80 °C there was an increase in viscosity and simultaneous elimination of water and NOx. The initial thermal decomposition of the precursor was carried out at 250 °C for 1 H in air and the resulting ash-like material was calcined at 500 °C for 1 H in air.
Synthesis of spherical particles of nanostructured GDC
Ce(NO3)3·6H2O (99.99%, Alfa Aesar) and Gd(NO3)3·6H2O (99.9%, Alfa Aesar) were employed as precursors. Each nitrate was dissolved in pure deionized H2O separately and then the solutions were mixed to obtain a 0.1 M nitrate solution with molar ratios of Ce:Gd and Ce:Pr appropriate for the preparation of GDC and PrDC, respectively. Urea was added in order to give a molar ratio of urea:final oxide of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 60 ml of the resulting solution was placed in a Teflon lined autoclave. A Milestone ETHOS 1 Advanced Microwave Digestion system was employed. The sealed autoclave was placed in the oven and was heated to 120 °C with a ramp rate of 30 °C min−1 – with oven power set to 550 W – and then kept at this temperature for 1 h. After cooling, the white powder product was collected by centrifugation (2.5 min at 7830 rpm) and dried at 37 °C for 12 H. After calcination at 500 °C in air for 1 H, the nanostructured GDC spheres were obtained.39
1 and 60 ml of the resulting solution was placed in a Teflon lined autoclave. A Milestone ETHOS 1 Advanced Microwave Digestion system was employed. The sealed autoclave was placed in the oven and was heated to 120 °C with a ramp rate of 30 °C min−1 – with oven power set to 550 W – and then kept at this temperature for 1 h. After cooling, the white powder product was collected by centrifugation (2.5 min at 7830 rpm) and dried at 37 °C for 12 H. After calcination at 500 °C in air for 1 H, the nanostructured GDC spheres were obtained.39
Synthesis of nanostructured LSC powders
LSC nanopowders were synthesized by the gel-combustion (GC) method, starting from Sr(NO3)2 (Aldrich, 99%), La(NO3)3·6H2O (Aldrich, 99.999%) and Co(NO3)2·6H2O (Aldrich, >98%) as precursors. Glycine (C2H5O2N) (Merck, ≥99%) was used as the complexing agent and the fuel for the combustion step.8Synthesis of Co(III) and Co(IV) standards
LaCoO3 was synthesized from La(NO3)3·6H2O (Aldrich, 99.999%) and Co(NO3)2·6H2O (Aldrich, >98%) precursors and citric acid (Aldrich, 99%) following the procedure described by Taguchi et al.40 BaCoO3 was synthesized using Ba(NO3)2 (Sigma-Aldrich, 99.999%) and Co(NO3)2.6H2O following the procedure described by Milt et al.41 Commercial CoO (Sigma-Aldrich, ≥99.99%) was employed as Co(II) reference material.Preparation of symmetrical cell for EIS
Slurries of nanostructured LSC–GDC composite cathodes with different weight ratios of LSC:GDC (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 75
0, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50
25, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 25
50 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 wt%)and DECOFLUX vehicle were prepared using a weight ratio powder:vehicle close to 3
75 wt%)and DECOFLUX vehicle were prepared using a weight ratio powder:vehicle close to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pursuing a viscosity similar to that exhibited by commercial paints. As an example of the nomenclature used in this work, cathode composite with a 75 wt% of nanostructured LSC powder and 25 wt% of nanostructured GDCCC or GDCMW powder will be denoted as LSC75–GDCCC25 or LSC75–GDCMW25, respectively.
1, pursuing a viscosity similar to that exhibited by commercial paints. As an example of the nomenclature used in this work, cathode composite with a 75 wt% of nanostructured LSC powder and 25 wt% of nanostructured GDCCC or GDCMW powder will be denoted as LSC75–GDCCC25 or LSC75–GDCMW25, respectively.
Dense electrolytes of 1 mm thick and 10 mm diameter were obtained from commercial Sm0.2Ce0.8O1.9 powder (SDC, NexTech Materials) uniaxially pressed under 20 kPa and then sintered in a tubular furnace at 1400 °C for 5 H. Thick films of area 0.24 cm2 and thickness of near 75 μm were then screen printed onto both faces of the SDC electrolytes and fired in a range of temperatures between 800 °C and 950 °C for 2 H. As LSC phase is an excellent electronic conductor in the range of temperatures of interest for this study, no other current collector was used (such as Pt paste), as LSC itself acts as current collector.42 This approach has the advantage of eliminate any possible contribution of the current collector phase to the EIS spectra or any interaction between the noble metal and the cathode phase itself that could modify the electrode performance.
Characterization of LSC–GDC cathodes
X-ray powder diffraction (XPD) was employed for preliminary structural characterization using a Philips PW 3710 diffractometer with Cu K-α radiation at room temperature (RT). Scans were collected in the range of 2θ = 20–80° with steps of 0.02° and step counting time of 4 s.The morphology of nanostructured LSC–GDC cathodes was studied with a scanning electron microscope SEM FEI Quanta 200.
Electrochemical measurements
Electrochemical properties of LSC–GDC cathodes were studied by EIS using the symmetrical cell configuration, cathode/electrolyte/cathode. EIS measurements were carried out using an Autolab PGSTAT302N instrument (Metrohm, Echo Chemie) in the temperature range of 500–700 °C under a synthetic air flow and 10 and 5% O2/N2 flow (100 ml min−1). The symmetric cells were excited with a sine wave of 20–50 mV amplitude in the frequency range of 0.1 Hz to 1 MHz sampling ten points per decade on a log scale. The EIS spectra were fitted by means of the equivalent circuit method using the ZView 2 software (Version 3.0, Scribner Associates, Inc.). The fittings were performed in the confidence range for this equipment, at frequencies below 30 kHz, according to manufacturer's recommendation and the quality of experimental data.X-ray absorption measurements
X-ray Absorption Near Edge Structure (XANES) experiments were carried out at the D08B-XAFS2 beam line of the Brazilian Synchrotron National Laboratory (LNLS), Brazil, in transmission mode.43 The energy was selected with a Si(111) monochromator. 8 mg of LSC, LaCoO3, BaCoO3 and CoO powders were mixed with 92 mg of boron nitride and uniaxially pressed applying 0.2 kg m−2 to a 13 mm diameter die. The pellets were placed in an iron sample holder and introduced in the tubular quartz chamber of a furnace with refrigerated KaptonTM (DuPont) windows. Experiments were performed under synthetic air flow and 5% O2/N2 flow (50 ml min−1). The entrance and exit of the gas flow were at the ends of the tube. Samples were heated from room temperature to 700 °C at a rate of 10 °C min−1 and a stabilization time of 10 min. XAS spectra of Co-K edge were measured from 6900 to 8700 eV. Energy step of 0.3 eV and counting time of 4 s was used for the XANES region. The data reduction was done following the standard procedure8,44 using WinXAS 3.1 software.45 A detailed description of the procedure can be found in the ESI's XANES section.No XAS experiments we carried out on LSC–GDC mixtures because the XAS signal of Gd-L2 (with edge energy E0 = 7898 eV), that lies within the energy range of the EXAFS signal of Co-K edge, and the EXAFS oscillations of Gd-L3 edge (E0 = 7211 eV), may complicate the XANES analysis of the Co-K edge. In addition, self-absorption in LSC–GDC powders considerably diminishes XAS signal of the Co-K edge (between 30 and 70% of signal loss). The XANES of Sr-K and La-L3 edges were not measured because no relevant information for this study was expected, as these cations exhibit only one oxidation state (+2 and +3, respectively).
Results and discussion
In this section is analyzed the effect of GDC particle's morphology on the electrochemical activity of LSC–GDC composite cathodes and a model describing reactions that involve molecular and atomic oxygen on the cathode's surface will be developed, based on the chemical properties of cobalt atoms in LSC. The effect that the morphology of LSC particles (nanopowders of irregular shape, nanotubes of different diameters, nanowires and others) may have on the electrochemical performance of LSC–GDC composite cathodes is a very interesting question that deserves to be developed in another work, due to the amount of data that it is needed to be analyzed and the different issues to be discussed.Morphological and structural characterization
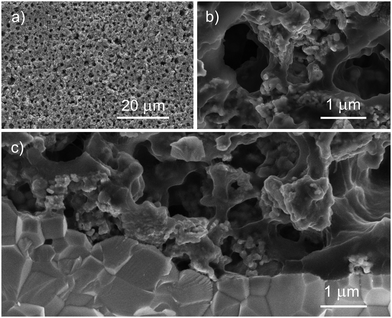
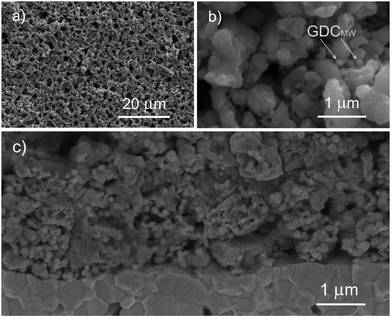
The thickness of the porous thick electrodes was approximately 75 μm in every case. The microstructure of the electrode layer is highly porous and the interface between the electrode and the electrolyte shows good contact between these two layers (Fig. 1 and 2). LSC75–GDCCC25 cathode consists of particles with two distinct particle size. The larger ones, in the scale of the μm can be ascribed to the LSC phase, which are partially covered with smaller ones that can be related to the GDC phase (Fig. 1 and Fig. S4, ESI†).LSC75–GDCMW25 cathode is made of two types of particles with well differentiated particle shapes: particles of irregular shape with particle size on the order of the μm, ascribable to LSC, and spherical particles of about 200 nm diameter, corresponding to GDCMW (Fig. 2 and Fig. S4, S5, ESI†). From now on, we will refer to the latter simply as GDCMW spheres, in opposition to GDCCC nanopowder. At high magnifications it is possible to observe GDCMW particles of spherical shape (Fig. 2b and c) embedded in the LSC powder. Some trend to agglomeration in GDC spheres is observed and may play an important role in composite cathode's performance.
All the reflections observed in the XRD patterns of LSC–GDC cathodes correspond to the LSC (rhombohedral phase: R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group, No. 167,) or to the GDC phase (cubic phase: Fm
c space group, No. 167,) or to the GDC phase (cubic phase: Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group) and no reaction or segregated phases are observed neither in the fresh cathodes (Fig. 3) nor in the LSC75–GDCMW25 cathode after the long term stability test (Fig. S6, ESI†). Detailed Rietveld refinement results are listed in Tables S1 and S2 (ESI†). The lattice parameters obtained by means of the Rietveld refinement method are in excellent agreement with reference data found in the literature (Tables S3 and S4, ESI†). The exception is the work of Gwon et al. (later reproduced in the review of Jun et al.), who studied the La1−xSrxCoO3−δ phases as function of Sr content x.46,47 While Gwon et al. reported that the cubic phase is present at room temperature in the La1−xSrxCoO3−δ samples with low Sr content (x ≤ 0.4), Petrov et al. and Closset et al., among others, have identified at room temperature the rhombohedral phase for 0 ≤ x < 0.6 and the cubic phase for 0.6 ≤ x ≤ 1.3,31,48,49
m space group) and no reaction or segregated phases are observed neither in the fresh cathodes (Fig. 3) nor in the LSC75–GDCMW25 cathode after the long term stability test (Fig. S6, ESI†). Detailed Rietveld refinement results are listed in Tables S1 and S2 (ESI†). The lattice parameters obtained by means of the Rietveld refinement method are in excellent agreement with reference data found in the literature (Tables S3 and S4, ESI†). The exception is the work of Gwon et al. (later reproduced in the review of Jun et al.), who studied the La1−xSrxCoO3−δ phases as function of Sr content x.46,47 While Gwon et al. reported that the cubic phase is present at room temperature in the La1−xSrxCoO3−δ samples with low Sr content (x ≤ 0.4), Petrov et al. and Closset et al., among others, have identified at room temperature the rhombohedral phase for 0 ≤ x < 0.6 and the cubic phase for 0.6 ≤ x ≤ 1.3,31,48,49
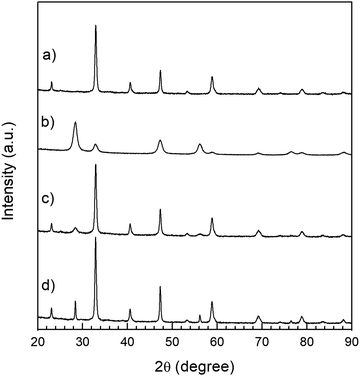 | ||
| Fig. 3 XRD patterns. (a) LSC nanopowders; (b) GDC powder with particles of spherical shape; (c) LSC75–GDCMW25 cathode before the sintering process; (d) LSC75–GDCMW25 cathode after 2H at 850 °C. | ||
EIS studies
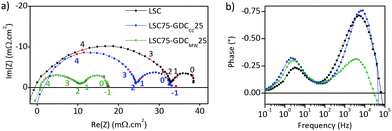
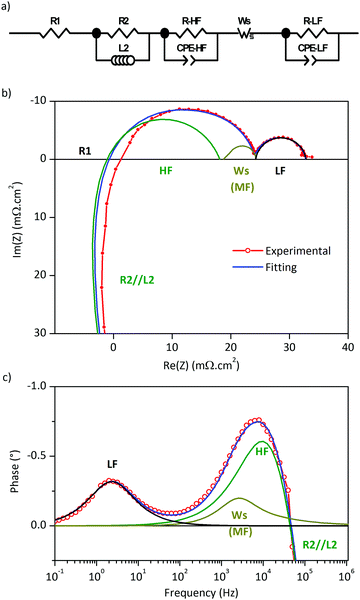
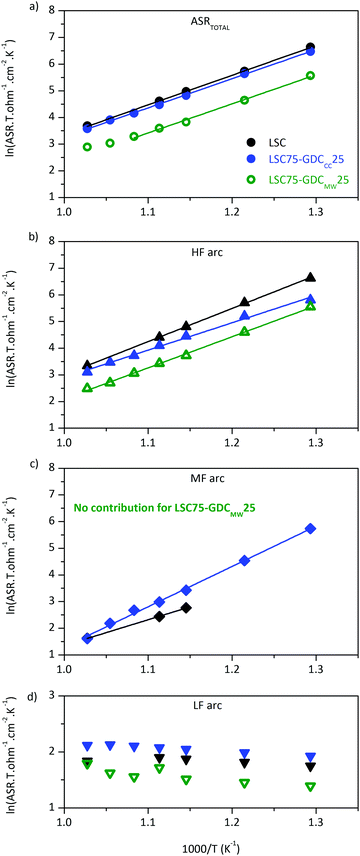
