Open Access Article
Open Access ArticleInterfacial passivation with 4-chlorobenzene sulfonyl chloride for stable and efficient planar perovskite solar cells†
Foo
Shini‡
abc,
M.
Thambidurai‡
 ab,
Herlina Arianita
Dewi
b,
Nur Fadilah
Jamaludin
b,
Annalisa
Bruno
ab,
Herlina Arianita
Dewi
b,
Nur Fadilah
Jamaludin
b,
Annalisa
Bruno
 b,
Anil
Kanwat
b,
Anil
Kanwat
 b,
Nripan
Mathews
b,
Nripan
Mathews
 bc,
Cuong
Dang
bc,
Cuong
Dang
 *ab and
Hung D.
Nguyen
*ab
*ab and
Hung D.
Nguyen
*ab
aCentre for OptoElectronics and Biophotonics (COEB), School of Electrical and Electronic Engineering, The Photonics Institute (TPI), Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore. E-mail: hcdang@ntu.edu.sg; hunghtd@ntu.edu.sg
bEnergy Research Institute @NTU (ERI@N), Research Techno Plaza, X-Frontier Block, Level 5, 50 Nanyang Drive, 637553, Singapore
cSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore
First published on 12th May 2022
Abstract
The interfacial passivation technique is an effective method to improve the stability and photovoltaic performance of perovskite solar cells. Here, we demonstrate the importance of passivating undercoordinated halide ions in minimizing carrier losses at the perovskite/spiro-OMeTAD interface. 4-Chlorobenzene sulfonyl chloride (CBSC) has been utilized as a Lewis acid passivation material. CBSC molecules act as electron acceptors, which bind to the negatively charged undercoordinated halide ions and Pb–I antisite defects (PbI3−). The champion CBSC-passivated perovskite device shows a high power conversion efficiency (PCE) of 20.02%, unlike the pristine device with an efficiency of 18.29%. Significant long term-stability in the CBSC passivated device is also observed, maintaining 93% of the initial PCE after 768 h stored in ambient conditions with 30% relative humidity.
1. Introduction
Organic–inorganic perovskite solar cells (PSCs) have attracted significant attention due to their low-cost and high-power conversion efficiency (PCE) achieved to date.1–5 Despite this, the maximum PCE of current state-of-the-art PSCs (>25%) remains far below the Shockley–Quisser limit of over 30%, assuming no other losses apart from radiative recombination of solar cells.6–8 Therefore, it is critical that defects (bulk and interfacial) – primary contributors to non-radiative recombination losses – be effectively managed to improve device efficiency. While bulk defects can be mitigated with compositional or additive engineering, it is noted that major losses in PSCs arise from surface defects, especially at the interfaces between the perovskite absorber and adjacent charge selective transport layers.9–11 Despite the facile fabrication of compact and uniform perovskite films via solution-based processing, the tendency for surface defect formation – a result of rapid crystallization and post-deposition annealing – is high. These defects, including pinholes, grain boundaries, under-coordinated ions, dangling bonds, and non-stoichiometric composition on the surface, could lead to non-radiative charge recombination, which can severely hamper the photovoltaic performance of PSCs and may simultaneously induce perovskite degradation.12–16One of the more direct and efficient strategies of managing perovskite surface defects and ensuring efficient charge transport into the adjacent interlayers is through interface passivation.17–20 Several works on interfacial passivation in PSCs have been reported previously. For example, post-treatment on the perovskite surface using a two-dimensional (2D) perovskite, polymer, or organic ammonium salt, as well as inducing Lewis acid or base reactions, have shown the capability of improving both the PSC efficiency and stability.21–26 The mitigation of surface defects translates to an increase in open circuit voltage (Voc) arising from enhanced charge extraction. Liu and coworkers applied 2-aminoterephthalic acid as the passivation layer between the perovskite and Spiro-OMeTAD, leading to improved hydrophobicity of the perovskite film and PCE of 21.09%.27 Yi and coworkers utilized phenylhydroxylammonium halide (PBABr) salts to passivate the perovskite surface, and it was found that PBABr treatment can reduce the trap density and give a longer carrier lifetime.28 Recently, Huang et al., reported that 4-chlorobenzene sulfonyl chloride (CBSC) as a hydro-stable self-assembled small-molecule was utilized in organic solar cells, which enhanced the surface energy, gave a higher absorption coefficient, and tuned the work function, resulting in a PCE of 10.6%.29 Hence, CBSC passivation materials could be expected to improve the PCE and stability of perovskite devices. Nevertheless, it is still challenging to determine the several effects of suitable morphology, surface defect passivation, hydrophobicity, and optoelectronic properties to further boost the PCE and stability of perovskite devices. Here, spin coating of a thin CBSC (ClC6H4SO2Cl) layer on the Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 perovskite film yielded enhanced PCEs. The PCE of the CBSC passivated device was 20.02%, versus 18.29% in the control device. The CBSC-passivated PSCs show a ∼50 mV increase in Voc as compared to the pristine sample, confirming successful surface defect passivation. The champion CBSC-passivated PSC exhibited an efficiency of 20.02% with a high PCE retention of ∼93% when stored under a controlled environment (relative humidity of 30% and room temperature) for 768 hours.
2. Results and discussion
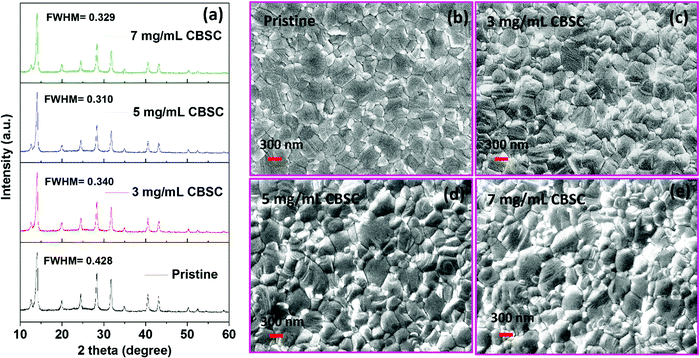
X-Ray diffraction (XRD) measurement was used to investigate the pristine and different concentrations (3, 5, and 7 mg mL−1) of CBSC-passivated perovskite films. As seen in Fig. 1a, the films exhibit similar diffraction patterns corresponding to a tetragonal crystal structure despite the various CBSC concentrations employed. Furthermore, all samples display a characteristic PbI2 peak at 12.5° due to the presence of excess PbI2 in the perovskite precursor solution.30–33 As depicted in Fig. 1a, the full width at half maximum (FWHM) decreases from 0.428 for pristine perovskite to 0.340, 0.310, and 0.329 for the 3, 5 and 7 mg mL−1 CBSC passivated perovskite film. The smaller FWHM represents good crystallinity of the perovskite. A field emission scanning electron microscope (FESEM) was used to study the effect of the CBSC passivation layer on the film morphologies, as displayed in Fig. 1b–e. Both pristine and passivated films exhibit dense, uniform, and smooth surface morphologies. That being said, CBSC passivated films exhibit slightly larger grain sizes when compared to the pristine perovskite film. The average grain sizes of the 0 mg mL−1 (prisitine), 3 mg mL−1, 5 mg mL−1 and 7 mg mL−1 CBSC passivated films are 220, 280, 310 and 290 nm, respectively.To understand the effects of CBSC passivation on the perovskite films, UV-vis spectroscopy was employed. Specifically, perovskite films were treated with CBSC of concentrations up to 7 mg mL−1 prior to testing. As seen in Fig. 2a, the absorption spectra of CBSC treated perovskite films exhibit a redshift when compared to that of the pristine film. As presented in Fig. S1, (ESI†) the band gaps of pristine and 5 mg mL−1 CBSC treated perovskite were estimated to be 1.63 eV and 1.62 eV, respectively, indicating a little improvement of light absorption after CBSC passivation. Fig. 2b presents the steady-state photoluminescence (PL) spectra of the pristine and CBSC-passivated perovskite films. It is evident that the addition of CBSC, regardless of concentration, provides effective passivation of defects. All the CBSC-passivated films showed increased PL intensities with the highest observed in the 5 mg mL−1 CBSC passivated film while retaining a similar PL emission profile (757 nm). The drop of PL intensity for the 7 mg mL−1 CBSC-passivated perovskite films suggests that the employment of CBSC concentration beyond 5 mg mL−1 is detrimental to the film's optical properties. Moreover, utilization of excessive passivation material has previously been shown to induce more trap states at the perovskite/HTL interface.34–37 Henceforth, it is essential that an optimal concentration of passivation material is employed to yield the desired defect passivation effect.
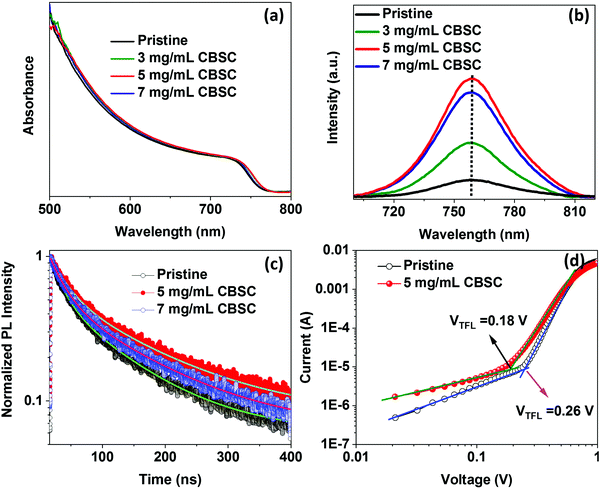 | ||
| Fig. 2 (a) Absorbance, (b) photoluminescence, and (c) TRPL spectra of pristine and CBSC passivated perovskite films. (d) Dark current–voltage (I–V) curves of electron only devices. | ||
The time-resolved photoluminescence (TRPL) measurements were characterized to further analyze charge carrier dynamics and defect states of pristine and CBSC passivated perovskite films, as depicted in Fig. 2c. The TRPL decay curves are fitted by a bi-exponential function and the corresponding fitted data are displayed in Table S1 (ESI†). The average carrier lifetime of pristine, 5 mg mL−1, and 7 mg mL−1 CBSC passivated perovskite films are 48.40, 72.42 and 61.73 ns, respectively. Both PL and TRPL results indicate reduced surface defects and improved PCE in the CBSC passivated film. To investigate defect state densities of pristine and passivated perovskite film, space charge-limited current (SCLC) measurement was performed. Depicted in Fig. 2d are the dark current–voltage (I–V) characteristics of the electron only device with a structure of FTO/TiO2/SnO2/perovskite with or without CBSC/PCBM/Ag. The trap density (Ntrap) of the pristine perovskite without and with a CBSC passivation device was determined using the equation Ntrap = 2εε0VTFL/eL2, where ε,ε0, VTFL, e and L represent the relative dielectric constant of the perovskite, vacuum permittivity, trap filled limit voltage, elemental charge, and film thickness of the perovskite, respectively.38 The trap filled limit voltage (VTFL) values were obtained to be 0.26 V and 0.18 V for pristine and CBSC based devices, respectively, and the corresponding Ntrap values were estimated to be 2.66 × 1015 cm−3 and 1.84 × 1015 cm−3, respectively.
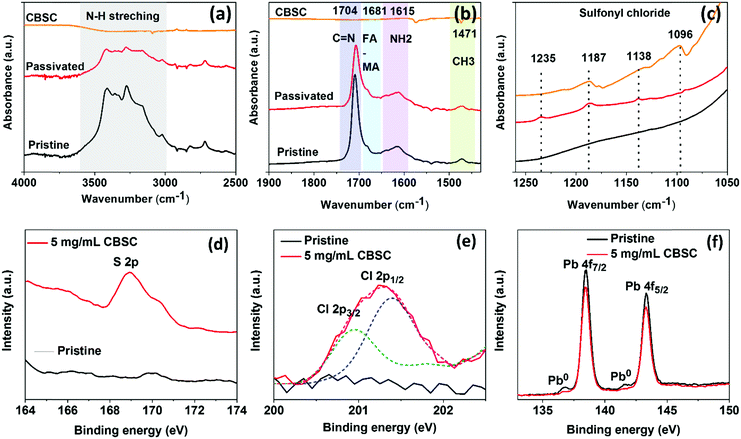
In order to disclose the CBSC molecular interaction in the mixed cation perovskite, we perform attenuated reflectance Fourier transform infrared spectroscopy (ATR-FTIR). The FTIR spectra of the as-prepared pristine perovskite film, CBSC passivated film, and CBSC powder were obtained as shown in Fig. 3(a–c). The characteristic peaks depict N–H stretching at higher wavenumber, confirming the perovskite phase formation. The peaks at 1705, 1681, and 1615 cm−1 are characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching of the FA+ cation, FA–MA complex, and NH2 scissoring vibration, respectively.39,40 The CBSC molecule in the passivated films is confirmed by the presence of sulfonyl chloride characteristic peaks found at lower wavenumber (1250–1050 cm−1). We found slight displacement of the C
N stretching of the FA+ cation, FA–MA complex, and NH2 scissoring vibration, respectively.39,40 The CBSC molecule in the passivated films is confirmed by the presence of sulfonyl chloride characteristic peaks found at lower wavenumber (1250–1050 cm−1). We found slight displacement of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching peaks in the CBSC passivated film, indicating possible interaction of the CBSC molecule with the perovskite.
N stretching peaks in the CBSC passivated film, indicating possible interaction of the CBSC molecule with the perovskite.
To confirm the presence and effects of CBSC passivation, X-ray photoelectron spectroscopy (XPS) measurements were carried out on the as-prepared perovskite films. In Fig. 3d, the sulfur (S 2p) spectrum shows a peak at 168.95 eV. Fig. 3e shows the deconvoluted Cl characteristic peaks at 200.9 eV and 201.3 eV representing the Cl 2p3/2 and 2p1/2 peaks. Cheng and coworkers demonstrated a series of chlorobenzoic acids with varied positions and degrees of chlorination on the molecules and it was found that the binding energies of the Cl (organic molecule) peaks at 201.2 and 202.8 eV were associated with Cl 2p3/2 and Cl 2p1/2, respectively. In addition, the chloride (Cl 2p3/2) binding energy is 198.9 eV, which is considered as Cl-ITO.41 The presence of a Cl peak in the CBSC-passivated perovskite films, contrary to the pristine ones, strongly supports the successful addition of CBSC molecules. The highly electronegative chlorine atoms in the CBSC molecule inductively withdraw electron density from the sulfur (S) atom within the sulfonyl group (R–S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2–R′), leaving behind a partial positive charge on the sulfur atom. Hence, the sulfur atom acts as an electron acceptor to form coordination bonds with negatively charged defects such as Pb–I antisites (PbI3−) and/or undercoordinated I− ions.42–44Fig. 3f and Fig. S2 (ESI†) show the signals of the lead (Pb) 4f and iodine (I) 2p peaks, respectively. The Pb element is represented by two main peaks, namely, Pb 4f7/2 and Pb 4f5/2 at respective binding energies of 138.2 and 143.3 eV. On the other hand, the I element is represented by two main peaks, namely, I 3d5/2 and I 3d3/2 at respective binding energies of 619.2 and 630.6 eV. With the reduction in peak intensity for both the Pb and I spectra in the CBSC-passivated perovskite film, it is clear that the addition of CBSC molecules at the perovskite/spiro–OMeTAD does passivate the defects. It is interesting to note that while peaks at 136.8 and 141.5 eV corresponding to metallic Pb0 are present in the pristine perovskite film, neither were observed on the addition of CBSC, further justifying its role as a passivator.45,46
O)2–R′), leaving behind a partial positive charge on the sulfur atom. Hence, the sulfur atom acts as an electron acceptor to form coordination bonds with negatively charged defects such as Pb–I antisites (PbI3−) and/or undercoordinated I− ions.42–44Fig. 3f and Fig. S2 (ESI†) show the signals of the lead (Pb) 4f and iodine (I) 2p peaks, respectively. The Pb element is represented by two main peaks, namely, Pb 4f7/2 and Pb 4f5/2 at respective binding energies of 138.2 and 143.3 eV. On the other hand, the I element is represented by two main peaks, namely, I 3d5/2 and I 3d3/2 at respective binding energies of 619.2 and 630.6 eV. With the reduction in peak intensity for both the Pb and I spectra in the CBSC-passivated perovskite film, it is clear that the addition of CBSC molecules at the perovskite/spiro–OMeTAD does passivate the defects. It is interesting to note that while peaks at 136.8 and 141.5 eV corresponding to metallic Pb0 are present in the pristine perovskite film, neither were observed on the addition of CBSC, further justifying its role as a passivator.45,46
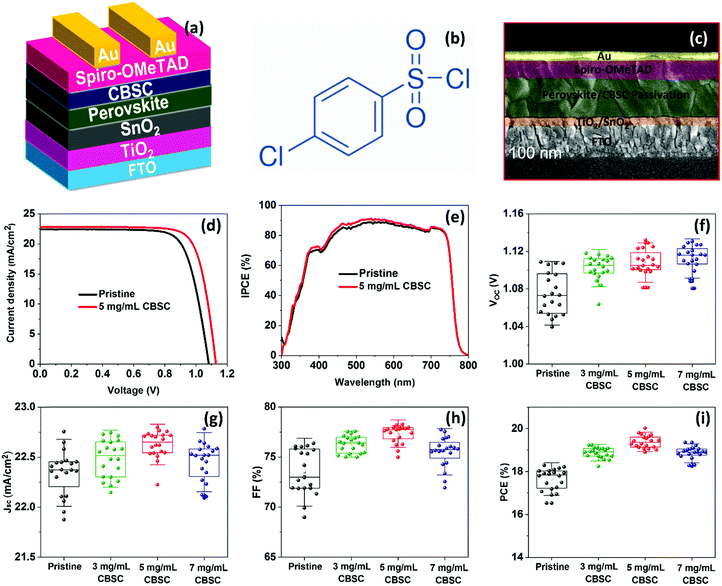
Fig. 4a shows the complete CBSC-passivated PSC adopting a planar n–i–p structure device configuration consisting of FTO/c-TiO2/SnO2/perovskite/CBSC/spiro–OMeTAD/Au. The chemical structure of the CBSC molecule is shown in Fig. 4b. Although the chlorine (Cl) atoms found in the CBSC molecule have non-bonding valence electron pairs, the highly electronegative nature of the electron-withdrawing chlorine atoms far outweighs the donation of electron density from the lone pair. The cross-sectional SEM image of the PSC device is shown in Fig. 4c, with the thickness of the compact c-TiO2/SnO2, perovskite/CBSC, spiro-OMeTAD, and Au films noted to be 80, 520, 250, and 80 nm, respectively. To understand the effectiveness of the passivated perovskite devices for photovoltaic applications, current density against voltage (J–V) measurements tested at AM 1.5 G illumination (class AAA solar simulator, Newport Oriel Sol3A, 100 mW cm−2) were conducted for the pristine and CBSC-passivated devices. Fig. 4d and Fig. S3 (ESI†) represent the J–V curves of the pristine and CBSC-passivated devices collected under reverse scan. The highest PCE was achieved with the 5 mg mL−1 CBSC-passivated device, suggesting optimal passivator concentration for photovoltaic performance. As seen in Table 1, the nonpareil photovoltaic performance observed in the 5 mg mL−1 CBSC device attained short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF) and PCE of 22.80 mA cm−2, 1.13 V, 77.79 and 20.02%, respectively. While lower concentrations of CBSC do not offer sufficient passivating effect, higher concentrations, on the other hand, are detrimental for device performance owing to the introduction of undesired trapped sites, resulting in lower Jsc and FF values.47,48 This is further supported by the PL trend mentioned previously (Fig. 2b). Given these points, the use of the optimal concentration of passivation material is of paramount importance for superior device performance to be achieved. In accordance with the results obtained from the J–V measurements, the pristine device showed the poorest PCE of 18.29% with Jsc, Voc and FF of 22.43 mA cm−2, 1.08 V and 75.4, respectively. Enhancement to Voc and FF suggests effective defect passivation provided by the CBSC molecules.49Fig. 4e is the incident photon-to-current conversion efficiency (IPCE) spectra of pristine and CBSC passivated devices. The integrated Jsc value of the CBSC passivated device is 20.82 mA cm−2, higher than that of the pristine device (20.43 mA cm−2), and is in good agreement with the Jsc value extracted from the J–V curve. Fig. S4(a and b) (ESI†) presents the J–V curves of the pristine and 5 mg mL−1 CBSC passivated PSC devices when reverse and forward bias were applied. Unsurprisingly, a drop in hysteresis was noted when 5 mg mL−1 of CBSC was deposited on top of the perovskite film (Table S2, ESI†). Based on the calculation of hysteresis index (HI) 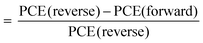 , HI for the 5 mg mL−1 CBSC-passivated device is approximately 0.07 while that of the pristine device is 0.17.50 The smaller degree of hysteresis noted for the 5 mg mL−1 CBSC-passivated perovskite device could be attributed to the successful passivation of defects, which reduces the susceptibility of charge trapping and de-trapping phenomena thought to play a part in hysteresis behavior.51 The steady-state photocurrent and PCE of the champion devices measured at the maximum power point tracking (MPPT) under AM 1.5 G illumination are displayed in Fig. S5(a and b). The CBSC passivated device shows a Jsc of 20.69 mA cm−2, and a PCE of 19.02% under an applied voltage bias of 0.92 V. On the other hand, the pristine device exhibits a Jsc of 19.69 mA cm−2, and a PCE of 17.38% under a voltage bias of 0.88 V.
, HI for the 5 mg mL−1 CBSC-passivated device is approximately 0.07 while that of the pristine device is 0.17.50 The smaller degree of hysteresis noted for the 5 mg mL−1 CBSC-passivated perovskite device could be attributed to the successful passivation of defects, which reduces the susceptibility of charge trapping and de-trapping phenomena thought to play a part in hysteresis behavior.51 The steady-state photocurrent and PCE of the champion devices measured at the maximum power point tracking (MPPT) under AM 1.5 G illumination are displayed in Fig. S5(a and b). The CBSC passivated device shows a Jsc of 20.69 mA cm−2, and a PCE of 19.02% under an applied voltage bias of 0.92 V. On the other hand, the pristine device exhibits a Jsc of 19.69 mA cm−2, and a PCE of 17.38% under a voltage bias of 0.88 V.
| Devices | Devices | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] |
|---|---|---|---|---|---|
| Pristine | Best | 1.08 | 22.43 | 75.40 | 18.29 |
| Average | 1.07 ± 0.02 | 22.34 ± 0.22 | 73.49 ± 2.25 | 17.65 ± 0.50 | |
| 3 mg mL−1 CBSC | Best | 1.11 | 22.66 | 76.23 | 19.23 |
| Average | 1.10 ± 0.01 | 22.48 ± 0.18 | 76.28 ± 0.86 | 18.88 ± 0.25 | |
| 5 mg mL−1 CBSC | Best | 1.13 | 22.80 | 77.79 | 20.02 |
| Average | 1.10 ± 0.01 | 22.62 ± 0.13 | 77.33 ± 0.91 | 19.38 ± 0.30 | |
| 7 mg mL−1 CBSC | Best | 1.13 | 22.48 | 76.15 | 19.35 |
| Average | 1.11 ± 0.01 | 22.44 ± 0.19 | 75.51 ± 1.54 | 18.85 ± 0.29 |
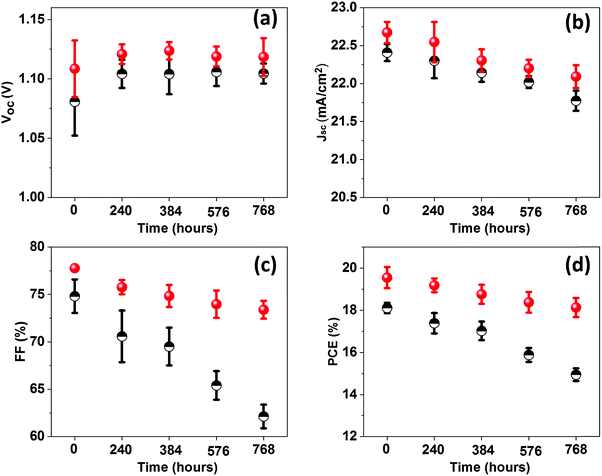
To understand the trends and device reproducibility of the pristine and CBSC-passivated devices, 20 devices were prepared for each concentration and the performance is presented in the statistical distribution shown in Fig. 4f–i. It is obvious that the passivation with CBSC, regardless of concentration employed, achieved superior efficiency attributed to the heightened Voc and FF. The average PCEs of the 0 mg mL−1 (pristine), 2 mg mL−1, 5 mg mL−1, and 7 mg mL−1 CBSC based devices are 17.65%, 18.88%, 19.38% and 18.65%, respectively. Long-term stabilities of the pristine and CBSC passivated devices were tested under 30% relative humidity and room temperature for 768 hours, as shown in Fig. S6 and Table S3 (ESI†). The pristine device shows a large drop in PCE (from 18.29% to 15.09%), while the CBSC-passivated device exhibits a slight decrease in PCE from 20.02% to 18.66%. Fig. 5(a–d) present the long–term stability of the devices categorized according to the different photovoltaic parameters. The pristine device retains 82% of its initial PCE after 768 hours, while the CBSC-passivated device maintains 93% of its initial PCE under the same conditions.
3. Conclusion
In summary, deposition of the CBSC film at the perovskite/spiro-OMeTAD interface demonstrated effective passivation, which reduces surface defects. When the optimal concentration of CBSC was employed, a nonpareil PCE, reproducibility, and device stability were attained. The 5 mg mL−1 CBSC passivated perovskite device achieved a maximum PCE of 20.02%, attributed to the improved FF and Voc attained. Excellent long-term stability was also exhibited, retaining 93% of its initial PCE after 768 hours in the absence of encapsulation.4. Experimental section
Device fabrication
The fluorine–doped tin oxide (FTO) glass substrates were cleaned (detergent, deionized water, acetone, ethanol, and 2-propanol) and treated with ultraviolet ozone (20 min). The compact-TiO2 film was deposited on the FTO substrates at 3000 rpm for 40 s and annealed at 500 °C for 1 h. Then, a SnO2 layer was spin-coated on the TiO2 surface at 5000 rpm for 20 sec and heated at 200 °C for 1 h. The triple cation perovskite precursor solution was prepared by dissolving MABr (28 mg), PbBr2 (101) mg, FAI (215 mg), PbI2 (633.8 mg), and 52.6 μL (39 mg of CsI in 100 μL of DMSO) in 1 mL of a mixture of DMF and DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio). The perovskite precursor (40 μL) solution was deposited on TiO2/SnO2 substrates at 1000 rpm for 10 s and 6000 rpm for 25 s and 100 μL chlorobenzene was added dropwise in the last 10 s and heated at 100 °C for 1 h. For 4-chlorobenzene sulfonyl chloride (CBSC, ClC6H4SO2Cl) passivation, various concentrations of CBSC (3, 5, and 7 mg mL−1 in chlorobenzene) were deposited on the perovskite films and then heated at 100 °C for 10 min. The hole transport layer was deposited on the perovskite film at 4000 rpm for 30 sec using 1 mL chlorobenzene, Spiro-OMeTAD (70 mg mL−1), 4-tert-butylpyridine (28 μL), bis(trifluoromethylsulfonyl)amine lithium salt (16.94 μL; 520 mg mL−1 in acetonitrile) and FK209 Co(III) TFSI Salt (35 μL; 37.6 mg/100 μL in acetonitrile). Finally, an 80 nm gold electrode was deposited by thermal evaporation under a high vacuum.
1 volume ratio). The perovskite precursor (40 μL) solution was deposited on TiO2/SnO2 substrates at 1000 rpm for 10 s and 6000 rpm for 25 s and 100 μL chlorobenzene was added dropwise in the last 10 s and heated at 100 °C for 1 h. For 4-chlorobenzene sulfonyl chloride (CBSC, ClC6H4SO2Cl) passivation, various concentrations of CBSC (3, 5, and 7 mg mL−1 in chlorobenzene) were deposited on the perovskite films and then heated at 100 °C for 10 min. The hole transport layer was deposited on the perovskite film at 4000 rpm for 30 sec using 1 mL chlorobenzene, Spiro-OMeTAD (70 mg mL−1), 4-tert-butylpyridine (28 μL), bis(trifluoromethylsulfonyl)amine lithium salt (16.94 μL; 520 mg mL−1 in acetonitrile) and FK209 Co(III) TFSI Salt (35 μL; 37.6 mg/100 μL in acetonitrile). Finally, an 80 nm gold electrode was deposited by thermal evaporation under a high vacuum.
Characterization
The X-ray diffraction (XRD) measurements were measured with a Bruker D8 Advance diffractometer. The field emission scanning electron microscope (FESEM) characterization was performed by a JEOL JSM-7600F. X-ray photoelectron spectroscopy (XPS) was performed by a Kratos - AXIS Supra photoelectron spectrometer. The UV-Vis absorption spectra were recorded by a UV-1800 Shimadzu Spectrophotometer. The photoluminescence (PL) measurements were performed by RF-5301PC Shimadzu spectrophotometer. TRPL decay spectra were measured using a time-correlated single photon counting system (PicoQuant, PicoHarp 300). ATR-FTIR measurements were performed by using a Frontier Perkin Elmer instrument. The dark I–V characteristics of electron only devices were measured using a Keithley 2400 source meter. The current density- voltage (J–V) characteristics of the devices were determined by a Keithley 2612A source meter with a scan rate of 300 mV s−1. The incident photon-to-current efficiency (IPCE) spectra were tested on a PVE300 (Bentham) measurement system.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research is supported by an AcRF Tier2 grant (MOE-T2EP50121-0012) from Singapore Ministry of Education, and EMA-EP004-EKJGC-0003 grant from the Energy Market Authority (EMA) and National Research Foundation (NRF) Singapore and NRF2018-ITC001-001. We would like to thank Prof. Subodh Mhaisalkar, Executive Director of Energy Research Institute @ NTU (ERI@N) for supporting this work.References
- M. Shao, T. Bie, L. Yang, Y. Gao, X. Jin, F. He, N. Zheng, Y. Yu and X. Zhang, Adv. Mater., 2021, 34, 2107211 CrossRef PubMed
.
- Q. Zhou, Y. Gao, C. Cai, Z. Zhang, J. Xu, Z. Yuan and P. Gao, Angew. Chem., 2021, 133, 8384 CrossRef
.
- B. Wang, H. Li, Q. Dai, M. Zhang, Z. Zou, J. Brédas and Z. Lin, Angew. Chem., 2021, 133, 17805 CrossRef
.
- C. Y. Chang and C. C. Wang, J. Mater. Chem. A, 2020, 8, 8593 RSC
.
- D. G. Lee, D. H. Kim, J. M. Lee, B. J. Kim, J. Y. Kim, S. S. Shin and H. S. Jung, Adv. Funct. Mater., 2021, 31, 2006718 CrossRef CAS
.
- J. J. Yoo, G. Seo, M. R. Chua, T. G. Park, Y. Lu, F. Rotermund, Y. K. Kim, C. S. Moon, N. J. Jeon, J. P. Correa-Baena, V. Bulović, S. S. Shin, M. G. Bawendi and J. Seo, Nature, 2021, 590, 587 CrossRef CAS PubMed
.
- W. E.-I. Sha, X. Ren, L. Chen and W. C.-H. Choy, Appl. Phys. Lett., 2015, 106, 221104 CrossRef
.
- C. Ma and N. G. Park, Chem, 2020, 6, 1254 CAS
.
- C. M. Wolff, P. Caprioglio, M. Stolterfoht and D. Neher, Adv. Mater., 2019, 31, 1902762 CrossRef CAS PubMed
.
- M. Stolterfoht, P. Caprioglio, C. M. Wolff, J. A. Márquez, J. Nordmann, S. Zhang, D. Rothhardt, U. Hörmann, Y. Amir, A. Redinger, L. Kegelmann, F. Zu, S. Albrecht, N. Koch, T. Kirchartz, M. Saliba, T. Unold and D. Neher, Energy Environ. Sci., 2019, 12, 2778 RSC
.
- Y. Pei, Y. Liu, F. Li, S. Bai, X. Jian and M. Liu, iScience, 2019, 15, 165 CrossRef CAS PubMed
.
- W. Shen, Y. Dong, F. Huang, Y.-B. Cheng and J. Zhong, Mater. Rep. Energy, 2021, 1, 100060 Search PubMed
.
- J. Ye, M. M. Byranvand, C. O. Martínez, R. L.-Z. Hoye, M. Saliba and L. Polavarapu, Angew. Chem., Int. Ed., 2021, 60, 21636 CrossRef CAS PubMed
.
- W. Kong, T. Ding, G. Bi and H. Wu, Phys. Chem. Chem. Phys., 2016, 18, 12626 RSC
.
- A. R. Bin Mohd Yusoff, M. Vasilopoulou, D. G. Georgiadou, L. C. Palilis, A. Abate and M. K. Nazeeruddin, Energy Environ. Sci., 2021, 14, 2906 RSC
.
- M. Yang, T. Tian, W. Feng, L. Wang and W.-Q. Wu, Accounts Mater. Res., 2021, 2, 1141 CrossRef CAS
.
- S. Rahmany and L. Etgar, Mater. Adv., 2021, 2, 2617 RSC
.
- Z. Zhang, Y. Gao, Z. Li, L. Qiao, Q. Xiong, L. Deng, Z. Zhang, R. Long, Q. Zhou, Y. Du, Z. Lan, Y. Zhao, C. Li, K. Müllen and P. Gao, Adv. Mater., 2021, 33, 2008405 CrossRef CAS PubMed
.
- X. Li, W. Sheng, X. Duan, Z. Lin, J. Yang, L. Tan and Y. Chen, ACS Appl. Mater. Interfaces, 2021 DOI:10.1021/acsami.1c08539
.
- W. Zhang, Q. S. Li and Z. S. Li, Appl. Surf. Sci., 2021, 563, 150267 CrossRef CAS
.
- C. C. Chueh, C. Z. Li and A. K.-Y. Jen, Energy Environ. Sci., 2015, 8, 1160 RSC
.
- E. Ochoa-Martinez, M. Ochoa, R. D. Ortuso, P. Ferdowsi, R. Carron, A. N. Tiwari, U. Steiner and M. Saliba, ACS Energy Lett., 2021, 6, 2626 CrossRef CAS
.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Nat. Photonics, 2019, 13, 460 CrossRef CAS
.
- J. Peng, Y. Wu, W. Ye, D. A. Jacobs, H. Shen, X. Fu, Y. Wan, T. Duong, N. Wu, C. Barugkin, H. T. Nguyen, D. Zhong, J. Li, T. Lu, Y. Liu, M. N. Lockrey, K. J. Weber, K. R. Catchpole and T. P. White, Energy Environ. Sci., 2017, 10, 1792 RSC
.
- D. H. Kang, S. Y. Kim, J. W. Lee and N. G. Park, J. Mater. Chem. A, 2021, 9, 3441 RSC
.
- E. A. Alharbi, A. Y. Alyamani, D. J. Kubicki, A. R. Uhl, B. J. Walder, A. Q. Alanazi, J. Luo, A. Burgos-Caminal, A. Albadri, H. Albrithen, M. H. Alotaibi, J. E. Moser, S. M. Zakeeruddin, F. Giordano, L. Emsley and M. Grätzel, Nat. Commun., 2019, 10, 3008 CrossRef PubMed
.
- Z. Liu, F. Cao, M. Wang, M. Wang and L. Li, Angew. Chem., Int. Ed., 2020, 59, 4161 CrossRef CAS PubMed
.
- X. Yi, Y. Mao, L. Zhang, J. Zhuang, Y. Zhang, N. Chen, T. Lin, Y. Wei, F. Wang, J. Wang and C. Li, Small, Methods, 2021, 5, 2000441 CAS
.
- L. Huang, G. Wang, W. Zhou, B. Fu, X. Cheng, L. Zhang, Z. Yuan, S. Xiong, L. Zhang, Y. Xie, A. Zhang, Y. Zhang, W. Ma, W. Li, Y. Zhou, E. Reichmanis and Y. Chen, ACS Nano, 2018, 12, 4440 CrossRef CAS PubMed
.
- B. Roose, K. Dey, Y. H. Chiang, R. H. Friend and S. D. Stranks, J. Phys. Chem. Lett., 2020, 11, 6505 CrossRef CAS PubMed
.
- B. Wook Park, N. Kedem, M. Kulbak, D. Y. Lee, W. S. Yang, N. J. Jeon, J. Seo, G. Kim, K. J. Kim, T. J. Shin, G. Hodes, D. Cahen and S. Il Seok, Nat. Commun., 2018, 9, 93301 Search PubMed
.
- T. Meier, T. P. Gujar, A. Schönleber, S. Olthof, K. Meerholz, S. Van Smaalen, F. Panzer, M. Thelakkat and A. Köhler, J. Mater. Chem. C, 2018, 6, 7512 RSC
.
- F. Liu, Q. Dong, M. K. Wong, A. B. Djurišić, A. Ng, Z. Ren, Q. Shen, C. Surya, W. K. Chan, J. Wang, A. M.-C. Ng, C. Liao, H. Li, K. Shih, C. Wei, H. Su and J. Dai, Adv. Energy Mater., 2016, 6, 1502206 CrossRef
.
- X. Wang, Y. Qiu, L. Wang, T. Zhang, L. Zhu, T. Shan, Y. Wang, J. Jiang, L. Kong, H. Zhong, H. Yu, F. Liu, F. Gao, F. Wang and C. C. Chen, Nano Energy, 2021, 89, 106445 CrossRef CAS
.
- F. Zhang, J. Song, R. Hu, Y. Xiang, J. He, Y. Hao, J. Lian, B. Zhang, P. Zeng and J. Qu, Small, 2018, 14, 1704007 CrossRef PubMed
.
- T. S. Sherkar, C. Momblona, L. Gil-Escrig, J. Ávila, M. Sessolo, H. J. Bolink and L. J.-A. Koster, ACS Energy Lett., 2017, 2, 1214 CrossRef CAS PubMed
.
- H. Pan, H. Shao, X. L. Zhang, Y. Shen and M. Wang, J. Appl. Phys., 2021, 129, 130904 CrossRef CAS
.
- R. Zheng, S. Zhao, H. Zhang, H. Li, J. Zhuang, X. Liu, H. Li and H. Wang, Sol. Energy, 2021, 224, 472 CrossRef CAS
.
- A. Kanwat, B. Ghosh, S. E. Ng, P. J.-S. Rana, Y. Lekina, T. J.-N. Hooper, N. Yantara, M. Kovalev, B. Chaudhary, P. Kajal, B. Febriansyah, Q. Y. Tan, M. Klein, Z. X. Shen, J. W. Ager, S. G. Mhaisalkar and N. Mathews, ACS Nano, 2022, 16, 2942 CrossRef CAS PubMed
.
- M. Vásquez-Montoya, J. F. Montoya, D. Ramirez and F. Jaramillo, J. Energy Chem., 2021, 57, 386 CrossRef
.
- X. Cheng, L. Huang, L. Zhang, Q. Ai, L. Chen and Y. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 9204 CrossRef CAS PubMed
.
- X. Liu, Z. Yu, T. Wang, K. L. Chiu, F. Lin, H. Gong, L. Ding and Y. Cheng, Adv. Energy Mater., 2020, 10, 2001958 CrossRef CAS
.
- B. Chen, P. N. Rudd, S. Yang, Y. Yuan and J. Huang, Chem. Soc. Rev., 2019, 48, 3842 RSC
.
- X. Zheng, B. Chen, J. Dai, Y. Fang, Y. Bai, Y. Lin, H. Wei, X. C. Zeng and J. Huang, Nat. Energy, 2017, 2, 17102 CrossRef CAS
.
- J. Hieulle, X. Wang, C. Stecker, D. Y. Son, L. Qiu, R. Ohmann, L. K. Ono, A. Mugarza, Y. Yan and Y. Qi, J. Am. Chem. Soc., 2019, 141, 3515 CrossRef CAS PubMed
.
- G. Sadoughi, D. E. Starr, E. Handick, S. D. Stranks, M. Gorgoi, R. G. Wilks, M. Bär and H. J. Snaith, ACS Appl. Mater. Interfaces, 2015, 7, 13440 CrossRef CAS PubMed
.
- M. Thambidurai, B. Febriansyah, S. Foo, P. C. Harikesh, K. T. Ming, N. Mathews and C. Dang, J. Power Sources, 2020, 479, 228811 CrossRef CAS
.
- X. Gong, L. Guan, H. Pan, Q. Sun, X. Zhao, H. Li, H. Pan, Y. Shen, Y. Shao, L. Sun, Z. Cui, L. Ding and M. Wang, Adv. Funct. Mater., 2018, 28, 1804286 CrossRef
.
- S. Gharibzadeh, P. Fassl, I. M. Hossain, P. Rohrbeck, M. Frericks, M. Schmidt, T. Duong, M. R. Khan, T. Abzieher, B. A. Nejand, F. Schackmar, O. Almora, T. Feeney, R. Singh, D. Fuchs, U. Lemmer, J. P. Hofmann, S. A.-L. Weber and U. W. Paetzold, Energy Environ. Sci., 2021, 14, 5875 RSC
.
- S. Li, Z. He, Y. Li, K. Liu, M. Chen, Y. Yang and X. Li, J. Alloys Compd., 2022, 889, 161561 CrossRef CAS
.
- W. Tress, N. Marinova, T. Moehl, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Grätzel, Energy Environ. Sci., 2015, 8, 995 RSC
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc00982j |
| ‡ Foo Shini and M. Thambidurai contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |