Open Access Article
Open Access ArticlePhotostable polymorphic organic cages for targeted live cell imaging†
Dana
Al Kelabi‡
a,
Avishek
Dey‡
a,
Lukman O.
Alimi
a,
Hubert
Piwoński
 b,
Satoshi
Habuchi
b,
Satoshi
Habuchi
 b and
Niveen M.
Khashab
b and
Niveen M.
Khashab
 *a
*a
aSmart Hybrid Materials (SHMs) Laboratory, Advanced Membranes and Porous Materials Center, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: niveen.khashab@kaust.edu.sa
bKing Abdullah University of Science and Technology, Biological and Environmental Science and Engineering Division, Thuwal 23955-6900, Saudi Arabia
First published on 1st June 2022
Abstract
Fluorescent microscopy is a powerful tool for studying the cellular dynamics of biological systems. Small-molecule organic fluorophores are the most commonly used for live cell imaging; however, they often suffer from low solubility, limited photostability and variable targetability. Herein, we demonstrate that a tautomeric organic cage, OC1, has high cell permeability, photostability and selectivity towards the mitochondria. We further performed a structure–activity study to investigate the role of the keto–enol tautomerization, which affords strong and consistent fluorescence in dilute solutions through supramolecular self-assembly. Significantly, OC1 can passively diffuse through the cell membrane directly targeting the mitochondria without going through the endosomes or the lysosomes. We envisage that designing highly stable and biocompatible self-assembled fluorophores that can passively diffuse through the cell membrane while selectively targeting specific organelles will push the boundaries of fluorescent microscopy to visualize intricate cellular processes at the single molecule level in live samples.
Organic fluorophores are of great interest in many research fields especially live cell imaging, which allows for noninvasive observation and monitoring of biological processes. Small molecule fluorophores can be functionalized and tuned with high precision to tag a diverse variety of biological targets; however, limited water solubility, cell-permeability, photostability and targetability have halted the biomedical translation of many promising fluorescent molecules.1 Ultimately, new generations of fluorescent tags were designed to include new functionalities that can improve their physical properties and cell penetration such as long aliphatic side chains and cell penetrating peptides.2–6 In addition, various strategies have been employed to improve the photostability of these compounds by the addition of electron withdrawing groups, conformational changes, encapsulation or incorporation within host macrocycles.7–13
Major efforts in the organic fluorophores development have been directed towards designing stable probes targeting the mitochondria as it plays a central role in the generation of adenosine triphosphate (ATP), central metabolism, and apoptosis.14,15 Successful examples of mitochondria targeting fluorophores include triphenyl phosphonium cations, heterocyclic aromatic cations, macrocyclic amphiphiles, BODIPY derivatives and mitochondria targeted peptides that have limited solubility.16–21 Commercially available dyes for mitochondrial imaging such as Mito Tracker Red have a high photobleaching tendency and can form covalent bonds with the mitochondrial respiratory chain complex I resulting in increased cytotoxicity. Consequently, various organic, inorganic and hybrid platforms were developed ranging from small nanoparticles to self-assembled frameworks on the quest for improved mitochondrial imaging agents.22–27
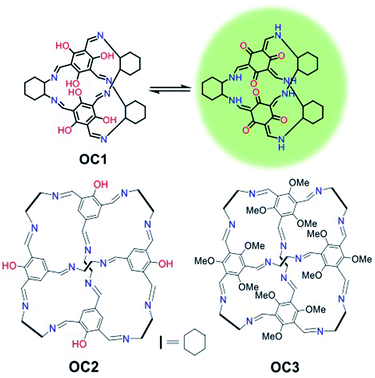
Porous organic cages have received major attention over the last decade as they are easily prepared, soluble in a range of solvents and are intrinsically porous.28–31 They have shown excellent applicability in molecular recognition, sensing and hydrocarbon separation but their biomedical imaging applications have never been investigated.32–35 Recently, self-assembled organic imine stacks showed impressive electroluminescence and live cell imaging.36 Moreover, an intracellular targeted macrocyclic nanohoop showed fast cell uptake and two-photon live cell fluorescence imaging.37 In this work, a tautomeric organic cage (OC1) that shows unprecedented photostability for live-cell imaging with high biocompatibility, cell permeability and mitochondrial targetability is reported (Fig. 1). We hypothesize that the keto–enol tautomerism is playing a major role in promoting the stability and sustaining the fluorescence of this platform. Such effect has been previously reported in other organic dyes such as oxyluciferin and HDFL.38,39
Investigating other organic cages with less hydroxyl groups (OC2) or replacing the hydroxyl groups with methoxy substituents (OC3) resulted in the loss of cell stability, retention and targetability (Fig. 1). The presence of the –OH groups in OC1 not only enables keto–enol tautomerization but also promotes H-bonding interactions, which ultimately improves solubility and cell permeability. We envision that understanding the nature of the intra- and intermolecular interactions in organic probes can lead to a profound enhancement in the photobleaching efficacy in addition to cell permeability. OC1 can effectively image the mitochondria over 3 h with high selectivity and without any loss of efficacy (no photo bleaching), which is comparable to the commercially used MitoTracker RedFM.