Electrochemical impedance spectra were measured in the temperature range of 500–700 °C for the half-cells constructed from LSC–GDC cathodes with various LSC:GDC weights and different sintering temperatures. Every Nyquist plot exhibits two distinct arcs, and the Bode phase plots, two well resolved peaks (Fig. 4). The plots of polarization area specific resistance (ASRpol) as function of sintering temperature show that the lowest value of ASRpol is obtained for LSC75–GDC25, LSC50–GDC50 and LSC25–GDC75 sintered at 850, 875 and 925 °C, respectively (Fig. S7, ESI†). The best performance is achieved with a GDC load of 25 wt% (powder or spheres). This is in agreement with several works dedicated to LSC–GDC composites, that obtained the best results with the 30 wt% GDC load (among 20, 30, 40 wt% GDC).18,20,21 Nevertheless, Kim and Shikazono, in a combined theoretical and experimental work, reported that the lowest polarization resistance is achieved with a 70 wt% GDC load.50EIS spectra were fitted with different equivalent circuit models. Fig. 5a shows a scheme of the equivalent circuit that will be discussed here, while Fig. 5b and c are examples corresponding to LSC75–GDCCC25 sample at 700 °C under synthetic air flow along with the fitting curve and the contribution of each circuit element.
Between 600 and 700 °C the electrolyte contribution to the EIS spectra is pure resistive and it was fitted to the R1 resistance (Fig. 5a). The inductance contribution of the wires was modelled with a parallel L2//R2 (L2 in the order of 10−7 H and R2 ≈ 1.9 Ω). The high frequency (HF) and low frequency (LF) arcs where represented with two parallel R//CPE. A middle frequency (MF) circuit was also used to improve the fit to EIS spectra in LSC and LSC75–GDCCC25 cathodes. It was tested an R//CPE and a Finite Length Warburg-short (WS) elements with similar quality of fit.51–53 The use of either model didn’t affect the values obtained for the parameters of other circuit elements. Here we present the results obtained for the WS element and the reason for this will become apparent in the course of the discussion of the results.
Examination of Arrhenius plot indicates that LSC75–GDCMW25 exhibits the lowest polarization losses in the whole temperature range (Fig. 6a), where ASRTOTAL = ASRHF + ASRMF + ASRLF. The analysis of each contribution points out that the improvement is related to the processes represented by the HF and MF arcs (Fig. 6b and c, respectively). In fact, ASRMF in LSC75–GDCMW25 is negligible (Table 1).
| Cathode | ASR at T = 700 °C (mΩ cm2) | |||
|---|---|---|---|---|
| Total | HF | MF | LF | |
| LSC | 41(2) | 29.2(1) | 5.2(8) | 6.4(1) |
| LSC–GDCCC | 39(1) | 24.7(7) | 5.5(8) | 8.6(1) |
| LSC–GDCMW | 18.6(2) | 12.4(1) | — | 6.2(1) |
The WS contribution in LSC (Fig. 6c) is truncated at temperatures below 625 °C, due to the decreasing weight that the WS element has on the overall ASR with decreasing temperature. As consequence, the statistical error on the related parameters WR (Warburg resistance) and WT (Warburg characteristic time) is higher at lower temperatures. Therefore, we chose to focus our analysis at T = 700 °C. At this temperature, every arc is resolved more easily and, in consequence, results are more reliable. Within LSC-based thick cathodes, the ASRTOTAL values achieved in this work are among the best reported in literature to the best of our knowledge (Table S5, ESI†).
The WS contribution to the total ASR of LSC–GDCCC is very low at high temperatures but, at 500 °C, it became comparable to that of the HF arc (Fig. 6b and c) of the same sample. This fact is in opposition to the trend observed in the case of LSC cathode. The total Ea (calculated from the analysis of Fig. 6) is in good agreement with Hjalmarsson et al (Table 2).31 The origin of the different activation energy of each arc will be discussed later in this section.
| Cathode | E a (eV) | ||
|---|---|---|---|
| Total | HF | MF | |
| LSC | 0.96(1) | 1.07(1) | 0.83(1) |
| LSC–GDCCC | 0.94(1) | 0.88(4) | 1.30(3) |
| LSC–GDCMW | 0.94(3) | 1.01(2) | — |
The low frequency arc proved to be weak dependent on temperature (Fig. 6d). As the temperature decreases, it gets more difficult to resolve this LF arc from the HF or MF arcs. This is the reason why we chose to fix RLF to the value obtained from the fit to the spectra at 700 °C in air for LSC and LSC–GDCCC. This weak dependency on temperature makes its relative contribution more important as ASRHF and ASRMF decreases with increasing temperature. This is the reason why the Arrhenius plot of the ASRTOTAL of LSC75–GDCMW25 departs from the straight line above 650 °C (Fig. 6a). Above this temperature, the HF and LF arcs become comparable.
Several works found in the literature have stated a relationship between the value of n (the slope of the linear function that fits the plot of log(1/ASR) as function of the logarithm of the oxygen partial pressure, log(Po2)), and specific processes in cathodes (Table 3 and Fig. S8, ESI†). According to Takeda et al,36 the HF process (that exhibits n ≈ 0.25 in every case) would be related to Langmuir's type oxygen atom adsorption, where there is a very low fraction of adsorption sites occupied by O atoms. This reaction is represented by the equation:
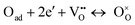 | (1) |
On the other side, the low frequency arc in Fig. 5b exhibits a simple inverse law (n ≈ 1) as function of Po2. Always according to Takeda, this corresponds to a reaction in which molecular oxygen is involved in this rate limiting process, which consists of three steps described by the equations:
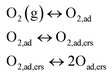 | (2) |
The process that contributes to the middle frequency arc is more difficult to analyse because of its low ASR value (close to 15% of the total ASR at 700 °C in air) and its high associated uncertainty. Its dependency with Po2 in LSC cathode is n = 0.5. This is in accordance with an atomic oxygen diffusion and reduction process, described by36
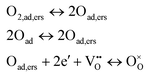 | (3) |
The lower value of n = 0.32 calculated for the LSC75–GDCCC25 cathode (Fig. S8b, ESI†) and the higher value of n = 0.9 for LSC75–GDCMW25 cathode would indicate that the weight of each step in eqn (3) is different in them. We can see in eqn (3) that the first step describes the same reaction observed in the last step of eqn (2). On the other hand, the last step in eqn (3) consists in the same reaction described by eqn (1). So, it is possible that n = 0.32 is indicating that the CT step in eqn (3) is the major contributor to the ASRMF in LSC75–GDCCC25. Following the same reasoning, n = 0.9 would be indicating that the two last reactions in eqn (3) have a lower weight than the first one, the dissociation of adsorbed O2.
These results would indicate that the ad-atomic diffusion process has more active paths in the composite than in the LSC cathode. Maybe, the diffusion path to the electrochemical reaction sites is shorter (or faster) in the composites. This, along with a lower contribution of the adsorbed O2 dissociation (1st) step in eqn (3) to the ASRMF of LSC75–GDCCC25, makes the whole process more similar to that described in eqn (1) (involving a Langmuir's type oxygen atom adsorption). This hypothesis is consistent with the values obtained for the diffusion characteristic time, WS–T, in LSC (4.3 × 10−4 s) and in LSC75–GDCCC25 (1.5 × 10−4 s) cathodes. Another possibility is related to the fact that the ionic conduction in GDC at 700 °C is high compared to that exhibited by LSC perovskite,54 suggesting that the GDC phase is a possible path for oxide ions. This would contribute to deplete the LSC surface of oxygen species, maintaining in this way the electrochemical reaction sites active. This is more apparent in LSC75–GDCMW25 cathode, where the contribution of this middle frequency arc is negligible at 20% O2/N2. The value it would take under these conditions is represented by the extended line that fits the experimental data in Fig. S8b (ESI†). The extrapolated ASR value is 0.81 mΩ cm2, which is relatively very low if it is compared to the other contributions and explains the difficulty of including the WS element in the fitting of the data under these conditions. This result, along with its lower ASRTOTAL (Table 1), means that the LSC75–GDCMW25 cathode has more active sites on its surface compared to the other film.
The higher value of n = 0.9 for the MF arc of LSC75–GDCMW25 (Fig. S8b, ESI†), along with its lower ASRMF and the lower ASRTOTAL, may rest on the possibility that the combination of GDCMW spheres with LSC powder optimizes the connectivity between the two phases and also improves the percolation of GDC spheres, resulting in faster CT reaction in eqn (3). This is also consistent with the lower ASR values of the HF arc that this cathode exhibits (Fig. 6b).
Factually, the only difference between the two cathodes is the shape of GDC particles. Therefore, the origin of the improvement in LSC–GDC composites is possibly related to the spherical morphology of GDCMW. In fact, the area of the contact surface between one LSC particle and one GDCMW sphere is smaller than that between two particles LSC–LSC or LSC–GDCCC. This has at least two positive aspects. One, GDCMW spheres would prevent that two LSC particles be in contact and sinter during the sintering procedure. In that way, they would keep available more superficial area for reaction (eqn (1)–(3)). This picture is consistent with the differences exhibited by each cathode in the values of the ASRLF. They would suggest that a greater LSC surface is covered by GDCCC powder than by LSC itself or GDCMW spheres. But it has to be mentioned that the variations observed in ASRLF are small enough to be conclusive. The other, already mentioned, is that the GDC phase would contribute to deplete the LSC surface of oxygen anions, keeping low the number of occupied active sites with oxygen species. We will explain this issue in detail after discussing XANES results.
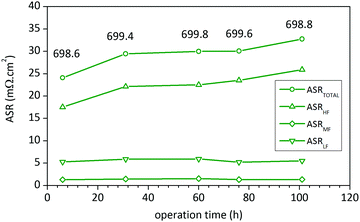
Finally, in order to check the stability of LSC75–GDCMW25 cathode, EIS spectra were recorded at 700 °C after several dwell-times. The experimental data was analysed using the equivalent circuit shown in Fig. 5a. After 101 H at 700 °C in air, ASRTOTAL of LSC75–GDCMW25 cathode showed an increment of 30%, due to the growth of the HF arc (Fig. 7 and Fig. S9, ESI†). The LF arc, associated to diffusion of adsorbed O2 molecules, remained essentially unchanged, suggesting that no sintering process took place during the test. The small contribution of the MF arc became noticeable and was fit to the WS element (WR = 1.1(1) mohm cm2), and it was constant with time. The increment in the HF arc was observed during the very first hours of the experiment, remaining without any noticeable change for the next 70 hours, in accordance with Tao et al.18 The increment observed in ASRTOTAL and ASRW at the end of the test (Fig. 7) is more likely to be due to the fall of 0.8 °C in sample's temperature. All these evidences point to loss of efficiency in the CT process, probably due to a small surface reaction between LSC and GDC phases or to element segregation in the surface of the particles.18 This was not possible to detect by means of XRD analysis. Also, it was observed a small shift in the HF pure resistance R1 towards higher values (ΔR1 = 3 mohm cm2, what represents 0.5% of its initial value of 639 mohm cm2, or 12% of the initial ASRTOTAL), probably due to the same causes.
XANES of Co-K edge
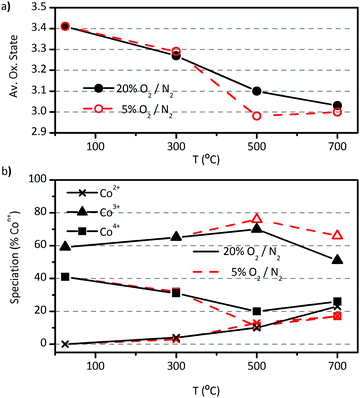
In this section we offer new insight on cobalt's speciation in LSC powders. The sensibility of Co atoms to changes in Po2 was studied by linear combination fitting (LCF) of Co(II,III,IV) experimental standards to cobalt's XANES spectra in LSC (Fig. S1, ESI†).8XANES experiments showed that the average oxidation state of Co atoms decreases from +3.4 to +3.0 as temperature increases from 20 to 700 °C in air, starting with a mixture of 60% Co3+ and 40% Co4+ (Fig. 8a and b). This result is in agreement with indirect calculations made by others researchers through, for example, iodometric titration experiments.27,31 For the first time, it was observed that the Co speciation then gradually evolves to approximately 25% Co2+ and Co4+ and 50% Co3+ with increasing temperature in air. This balance is modified to 17% Co2+ and Co4+ and 66% Co3+ when Po2 decreases to 5% O2/N2 at 700 °C.
The average oxidation state of Co (Fig. 8a), calculated from the cobalt's speciation, is in good agreement whit previous works found in the literature.27,31,35 In addition, the initial amount of 40% Co4+ exactly compensates the 40% of Sr2+ cations in the A-site, what leads to the conclusion that no vacancies are present in the fresh LSC powder at room temperature. Mineshigue et al. have found metal-like behaviour in La1−xSrxCoO3−δ (x ≥ 0.3 and δ = 0) in the temperature range of 20–1200 °C when no oxygen vacancies are found in the lattice.55,56 On the other hand, when x = 0.3 and δ ≥ 0.06 they observed insulator-like behaviour at low temperatures (below 200 °C). Mineshigue et al. related the oxidation of Co3+ to Co4+ (as response to Sr2+ doping), to electronic conductivity by means of the creation of holes in the electronic band of Co atoms. In the same way, the loss of Co4+ with increasing temperature (Fig. 8b) is in accordance with the metallic-like behaviour.31
The low intensity peak between 7709 and 7712 eV in Co K-edge XANES spectra contains information about the electronic configuration and spin state of cobalt atoms and it is originated in the electronic excitation from Co-1s to Co-3d orbitals. This forbidden transition is possible due to the hybridization between the Co-3d and O-2p orbitals, though some researchers have found evidence for quadrupolar transition nature.33,35,57,58 In any case, it is clear that its features are closely related to the electronic conductivity properties of LSC and that it is strongly affected by the crystal field.58,59 The reader is invited to consult the ESI,† for a more detailed discussion about this topic. It is worth to mention that the detailed analysis of this peak regarding the electronic conductivity of LSC is beyond the scope of the present study.
The differences observed between both XANES spectra of LSC (Fig. 9) would be related to desorption of O2.60 On going from 20 to 5% O2/N2, the slight shift towards higher energies and the disappearance of the weak peak at 7712 eV would be associated to the mentioned loss of oxygen in LSC bulk or surface with decreasing Po2 as, in this case, the hybridization between Co 3d and O 2p orbitals also decreases.59,61,62 This is because when these orbitals are hybridized a charge transfer from Co 3d to O 2p orbitals takes place, creating empty states of lower energy in the hybridized Co 3d orbitals. A very detailed and complementary analysis of this peak can be found in the work of Itoh et al.35
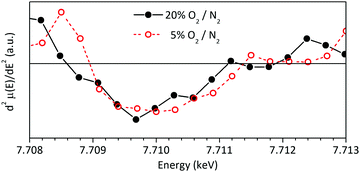 | ||
| Fig. 9 Analysis of the low intensity peak at E < E0 in the Co-K edge XANES of LSC at 700 °C under synthetic air and 5% O2/N2. | ||
One final consideration regarding the results in Fig. 8 is that, as temperature increases and O2− vacancies are created, the system seems to evolve to a speciation in which Co4+ is exactly compensated by Co2+ at 700 °C in air (Fig. 8b). Even though the growth rate followed by the population of each species leaves open the possibility that Co2+ overcomes the population of Co4+, an examination of the evolution under low oxygen content shows that, once both species are balanced, they continue in that way at higher temperatures. This result may point out some kind of thermodynamical equilibrium in which, once reached, the disproportionation reaction rules the evolution of the speciation. The analysis suggests that this change of behaviour would be triggered by an upper limit for O2− vacancy concentration in the lattice (Fig. 8a and b). This hypothesis is based on the fact that cobalt's average oxidation state remains essentially constant once it reaches the value of +3 under low oxygen content, while speciation continues evolving by means of the disproportionation reaction. The limit for oxygen vacancy concentration was determined by Petrov et al. in microstructured La0.7Sr0.3CoO3−δ,48 They found a value of δ = 0.2 at 1160 °C and Po2 ≈ 0.01 beyond which, the sample decomposes. The limit δ = 0.2 is the same that our results suggest. The lower temperature (500 °C) and higher Po2 (0.05) at which we observed it in our sample may be due to its smaller grain and crystallite size. These results point out the outstanding feature nanostructured LSC has, namely, once nanostructured LSC reaches the maximum oxygen vacancy concentration, it uses the disproportionation reaction as a mechanism to keep stabilized the perovskite phase against decomposition. To the best of our knowledge, this is the first time that this mechanism is observed. In addition, the reaction 2Co3+ → Co2+ + Co4+ would help to improve the electrical conductivity by increasing the Co4+ concentration, as suggested by Mineshigue et al.55,59 This last hypothesis would be very interesting to test. Our results are also in agreement with Abbate el al., who have seen no disproportionation reaction below 360 °C in LaCoO3.58
This experiment was intended to have an approach to the behaviour of cobalt atoms in LSC under oxygen demanding conditions (such as in the electrode/electrolyte interface in a SOFC with high oxygen vacancy concentration on the electrolyte side) or under oxygen excess conditions (such as on the electrode/gas interface). The experimental results show that Co atoms in LSC are very sensitive to changes in oxygen concentration in the gas in contact with the LSC particle's surface. This property can be extrapolated to the case of LSC particles in LSC–GDC composite cathodes. Under these conditions, LSC particles are in contact with other particles of LSC or GDC (Scheme S1, ESI†). It is possible that atoms in the vicinity of the contact area with GDC phase exhibit a speciation that resembles that observed during the low oxygen concentration experiment. On the other hand, cobalt atoms near the LSC surface exposed to the gas phase would adopt a chemical speciation resembling that observed under synthetic air conditions. Hence, the improvement in the performance is not only because of more superficial active sites for the electrochemical reactions (favoured by the spherical shape of the GDC particles and the possible agglomeration of them, that keeps apart the LSC particles), and better connectivity between GDC spherical particles, but also because of the action of a breathing structure composed of Co2+, Co3+ and Co4+ that facilitates anionic conduction to the LSC/GDC interface. The fast paths created by the better GDC connectivity complete the cycle, taking away the oxide species from the LSC phase, preventing the modification of the balance of cobalt species, the loss of oxygen vacancies, and active sites blocking by accumulation of adsorbed oxygen. This subtle relationship between GDC morphology, cobalt speciation and LSC electrochemical activity represents the key feature in the enhanced performance of LSC–GDC composite cathode with control of morphology. This hypothesis, however, needs to be tested by more specific experiments. Our work aims to highlight this relationship and encourage further investigations on this issue.
In consequence, the XANES results of our work substitutes the static picture with all cobalt atoms in a unique oxidation state (near +3), for a more dynamical model in which cobalt atoms reacts in a very specific way in response to changes in their surroundings (Scheme 1). The local cycling of cobalt atoms between +3 and +2/+4 oxidation states would be important for the O2(g) adsorption, O2 dissociation and O reduction reactions, which were identified as the limiting process by our EIS study. This is in agreement with Tsvetkov et al., who showed that oxygen vacancies play a secondary role in surface activity.13 In addition, this local redox cycling (probably favoured by the electronic conduction of the material), along with the co-existence of the three cobalt cations (with their different ionic radius), would be beneficial for O2− transport and transfer to the electrolyte phase. Some researchers have guessed this possibility through indirect methods, such as neutron powder diffraction.63 Our results represent strong new evidence in favour of this picture.
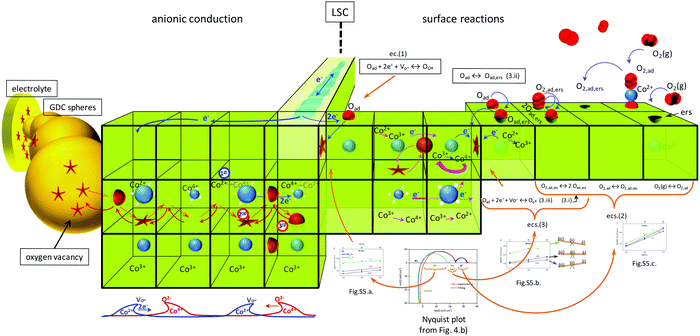 | ||
| Scheme 1 Dynamical model for surface reactions and ionic conduction in LSC–GDC composite cathode based on the most important results found in this work. The ease of Co atoms for changing oxidation state, and disproportionation reaction, along with electronic conduction, are the key features to explain the LSC electrochemical properties. Better percolation of GDC spherical particles yield fast and efficient ionic paths from LSC to the electrolyte. This enhanced ionic flow depletes LSC surface electrochemical reaction sites and favours higher oxygen vacancy concentration, which lower the polarization losses of LSC–GDC composite cathode. “1st” indicates that charge transfer from Co2+ to Co4+ promotes vacancy conduction (2nd) in one way, and anionic conduction in the inverse direction (3rd). Inserted Fig. 4b and 5a–c, and eqn (1)–(3) are explained in terms of this schematic representation. | ||
In our case, EIS analysis suggests that the limiting processes in LSC–GDC composite cathodes take place on the surface of the LSC phase. Consequently, the existence of vacancies in bulk is not as important as the ease of cobalt atom for changing its oxidation state for the charge transfer process that takes place in the surface during oxygen reduction reaction or at the LSC/GDC interface. Our analysis cannot answer the question whether there is mass transport through the bulk of LSC phase, (mass transport would be fast enough so to not contribute significantly to the total ASR), or whether surface diffusion is favoured against bulk diffusion.
XANES and EIS analyses suggest that ease of cobalt atoms for changing its oxidation state along with surface oxygen vacancies are complementary key features that prompt oxygen surface diffusion.
Conclusions
In the present study, the electrochemical performance of nanostructured La0.6Sr0.4CoO3−δ–Gd0.1Ce0.9O1.95 (LSC–GDC) composite cathodes for solid oxide fuel cell (SOFC) with different morphologies (LSC: nanostructured powders; GDC: nanostructured powders composed of particles with irregular shape or spherical particles) were experimentally evaluated.The results obtained from complementary techniques suggest that the LSC phase in LSC–GDC composite cathodes is the active phase for O2(g) adsorption, diffusion, dissociation and reduction of atomic oxygen reactions, which seem to be the limiting processes. Composite cathodes made of 75 wt% LSC and 25 wt% GDC nanostructured spheres exhibited an enhanced performance as cathode in symmetrical cells using Sm0.2Ce0.8O2−d electrolytes. The decrease in the polarization resistance (ASRTOTAL = 18.6(2) mΩ cm2) was attributed to a faster depopulation of oxygen species on LSC surface, which kept active a high number of electrochemical reaction sites. This was favoured by the spherical shape of GDC particles, that provided good electrical contact and high speed paths for oxygen diffusion to the electrolyte (through a good percolation between GDC spherical particles), along with a minimum area of contact between LSC and GDC phases.
X-Ray absorption spectroscopy experiments proved to be a powerful probe to unveil the atomic origin of the electrochemical properties that cathode materials exhibit. Our study suggests that the electrochemical activity of LSC reported in this paper is based on the chemical properties exhibited by cobalt atoms. Novel information about cobalt's speciation in LSC under working-like conditions was presented in this work. The easiness with which cobalt atoms change their oxidation state according to the environment conditions of temperature and O2(g) concentration would be a key feature for adsorption of O2(g), diffusion and dissociation of O2 and reduction of O atoms reactions, on one hand, and for O2− transfer to the electrolyte phase, on the other. The spherical shape of the GDC particles may indirectly modify the chemical speciation of cobalt atoms, favouring the existence of Co2+ and Co4+ species and the local cycling of cobalt atoms between the three oxidation states. Importantly, this effect would contribute to the creation of a “breathing structure” in the LSC phase that favours the anionic conduction. A model describing the underlying mechanism is proposed.
In addition, and to the best of our knowledge, this is the first time that a transition from the regime of O2− vacancy creation to disproportionation reaction in cobalt atoms was observed in nanostructured LSC upon heating, between 500 and 700 °C. After reaching a maximum oxygen vacancy concentration in the LSC lattice (associated to δ ≈ 0.2), the onset of the disproportionation reaction was interpreted as a mechanism that prevents nanostructured LSC from phase instability at higher temperatures and that would favour the electrochemical reactions between LSC and O2(g) on surface, and diminish the electronic resistance growth with increasing temperature. The coexistence of Co2+,3+,4+ species would mechanically favour the oxide anion diffusion through the LSC lattice due to the different ionic radii, and the cycling in the local order of Co species between +2, +3 and +4 oxidation states would also dynamically drive the transport of O2− anions.
As it was observed that for temperatures higher than 500 °C the amount of Co4+ starts to grow, exactly compensating the amount of Co2+, it would be interesting to prove the hypothesis that the existence of Co4+ cations is the responsible for or, at least, contributes to, the electronic conduction in nanostructured LSC at high temperatures.
The effect of LSC particle's morphology (irregular nanopowders, nanotubes of different diameter, nanowires and others) on the electrochemical and thermomechanical properties of LSC–GDC composite cathodes represents an interesting issue to be explored in future works.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the Agency for Science and Technology (PICT 2016-1921, ANPCyT, Ministry of Science, Technology and Innovation, Argentine). This research used resources of the Brazilian Synchrotron Light Laboratory (LNLS), an open national facility operated by the Brazilian Centre for Research in Energy and Materials (CNPEM) for the Brazilian Ministry for Science, Technology, Innovations and Communications (MCTIC). The D08B-XAFS2 beam line staff, Fávio Sambello, and Simone Baú Betim and Tamiris Bouças Piva from the Laboratory of Chemistry (LQU) are acknowledged for the assistance during the experiments. Dr D. Vega (CAC-CNEA, Argentina) and the X-Ray National System (SNRX, MINCYT, Argentina) for the XRD facilities. The authors are members of CIC-CONICET, Argentina.Notes and references
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345–352 CrossRef CAS PubMed.
- Z. Gao, L. V. Mogni, E. C. Miller, J. G. Railsback and S. A. Barnett, Energy Environ. Sci., 2016, 9, 1602–1644 RSC.
- S. J. Skinner, Int. J. Inorg. Mater., 2001, 3, 113–121 CrossRef CAS.
- S. B. Adler, Solid State Ionics, 1998, 111, 125–134 CrossRef CAS.
- H. Uchida, S. Arisaka and M. Watanabe, Solid State Ionics, 2000, 135, 347–351 CrossRef CAS.
- D. Prieur, W. Bonani, K. Popa, O. Walter, K. W. Kriegsman, M. H. Engelhard, X. Guo, R. Eloirdi, T. Gouder, A. Beck, T. Vitova, A. C. Scheinost, K. Kvashnina and P. Martin, Inorg. Chem., 2020, 59, 5760–5767 CrossRef CAS PubMed.
- L. M. Acuña, J. Peña-Martínez, D. Marrero-López, R. O. Fuentes, P. Nuñez and D. G. Lamas, J. Power Sources, 2011, 196, 9276–9283 CrossRef.
- L. M. Acuña, F. F. Muñoz and R. O. Fuentes, J. Phys. Chem. C, 2016, 120, 20387–20399 CrossRef.
- J. Sacanell, A. G. Leyva, M. G. Bellino and D. G. Lamas, J. Power Sources, 2010, 195, 1786–1792 CrossRef CAS.
- M. G. Bellino, J. G. Sacanell, D. G. Lamas, A. G. Leyva and N. E. Walsöe De Reca, J. Am. Chem. Soc., 2007, 129, 3066–3067 CrossRef CAS PubMed.
- L. Dieterle, P. Bockstaller, D. Gerthsen, J. Hayd, E. Ivers-Tiffée and U. Guntow, Adv. Energy Mater., 2011, 1, 249–258 CrossRef CAS.
- K. Develos-Bagarinao, R. A. Budiman, S. S. Liu, T. Ishiyama, H. Kishimoto and K. Yamaji, J. Power Sources, 2020, 453, 227894 CrossRef CAS.
- N. Tsvetkov, Q. Lu, L. Sun, E. J. Crumlin and B. Yildiz, Nat. Mater., 2016, 15, 1010–1016 CrossRef CAS PubMed.
- S. Wang, J. Yoon, G. Kim, D. Huang, H. Wang and A. J. Jacobson, Chem. Mater., 2010, 22, 776–782 CrossRef CAS.
- L. Dos Santos-Gómez, S. Sanna, P. Norby, N. Pryds, E. R. Losilla, D. Marrero-López and V. Esposito, Nanoscale, 2019, 11, 2916–2924 RSC.
- A. Heel, P. Holtappels and T. Graule, J. Power Sources, 2010, 195, 6709–6718 CrossRef CAS.
- S. Wang, T. Kobayashi, M. Dokiya and T. Hashimoto, J. Electrochem. Soc., 2000, 147, 3606 CrossRef CAS.
- Y. Tao, J. Shao, W. G. Wang and J. Wang, Fuel Cells, 2009, 9, 679–683 CrossRef CAS.
- S. Park, S. Choi, J. Shin and G. Kim, Electrochim. Acta, 2014, 125, 683–690 CrossRef CAS.
- F. Wei, H. Cao and X. Chen, J. Mater. Sci., 2016, 51, 2160–2167 CrossRef CAS.
- C. Benel, A. J. Darbandi, R. Djenadic, A. Evans, R. Tölke, M. Prestat and H. Hahn, J. Power Sources, 2013, 229, 258–264 CrossRef CAS.
- H. Yokokawa, N. Sakai, T. Horita, K. Yamaji, M. E. Brito and H. Kishimoto, J. Alloys Compd., 2008, 452, 41–47 CrossRef CAS.
- R. Knibbe, J. Hjelm, M. Menon, N. Pryds, M. Søgaard, H. J. Wang and K. Neufeld, J. Am. Ceram. Soc., 2010, 93, 2877–2883 CrossRef CAS.
- M. Shiono, K. Kobayashi, T. L. Nguyen, K. Hosoda, T. Kato, K. Ota and M. Dokiya, Solid State Ionics, 2004, 170, 1–7 CrossRef CAS.
- S. B. Adler, X. Y. Chen and J. R. Wilson, J. Catal., 2007, 245, 91–109 CrossRef CAS.
- Y. C. Chen, M. Yashima, T. Ohta, K. Ohoyama and S. Yamamoto, J. Phys. Chem. C, 2012, 116, 5246–5254 CrossRef CAS.
- Y. Orikasa, T. Ina, T. Nakao, A. Mineshige, K. Amezawa, M. Oishi, H. Arai, Z. Ogumi and Y. Uchimoto, J. Phys. Chem. C, 2011, 115, 16433–16438 CrossRef CAS.
- M. H. R. Lankhorst, H. J. M. Bouwmeester and H. Verweij, J. Solid State Chem., 1997, 133, 555–567 CrossRef CAS.
- A. N. Petrov, V. A. Cherepanov, O. F. Kononchuk and L. Y. Gavrilova, J. Solid State Chem., 1990, 87, 69–76 CrossRef CAS.
- J. L. Hueso, J. P. Holgado, R. Pereñíguez, V. M. Gonzalez-Delacruz and A. Caballero, Mater. Chem. Phys., 2015, 151, 29–33 CrossRef CAS.
- P. Hjalmarsson, M. Søgaard, A. Hagen and M. Mogensen, Solid State Ionics, 2008, 179, 636–646 CrossRef CAS.
- V. V. Efimov, E. Efimova, D. Karpinsky, D. I. Kochubey, V. Kriventsov, A. Kuzmin, S. Molodtsov, V. Sikolenko, J. Purans, S. Tiutiunnikov, I. O. Troyanchuk, A. N. Shmakov and D. Vyalikh, Nucl. Instrum. Methods Phys. Res., Sect. A, 2007, 575, 176–179 CrossRef CAS.
- O. Haas, C. Ludwig, U. Bergmann, R. N. Singh, A. Braun and T. Graule, J. Solid State Chem., 2011, 184, 3163–3171 CrossRef CAS.
- V. Sikolenko, A. Sazonov, V. Efimov, V. Krivencov, N. Darowski and D. Vyalikh, J. Magn. Magn. Mater., 2007, 310, 2006–2008 CrossRef.
- T. Itoh, M. Inukai, N. Kitamura, N. Ishida, Y. Idemoto and T. Yamamoto, J. Mater. Chem. A, 2015, 3, 6943–6953 RSC.
- Y. Takeda, R. Kanno, M. Noda, Y. Tomida and O. Yamamoto, J. Electrochem. Soc., 2019, 134, 2656–2661 CrossRef.
- D. Baskar and S. B. Adler, ECS Trans., 2019, 7, 1279–1286 CrossRef.
- R. O. Fuentes and R. T. Baker, J. Power Sources, 2009, 186, 268–277 CrossRef CAS.
- F. F. Muñoz, L. M. Acuña, C. A. Albornoz, A. G. Leyva, R. T. Baker and R. O. Fuentes, Nanoscale, 2015, 7, 271–281 RSC.
- H. Taguchi, S. Yamada, M. Nagao, Y. Ichikawa and K. Tabata, Mater. Res. Bull., 2002, 37, 69–76 CrossRef CAS.
- V. G. Milt, M. A. Ulla and E. E. Miró, Appl. Catal. B: Environ., 2005, 57, 13–21 CrossRef CAS.
- H. Kozuka, H. Yamada, T. Hishida, K. Yamagiwa, K. Ohbayashi and K. Koumoto, J. Mater. Chem., 2012, 22, 20217–20222 RSC.
- S. J. A. Figueroa, J. C. Mauricio, J. Murari, D. B. Beniz, J. R. Piton, H. H. Slepicka, M. F. De Sousa, A. M. Espíndola and A. P. S. Levinsky, J. Phys.: Conf. Ser., 2016, 712, 1–6 Search PubMed.
- S. Calvin, XAFS for Everyone, CRC Press, 2013 Search PubMed.
- T. Ressler, J. Synchrotron Radiat., 1998, 5, 118–122 CrossRef CAS PubMed.
- O. Gwon, S. Yoo, J. Shin and G. Kim, Int. J. Hydrogen Energy, 2014, 39, 20806–20811 CrossRef CAS.
- A. Jun, J. Kim, J. Shin and G. Kim, ChemElectroChem, 2016, 3, 511–530 CrossRef CAS.
- A. N. Petrov, O. F. Kononchuk, A. V. Andreev, V. A. Cherepanov and P. Kofstad, Solid State Ionics, 1995, 80, 189–199 CrossRef CAS.
- N. M. L. N. P. Closset, R. H. E. Van Doorn and H. Kruidhof, Powder Diffr., 1996, 11, 31–34 CrossRef CAS.
- Y. T. Kim and N. Shikazono, Solid State Ionics, 2017, 309, 77–85 CrossRef CAS.
- J. R. Macdonald and D. R. Franceschetti, J. Chem. Phys., 1978, 68, 1614–1637 CrossRef CAS.
- J. M. Lattimer and M. Prakash, Science, 2004, 304, 536–542 CrossRef CAS PubMed.
- A. Ringuedé and J. Fouletier, Solid State Ionics, 2001, 139, 167–177 CrossRef.
- V. A. Sadykov, S. N. Pavlova, V. S. Kharlamova, V. S. Muzykantov, N. F. Uvarov, Y. S. Okhupin, A. V. Ishchenko, A. S. Bobin, N. V. Mezentseva, G. M. Alikina, A. I. Lukashevich, T. A. Krieger, T. V. Larina, N. N. Bulgakov, V. M. Tapilin, V. D. Belyaev, E. M. Sadovskaya, A. I. Boronin, V. A. Sobyanin, A. F. Bobrenok, A. L. Smirnova, O. L. Smorygo and J. A. Kilner, in Perovskites: Structure, Properties and Uses, ed. M. Borowski, Nova Science Publishers, Inc., 2010, pp. 67–178 Search PubMed.
- A. Mineshige, M. Kobune, S. Fujii, Z. Ogumi and M. Inaba, J. Solid State Chem., 1999, 381, 374–381 CrossRef.
- M. a Senaris-Rodriguez and J. B. Goodenough, J. Solid State Chem., 1995, 118, 323–336 CrossRef.
- O. Toulemonde, N. N’Guyen, F. Studer and A. Traverse, J. Solid State Chem., 2001, 158, 208–217 CrossRef CAS.
- M. Abbate, J. C. Fuggle, A. Fujimori, L. H. Tjeng, C. T. Chen, R. Potze, G. A. Sawatzky, H. Eisaki and S. Uchida, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 16124–16130 CrossRef CAS PubMed.
- B. Raveau and M. M. Seikh, Cobalt Oxides, 2012, 3–70 Search PubMed.
- M. Søgaard, P. V. Hendriksen, M. Mogensen, F. W. Poulsen and E. Skou, Solid State Ionics, 2006, 177, 3285–3296 CrossRef.
- Y. Tokura and N. Nagaosa, Science, 2000, 288, 462 LP–468 CrossRef PubMed.
- H. Kozuka, H. Yamada, T. Hishida, K. Yamagiwa, K. Ohbayashi and K. Koumoto, J. Mater. Chem., 2012, 22, 20217–20222 RSC.
- Y. Gong, C. Sun, Q. A. Huang, J. A. Alonso, M. T. Fernández-Díaz and L. Chen, Inorg. Chem., 2016, 55, 3091–3097 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XANES procedure and analysis, XRD, EIS and SEM analysis. See DOI: https://doi.org/10.1039/d2ya00085g |
| This journal is © The Royal Society of Chemistry 2022 |