Open Access Article
Open Access ArticleStabilizing all-inorganic CsPbI3 perovskite films with polyacrylonitrile for photovoltaic solar cells†
Guanghui
Yu
ab,
Ke-Jian
Jiang
 *b,
Wei-Min
Gu
bc,
Yue
Zhang
b,
Yanting
Xu
b,
Tangyue
Xue
ab,
Xinning
Jiao
b,
Yiqiang
Zhang
*b,
Wei-Min
Gu
bc,
Yue
Zhang
b,
Yanting
Xu
b,
Tangyue
Xue
ab,
Xinning
Jiao
b,
Yiqiang
Zhang
 *a and
Yanlin
Song
*a and
Yanlin
Song
 *b
*b
aCollege of Chemistry, Zhengzhou University, Zhengzhou, 450001, P. R. China. E-mail: yqzhang@zzu.edu.cn
bKey Laboratory of Green Printing, Institute of Chemistry, CAS, Beijing, 100190, P. R. China. E-mail: ylsong@iccas.ac.cn; kjjiang@iccas.ac.cn
cSchool of Chemical Science, University of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 23rd December 2021
Abstract
A solution-processed CsPbI3 perovskite film usually suffers from nonuniform crystallization owing to its inorganic components, leading to its poor surface morphology and various defects on the surface and grain boundaries. Herein, polyacrylonitrile (PAN) was employed as a defect passivator for CsPbI3 perovskite films. The nitrile (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) groups on the side chains of the PAN chain skeleton could passivate an undercoordinated Pb ion with a partial positive charge by donating their lone pair of electrons from the N atom, decreasing the density of defect states and enhancing the energy barrier of phase transition. In addition, the incorporation of PAN triggered the nucleation of the perovskite precursor, leading to the formation of a dense CsPbI3 perovskite film with high coverage. Moreover, the robust PAN in the perovskite film enhanced the thermal and water resistance properties of the perovskite film. As a result, the unencapsulated CsPbI3 perovskite cells exhibited superior thermal and humidity stability with 16.21% power conversion efficiency. This work provides a liable polymer passivator to further improve both the efficiency and stability of CsPbI3-based solar cells.
N) groups on the side chains of the PAN chain skeleton could passivate an undercoordinated Pb ion with a partial positive charge by donating their lone pair of electrons from the N atom, decreasing the density of defect states and enhancing the energy barrier of phase transition. In addition, the incorporation of PAN triggered the nucleation of the perovskite precursor, leading to the formation of a dense CsPbI3 perovskite film with high coverage. Moreover, the robust PAN in the perovskite film enhanced the thermal and water resistance properties of the perovskite film. As a result, the unencapsulated CsPbI3 perovskite cells exhibited superior thermal and humidity stability with 16.21% power conversion efficiency. This work provides a liable polymer passivator to further improve both the efficiency and stability of CsPbI3-based solar cells.
Introduction
Organic–inorganic hybrid perovskites have attracted tremendous attention because of their excellent optoelectronic properties and low-temperature solution-processing ability.1–4 The power conversion efficiency (PCE) of solution processed perovskite solar cells (PSCs) has increased from an initial 3.8% to a certified 25.5%.1,3 Although continuous progress in improving efficiency has been made in organic–inorganic hybrid perovskites, the long-term stability issue from the degradation and volatilization of organic groups, e.g., methylammonium (MA+) and formamidinium (FA+) cations, is still one of the key challenges for their practical applications. Replacing the organic cations with Cs+ ions for all-inorganic perovskites, especially CsPbI3 with a suitable optical bandgap (≈1.7 eV), has attracted intense research attention recently because of their intrinsic chemical stability. However, CsPbI3 suffers from a serious phase stability problem; photoactive black-phase CsPbI3 tends to convert into a nonperovskite yellow phase (δ-CsPbI3) under thermal or humid stress.5–8 In 2014, Choi et al. first reported CsPbI3-based PSCs. However, the PCE is very low (0.09%) due to the presence of nonperovskite δ-CsPbI3 in the devices.5 Thereafter, various methods were developed to stabilize the black CsPbI3 phase at room temperature via distortion of PbI6 octahedra without breaking the three-dimensional (3D) Pb–I network. Swarnkar et al. utilized stable cubic-phase CsPbI3 perovskite quantum dots (QDs) to fabricate photovoltaic devices with a PCE of 10.77%.6 Wang et al. developed a solvent controlled growth method to prepare a CsPbI3 film, and the resultant solar cell showed a PCE of 15.7% in a N2-filled glovebox.7 In 2015, Eperon et al. introduced hydroiodic acid (HI) into CsPbI3 precursor solution in N,N-dimethylformamide (DMF) to stabilize the black perovskite phase at room temperature.8 Recently, great efforts have been devoted to the fabrication of HI-related CsPbI3 films, and the device exhibited high PCEs of ∼20% with enhanced stability. It has been known that the introduction of dimethylammonium iodide (DMAI), generated through the reaction between HI and DMF, plays a key role in tuning the intermediate phase and crystallization processes of the CsPbI3 film.9–20 Moreover, defect passivation is found to be another effective method for further improvement of both the efficiency and stability of CsPbI3 solar cells.18–28In the past years, small-molecule organic ammonium salts have been employed to passivate the defects in CsPbI3 films.18–20 Zhao et al. introduced phenyltrimethylammonium chloride (PTACl) to passivate the defects at the surfaces and grain boundaries of CsPbI3 films by a solution post-treatment method, and the charge-carrier lifetime of the device was greatly increased with a PCE of up to 19%.18 Recently, the Seok group achieved a high PCE of up to 20.37% through surface-defect passivation of CsPbI3 films using octylammonium iodide.19 Different from the surface passivation reported above, bulk defect passivation was developed by the Meng group, where a urea-ammonium thiocyanate (UAT) molten salt was directly introduced into CsPbI3 precursor solution. It was found that SCN− anions could be fully released from UAT and strongly coordinate with the Pb–I octahedron, improving the CsPbI3 film morphology and significantly suppressing the defects and the non-radiative charge recombination of the film. With the optimal UAT component, the CsPbI3 solar cell exhibited a high efficiency of over 20.0%.20 However, the high diffusion coefficient and strong volatility of such organic small-molecule passivation agents could weaken the passivation effect and the thermal stability of PSCs. Thus, polymer passivation agents with the same functional groups are expected to overcome these shortcomings.21–25 Previously, Li et al. introduced poly(4-vinylpyridine) (PVP) as a passivation agent to synthesize long-term stable cubic CsPbI3.21 The acylamino groups in PVP enhanced the electron cloud density on the surface of CsPbI3, thus reducing the surface energy. The resultant device showed excellent thermal/moisture stability, but a low PCE of 10.7%. In addition, Wei et al. introduced a PMMA (poly(methyl methacrylate)) buffer layer in CsPbI3-based solar cells to passivate interface defects, and suppress defect state-induced carrier recombination. The devices with PMMA showed enhanced stability with a PCE of 10.99%.23 Recently, Yang et al. employed poly(styrene-co-acrylonitrile) polymer (PS-PAN polymer) to modify the surfaces of organic–inorganic hybrid perovskite films.25 The nitrile (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) groups in the side chain could coordinate with Pb2+ defects at the surface and the grain boundaries of the perovskite, similar to CN-containing small molecules reported previouly.25–28
N) groups in the side chain could coordinate with Pb2+ defects at the surface and the grain boundaries of the perovskite, similar to CN-containing small molecules reported previouly.25–28
In this work, polyacrylonitrile (PAN) was employed as a passivation agent to passivate bulk CsPbI3 perovskite defects and improve the device stability. It was found that the nitrile (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) groups in the side chain could coordinate with Pb2+ defects at the surface and the grain boundaries. In addition, the incorporation of PAN could trigger the nucleation of the perovskite precursor, leading to the formation of a denser CsPbI3 perovskite film with high coverage. Moreover, the robust PAN in the perovskite films effectively enhanced the thermal and water resistance properties of the perovskite film. As a result, the PAN modified CsPbI3 solar cells exhibited a high PCE of 16.21% with significantly enhanced stabilities.
N) groups in the side chain could coordinate with Pb2+ defects at the surface and the grain boundaries. In addition, the incorporation of PAN could trigger the nucleation of the perovskite precursor, leading to the formation of a denser CsPbI3 perovskite film with high coverage. Moreover, the robust PAN in the perovskite films effectively enhanced the thermal and water resistance properties of the perovskite film. As a result, the PAN modified CsPbI3 solar cells exhibited a high PCE of 16.21% with significantly enhanced stabilities.
Results and discussion
Polyacrylonitrile (PAN) was commercially available with average Mw 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, where the functional nitrile groups (C
000, where the functional nitrile groups (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) are attached on the side chains of the C–C chain skeleton (Fig. S1, ESI†). It exhibits high thermal stability with a decomposition temperature of up to 300 °C, much higher than the processing temperatures (∼200 °C) of the CsPbI3 films, ensuring that the PAN remains in the CsPbI3 films after the processing (Fig. S2, ESI†).
N) are attached on the side chains of the C–C chain skeleton (Fig. S1, ESI†). It exhibits high thermal stability with a decomposition temperature of up to 300 °C, much higher than the processing temperatures (∼200 °C) of the CsPbI3 films, ensuring that the PAN remains in the CsPbI3 films after the processing (Fig. S2, ESI†).
CsPbI3 perovskite thin films were fabricated by the one-step spin coating method reported by Wang et al.,18 where the precursor solution is composed of stoichiometric cesium iodide (CsI), lead iodide (PbI2), and dimethylammonium iodide (DMAI) in DMF with a concentration of 0.6 M. For a PAN-modified film, a certain amount of PAN was added to the solution. The resulting film is denoted as xPAN–CsPbI3 (x: the mass percent of PAN to CsPbI3). Fig. 1a and Fig. S3 (ESI†) show the ultraviolet-visible (UV-vis) absorption spectra of the xPAN–CsPbI3 films (x: 0–0.3). It was found that all the films showed the same absorption properties with the absorption edge at ∼736 nm, corresponding to band gaps of about 1.68 eV (Fig. S4, ESI†). The result indicated that the incorporation of PAN did not affect light absorption of the resultant films. In addition, the X-ray diffraction (XRD) patterns (Fig. 1b and Fig. S5, ESI†) showed that all the films exhibited the same diffraction peaks, indicating that the PAN did not enter into the perovskite crystal, but remained at the surface and grain boundaries of the perovskite films. Fig. 1c and d and Fig. S6 (ESI†) show the scanning electron microscopy (SEM) images of the xPAN–CsPbI3 films. It could be observed that the control film without PAN exhibited a poor morphology with a lot of pin holes, probably due to the fast crystallization of the inorganic perovskite commonly observed previously.29–34 In addition, the removal of a large amount of DMAI from the films at high temperature could contribute to the formation of pinholes. It was observed that the pinhole density decreased with the addition of PAN, and almost disappeared when the x was increased to 0.2 (0.2PAN-CsPbI3 film). However, the pinhole density increased with the further addition of PAN (x = 0.3) in the films. This result indicates that the appropriate amount of PAN would favour the nucleation and crystal growth of the perovskite for the formation of pin hole-free CsPbI3 films.35
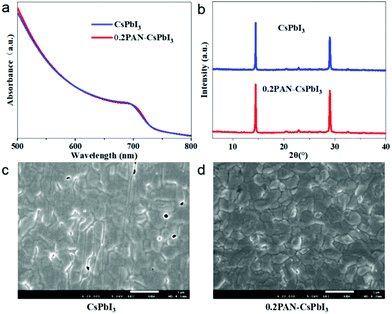 | ||
| Fig. 1 UV-vis (a) and XRD (b) spectra of CsPbI3 and 0.2PAN–CsPbI3 thin films, and top-surface SEM images of CsPbI3 (c) and 0.2PAN–CsPbI3 films (d). Scale bar, 1 μm. | ||
To confirm the existence of PAN and the interaction with CsPbI3 in the films, Fourier transform infrared spectroscopy (FTIR) measurement was performed. As shown in Fig. 2a, and Fig. S7 (ESI†), pure PAN exhibited absorption bands in the regions of 2941 cm−1 and 2247 cm−1, which were attributed to the functional C–H and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N in PAN, respectively. To check the interaction between PAN and CsPbI3, excess PAN was incorporated in CsPbI3 (PAN
N in PAN, respectively. To check the interaction between PAN and CsPbI3, excess PAN was incorporated in CsPbI3 (PAN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CsPbI3, 1
CsPbI3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, by wt) for FTIR. It was found that the two characteristic vibrations of PAN were blue-shifted to 2931 cm−1 for the C–H stretching, and 2243 cm−1 for the C
100, by wt) for FTIR. It was found that the two characteristic vibrations of PAN were blue-shifted to 2931 cm−1 for the C–H stretching, and 2243 cm−1 for the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching, respectively, indicating the interaction between PAN and CsPbI3. In order to investigate the interaction mechanism, solid-state 13C magic angle spinning (MAS) NMR spectroscopy was performed. As shown in Fig. 2b, two broad peaks in the pristine PAN were observed at 30.1 and 121.6 ppm, which were ascribed to the main chain C–C and C
N stretching, respectively, indicating the interaction between PAN and CsPbI3. In order to investigate the interaction mechanism, solid-state 13C magic angle spinning (MAS) NMR spectroscopy was performed. As shown in Fig. 2b, two broad peaks in the pristine PAN were observed at 30.1 and 121.6 ppm, which were ascribed to the main chain C–C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups, respectively, and well consistent with a previous report.36 After the incorporation of PAN in the CsPbI3 film, the C signals were shifted to 29.3 ppm for the C–C group and 122.4 ppm for the C
N groups, respectively, and well consistent with a previous report.36 After the incorporation of PAN in the CsPbI3 film, the C signals were shifted to 29.3 ppm for the C–C group and 122.4 ppm for the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group, further demonstrating the chemical action between the PAN and the perovskite. To further confirm the interaction between the PAN and the perovskite film, X-ray photoelectron spectroscopy (XPS) was performed to study the chemical states of the samples. As shown in Fig. 2c, two main peaks for the Pb4f were located at 143.20 eV for Pb 4f5/2 and 138.30 eV for Pb 4f7/2 in the pristine perovskite film. After the incorporation of PAN, both the peaks were shifted towards low binding energies of 143.05 and 138.15 eV, respectively, suggesting an increased electron cloud density on Pb from the N lone pair electrons in the C
N group, further demonstrating the chemical action between the PAN and the perovskite. To further confirm the interaction between the PAN and the perovskite film, X-ray photoelectron spectroscopy (XPS) was performed to study the chemical states of the samples. As shown in Fig. 2c, two main peaks for the Pb4f were located at 143.20 eV for Pb 4f5/2 and 138.30 eV for Pb 4f7/2 in the pristine perovskite film. After the incorporation of PAN, both the peaks were shifted towards low binding energies of 143.05 and 138.15 eV, respectively, suggesting an increased electron cloud density on Pb from the N lone pair electrons in the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group. In addition, the N 1s peak from the C
N group. In addition, the N 1s peak from the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group observed at 399.6 eV in the neat PAN was shifted to 400.1 eV after the PAN incorporation (Fig. 2d), indicating decreased electron density near the N in the C
N group observed at 399.6 eV in the neat PAN was shifted to 400.1 eV after the PAN incorporation (Fig. 2d), indicating decreased electron density near the N in the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group, consistent with the binding energy change from the Pb 4f.
N group, consistent with the binding energy change from the Pb 4f.
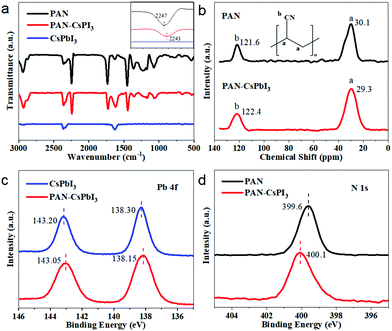 | ||
| Fig. 2 (a) FTIR spectra of PAN, PAN–CsPbI3, and the CsPbI3. (b) 13C NMR spectra of PAN and PAN–CsPbI3. (c) Pb 4f and (d) N 1s XPS spectra of PAN and PAN–CsPbI3. | ||
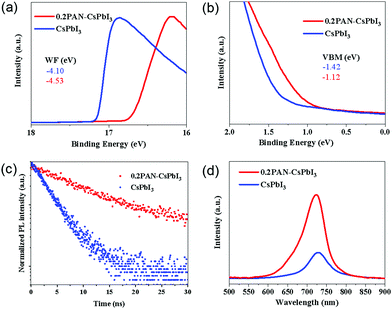
Ultra-violet photoelectron spectra (UPS) were recorded for CsPbI3 films with and without PAN, as shown in Fig. 3a and b. The valence and conduction bands of 0.2PAN–CsPbI3 were found to be slightly up-shifted as compared those of the pristine CsPbI3 according to the UPS and the Tauc plots shown in Fig. S8 and S9 (ESI†). The shift could help carrier extraction at the interface between the CsPbI3 and Spiro-OMeTAD hole transport material, contributing to an enhanced open-circuit voltage (VOC) in the devices. To further investigate the effect of PAN on the electronic properties of the perovskite film, steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) measurements were performed. As shown in Fig. 3c, the 0.2PAN–CsPbI3 film exhibited a slower decay behaviour than the pristine film. Both the curves could be fit to a biexponential function, giving a fast time constant τ1 (corresponding to the fast nonradiative recombination induced by defects or impurities) and a slow time constant τ2 (representing the radiative recombination of the charge carriers in the bulk). The τave value was found to increase from 3.42 to 6.93 ns after the incorporation of PAN in the 0.2PAN–CsPbI3 film. In addition, the PL intensity was increased about 3 times after the PAN inclusion (Fig. 3d), which was consistent with the TRPL result. The results indicate lower defect density in the 0.2PAN–CsPbI3 film, which mainly originates from the efficient passivation effect due to the PAN.
It is known that black β-CsPbI3 is unstable thermodynamically, and could be converted to δ-CsPbI3 at room temperature, especially in a humid environment. With the inclusion of PAN polymer on the surface and the grain boundaries, the resulting film resistance to the moisture could be enhanced to improve the stability of the black β-CsPbI3. To check the role of PAN in the CsPbI3 stability, perovskite films without and with PAN (x = 0.2) were fabricated on glass substrates, and stored under ambient conditions with 30 ± 5% RH and at 25 ± 5 °C for the stability test. As shown in the XRD spectra in Fig. S10 (ESI†), a significant diffraction peak at 9.9° emerged in the control film after 24 h aging, indicating the formation of yellow δ-CsPbI3.37 In contrast, the target sample retained the original diffraction patterns under the same conditions. In addition, the high stability of the 0.2PAN–CsPbI3 film was also confirmed by the UV-vis absorption spectra shown in Fig. S11 (ESI†).
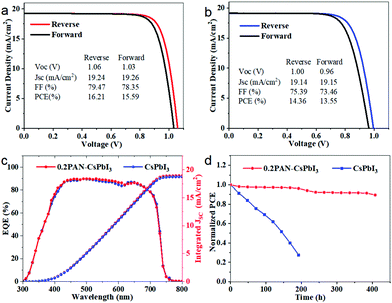
To verify the effect of the PAN inclusion in the CsPbI3 layer on the photovoltaic performance of PSCs, we prepared solar cells with a glass/fluorine doped tin oxide (FTO)/TiO2/xPAN–CsPbI3/Spiro-OMeTAD/Au configuration, where the CsPbI3 perovskite films were fabricated by the one-step spin coating method reported previously,18 and the x value is 0 and 0.2 for the control device and the target device, respectively. Fig. 4a and b show the optimized current density (J)–voltage (V) curves of the control and target devices, respectively, measured under both reverse and forward scans (the scan rate is 0.1 V s−1) under 100 mW cm−2 AM1.5G illumination. The corresponding PV performance parameters are listed in the figures. The control device without PAN showed a PCE of 14.36 (13.55)% with a fill factor (FF) of 75.39 (73.46)%, a short-circuit current (Jsc) of 19.14 (19.15) mA cm−2, and an open-circuit voltage (VOC) of 1.00 (0.96) V in the reverse (forward) scan. In contrast, the target device with PAN exhibited a higher PCE of 16.21 (15.59)% with a FF of 79.47 (78.35)%, a Jsc of 19.24 (19.26) mA cm−2 and a VOC of 1.06 (1.03) V under the same conditions. The integrated Jsc values obtained from the external quantum efficiency (EQE) spectra matched well with the J–V measured values (Fig. 4c). The increase in PCE for the target device is mainly due to the improved VOC and FF, suggesting a satisfactory passivation effect from the PAN.18–20 The stability of the unencapsulated devices was preliminarily tested in air at 25 °C and 20–30% relative humidity. As shown in Fig. 4d, the control device lost 72% of its initial PCE after 200 h aging, while the target device still retained 89% of its initial PCE after 400 h aging. The result clearly indicates that the incorporation of PAN can enhance both the efficiency and stability of the photovoltaic solar cells.
Conclusion
In summary, polyacrylonitrile (PAN) was employed for the fabrication of all-inorganic CsPbI3 perovskite films. It was found that the nitrile group in PAN could passivate the defects on the surface and grain boundaries of the CsPbI3 perovskite films. In addition, the PAN assisted the nucleation and the crystal growth for the formation of pinhole-free CsPbI3 perovskite films. Moreover, the polymer properties of PAN can enhance the thermal and humidity stability of the CsPbI3 film. With the optimization of PAN in the perovskite film, the efficiency of the device was increased by about 13% compared with the control samples. Moreover, the device stability was significantly enhanced under ambient conditions. This result provides an effective and robust approach for the fabrication of all-inorganic CsPbI3 perovskite solar cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (Grant No. 2018YFA0208501), the National Natural Science Foundation of China (Grant No. 51803217, 51773206, 61874123, 91963212, 21401167), the External Cooperation Program of Chinese Academy of Sciences (Grant No. GJHZ201948) and the Key R&D and Promotion Project of Henan Province (Grant No. 192102210032). The authors also thank the Advanced Analysis & Computation Center at Zhengzhou University for materials and device characterization support.Notes and references
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- P. Li, Y. Zhang, C. Liang, G. Xing, X. Liu, F. Li, X. Liu, X. Hu, G. Shao and Y. Song, Adv. Mater., 2018, 30, 1805323 CrossRef PubMed.
- National Renewable Energy Laboratory (NREL), Best Research-Cell Efficiencies Chart, https://www.nrel.gov/pv/cell-efficiency.html.
- J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat, A. Mishra, Y. Yang, M. A. Hope, F. T. Eickemeyer, M. Kim, Y. J. Yoon, I. W. Choi, B. P. Darwich, S. J. Choi, Y. Jo, J. H. Lee, B. Walker, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, A. Hagfeldt, D. S. Kim, M. Grätzel and J. Y. Kim, Nature, 2021, 592, 381–385 CrossRef CAS PubMed.
- H. Choi, J. Jeong, H.-B. Kim, S. Kim, B. Walker, G.-H. Kim and J. Y. Kim, Nano Energy, 2014, 7, 80–85 CrossRef CAS.
- A. Swarnkar, A. R. Marshall, E. M. Sanehira, B. D. Chernomordik, D. T. Moore, J. A. Christians, T. Chakrabarti and J. M. Luther, Science, 2016, 354, 92–95 CrossRef CAS PubMed.
- P. Wang, X. Zhang, Y. Zhou, Q. Jiang, Q. Ye, Z. Chu, X. Li, X. Yang, Z. Yin and J. You, Nat. Commun., 2018, 9, 2225 CrossRef PubMed.
- G. E. Eperon, G. M. Paternò, R. J. Sutton, A. Zampetti, A. A. Haghighirad, F. Cacialli and H. J. Snaith, J. Mater. Chem. A, 2015, 3, 19688–19695 RSC.
- P. Luo, W. Xia, S. Zhou, L. Sun, J. Cheng, C. Xu and Y. Lu, J. Phys. Chem. Lett., 2016, 7, 3603–3608 CrossRef CAS PubMed.
- Y. Jiang, J. Yuan, Y. Ni, J. Yang, Y. Wang, T. Jiu, M. Yuan and J. Chen, Joule, 2018, 2, 1356–1368 CrossRef.
- F. Li, Y. Pei, F. Xiao, T. Zeng, Z. Yang, J. Xu, J. Sun, B. Peng and M. Liu, Nanoscale, 2018, 10, 6318–6322 RSC.
- B. Zhao, S.-F. Jin, S. Huang, N. Liu, J.-Y. Ma, D.-J. Xue, Q. Han, J. Ding, Q.-Q. Ge, Y. Feng and J.-S. Hu, J. Am. Chem. Soc., 2018, 140, 11716–11725 CrossRef CAS PubMed.
- Y. Wang, T. Zhang, F. Xu, Y. Li and Y. Zhao, Sol. RRL, 2018, 2, 1700180 CrossRef.
- Y. Wang, M. I. Dar, L. K. Ono, T. Zhang, M. Kan, Y. Li, L. Zhang, X. Wang, Y. Yang, X. Gao, Y. Qi, M. Grätzel and Y. Zhao, Science, 2019, 365, 591–595 CrossRef CAS PubMed.
- H. Bian, H. Wang, Z. Li, F. Zhou, Y. Xu, H. Zhang, Q. Wang, L. Ding, S. Liu and Z. Jin, Adv. Sci., 2020, 7, 1902868 CrossRef CAS PubMed.
- W. Ke, I. Spanopoulos, C. C. Stoumpos and M. G. Kanatzidis, Nat. Commun., 2018, 9, 4785 CrossRef PubMed.
- H. Meng, Z. Shao, L. Wang, Z. Li, R. Liu, Y. Fan, G. Cui and S. Pang, ACS Energy Lett., 2020, 5, 263–270 CrossRef CAS.
- Y. Wang, Xi Liu, T. Zhang, X. Wang, M. Kan, J. Shi and Y. Zhao, Angew. Chem., Int. Ed., 2019, 58, 16691–16696 CrossRef CAS PubMed.
- S. M. Yoon, H. Min, J. B. Kim, G. Kim, K. S. Lee and S. Seok II, Joule, 2021, 5, 183–196 CrossRef CAS.
- B. Yu, J. Shi, S. Tan, Y. Cui, W. Zhao, H. Wu, Y. Luo, D. Li and Q. Meng, Angew. Chem., Int. Ed., 2021, 60, 13436–13443 CrossRef CAS PubMed.
- B. Li, Y. Zhang, L. Fu, T. Yu, S. Zhou, L. Zhang and L. Yin, Nat. Commun., 2018, 9, 1076 CrossRef PubMed.
- R. Saraf and V. Maheshwari, ACS Appl. Energy Mater., 2019, 2, 2214–2222 CrossRef CAS.
- W. Wei, W. Chen, X. Zhao, Z. Yang and Y. Liu, J. Alloys Compd., 2021, 891, 161985 CrossRef.
- Z. Huang, X. Hu, C. Liu, L. Tan and Y. Chen, Adv. Funct. Mater., 2017, 27, 1703061 CrossRef.
- J. Yang, Q. Cao, Z. He, X. Pu, T. Li, B. Gao and X. Li, Nano Energy, 2021, 82, 105731 CrossRef CAS.
- T. Wu, Y. Wang, Z. Dai, D. Cui, T. Wang, X. Meng, E. Bi, X. Yang and L. Han, Adv. Mater., 2019, 31, 1900605 CrossRef PubMed.
- Y. Lin, L. Shen, J. Dai, Y. Deng, Y. Wu, Y. Bai, X. Zheng, J. Wang, Y. Fang, H. Wei, W. Ma, X. C. Zeng, X. Zhan and J. Huang, Adv. Mater., 2017, 29, 1604545 CrossRef PubMed.
- J. Wang, J. Zhang, Y. Zhou, H. Liu, Q. Xue, X. Li, C.-C. Chueh, H.-L. Yip, Z. Zhu and A. K. Y. Jen, Nat. Commun., 2020, 11, 177 CrossRef CAS PubMed.
- T. Zhang, M. I. Dar, G. Li, F. Xu, N. Guo, M. Grätzel and Y. Zhao, Sci. Adv., 2017, 3, e1700841 CrossRef PubMed.
- Y. Wang, T. Zhang, M. Kan and Y. Zhao, J. Am. Chem. Soc., 2018, 140, 12345–12348 CrossRef CAS PubMed.
- X. Ding, M. Cai, X. Liu, Y. Ding, X. Liu, Y. Wu, T. Hayat, A. Alsaedi and S. Dai, ACS Appl. Mater. Interfaces, 2019, 11, 37720–37725 CrossRef CAS PubMed.
- Y. Wang, G. Chen, D. Ouyang, X. He, C. Li, R. Ma, W.-J. Yin and W. C. H. Choy, Adv. Mater., 2020, 32, 2000186 CrossRef CAS PubMed.
- S. Fu, L. Wan, W. Zhang, X. Li, W. Song and J. Fang, ACS Energy Lett., 2020, 5, 3314–3321 CrossRef CAS.
- T. Moot, A. R. Marshall, L. M. Wheeler, S. N. Habisreutinger, T. H. Schloemer, C. C. Boyd, D. R. Dikova, G. F. Pach, A. Hazarika, M. D. McGehee, H. J. Snaith and J. M. Luther, Adv. Energy Mater., 2020, 10, 1903365 CrossRef CAS.
- D. Bi, C. Yi, J. Luo, J.-D. Décoppet, F. Zhang, S. M. Zakeeruddin, X. Li, A. Hagfeldt and M. Grätzel, Nat. Energy, 2016, 1, 16142 CrossRef CAS.
- Y. H. Choi, C. M. Choi, D. H. Choi, Y. Paik, B. J. Park, Y. K. Joo and N. J. Kim, J. Membr. Sci., 2011, 371, 84–89 CrossRef CAS.
- J. Zhang, J. Liu, A. Tan, J. Piao and Z. Fu, Chem. Commun., 2020, 56, 13816–13819 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ya00068c |
| This journal is © The Royal Society of Chemistry 2022 |