OC1, a [2 + 3] cage, was synthesized following a reported procedure and its structure was confirmed by 1H Nuclear Magnetic Resonance (NMR) and TOF-Mass analysis (Fig. S1–S4†).32 Dynamic light scattering (DLS) of OC1 revealed an average size of 30–50 nm in DMSO–H2O (2–5%) with a negative zeta potential of −5 mV (Fig. S5†). Single crystal X-ray analysis revealed that OC1 crystallizes in a triclinic crystal system with chiral P1 space group and the asymmetric unit consists of two molecules of [2 + 3] cages (Fig. S6†). Crystal structure analysis suggests that the imine cage crystallizes as the keto tautomeric form (Fig. 2a). This results in the formation of intramolecular N–H⋯O hydrogen bonding between the imine (N–H) hydrogen and the keto (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) oxygen (dC–OH actual = 1.36 Å; dC
O) oxygen (dC–OH actual = 1.36 Å; dC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O actual = 1.25–1.26 Å vs. dC
O actual = 1.25–1.26 Å vs. dC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O for OC1 = 1.260 Å and dC
O for OC1 = 1.260 Å and dC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N actual = 1.25 Å vs. dC–NH for OC1 = 1.30–1.35 Å) (Fig. S6†). Three different windows of each cage are connected with three different cages in the same plane through weak C–H⋯O hydrogen bonding (d = 2.414 Å, and θ = 60°), which creates isolated pores at the center of the cage (Fig. 2b). Furthermore, the packing diagram suggests the formation of one-dimensional infinite extrinsic pores along the b-axis (Fig. 2c and S6†). OC1 is stable in aqueous solutions even at low pH as well as in cell media (Fig. S7–S10†).
N actual = 1.25 Å vs. dC–NH for OC1 = 1.30–1.35 Å) (Fig. S6†). Three different windows of each cage are connected with three different cages in the same plane through weak C–H⋯O hydrogen bonding (d = 2.414 Å, and θ = 60°), which creates isolated pores at the center of the cage (Fig. 2b). Furthermore, the packing diagram suggests the formation of one-dimensional infinite extrinsic pores along the b-axis (Fig. 2c and S6†). OC1 is stable in aqueous solutions even at low pH as well as in cell media (Fig. S7–S10†).
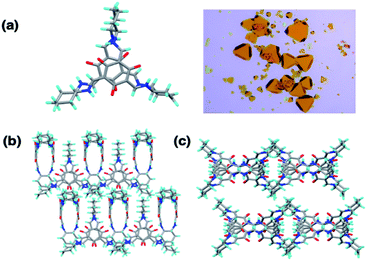 | ||
| Fig. 2 (a) SCXRD and crystal image of OC1. (b) OC1 self-assembled through hydrogen bonding interactions. (c) Packing of OC1 along b-axis. | ||
To test our original proposal that organic cages can act as effective imaging agents, we first investigated the UV-visible spectrum of OC1 in DMSO, which exhibited two characteristic peaks at λmax = 286 and 330 nm that can be assigned to π–π* and n–π* transitions, respectively (Fig. S11†). Fluorescence emission spectra showed that OC1 exhibits two major peaks at around 411 and 497 nm upon excitation at 330–350 nm in DMSO, while excitation at 400 and 480 nm showed characteristic emission peaks at 503 and 538 nm, respectively (Fig. S12†). This behavior can be explained by the presence of two emissive forms due to the keto–enol tautomerization. The quantum yield (QY) of OC1 in DMSO (0.25) and chloroform (0.4) was also tested. Molar extinction coefficient of OC1 was estimated as 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 806 M−1 cm−1 in DMSO (Fig. S13–S14†). The quantum yield of OC1 in DMSO are about half that of in chloroform that is significantly matched with the reported intracellular targeted macrocyclic nanohoop structure.37 The fluorescent properties of OC1 were maintained at different pH values (4, 7 and 10) (Fig. S15†). We hypothesize that this mode of assembly, as supported by SCXRD, promotes stability and prevents aggregation-induced quenching that is very common in small molecule dye fluorophores. The keto–enol tautomerism is boosting intra- and intermolecular hydrogen bonding interactions that can be contributing to the emissive characteristics of this system. In fact, the presence of the phenolic OH groups have been reported to have critical effects on the structural and spectroscopic properties of organic cages.31,40
806 M−1 cm−1 in DMSO (Fig. S13–S14†). The quantum yield of OC1 in DMSO are about half that of in chloroform that is significantly matched with the reported intracellular targeted macrocyclic nanohoop structure.37 The fluorescent properties of OC1 were maintained at different pH values (4, 7 and 10) (Fig. S15†). We hypothesize that this mode of assembly, as supported by SCXRD, promotes stability and prevents aggregation-induced quenching that is very common in small molecule dye fluorophores. The keto–enol tautomerism is boosting intra- and intermolecular hydrogen bonding interactions that can be contributing to the emissive characteristics of this system. In fact, the presence of the phenolic OH groups have been reported to have critical effects on the structural and spectroscopic properties of organic cages.31,40
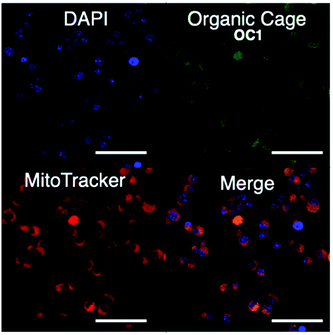
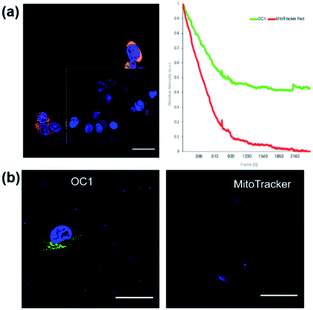
We then proceeded to test OC1 for cell imaging by first testing the cytotoxicity of OC1 in MCF-7 cells (Fig. S16a†). The cells were treated with a solution of concentrations ranging from 3.9–500 μg ml−1 for 24 h. Cell death was measured using MTT assay demonstrating the excellent biocompatibility of OC1 with an IC50 of > 500 μg ml−1. MCF-7 cells were then either co-incubated with OC1 and LysoTracker Red or MitoTracker RedFM, the cells are then fixed with 4% formaldehyde (w/v) and imaged on a confocal laser-scanning microscope (CLSM). Interestingly, OC1 showed strong colocalization in the mitochondria with a Pearson's correlation coefficient of 0.9751 (Fig. S16b and c†). Real time imaging of MCF-7 cells at different time intervals after co-internalization of OC1 and MitoTracker showed a clear signal overlap on a subcellular level (Fig. 3 and MOV1), which ultimately supports that OC1 can selectively target the mitochondria.
These encouraging results piqued our interest to investigate other organic cages in order to have a better understanding of the mechanistic factors in play. As the tautomeric activity is well reported to affect the photophysical properties of organic compounds, we compared OC1 to an organic cage with less phenolic OHs (OC2) and an organic cage with only methoxy substituents (OC3). OC2 and OC3 were synthesized and characterized according to reported procedures (Fig. S17–S25†).32,41 Absorbance, emission properties and stabilities of OC2 and OC3 in DMSO were also verified. OC2 showed one major characteristic emission peak at around 547 nm upon excitation at 350 and 440 nm. The quantum yield (QY) of OC2 in DMSO (6.7%) and chloroform (11.1%) was also tested (Fig. S13 and S14†). OC3 showed emission at 494 and 537 nm upon excitation at 320 nm and 480 nm upon excitation at 420 nm (Fig. S26†). The quantum yield (QY) and molar extinction coefficient of OC3 in DMSO is negligible. Cytotoxicity and biocompatibility studies of OC2 and OC3 showed a relatively higher cytotoxicity compared to OC1 (Fig. S27†). As to targetability and photostability, OC2 and OC3 showed less selectivity with colocalization in both mitochondria and lysosome where the fluorescent signal slowly disappeared, which implies the OC2 and OC3 are less stable than OC1 (Fig. S28†). Moreover, FACS data revealed that OC1 showed the highest uptake percentage in comparison to OC2, and OC3 with an uptake percentage of 86.4%, 78%, and 69%, respectively (Fig. S29†). Based on this data, we can conclude that indeed the hydroxyl groups, and their prompted keto–enol tautomerism, are playing a major role in stabilizing the overall structure through hydrogen bonding while simultaneously improving photostability and targetability. As a control, we tested the aldehyde starting material, 1,3,5-triformylphloroglucinol, to verify that OC1 is being uptaken as a whole and not as the aldehyde building block or a possible decomposition product. The experiment clearly demonstrates that 1,3,5-triformylphloroglucinol as a fluorophore had no selectivity towards the mitochondria and is initially distributed in the cytoplasm and finally localizes in the lysosomes for degradation (Fig. S30†).
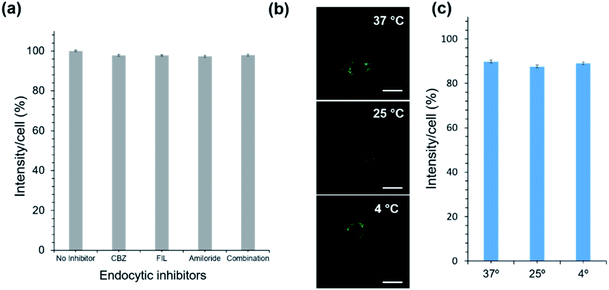
As for the uptake mechanism, we initially hypothesized that OC1 will be taken up by cells through endocytosis, which is the typical biological pathway of nanoparticles and nanoassemblies.42,43 Surprisingly, we discovered that OC1 can passively diffuse rather than being actively transported through the cell membrane. Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes as it mainly relies on the second law of thermodynamics. We tested OC1 uptake in the presence of different endocytic inhibitors including chlorpromazine (CPZ), filipin (FIL), and amiloride to inhibit clathrin-mediated, caveolae mediated, and macropinocytotic endocytosis pathways, respectively.44 All cells were treated with the inhibitors for 30 min, followed by 3 h incubation with OC1. The cellular uptake data showed that there was no significant reduction in the uptake using the active transport inhibitors (Fig. 4a). Moreover, the passive transport was tested by incubating OC1 (125 μg ml−1) with MCF-7 for 3 h at different temperatures (4, 27, or 37 °C). The uptake was monitored by confocal microscopy (Fig. 4b) and quantified by FACS (Fig. 4c). Interestingly, no obvious reduction of uptake was observed in all the cases.
After verifying the targetability and the uptake mechanism of OC1, we focused our attention on photostability. Since the commercially available mitochondrion tracker dyes normally have moderate photostability, it is critical to evaluate the photostability of OC1 in live cells. We first compared the photostability of OC1 with the commercially available MitoTracker RedFM. Since the excitation/emission wavelengths of OC1 (abs/em ∼495/519 nm) and MitoTracker RedFM (abs/em ∼581/644 nm) are distant from each other, we performed simultaneous scans for both dyes for 100 scans at 1.5 s per scan by incubating OC1 and MitoTracker RedFM with MCF-7 cells at 37 °C for 48 h. The results showed that at a laser power density of P ∼ 0.4132 W cm−2OC1 (ε = 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 806 M−1 cm−1) had higher retention and photostability than that of the MitoTracker RedFM where OC1 retained 54% of its initial fluorescence intensity while the fluorescence of MitoTracker RedFM dropped below 15% (Fig. 5a). Moreover, OC1 showed high photostability even at 72 h post-staining (Fig. 5b). Finally, it was noted that throughout the 72 h of incubation of OC1 with MCF-7, over three generations occurred in the cell culture and showed fluorescent labeling of the mitochondria suggesting that OC1 can be passed through to daughter cells upon cell division.
806 M−1 cm−1) had higher retention and photostability than that of the MitoTracker RedFM where OC1 retained 54% of its initial fluorescence intensity while the fluorescence of MitoTracker RedFM dropped below 15% (Fig. 5a). Moreover, OC1 showed high photostability even at 72 h post-staining (Fig. 5b). Finally, it was noted that throughout the 72 h of incubation of OC1 with MCF-7, over three generations occurred in the cell culture and showed fluorescent labeling of the mitochondria suggesting that OC1 can be passed through to daughter cells upon cell division.
Conclusions
In summary, we demonstrated that a biocompatible organic cage (OC1) can be efficiently employed as a mitochondria-targeted fluorescent probe with high cell permeability and impressive photostability. We investigated the role of the keto–enol tautomerism in improving the stability, fluorescence and cell retention of OC1 where it outperformed the commercially used MitoTracker RedFM. This work demonstrates the relatively unexplored potential of organic cages for use in biomedical applications. Moreover, it highlights the importance of inter- and intramolecular interactions to stabilize and improve the performance of current fluorophores. Understanding the interactions and assemblies at a molecular level can provide a solid base for the rational design and synthesis of the next generation fluorescent tags with superior physiological profiles and exceptional photostability.Data availability
Full experimental and crystallographic details are provided as part of the ESI. CCDC 2144153.†Author contributions
DK: investigation, validation, data collection, formal analysis, writing original draft. AD: conceptualization, synthesis, validation, investigation, data collection, formal analysis, writing original draft, review & editing. LA: formal analysis. HP: formal analysis, data collection. SH: discussion. NMK: supervision the project, funding acquisition, writing, review & editing.Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Chan, S. C. Dodani and C. J. Chang, Reaction-based small-molecule fluorescent probes for chemoselective bioimaging, Nat. Chem., 2012, 4, 973 CrossRef CAS PubMed.
- S. Hapuarachchige, G. Montaño, C. Ramesh, D. Rodriguez, L. H. Henson, C. C. Williams, S. Kadavakkollu, D. L. Johnson, C. B. Shuster and J. B. Arterburn, Design and Synthesis of a New Class of Membrane-Permeable Triazaborolopyridinium Fluorescent Probes, J. Am. Chem. Soc., 2011, 133, 6780–6790 CrossRef CAS PubMed.
- A. N. Butkevich, G. Lukinavičius, E. D'Este and S. W. Hell, Cell-Permeant Large Stokes Shift Dyes for Transfection-Free Multicolor Nanoscopy, J. Am. Chem. Soc., 2017, 139, 12378–12381 CrossRef CAS PubMed.
- C. J. MacNevin, T. Watanabe, M. Weitzman, A. Gulyani, S. Fuehrer, N. K. Pinkin, X. Tian, F. Liu, J. Jin and K. M. Hahn, Membrane-permeant, environment-sensitive dyes generate biosen-sors within living cells, J. Am. Chem. Soc., 2019, 141, 7275–7282 CrossRef CAS PubMed.
- A. F. L. Schneider, L. S. Benz, M. Lehmann and C. P. R. Hackenberger, Cell-Permeable Nanobodies Allow Dual-Color Super-Resolution Microscopy in Untransfected Living Cells, Angew. Chem., Int. Ed., 2021, 60, 22075–22080 CrossRef CAS PubMed.
- T. Myochin, K. Hanaoka, T. Komatsu, T. Terai and T. Nagano, Design Strategy for a Near-Infrared Fluorescence Probe for Matrix Metalloproteinase Utilizing Highly Cell Permeable Boron Dipyrro-methene, J. Am. Chem. Soc., 2012, 134, 13730–13737 CrossRef CAS PubMed.
- M. Grzybowski, M. Taki, K. Senda, Y. Sato, T. Ariyoshi, Y. Okada, R. Kawakami, T. Imamura and S. Yamaguchi, A Highly Photostable Near-Infrared Labeling Agent Based on a Phospha-rhodamine for Long-Term and Deep Imaging, Angew. Chem., Int. Ed., 2018, 57, 10137–10141 CrossRef CAS PubMed.
- V. Glembockyte, M. Frenette, C. Mottillo, A. M. Durantini, J. Gostick, V. Štrukil, T. Friščić and G. Cosa, Highly Photostable and Fluorescent Microporous Solids Prepared via Solid-State Entrap-ment of Boron Dipyrromethene Dyes in a Nascent Metal–Organic Framework, J. Am. Chem. Soc., 2018, 140, 16882–16887 CrossRef CAS PubMed.
- X. Wu and W. Zhu, Stability enhancement of fluorophores for lighting up practical application in bioimaging, Chem. Soc. Rev., 2015, 44, 4179–4184 RSC.
- G. T. Nash, T. Luo, G. Lan, K. Ni, M. Kaufmann and W. Lin, Nanoscale Metal–Organic Layer Isolates Phthalocyanines for Effi-cient Mitochondria-Targeted Photodynamic Therapy, J. Am. Chem. Soc., 2021, 143, 2194–2199 CrossRef CAS PubMed.
- T. Luo, G. T. Nash, Z. Xu, X. Jiang, J. Liu and W. Lin, Na-noscale Metal–Organic Framework Confines Zinc-Phthalocyanine Photosensitizers for Enhanced Photodynamic Therapy, J. Am. Chem. Soc., 2021, 143, 13519–13524 CrossRef CAS PubMed.
- C. Grunwald, K. Schulze, G. Giannone, L. Cognet, B. Lounis, D. Choquet and R. Tampé, Quantum-Yield-Optimized Fluorophores for Site-Specific Labeling and Super-Resolution Imaging, J. Am. Chem. Soc., 2011, 133, 8090–8093 CrossRef CAS PubMed.
- R. Kimura, H. Kuramochi, P. Liu, T. Yamakado, A. Osuka, T. Tahara and S. Saito, Flapping Peryleneimide as a Fluorogenic Dye with High Photostability and Strong Visible-Light Absorption, Angew. Chem., Int. Ed., 2020, 59, 16430–16435 CrossRef CAS PubMed.
- K. Henze and W. Martin, Evolutionary biology: essence of mito-chondria, Nature, 2003, 426, 127 CrossRef CAS PubMed.
- D. C. Wallace, Mitochondrial Diseases in Man and Mouse, Science, 1999, 283, 1482–1488 CrossRef CAS PubMed.
- H. Zhu, J. Fan, J. Du and X. Peng, Fluorescent Probes for Sens-ing and Imaging within Specific Cellular Organelles, Acc. Chem. Res., 2016, 49, 2115–2126 CrossRef CAS PubMed.
- W. Xu, Z. Zeng, J.-H. Jiang, Y.-T. Chang and L. Yuan, Dis-cerning the Chemistry in Individual Organelles with Small-Molecule Fluorescent Probes, Angew. Chem., Int. Ed., 2016, 55, 13658–13699 CrossRef CAS PubMed.
- S. Luo, X. Tan, S. Fang, Y. Wang, T. Liu, X. Wang, Y. Yuan, H. Sun, Q. Qi and C. Shi, Mitochondria-Targeted Small-Molecule Fluorophores for Dual Modal Cancer Phototherapy, Adv. Funct. Mater., 2016, 26, 2826–2835 CrossRef CAS.
- W.-C. Geng, Z. Ye, Z. Zheng, J. Gao, J. –J. Li, M. R. Shah, L. Xiao and D. –S. Guo, Supramolecular Bioimaging through Sig-nal Amplification by Combining Indicator Displacement Assay with Förster Resonance Energy Transfer, Angew. Chem., Int. Ed., 2021, 60, 19614–19619 CrossRef CAS PubMed.
- D. Pendin, R. Norante, A. D. Nadai, G. Gherardi, N. Vajente, E. Basso, N. Kaludercic, C. Mammucari, C. Paradisi, T. Pozzan and A. Mattarei, A Synthetic Fluorescent Mitochondria-Targeted Sensor for Ratiometric Imaging of Calcium in Live Cells, Angew. Chem., Int. Ed., 2019, 58, 9917–9922 CrossRef CAS PubMed.
- Y. Han, M. Li, F. Qiu, M. Zhang and Y.-H. Zhang, Cell-permeable organic fluorescent probes for live-cell long-term super-resolution imaging reveal lysosome-mitochondrion interactions, Nat. Commun., 2017, 8, 1307 CrossRef PubMed.
- G. Hong, S. Diao, A. L. Antaris and H. Dai, Carbon Nano-materials for Biological Imaging and Nanomedicinal Therapy, Chem. Rev., 2015, 115, 10816–10906 CrossRef CAS PubMed.
- R. K. Pathak, N. Kolishetti and S. Dhar, Targeted Nanoparticles in Mitochondrial Medicine, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2015, 7, 315–329 CAS.
- H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS PubMed.
- H.-T. Feng, Y.-X. Yuan, J.-B. Xiong, Y.-S. Zheng and B. Z. Tang, Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect, Chem. Soc. Rev., 2018, 47, 7452–7476 RSC.
- Y. Kawazoe, H. Shimogawa, A. Sato and M. Uesugi, A Mito-chondrial Surface-Specific Fluorescent Probe Activated by Biocon-version, Angew. Chem., 2011, 123, 5592–5595 CrossRef.
- C. W. L. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam and B. Z. Tang, A Photostable AIE Luminogen for Specific Mitochon-drial Imaging and Tracking, J. Am. Chem. Soc., 2013, 135, 62–65 CrossRef CAS PubMed.
- T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Porous organic cages, Nat. Mater., 2009, 8, 973–978 CrossRef CAS PubMed.
- J. R. Holst, A. Trewin and A. I. Cooper, Porous organic molecules, Nat. Chem., 2010, 2, 915 CrossRef CAS PubMed.
- T. Hasell and A. I. Cooper, Porous organic cages: soluble, modu-lar and molecular pores, Nat. Rev. Mater., 2016, 1, 16053 CrossRef CAS.
- M. Mastalerz, Porous Shape-Persistent Organic Cage Com-pounds of Different Size, Geometry, and Function, Acc. Chem. Res., 2018, 51, 2411–2422 CrossRef CAS PubMed.
- S. Bera, A. Basu, S. Tothadi, B. Garai, S. Banerjee, K. Vanka and R. Banerjee, Porosity Switching in Polymorphic Po-rous Organic Cages with Exceptional Chemical Stability, Angew. Chem., 2019, 131, 4287–4291 CrossRef.
- M. Mastalerz, M. W. Schneider, I. M. Oppel and O. Presly, A Salicylbisimine Cage Compound with High Surface Area and Selective CO2/CH4 Adsorption, Angew. Chem., Int. Ed., 2011, 50, 1046–1051 CrossRef CAS PubMed.
- B. Moosa, L. O. Alimi, A. Shkurenko, A. Fakim, P. M. Bhatt, G. Zhang, M. Eddaoudi and N. M. Khashab, A Polymorphic Azo-benzene Cage for Energy-Efficient and Highly Selective p-Xylene Separation, Angew. Chem., Int. Ed., 2020, 59, 21367–21371 CrossRef CAS PubMed.
- A. Dey, S. Chand, B. Maity, P. M. Bhatt, M. Ghosh, L. Cavallo, M. Eddaoudi and N. M. Khashab, Adsorptive Molecular Sieving of Styrene over Ethylbenzene by Trianglimine Crystals, J. Am. Chem. Soc., 2021, 143, 4090–4094 CrossRef CAS PubMed.
- X. Zheng, W. Zhu, C. Zhang, Y. Zhang, C. Zhong, H. Li, G. Xie, X. Wang and C. Yang, Self-Assembly of a Highly Emissive Pure Organic Imine-Based Stack for Electroluminescence and Cell Imag-ing, J. Am. Chem. Soc., 2019, 141, 4704–4710 CrossRef CAS PubMed.
- T. C. Lovell, S. G. Bolton, J. P. Kenison, J. Shangguan, C. E. Otteson, F. Civitci, X. Nan, M. D. Pluth and R. Jasti, Subcellular Targeted Nanohoop for One- and Two-Photon Live Cell Imaging, ACS Nano, 2021, 15(9), 15285–15293 CrossRef CAS PubMed.
- Y. Cheng, G. Li, Y. Liu, Y. Shi, G. Gao, D. Wu, J. Lan and J. You, Unparalleled Ease of Access to a Library of Biheteroaryl Fluorophores via Oxidative Cross-Coupling Reactions: Discovery of Photostable NIR Probe for Mitochondria, J. Am. Chem. Soc., 2016, 138(14), 4730–4738 CrossRef CAS PubMed.
- T.-B. Ren, Q.-L. Zhang, D. Su, X.-X. Zhang, L. Yuan and X. –B. Zhang, Detection of analytes in mitochondria without inter-ference from other sites based on an innovative ratiometric fluoro-phore, Chem. Sci., 2018, 9, 5461–5466 RSC.
- P. Kieryk, J. Janczak, J. Panek, M. Miklitz and J. Lisowski, Chiral 2 + 3 Keto-Enamine Pseudocyclophanes Derived from 1,3,5-Triformylphloroglucinol, Org. Lett., 2016, 18, 12–15 CrossRef CAS PubMed.
- M. Petryk, J. Szymkowiak, B. Gierczyk, G. Spólnik, Ł. Popenda, A. Janiak and M. Kwit, Chiral, triformylphenol-derived salen-type [4 + 6] organic cages, Org. Biomol. Chem., 2016, 14, 7495–7499 RSC.
- J. Wagner, L. Li, J. Simon, K. Landfester, V. Mailänder, K. Müllen, D. Y. W. Ng, Y. Wu, T. Weil and L. Krutzke, Amphiphilic Polyphenylene Dendron Conjugates for Surface Remodeling of Adenovirus, Angew. Chem., Int. Ed., 2020, 59, 5712 CrossRef CAS PubMed.
- R. Stangenberg, Y. Wu, J. Hedrich, D. Kurzbach, D. Wehner, G. Weidinger, S. L. Kuan, M. I. Jansen, F. Jelezko, H. J. Luhmann, D. Hinderberger, T. Weil and K. Müllen, A Polyphenylene Den-drimer Drug Transporter with Precisely Positioned Amphiphilic Surface Patches, Adv. Healthcare Mater., 2015, 4, 377–384 CrossRef CAS PubMed.
- N. J. Yang and M. J. Hinner, Getting across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins, Methods Mol. Biol, 2015, 1266, 29–53 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2144153. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc00836j |
| ‡ D. K and A. D contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |